Abstract
To explore the control of thyroid hormone metabolism in brain during maturation, we have measured iodothyronine deiodination in homogenates of rat cerebrum, cerebellum, and hypothalamus from 1 d postnatally through adulthood. Homogenates were incubated with 125I-l-thyroxine (T4) + [131I]3,5,3′-l-triiodothyronine (T3) + 100 mM dithiothreitol. Nonradioactive T4, T3, and 3,3′,5′-triiodothyronine (rT3) were included, as appropriate. The net production rate of [125I]T3 from T4 in 1-d cerebral homogenates was similar to the rate in adult cerebral homogenates (9.9±2.5[SEM]% vs. 8.9±1.2% T4 to T3 conversion in 2 h). Production of T3 was not detectable in 1-d cerebellar and hypothalamic homogenates. The net T3 production rate in adult cerebellar homogenates was twice as great as, and that in adult hypothalamic homogenates similar to, the rate in cerebral homogenates.
Tyrosyl ring deiodination rates of T4 and T3 were more than three times as great in cerebral homogenates from 1-d-old rats as in adult cerebral homogenates. In cerebellar homogenates from 1-d-old rats, tyrosyl ring deiodination rates were much greater than the rates in adult cerebellar homogenates, but less than those in 1-d cerebral homogenates. In 1-d hypothalamic homogenates, tyrosyl ring deiodination rates were the highest of all the tissues tested, whereas rates in adult hypothalamic homogenates were similar to those in adult cerebral homogenates.
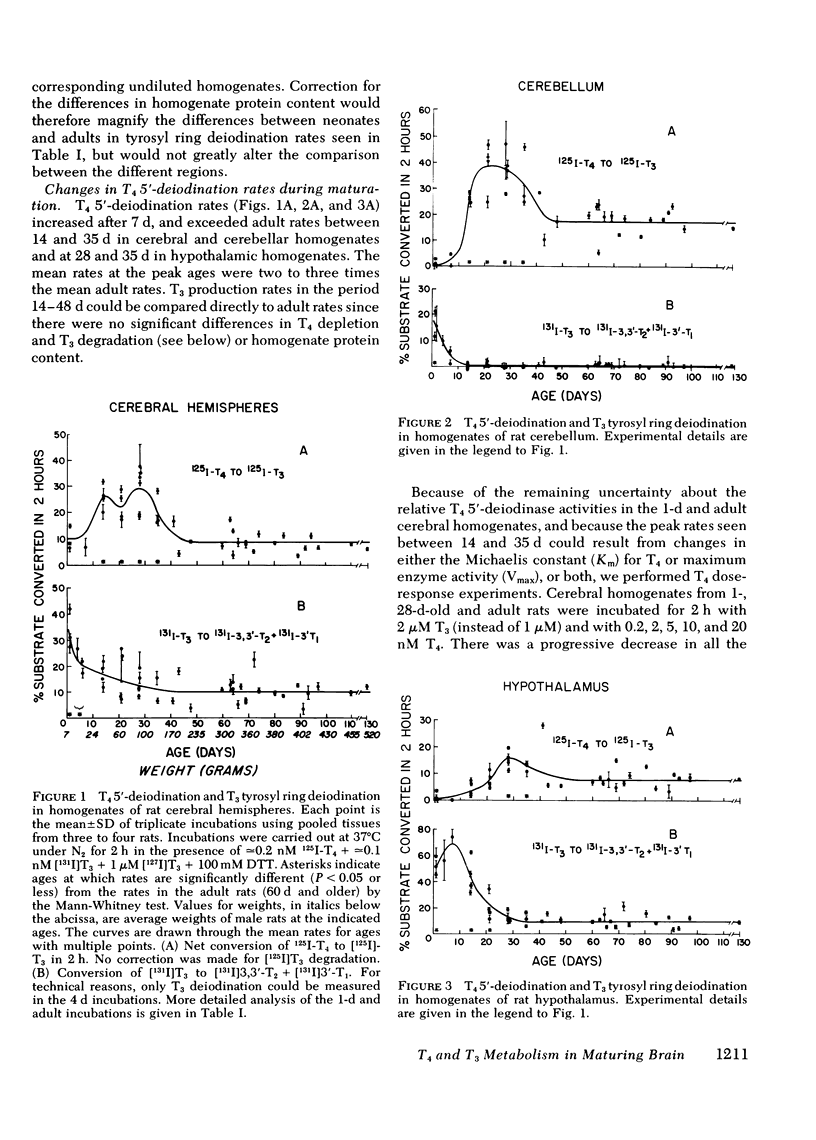
During maturation, T4 5′-deiodination rates increased after 7 d and exceeded adult rates between 14 and 35 d in cerebral and cerebellar homogenates, and at 28 and 35 d in hypothalamic homogenates. In cerebral homogenates, the peak in reaction rate at 28 d reflected an increase in the maximum enzyme activity (Vmax) of the reaction. T4 and T3 tyrosyl ring deiodination rates decreased progressively with age down to adult rates, which were attained at 14 d for cerebrum and cerebellum and at 28 d for hypothalamus.
These studies demonstrate quantitative differences in T4 5′-deiodinase activities in cerebrum, cerebellum, and hypothalamus at all ages, with the overall maturational pattern differing from the developmental patterns of both the pituitary and hepatic T4 5′-deiodinases. Iodothyronine tyrosyl ring deiodinase activities also vary quantitatively among these same brain regions and exhibit a pattern and a time-course of maturation different from that of the T4 5′-deiodinase. These enzymes could have important roles in the regulation of intracellular T3 concentrations and, hence, on the expression of thyroid hormone effects.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheron R. G., Kaplan M. M., Larsen P. R. Divergent changes of thyroxine-5'-monodeiodination in rat pituitary and liver during maturation. Endocrinology. 1980 May;106(5):1405–1409. doi: 10.1210/endo-106-5-1405. [DOI] [PubMed] [Google Scholar]
- Chiraseveenuprapund P., Buergi U., Goswami A., Rosenberg I. N. Conversion of L-thyroxine to triiodothyronine in rat kidney homogenate. Endocrinology. 1978 Feb;102(2):612–622. doi: 10.1210/endo-102-2-612. [DOI] [PubMed] [Google Scholar]
- Chopra I. J. A study of extrathyroidal conversion of thyroxine (T4) to 3,3',5-triiodothyronine (T3) in vitro. Endocrinology. 1977 Aug;101(2):453–463. doi: 10.1210/endo-101-2-453. [DOI] [PubMed] [Google Scholar]
- Coulombe P., Ruel J., Dussault J. H. Analysis of nuclear 5,5,3'-triiodothyronine-binding capacity and tissue response in the liver of the neonatal rat. Endocrinology. 1979 Oct;105(4):952–959. doi: 10.1210/endo-105-4-952. [DOI] [PubMed] [Google Scholar]
- Crantz F. R., Larsen P. R. Rapid thyroxine to 3,5,3'-triiodothyronine conversion and nuclear 3,5,3'-triiodothyronine binding in rat cerebral cortex and cerebellum. J Clin Invest. 1980 Apr;65(4):935–938. doi: 10.1172/JCI109749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dratman M. B., Crutchfield F. L. Synaptosomal [125I]triiodothyronine after intravenous [125I]thyroxine. Am J Physiol. 1978 Dec;235(6):E638–E647. doi: 10.1152/ajpendo.1978.235.6.E638. [DOI] [PubMed] [Google Scholar]
- El-Zaheri M. M., Braverman L. E., Vagenakis A. G. Enhanced conversion of thyroxine to triiodothyronine by the neonatal rat pituitary. Endocrinology. 1980 Jun;106(6):1735–1739. doi: 10.1210/endo-106-6-1735. [DOI] [PubMed] [Google Scholar]
- Fekkes D., van Overmeeren E., Hennemann G., Visser T. J. Solubilization and partial characterization of rat liver iodothyronine deiodinases. Biochim Biophys Acta. 1980;613(1):41–51. doi: 10.1016/0005-2744(80)90190-4. [DOI] [PubMed] [Google Scholar]
- Gavin L. A., Bui F., McMahon F., Cavalieri R. R. Sequential deiodination of thyroxine to 3,3'-diiodothyronine via 3,5,3'-triiodothyronine and 3,3',5'-triiodothyronine in rat liver homogenate. The effects of fasting versus glucose feeding. J Biol Chem. 1980 Jan 10;255(1):49–54. [PubMed] [Google Scholar]
- Glowinski J., Iversen L. L. Regional studies of catecholamines in the rat brain. I. The disposition of [3H]norepinephrine, [3H]dopamine and [3H]dopa in various regions of the brain. J Neurochem. 1966 Aug;13(8):655–669. doi: 10.1111/j.1471-4159.1966.tb09873.x. [DOI] [PubMed] [Google Scholar]
- HENINGER R. W., LARSON F. C., ALBRIGHT E. C. IODINE-CONTAINING COMPOUNDS OF EXTRATHYROIDAL TISSUES. J Clin Invest. 1963 Nov;42:1761–1768. doi: 10.1172/JCI104861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Hinerfeld L., Braverman L. E., Vagenakis A. G. The role of sulfhydryl groups on the impaired hepatic 3',3,5-triiodothyronine generation from thyroxine in the hypothyroid, starved, fetal, and neonatal rodent. J Clin Invest. 1979 Mar;63(3):516–524. doi: 10.1172/JCI109330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Prosky J., Braverman L. E., Vagenakis A. G. Decreased outer ring monodeiodination of thyroxine and reverse triiodothyronine in the fetal and neonatal rat. Endocrinology. 1978 Dec;103(6):2216–2222. doi: 10.1210/endo-103-6-2216. [DOI] [PubMed] [Google Scholar]
- Hüfner M., Grussendorf M., Ntokalou M. Properties of the thyroxine (T4) monodeiodinating system in rat liver homogenate. Clin Chim Acta. 1977 Jul 15;78(2):251–259. doi: 10.1016/0009-8981(77)90313-8. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M. Changes in the particulate subcellular component of hepatic thyroxine-5'-monodeiodinase in hyperthyroid and hypothyroid rats. Endocrinology. 1979 Aug;105(2):548–554. doi: 10.1210/endo-105-2-548. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Tatro J. B., Breitbart R., Larsen P. R. Comparison of thyroxine and 3,3',5'-triiodothyronine metabolism in rat kidney and liver homogenates. Metabolism. 1979 Nov;28(11):1139–1146. doi: 10.1016/0026-0495(79)90153-7. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M. Thyroxine 5'-monodeiodination in rat anterior pituitary homogenates. Endocrinology. 1980 Feb;106(2):567–576. doi: 10.1210/endo-106-2-567. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in rat liver homogenates. J Clin Invest. 1978 Feb;61(2):459–471. doi: 10.1172/JCI108957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M., Yaskoski K. A. Phenolic and tyrosyl ring deiodination of iodothyronines in rat brain homogenates. J Clin Invest. 1980 Sep;66(3):551–562. doi: 10.1172/JCI109887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leonard J. L., Rosenberg I. N. Iodothyronine 5'-deiodinase from rat kidney: substrate specificity and the 5'-deiodination of reverse triiodothyronine. Endocrinology. 1980 Nov;107(5):1376–1383. doi: 10.1210/endo-107-5-1376. [DOI] [PubMed] [Google Scholar]
- Neary J. T., Kieffer J. D., Federico P., Mover H., Maloof F., Soodak M. Thyrotropin releasing hormone: development of inactivation system during maturation of the rat. Science. 1976 Jul 30;193(4251):403–405. doi: 10.1126/science.819993. [DOI] [PubMed] [Google Scholar]
- Obregon M. J., Roelfsema F., Morreale de Escobar G., Escobar del Rey F., Querido A. Exchange of triiodothyronine derived from thyroxine with circulating triiodothyronine as studied in the rat. Clin Endocrinol (Oxf) 1979 Mar;10(3):305–315. doi: 10.1111/j.1365-2265.1979.tb02085.x. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Oppenheimer J. H. Ontogenesis of 3,5,3'-triiodothyronine receptors in neonatal rat brain: dissociation between receptor concentration and stimulation of oxygen consumption by 3,5,3'-triiodothyronine. Endocrinology. 1978 Sep;103(3):943–948. doi: 10.1210/endo-103-3-943. [DOI] [PubMed] [Google Scholar]
- Sorimachi K., Robbins J. Metabolism of thyroid hormones by cultured monkey hepatocarcinoma cells. Nonphenolic ring dieodination and sulfation. J Biol Chem. 1977 Jul 10;252(13):4458–4463. [PubMed] [Google Scholar]
- Strbak V., Greer M. A. Acute effects of hypothalamic ablation on plasma thyrotropin and prolactin concentrations in the suckling rat: evidence that early postnatal pituitary-thyroid regulation is independent of hypothalamic control. Endocrinology. 1979 Aug;105(2):488–489. doi: 10.1210/endo-105-2-488. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos T., Braverman L. E., Vagenakis A. G. Iodide-induced hypothyroidism: a potential hazard during perinatal life. Science. 1979 Aug 3;205(4405):502–503. doi: 10.1126/science.451615. [DOI] [PubMed] [Google Scholar]
- Theodoropoulos T., Braverman L. E., Vagenakis A. G. Thyrotropin-releasing hormone is not required for thyrotropin secretion in the perinatal rat. J Clin Invest. 1979 Apr;63(4):588–594. doi: 10.1172/JCI109340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonooka N., Greer M. A. Evidence that control of fetal thyrotropin secretion is independent of both the fetal and maternal hypothalamus. Endocrinology. 1978 Mar;102(3):852–858. doi: 10.1210/endo-102-3-852. [DOI] [PubMed] [Google Scholar]
- Valcana T., Timiras P. S. Nuclear triiodothyronine receptors in the developing rat brain. Mol Cell Endocrinol. 1978 Jun;11(1):31–41. doi: 10.1016/0303-7207(78)90030-8. [DOI] [PubMed] [Google Scholar]
- Vigouroux E., Clos J., Legrand J. Uptake and metabolism of exogenous and endogenous thyroxine in the brain of young rats. Horm Metab Res. 1979 Mar;11(3):228–232. doi: 10.1055/s-0028-1092714. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Fekkes D., Docter R., Hennemann G. Kinetics of enzymic reductive deiodination of iodothyronines. Effect of pH. Biochem J. 1979 Jun 1;179(3):489–495. doi: 10.1042/bj1790489. [DOI] [PMC free article] [PubMed] [Google Scholar]


