Abstract
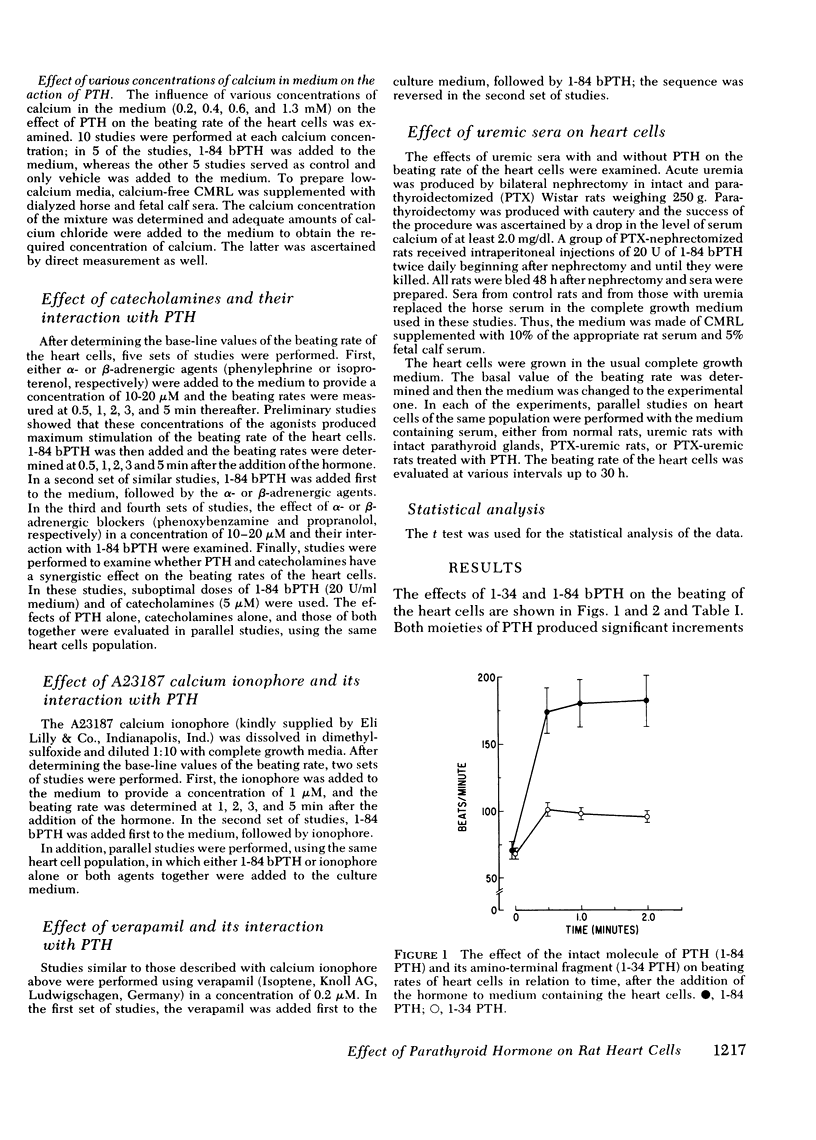
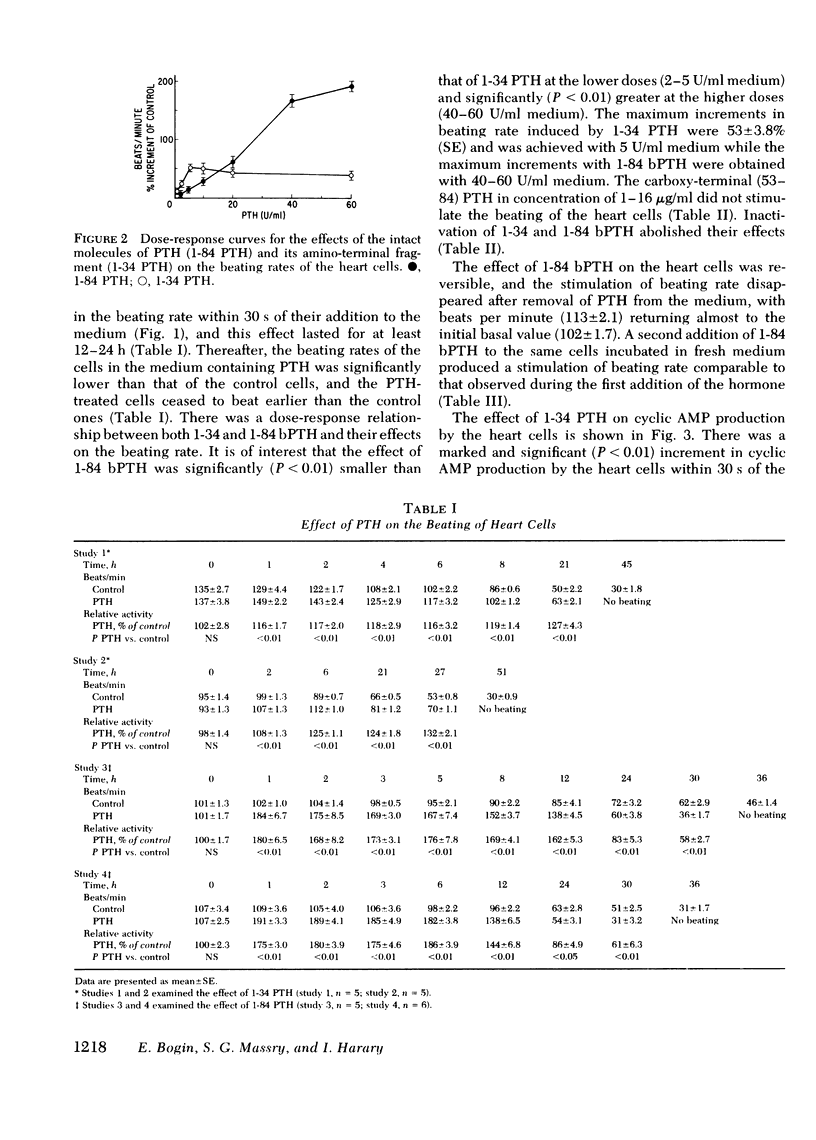
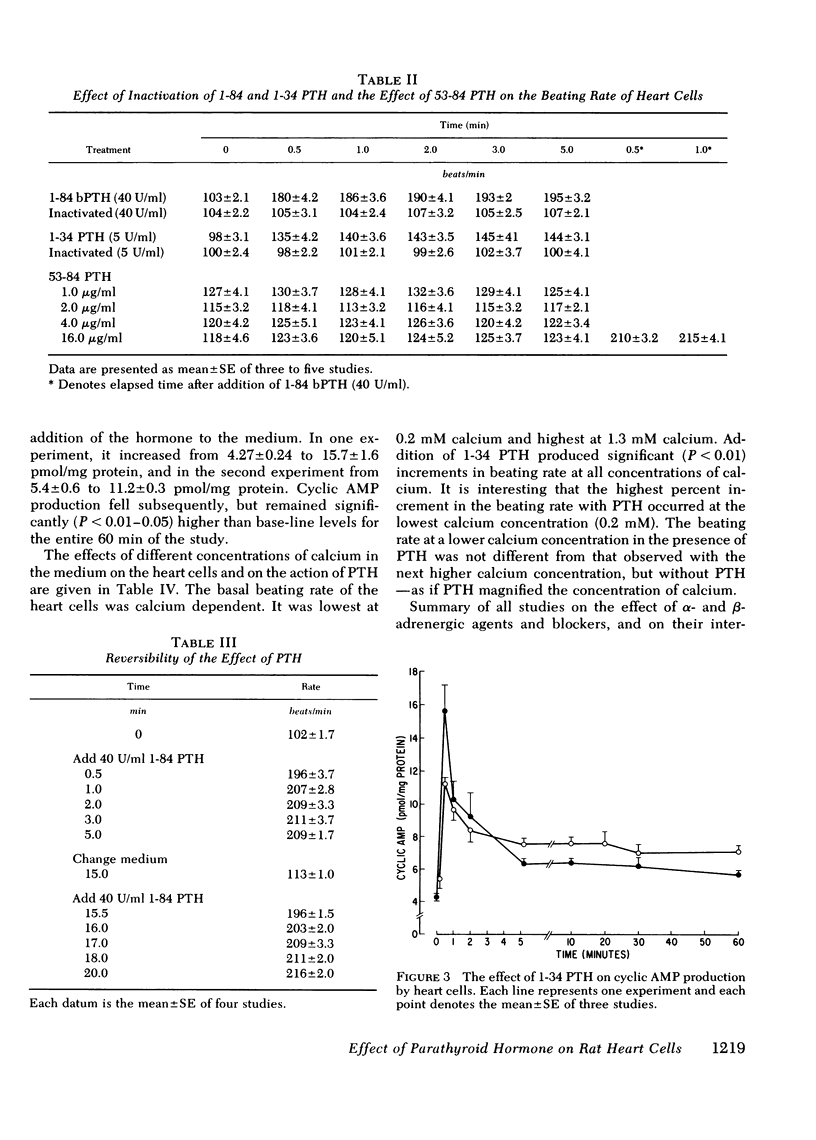
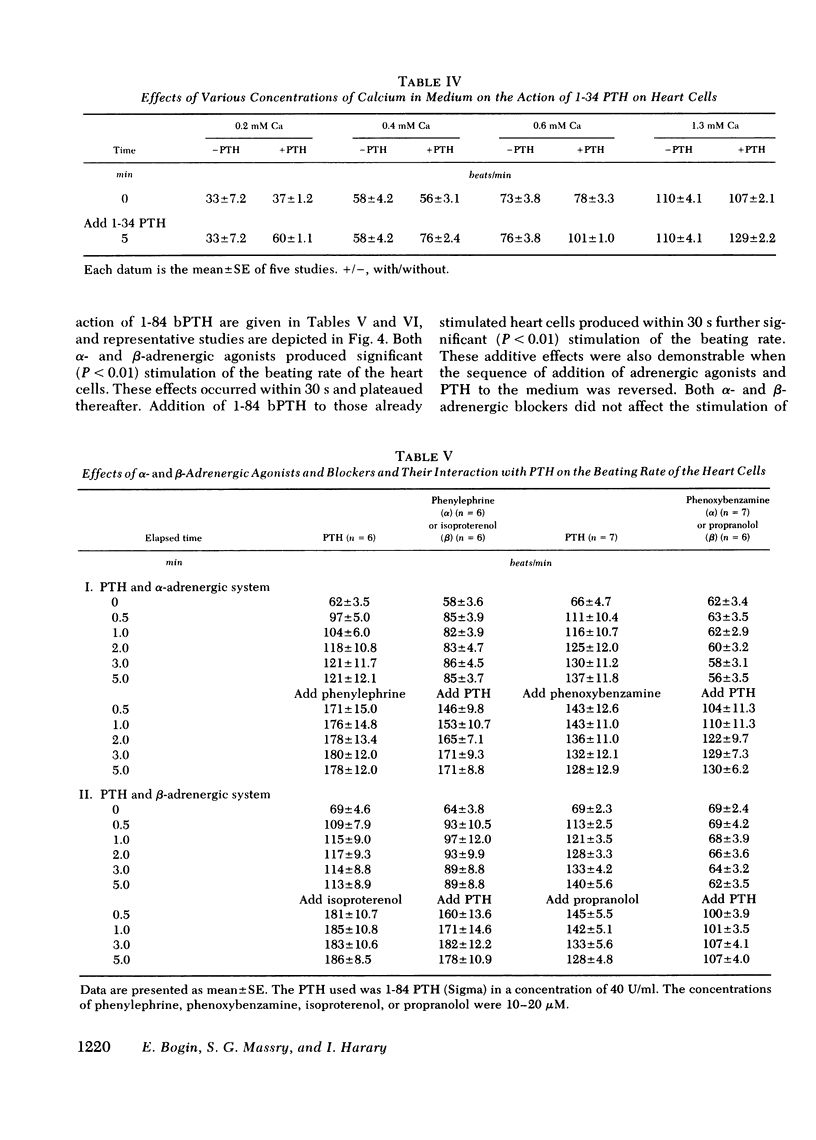
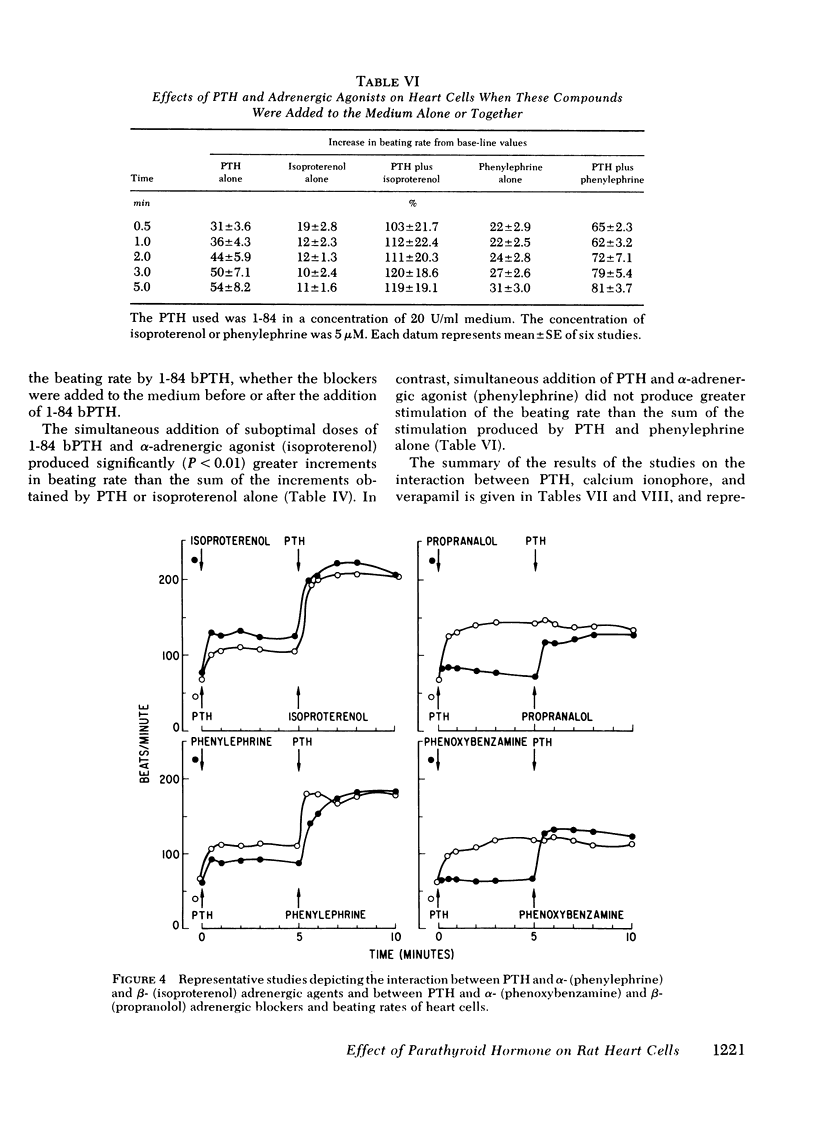
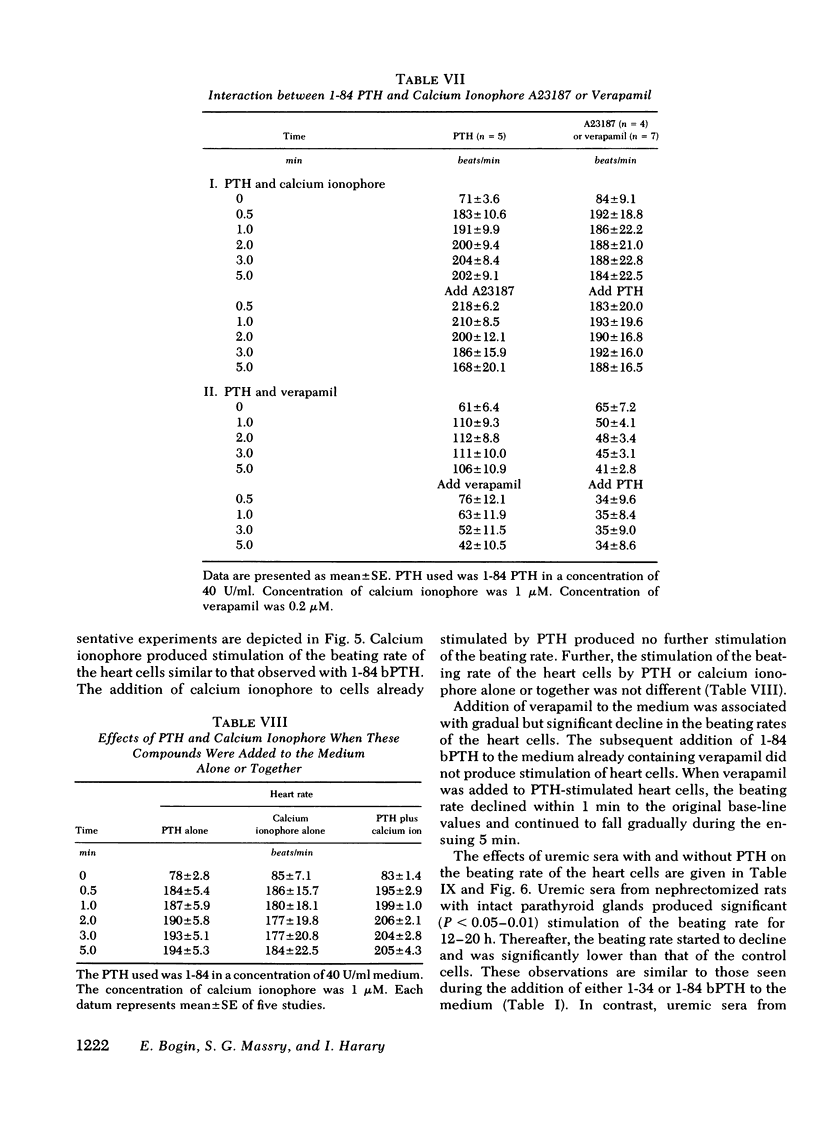
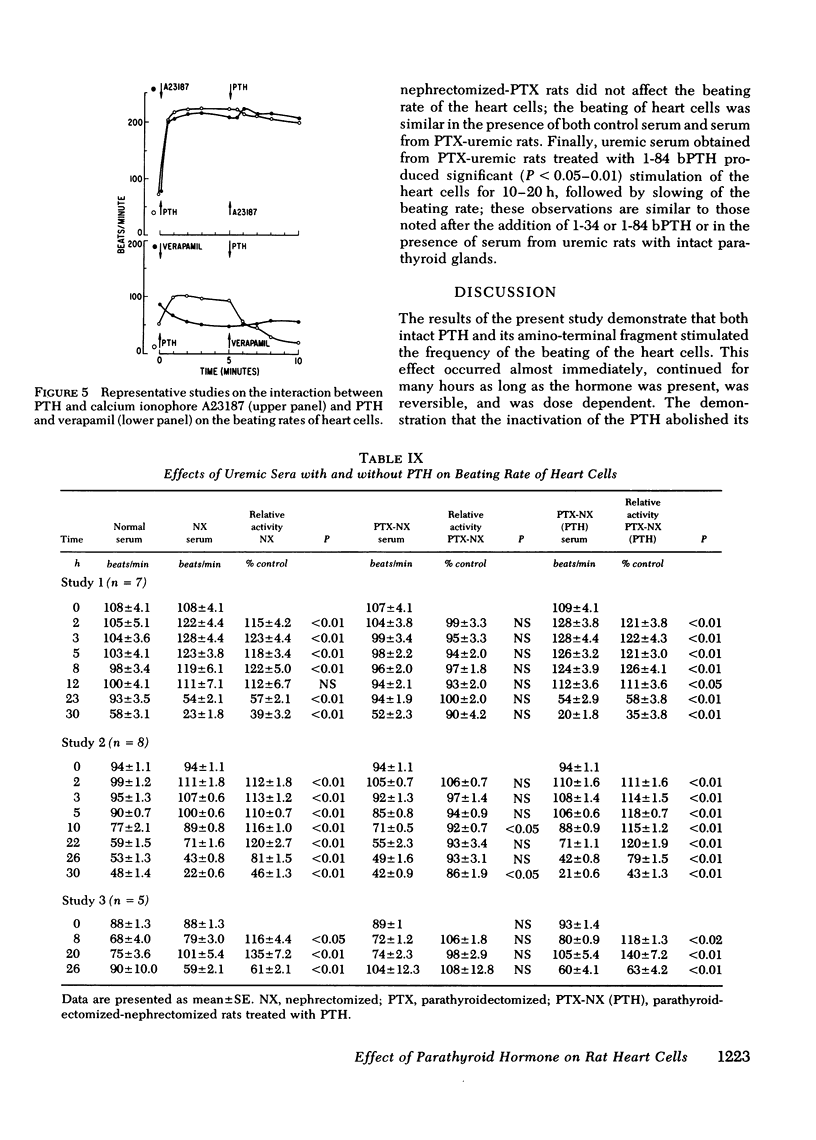
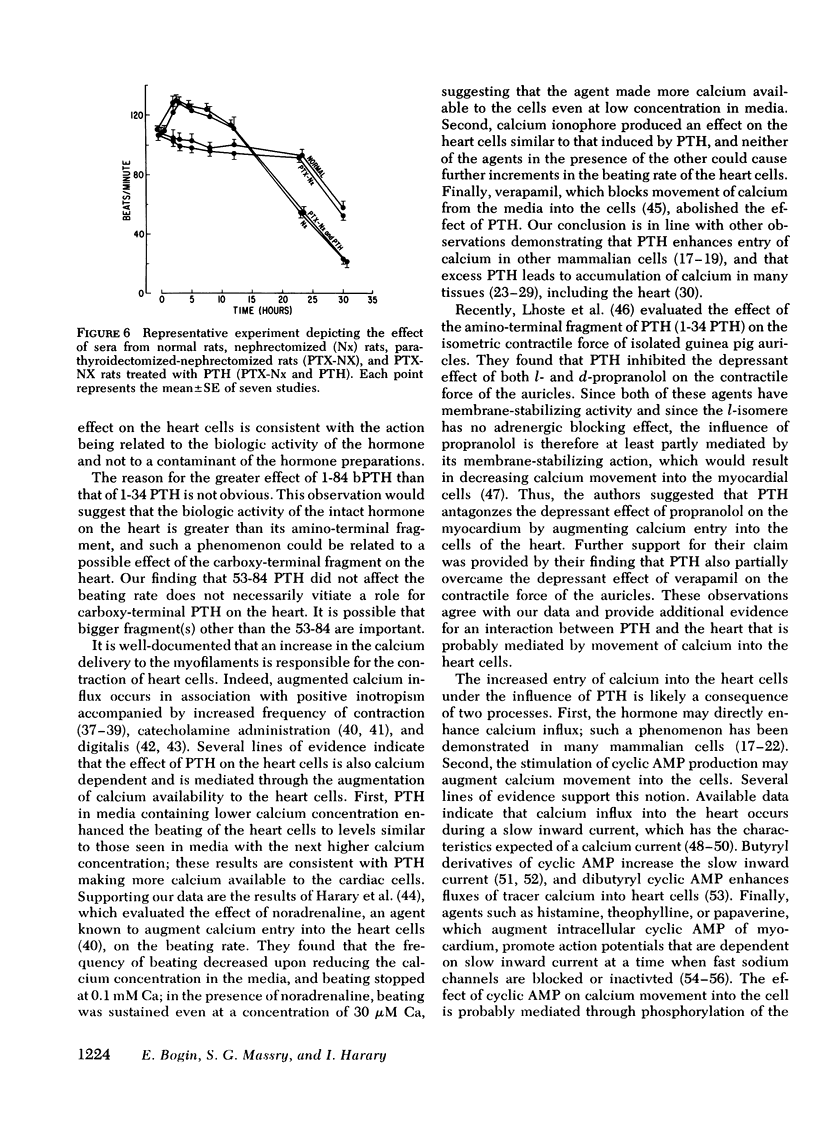
Myocardiopathy is common in uremia, but its cause in unknown. Excessive entry of calcium in heart cells by catecholamines has been shown to cause necrosis of myocardium. The high blood levels of parathyroid hormone (PTH) in uremia may also enhance entry of calcium into heart cells and exert deleterious effects on the heart. We examined the effect of PTH on rat heart cells grown in culture. Both amino-terminal (1-34) PTH and intact (1-84) PTH, but not the carboxy-terminal (53-84) PTH produced immediate and sustained significant rise in beats per minute and the cells died earlier than control. The effect was reversed if PTH was removed from medium, and was abolished by inactivation of the hormone. There was a dose-response relationship between both moieties of PTH and the rise in heart beats, but the effect of 1-84 PTH was significantly greater than that of 1-34 moiety. PTH stimulated cyclic AMP production within 1 min, and cyclic AMP remained significantly elevated thereafter. The effect of PTH required calcium, was mimicked by calcium ionophore, was prevented by verapamil and was not abolished by alpha- or beta-adrenergic blockers. PTH action was additive to phenylephrine and synergistic with isoproterenol. Sera from uremic parathyroidectomized rats did not effect heart beats, but sera from uremic rats with intact parathyroid glands or from uremic-parathyroidectomized rats treated with PTH had effects similar to PTH. Data indicate that (a) heart cell is a target organ for PTH and may have receptors for the hormone; (b) PTH increases beating rate of heart cells and causes early death of cells; (c) PTH effect appears to be due to calcium entry into heart cells; (d) the locus of action through which PTH induces calcium entry is different from that for catecholamines; and (e) uremic serum has no effect unless it contains PTH. Data suggest that myocardial damage may occur in uremia due to prolonged exposure to very high blood levels of PTH, and assign new dimensions to PTH toxicity in uremia.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arieff A. I., Massry S. G. Calcium metabolism of brain in acute renal failure. Effects of uremia, hemodialysis, and parathyroid hormone. J Clin Invest. 1974 Feb;53(2):387–392. doi: 10.1172/JCI107571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud C. D. Hyperparathyroidism and renal failure. Kidney Int. 1973 Aug;4(2):89–95. doi: 10.1038/ki.1973.87. [DOI] [PubMed] [Google Scholar]
- Atuk N. O., Bailey C. J., Turner S., Peach M. J., Westervelt F. B., Jr Red blood cell catechol-o-methyl transferase, plasma catecholamines and renin in renal failure. Trans Am Soc Artif Intern Organs. 1976;22:195–200. [PubMed] [Google Scholar]
- Bailey L. E., Sures H. A. The effect of ouabain on the washout and uptake of calcium in the isolated cat heart. J Pharmacol Exp Ther. 1971 Aug;178(2):259–270. [PubMed] [Google Scholar]
- Berkow J. W., Fine B. S., Zimmerman L. E. Unusual ocular calcification in hyperparathyroidism. Am J Ophthalmol. 1968 Nov;66(5):812–824. doi: 10.1016/0002-9394(68)92795-5. [DOI] [PubMed] [Google Scholar]
- Bernstein D. S., Pletka P., Hattner R. S., Hampers C. L., Merrill J. P. Effect of total parathyroidectomy and uremia on the chemical composition of bone, skin and aorta in the rat. Isr J Med Sci. 1971 Mar;7(3):513–514. [PubMed] [Google Scholar]
- Berson S. A., Yalow R. S. Parathyroid hormone in plasma in adenomatous hyperparathyroidism, uremia, and bronchogenic carcinoma. Science. 1966 Nov 18;154(3751):907–909. doi: 10.1126/science.154.3751.907. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Calcium metabolism at the cellular level. Fed Proc. 1973 Sep;32(9):1944–1950. [PubMed] [Google Scholar]
- Borle A. B. Calcium metabolism in HeLa cells and the effects of parathyroid hormone. J Cell Biol. 1968 Mar;36(3):567–582. doi: 10.1083/jcb.36.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borle A. B. Effects of purified parathyroid hormone on the calcium metabolism of monkey kidney cells. Endocrinology. 1968 Dec;83(6):1316–1322. doi: 10.1210/endo-83-6-1316. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Kinetic analyses of calcium movements in cell cultures. 3. Effects of calcium and parathyroid hormone in kidney cells. J Gen Physiol. 1970 Feb;55(2):163–186. doi: 10.1085/jgp.55.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht H. M., Ernst W., Koch K. M. Plasma noradrenaline levels in regular haemodialysis patients. Proc Eur Dial Transplant Assoc. 1976;12:281–290. [PubMed] [Google Scholar]
- Chausmer A. B., Sherman B. S., Wallach S. The effect of parathyroid hormone on hepatic cell transport of calcium. Endocrinology. 1972 Mar;90(3):663–672. doi: 10.1210/endo-90-3-663. [DOI] [PubMed] [Google Scholar]
- DEROW H. A. The heart in renal disease. Circulation. 1954 Jul;10(1):114–128. doi: 10.1161/01.cir.10.1.114. [DOI] [PubMed] [Google Scholar]
- Drüeke T., Fauchet M., Fleury J., Lesourd P., Toure Y., Le Pailleur C., de Vernejoul P., Crosnier J. Effect of parathyroidectomy on left-ventricular function in haemodialysis patients. Lancet. 1980 Jan 19;1(8160):112–114. doi: 10.1016/s0140-6736(80)90602-9. [DOI] [PubMed] [Google Scholar]
- Goldstein D. A., Chui L. A., Massry S. G. Effect of parathyroid hormone and uremia on peripheral nerve calcium and motor nerve conduction velocity. J Clin Invest. 1978 Jul;62(1):88–93. doi: 10.1172/JCI109118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisado R., Arieff A. I., Massry S. Muscle water and electrolytes in uremia and the effects of hemodialysis. J Lab Clin Med. 1977 Feb;89(2):322–331. [PubMed] [Google Scholar]
- HARARY I., FARLEY B. In vitro studies on single beating rat heart cells. I. Growth and organization. Exp Cell Res. 1963 Feb;29:451–465. doi: 10.1016/s0014-4827(63)80008-7. [DOI] [PubMed] [Google Scholar]
- Harary I., Renaud J. F., Sato E., Wallace G. A. Calcium ions regulate cyclic AMP and beating in cultured heart cells. Nature. 1976 May 6;261(5555):60–61. doi: 10.1038/261060a0. [DOI] [PubMed] [Google Scholar]
- Harary I., Wallace G. A. The effect of the reciprocal relationship of Ca2+ and cAMP on the control of beating in cultured rat heart cells. Recent Adv Stud Cardiac Struct Metab. 1976 May 26;12:635–643. [PubMed] [Google Scholar]
- Hui C. W., Drummond M., Drummond G. I. Calcium accumulation and cyclic AMP-stimulated phosphorylation in plasma membrane-enriched preparations of myocardium. Arch Biochem Biophys. 1976 Apr;173(2):415–427. doi: 10.1016/0003-9861(76)90279-4. [DOI] [PubMed] [Google Scholar]
- Katz A. I., Hampers C. L., Merrill J. P. Secondary hyperparathyroidism and renal osteodystrophy in chronic renal failure. Analysis of 195 patients, with observations on the effects of chronic dialysis, kidney transplantation and subtotal parathyroidectomy. Medicine (Baltimore) 1969 Sep;48(5):333–374. [PubMed] [Google Scholar]
- Kirchberger M. A., Tada M., Repke D. I., Katz A. M. Cyclic adenosine 3',5'-monophosphate-dependent protein kinase stimulation of calcium uptake by canine cardiac microsomes. J Mol Cell Cardiol. 1972 Dec;4(6):673–680. doi: 10.1016/0022-2828(72)90120-4. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Bauer B., Krause H., Fleckenstein A. Differentiation of the transmembrane Na and Ca channels in mammalian cardiac fibres by the use of specific inhibitors. Pflugers Arch. 1972;335(4):309–322. doi: 10.1007/BF00586221. [DOI] [PubMed] [Google Scholar]
- Krause E. G., Halle W., Kallabis E., Wollenberger A. Positive chronotropic response of cultured isolated rat heart cells to N6,2'-O-dibutryl-3',5'-adenosine monophosphate. J Mol Cell Cardiol. 1970 Mar;1(1):1–10. doi: 10.1016/0022-2828(70)90024-6. [DOI] [PubMed] [Google Scholar]
- LANGER G. A., BRADY A. J. Calcium flux in the mammalian ventricular myocardium. J Gen Physiol. 1963 Mar;46:703–719. doi: 10.1085/jgp.46.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer G. A., Serena S. D. Effects of strophanthidin upon contraction and ionic exchange in rabbit ventricular myocardium: relation to control of active state. J Mol Cell Cardiol. 1970 Mar;1(1):65–90. doi: 10.1016/0022-2828(70)90029-5. [DOI] [PubMed] [Google Scholar]
- Lindner A., Charra B., Sherrard D. J., Scribner B. H. Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med. 1974 Mar 28;290(13):697–701. doi: 10.1056/NEJM197403282901301. [DOI] [PubMed] [Google Scholar]
- Massry S. G., Coburn J. W., Peacock M., Kleeman C. R. Turnover of endogenous parathyroid hormone in uremic patients and those undergoing hemodialysis. Trans Am Soc Artif Intern Organs. 1972;18(0):416–422. doi: 10.1097/00002480-197201000-00103. [DOI] [PubMed] [Google Scholar]
- New W., Trautwein W. Inward membrane currents in mammalian myocardium. Pflugers Arch. 1972;334(1):1–23. doi: 10.1007/BF00585997. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Wilens S. L. Enlargement of the Parathyroid Glands in Renal Disease. Am J Pathol. 1935 Jan;11(1):73–91. [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H. Localization of beta adrenergic receptors, and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents and tension in mammalian cardiac muscle. J Physiol. 1974 Oct;242(2):429–451. doi: 10.1113/jphysiol.1974.sp010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth S. I., Marshall R. B. Pathology and ultrastructure of the human parathyroid glands in chronic renal failure. Arch Intern Med. 1969 Oct;124(4):397–407. [PubMed] [Google Scholar]
- Sands S. D., Winegrad S. Treppe and total calcium content of the frog ventricle. Am J Physiol. 1970 Mar;218(3):908–910. doi: 10.1152/ajplegacy.1970.218.3.908. [DOI] [PubMed] [Google Scholar]
- Seraydarian M. W., Harary I., Sato E. In vitro studies of beating-heart cells in culture. XI. The ATP level and contractions of the heart cells. Biochim Biophys Acta. 1968 Oct 1;162(3):414–423. doi: 10.1016/0005-2728(68)90127-8. [DOI] [PubMed] [Google Scholar]
- Solomon C., Roberts J. E., Lisa J. R. The Heart in Uremia. Am J Pathol. 1942 Jul;18(4):729–732. [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., Schneider J. A. A metabolic control mechanism for calcium ion influx that may protect the ventricular myocardial cell. Am J Cardiol. 1976 Jun;37(7):1079–1085. doi: 10.1016/0002-9149(76)90428-8. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Li H. C. Phosphoprotein phosphatase-catalyzed dephosphorylation of the 22,000 dalton phosphoprotein of cardiac sarcoplasmic reticulum. J Cyclic Nucleotide Res. 1975;1(5):329–338. [PubMed] [Google Scholar]
- Teo T. S., Wang J. H. Mechanism of activation of a cyclic adenosine 3':5'-monophosphate phosphodiesterase from bovine heart by calcium ions. Identification of the protein activator as a Ca2+ binding protein. J Biol Chem. 1973 Sep 10;248(17):5950–5955. [PubMed] [Google Scholar]
- Tsien R. W., Giles W., Greengard P. Cyclic AMP mediates the effects of adrenaline on cardiac purkinje fibres. Nat New Biol. 1972 Dec 6;240(101):181–183. doi: 10.1038/newbio240181a0. [DOI] [PubMed] [Google Scholar]
- Wallach S., Bellavia J. V., Schorr J., Schaffer A. Tissue distribution of electrolytes, 47ca and 28mg in experimental hyper- and hypoparathyroidism. Endocrinology. 1966 Jan;78(1):16–28. doi: 10.1210/endo-78-1-16. [DOI] [PubMed] [Google Scholar]


