Abstract
The gene expression of mTOR, autophagy-related ULK1, caspase 3, CDK-inhibitor p21, and TNF α was measured in the peripheral blood of osteoarthritic (OA) patients at different stages of the disease aiming to establish a gene expression profile that might indicate the activity of the disease and joint destruction. Whole blood of 65 OA outpatients, 27 end-stage OA patients, 27 healthy volunteers, and knee articular cartilages of 28 end-stage OA patients and 26 healthy subjects were examined. OA outpatients were subjected to clinical testing, ultrasonography, and radiographic and WOMAC scoring. Protein levels of p70-S6K, p21, and caspase 3 were quantified by ELISA. Gene expression was measured using real-time RT-PCR. Upregulation of mTOR gene expression was observed in PBMCs of 42 OA outpatients (“High mTOR expression subset”) and in PBMCs and articular cartilages of all end-stage OA patients. A positive correlation between mTOR gene expression in PBMCs and cartilage was observed in the end-stage OA patients. 23 OA outpatients in the “Low mTOR expression subset” exhibited significantly lower mTOR gene expression in PBMCs compared to healthy controls. These “Low mTOR” subset subjects experienced significantly more pain upon walking, and standing and increased total joint stiffness versus “High mTOR” subset, while the latter more often exhibited synovitis. The protein concentrations of p70-S6K, p21, and caspase 3 in PBMCs were significantly lower in the “Low” subset versus “High” subset and end-stage subjects. Increases in the expression of mTOR in PBMCs of OA patients are related to disease activity, being associated with synovitis more than with pain.
1. Introduction
Osteoarthritis (OA) is a systemic condition that can affect single or multiple joints and involves degenerative changes in the articular cartilage, remodeling of the subchondral bone, and limited synovial inflammation [1]. At present, the disease course is generally monitored by clinical and radiographic changes, which show poor sensitivity. Therefore, there is a need to identify new approaches in indicating disease activity.
Detection of gene expression changes measured in the whole blood is an emerging approach in OA research. Blood-based gene expression patterns recently obtained in transcriptome and microarray analyses appeared capable of distinguishing OA patients from control subjects [2, 3], already showing promising results. Moreover, the level of IL-1β gene expression in peripheral monocytes has been proposed for OA patient stratification, as upregulation of IL-1β was accompanied by increased pain and predicted higher risk of radiographic progression of the disease [4].
Recently evidence has been presented that disease manifestation is preceded by phenotypic modification (hypertrophy) of articular chondrocytes similar to that observed in fetal chondrocytes during their maturation in the growth plate [1, 5]. This was associated with the upregulation of genes involved in cartilage destruction and abnormal expression of regulatory proteins, such as growth and transcription factors, as well as apoptosis markers [6–8]. Other studies have reported that the majority of the identified genes involved in OA encode signal-transduction proteins [9].
Alteration in non-tissue-specific regulatory protein expression associated with disease manifestation may suggest differential gene expression in tissues other than cartilage, for example, blood. This is supported by the observation of modification in the expression of genes associated with fetal chondrocyte differentiation such as bone morphogenetic proteins 2, 4, and 6, as well as transcription factor Runx2, in the peripheral blood of OA patients [10].
Here we hypothesized that expression of genes associated with global cell survival and functioning, such as those involved in cell growth and proliferation, apoptosis, autophagy, and inflammation, measured from the whole blood of OA patients might point to the disease activity.
As extracellular matrix degradation in early OA is associated with chondrocyte hypertrophy [6], cessation of proliferative activity could involve changes in the expression of cyclin-dependent kinase (CDK) inhibitors, such as p21, whose overexpression is associated with the induction of genes expressed in different age-related disorders [11]. However, data on the activity of CDK inhibitors in chondrocytes of OA patients are inconclusive, as both activation [12, 13] and downregulation of p21 [14] have been reported in OA chondrocytes.
Alternatively, mTOR (mammalian target of rapamycin) is considered a key regulator of cell growth and proliferation [15], and its expression has been reported both in fetal chondrocytes in animal studies [16–18] and in human articular chondrocytes [19]. Moreover, treatment of mice with the mTOR inhibitor rapamycin or its analogs, has been recently shown to reduce the severity of experimental osteoarthritis [20, 21] and inflammatory arthritis [19, 22].
The effect of mTOR inhibition on cessation of growth is accompanied by the activation of autophagy [23], which has been observed both in OA articular chondrocytes [16, 20] and in peripheral blood cells [24]. This process occurs in lysosomes with membranes that contain proteins of the ULK (hATG) family and favors cellular survival [25].
Apoptosis represents a major cell death mechanism in eukaryotic cells. At present apoptotic activity in OA patients has been assessed only in articular chondrocytes [26, 27]. Some of these studies reported that cartilage destruction is accompanied by a significant increase in apoptotic activity [28, 29] while others observed only a few apoptotic cells in OA articular cartilage [30].
Although OA is not considered a classical inflammatory arthropathy because of the absence of neutrophils in the synovial fluid and the lack of marked systemic manifestations of inflammation, proinflammatory cytokines are involved in OA articular cartilage resorption [31, 32]. This is associated with increased expression of IL-1 and TNFα [32–34].
In the present study, we analyzed the expression of genes responsible for cell proliferation and growth (mTOR), regulation of cell cycle progression (p21), apoptosis (caspase 3), and autophagy (ULK1), as well as the proinflammatory cytokine TNFα,in the whole blood and articular cartilage of knee OA patients at different stages of their disease. Our results suggest that differences in the expression of these genes might serve as an indicator of disease activity, symptoms, and knee joint destruction and provide new insights into OA pathobiology.
2. Materials and Methods
2.1. Ethics
The study protocol was approved by the Local Committee on the Ethics of Human Research and informed consent was obtained from all subjects.
2.2. Patients
The Inclusion Criteria of the OA Patients and Control Subjects. The control group consisted of 27 postmenopausal healthy females (average age 58.6 ± 8.3 years, range 42–74 years) free of any serious diseases recruited from the Moscow area. The control subjects were of comparable age to the OA outpatient group. The exclusion criteria for control subjects included any knee pain and crepitus, as well as low bone mineral density (BMD) (T score < –2.5 SD). The recruited controls were subjected to blood testing, including analyses of biochemical and hematological parameters, and densitometry of the lumbar spine and femur.
The OA outpatient group consisted of 47 unrelated postmenopausal Russian women with primary knee OA who had visited the outpatient clinic of the Institute of Rheumatology, at the Russian Academy of Medical Sciences between December 2007 and June 2009 (set 1). The average age of set 1 OA outpatients was 60.3 ± 7.1 years (range 47–74 years). These patients had radiological Kellgren & Lawrence (K&L) OA grades of II–IV. Set 2 consisted of 18 unrelated postmenopausal Russian women with primary knee OA who had visited the outpatient clinic of the Institute of Rheumatology, at the Russian Academy of Medical Sciences between February 2012 and July 2012. The average age of set 2 OA outpatients was 61.6 ± 8.3 years (range 47–74 years), with K&L OA grades between II-III.
All of the OA outpatients had moderate rates of knee pain according to VAS (40–70 mm) and normal BMD. For pain medication the following NSAID were used: meloxicam (15 mg/day), nimesulide (200 mg/day), or aceclofenac (200 mg/day) (Table 1). Patients were also treated with the chondroprotective agent chondroitin sulfate (1 g/day) with or without glucosamine sulfate (1 g/day).
Table 1.
Demographic and clinical characteristics of the outpatients with knee OA.
| Set 1 patients | “Low mTOR” subset (n = 15) | “High mTOR” subset (n = 32) | P (Student's unpaired t-test) |
|---|---|---|---|
| Age, years | 60.7 ± 6.7 | 60.3 ± 6.9 | 0.86 |
| Disease duration, years | 12.0 ± 8.8 | 11.2 ± 10.3 | 0.80 |
| BMI, kg/m2 | 35.6 ± 5.4 | 32.3 ± 5.4 | 0.06 |
| Menopause, years | 11.4 ± 6.4 | 12.3 ± 7.2 | 0.69 |
| Average K&L radiological stage | |||
| II | 46.6% (7/15) | 68% (22/32) | 0.15 |
| III | 40% (6/15) | 18.7% (6/15) | 0.16 |
| IV | 13.4% (2/15) | 12.5% (4/32) | 0.93 |
| ESR, mm/h | 13.1 ± 8.8 | 11.7 ± 6.6 | 0.55 |
| Total WOMAC, mm | 1202 ± 285 | 1102 ± 309 | 0.29 |
| Pain on descending | 64.6 ± 15.7 | 58.8 ± 17.7 | 0.28 |
| Pain on ascending | 62.1 ± 16.1 | 57.0 ± 17.9 | 0.35 |
| Pain on move onset | 51.8 ± 12.3 | 48.9 ± 22.6 | 0.64 |
| Pain at rest | 35.0 ± 20.3 | 31.8 ± 15.8 | 0.55 |
| Pain on walking | 59.2 ± 12.5 | 49.6 ± 10.5 | 0.009 |
| Pain on standing | 62.5 ± 13.0 | 50.8 ± 16.4 | 0.02 |
| Pain at night | 28.8 ± 19.4 | 35.6 ± 23.4 | 0.34 |
| Total pain | 364.2 ± 82.0 | 332.4 ± 93.7 | 0.26 |
| Total stiffness | 101.3 ± 25.7 | 75.0 ± 27.0 | 0.003 |
| Total physical function | 737.4 ± 217.6 | 695.0 ± 229.8 | 0.55 |
| Heberden's nodes, % | 80.0 (12/15) | 59.0 (19/32) | 0.08 |
| Bouchard's nodes, % | 27.0 (4/15) | 18.7 (6/32) | 0.26 |
| Synovitis, % | 20 (3/15) | 62.5 (20/32) | 0.004 |
| BMD, g/cm2: | |||
| Lumbar spine (L1–L4) | 0.916 ± 0.1 | 0.960 ± 0.1 | 0.35 |
| Femoral neck | 0.843 ± 0.1 | 0.795 ± 0.1 | 0.16 |
| Total femur | 0.981 ± 0.1 | 0.884 ± 0.1 | 0.01 |
| Anti-inflammatory treatment (%): | |||
| Meloxicam | 53 (8/15) | 72 (23/32) | 0.20 |
| Nimesulide | 27 (4/15) | 22 (7/32) | 0.70 |
| Aceclofenac | 0 | 6 (2/32) | — |
| None | 20 (3/15) | 0 | — |
| Chondroprotective agents (%): | |||
| Chondroitin sulfate | 60 (9/15) | 22 (7/32) | 0.01 |
| Chondroitin sulfate + glucosamine sulphate | 40 (6/15) | 78 (25/32) | 0.01 |
Values given are mean ± SD. BMI: body mass index; K&L: Kellgren-Lawrence; ESR: erythrocyte sedimentation rate; WOMAC: Western Ontario and McMaster Universities osteoarthritis index; BMD: bone mineral density.
We also examined the peripheral blood of 14 end-stage postmenopausal female OA patients undergoing knee joint replacement surgery aged 49 to 71 years (average age 56.6 ± 8.9 years) (set 1). In addition, we examined the peripheral blood of another 13 end-stage postmenopausal female OA patients undergoing knee joint replacement surgery aged 46 to 72 years (average age 59.3 ± 8.9 years) (set 2). Knee articular cartilage was also obtained from the same set 2 end-stage patients for the studies of mTOR gene expression correlation in the blood versus cartilage. All end-stage patients had OA radiological K&L grades of III or IV, experienced severe pain, and had walking problems (lameness).
All of the examined patients fulfilled the criteria of the American College of Rheumatology regarding OA [35].
The Exclusion Criteria for OA Patients and Healthy Subjects. The exclusion criteria were rheumatoid arthritis; secondary arthritis associated with reactive arthritis, systemic inflammatory joint diseases, gout, pseudogout, Padgett's disease, intraarticular fractures, ochronosis, acromegaly, hemochromatosis, Wilson disease, primary synovial chondromatosis, chondrocalcinosis, aseptic necrosis of femoral or tibia condyles, orany type of knee surgery; and any abnormalities of bone metabolism including diabetes mellitus; renal diseases; thyroid, parathyroid, or other endocrinological diseases; uncontrolled arterial hypertension; instable angina; vascular insufficiency; gastric or duodenal ulcer; bleeding; or thrombophlebitis. Women who had taken drugs such as estrogen, progesterone, glucocorticoids, bisphosphonates, and alfacalcidol were not included in the study.
2.3. Cartilage
Human femoral condylar cartilage was obtained at total knee arthroplasty from 15 patients (4 men, mean age 64.5 ± 14.9 years; range 44 to 79 years and 11 women, mean age 57.8 ± 7.8 years; range 40 to 71 years) (set 1) and another 13 end-stage postmenopausal female patients aged 46 to 72 years (average age 59.3 ± 8.9 years) (set 2) with OA diagnosed according to the criteria of the American College of Rheumatology [35].
Human articular cartilage from 14 healthy individuals (9 men, mean age 45.0 ± 5.1 years; range 39 to 51 years and 5 women, mean age 31.5 ± 3.5 years; range 29 to 34 years) (set 1 controls) and another 12 healthy individuals (10 men, mean age 36.0 ± 7.7 years; range 25 to 45 years and 2 women, mean age 35.0 ± 2.1 years; range 34 to 37 years) (set 2 controls) was obtained in less than 12 hours post mortem at autopsy from the femoral condylar surfaces of the knee that articulate with the patella.
No patient had received chemotherapy or had diabetes or lower limb vascular insufficiency. In articular cartilage studies, we used specimens of both genders due to limitations in specimen availability.
In each knee, full-depth cartilage (to subchondral bone) was removed. The cartilage was analyzed for histology and gene expression. Cartilage samples from healthy patients contained normal cartilage, as revealed by a Mankin grade of 1-2. The degree of degeneration in the OA cartilage samples was 7.8 ± 2.5; range 5 to 12 [36, 37].
2.4. Clinical Testing
OA grade and osteophyte presence were determined by the analysis of the weight-bearing anteroposterior radiographs of the knees. These were scored on a five-point scale (0–4) according to Kellgren & Lawrence [38].
The WOMAC (Western Ontario and McMaster Universities osteoarthritis index) visual analogue scale was used to assess pain, stiffness, and physical function [39].
Synovitis registered in the medical history was diagnosed by ultrasonography and by joint effusion.
2.5. Ultrasound Examination for Synovitis
Knees were examined using a Voluson station (GE Medical Systems, Kretztechnik GmbH & Co. OHG, Zipf, Austria) with a multifrequency linear 4–13 MHz probe. The presence of synovitis was assessed according to the EULAR guidelines [40].
2.6. BMD Measurement
BMD at the lumbar spine (L1–L4), femoral neck, and total femur was measured by dual-energy X-ray absorptiometry using a QDR-4500w instrument (Hologic, USA). According to the criteria recommended by the World Health Organization [41], a T score of < –2.5 SD, all subjects examined in this study were diagnosed as free of osteoporosis.
2.7. Peripheral Blood Fractionation
Peripheral blood (10 mL) was collected in Vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA) (BDH, England). The blood samples were taken in a standardized manner in the morning (between 07:00 a.m. and 09:00 a.m.). Whole blood fractionation was performed using a Ficoll density gradient. Upon centrifugation, blood samples were separated into plasma enriched with thrombocytes, peripheral blood mononuclear cells (PBMCs) located in the interphase, and a pellet containing granulocytes on top of red blood cells [42]. Every cell fraction was collected and washed twice in phosphate-buffered saline (PBS). Erythrocytes were lysed using hypotonic buffer (1.6 mM EDTA, 10 mM KHCO3, and 153 mM NH4Cl, pH 7.4), which was added at a 3 : 1 volume ratio. The obtained cell fractions were frozen and kept at −70°C prior to protein extraction or were used immediately for RNA isolation.
2.8. Quantification of p70-S6K, p21, and Caspase 3 Protein Levels
Concentrations of total p70-S6K (KHO0571), phospho-p70-S6K (KHO0581), p21WAF1/Cip1 (KHO5421), and active caspase 3 (KHO1091) were determined in isolated PBMCs using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Invitrogen, Camarillo, CA, USA) according to the manufacturer's instructions. For mTOR protein expression, we evaluated levels of p70-S6K, an mTOR direct target for phosphorylation, which is usually used as an mTOR readout [43–45], as mTOR ELISA kits are not available in Russia.
Results were expressed per µg of protein measured in PBMC lysates. PBMC lysates were obtained using Cell Extraction Buffer containing 10 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM NaF, 20 mM Na4P2O7, 20 mM Na3VO4, 1% Triton X-100, 10% glycerol, 0.1% SDS, and 0.5% deoxycholate (Invitrogen, Camarillo, CA, USA) supplemented with Protease Inhibitor Cocktail (Sigma-Aldrich, Inc., St. Louis, USA) and 1 mM PMSF (Sigma-Aldrich, Inc., St. Louis, USA) according to the manufacturer's instructions. Total protein concentration in cell lysates was quantified by the Bradford method [46].
2.9. Total RNA Isolation and Reverse Transcriptase (RT) Reaction
For detection of gene expression total RNA was isolated from 100 μL of whole blood immediately, after withdrawal, from serum, from erythrocyte lysate, or from 107 freshly isolated cells using Ribo-zol-A kit (InterLabService, Moscow, Russia) in accordance with the manufacturer's recommendations. Total RNA had an A 260/290 > 1.9. Total RNA was also isolated from fresh knee articular cartilage using TRIzol reagent according to the manufacturer's recommendations (Invitrogen, Carlsbad, CA, USA). The RT reaction was performed using a Reverta kit containing M-MLV reverse transcriptase, random hexanucleotide primers, and total RNA according to the manufacturer's recommendations (InterLabService, Moscow, Russia).
2.10. Real-Time Quantitative PCR
Premade primers and probes for the TaqMan assay (Applied Biosystems, Foster City, CA, USA) of human genes used in this study were: mTOR (Hs00234522_m1), Unc-51-like kinase 1 (ULK1) (Hs00177504_m1), p21WAF1/Cip1 (p21) (Hs00355782_m1), caspase 3 (Hs00263337_m1), TNFα (Hs00174128_m1), COL10A1 (Hs00166657_m1), MMP-13 (Hs00233992_m1), and MMP-9 (Hs00234579_m1). β-Actin was used as an endogenous control.
Quantification of gene expression was conducted using a 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). A volume of 1 μL of RT product was subjected to real-time PCR in a 15 μL total reaction mixture containing 7.5 μL of TaqMan Universal PCR Master Mix (Applied Biosystems), 900 nM sense and antisense primers, 50 nM probe, and template cDNA. After a single step of 50°C for 2 min and initial activation at 95°C for 10 min, reaction mixtures were subjected to 40 amplification cycles (15 s at 95°C for denaturation and 1 min of annealing and extension at 60°C).
Relative mRNA expression was determined using the delta-delta C T method, as detailed by the manufacturer's guidelines (Applied Biosystems) [47]. The delta C T value was calculated by subtracting the C T value for the housekeeping gene β-actin from the C T value for each sample. A delta-delta C T value was then calculated by subtracting the delta C T value of the control (each healthy patient) from the delta C T value of each OA patient. Each PCR was performed in duplicate. Three “no template” controls were consistently negative for each reaction.
For subset division, we initially measured mTOR gene expression in relation to β-actin expression in healthy subjects. Then, we calculated mTOR gene expression in each of 27 healthy subjects using each of the same 27 healthy subjects as a calibrator. The average of all the obtained fold change values related to each healthy subject was considered the relative individual gene expression for each healthy control subject. We observed normal distributions for these relative gene expression values (1.04 ± 0.21 in the case of mTOR) (Supplementary Table 3 in Supplementary Material available online at http://dx.doi.org/10.1155/2013/461486). In further gene expressions testing, eight cDNAs from the healthy control subjects were placed on each PCR plate to reproduce initial relative gene expression results in the corresponding control subjects. The relative gene expression of each OA patient was compared to the pool of eight healthy controls using 7300 Sequence Detection Software Version 1.3.1 (Applied Biosystems) and the remainder 19 healthy controls using C T values obtained in the preliminary studies. The average of all the obtained fold change values related to each OA subject was considered the relative individual gene expression for each OA subject. The subjects whose mTOR relative gene expression was significantly lower than that of controls (P < 0.05) were registered to the “Low” subset; otherwise, they were registered to the “High” subset.
2.11. Statistical Analysis
A Kolmogorov-Smirnov normality test showed that the data were distributed according to a Gaussian distributive curve. Therefore, for statistical evaluations, Pearson's rank correlations and unpaired Student's t-test were used for comparisons between the control subjects and OA patient subsets. Quantitative data were expressed as the means ± SD. Differences in gene expression between the control group and the OA patient subsets were also tested using a two-way analysis of variance (ANOVA), followed by Scheffe's post-hoc test to confirm the results. Levene's test was used to assess a difference in variance between groups. Non-normally distributed data was expressed as median (quartiles) and Mann-Whitney U test was applied. To compare percentages, a two-tailed Z-test for percentages was applied. Statistica 6 Software (StatSoft, Tulsa, OK, USA) was used for all statistical analyses. P values less than 0.05 were considered significant.
3. Results
3.1. Clinical Parameters of OA Outpatients
Analysis of the demographic and clinical characteristics of 47 OA outpatients (set 1) revealed that the K&L OA grades of the examined subjects varied from II to IV (grade II, 29 patients; grade III, 12 patients; and grade IV, 6 patients). The average disease duration was 11.6 years (range 1–30 years). The majority of patients exhibited Heberden's nodes and an increased BMI (range 20.5–45.9). All the patients had normal bone mineral density (BMD). WOMAC scoring indicated moderate rates of knee pain (below 65 mm according to VAS) in these OA outpatients. The average joint stiffness was 83.4 (range 20–126). Synovitis at the knee joint was detected in half (48.9%) of the OA outpatients.
3.2. Whole Blood Gene Expression
Examination of gene expression in the whole blood of 47 OA outpatients (set 1) revealed that mTOR was significantly downregulated in 15 patients compared to the healthy controls, while the remaining 32 subjects exhibited mTOR gene upregulation (Figure 1(a)). As cellular metabolism has been shown to be dramatically affected by the level of mTOR expression [15, 17, 18], the examined OA outpatients were divided into 2 subsets: a “Low mTOR expression subset” (15 patients) and a “High mTOR expression subset” (32 patients). These subsets also demonstrated differences in clinical characteristics as presented in Table 1.
Figure 1.
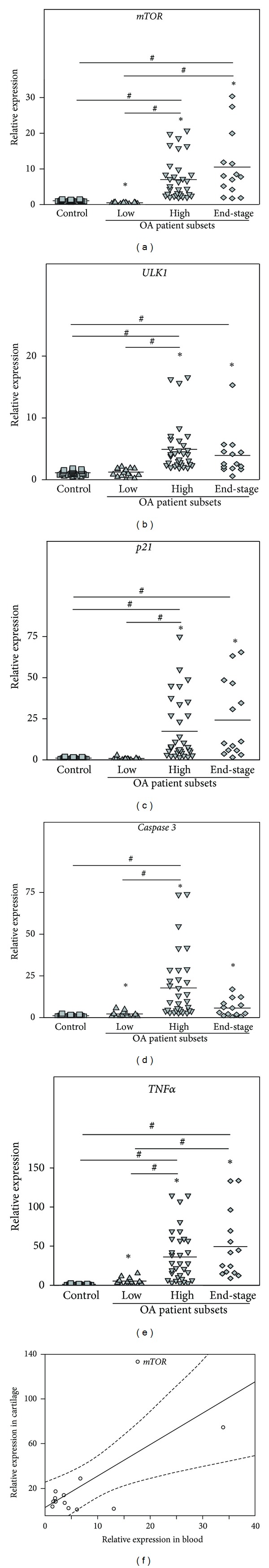
Relative expression of the genes mTOR (a), ULK1 (b), p21 (c), caspase 3 (d), and TNFα (e) with reference to β-actin determined by real-time PCR analyses in the whole blood of “Low mTOR subset” (n = 15), “High mTOR subset” (n = 32), and end-stage OA patients (n = 14) (set 1) compared with healthy controls (n = 27). (f) A relationship between mTOR gene expression measured in the blood or articular cartilage from end-stage OA patients (n = 13, set 2). Controls are shown as 1.0 as required for relative quantification with the real-time PCR protocol. Asterisks (∗) indicate significant differences from the control in pairwise comparisons (Student's unpaired t-test). Number signs (#) show significant differences in multigroup comparisons using two-way ANOVA followed by the Scheffe's post-hoc test.
Statistical analysis of the mTOR gene expression data for all of the OA outpatients did not result in a normal distribution (Kolmogorov-Smirnov test (K-S) d = 0.23, P < 0.05). Normal distribution of the mTOR gene expression values was observed in both the “Low mTOR expression subset” (K-S d = 0.163, P > 0.2) and the “High mTOR expression subset” (K-S d = 0.187, P < 0.2) of OA patients. The data for relative mTOR gene expression are presented in Supplementary Table 3.
Stratification of OA outpatients by disease stage did not show significant differences in the relative expression of mTOR, values of which were 5.7 ± 7.8 for grade II (n = 29), 5.1 ± 7.0 for grade III (n = 12), and 3.1 ± 2.7 for grade IV outpatients (n = 6).
Peripheral blood from the same subsets of OA outpatients was also examined for the expression of the autophagy marker ULK1, the regulator of cell cycle progression, a cyclin-dependent kinase inhibitor p21, the apoptosis indicator caspase 3, and the proinflammatory cytokine TNFα. These analyses demonstrated that the “Low mTOR expression subset” OA outpatients exhibited significant upregulation of caspase 3 and TNFα genes, while ULK1 and p21 expression remained similar to that in healthy individuals (Figures 1(b)–1(e)).
In contrast, the “High mTOR expression subset” OA outpatients exhibited significant upregulation of all the examined genes in comparison to healthy controls (Figure 1). Gene expression studies in the end-stage OA patients undergoing joint replacement surgery also demonstrated that all of the examined genes were overexpressed in their whole blood in comparison to healthy controls. A two-way analysis of variance (ANOVA) followed by Scheffe's post-hoc test of gene expression in the same control group and the OA patient subsets showed essentially similar results (Figure 1).
3.3. Association of Gene Expression with Peripheral Blood Mononuclear Cells (PBMCs)
The cellular origin of tested RNAs was confirmed by the examination of gene expression in cellular and noncellular elements of the whole blood. There was no expression of the examined genes in serum, erythrocyte, or thrombocyte fractions. In contrast, significantly higher expression of the examined genes was revealed in the PBMC fraction in comparison to isolated granulocytes in both the OA patients (Figure 2) and healthy subjects (data not shown).
Figure 2.
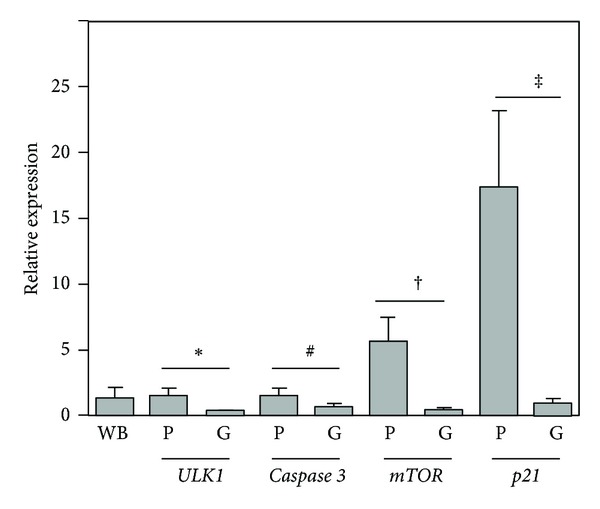
Relative expression of mTOR, ULK1, p21, and caspase 3 genes by real-time PCR with reference to β-actin in PBMCs (P) or granulocytes (G) compared with whole blood (WB) specimen of OA outpatients (n = 12). The control is shown as 1.0 as required for relative quantification with the real-time PCR protocol. The following symbols: ∗, #, †, and ‡ indicate significant differences in gene expression between PBMCs and granulocytes (Student's unpaired t-test).
3.4. Protein Levels of Total- and Phospho-p70-S6K, p21, and Active Caspase 3 in Isolated PBMCs
To further investigate the clinical significance of mTOR, p21, and caspase 3 relative gene expression in the whole blood of OA outpatients and end-stage OA subjects, we analyzed the protein levels of total- and phospho-p70-S6K serine/threonine kinase (a direct target for phosphorylation by mTOR [43–45]), p21, and active caspase 3 in the PBMC fraction. The “Low mTOR expression subset” OA outpatients possessed significantly lower total- and phospho-p70-S6K, p21, and caspase 3 protein concentrations in PBMCs compared to the “High mTOR expression subset” of OA outpatients and the end-stage OA subjects (Figures 3(a)–3(d)). When the amount of the examined proteins was evaluated compared to that in healthy subjects, we observed that the “Low mTOR subset” of OA outpatients possessed significantly lower total- and phospho-p70-S6K proteins, while p21 and caspase 3 levels were not significantly different. In contrast, protein concentrations of all of the examined genes in the “High mTOR subset” of OA outpatients and in the end-stage OA subjects significantly exceeded those in healthy individuals (Figures 3(a)–3(d)).
Figure 3.
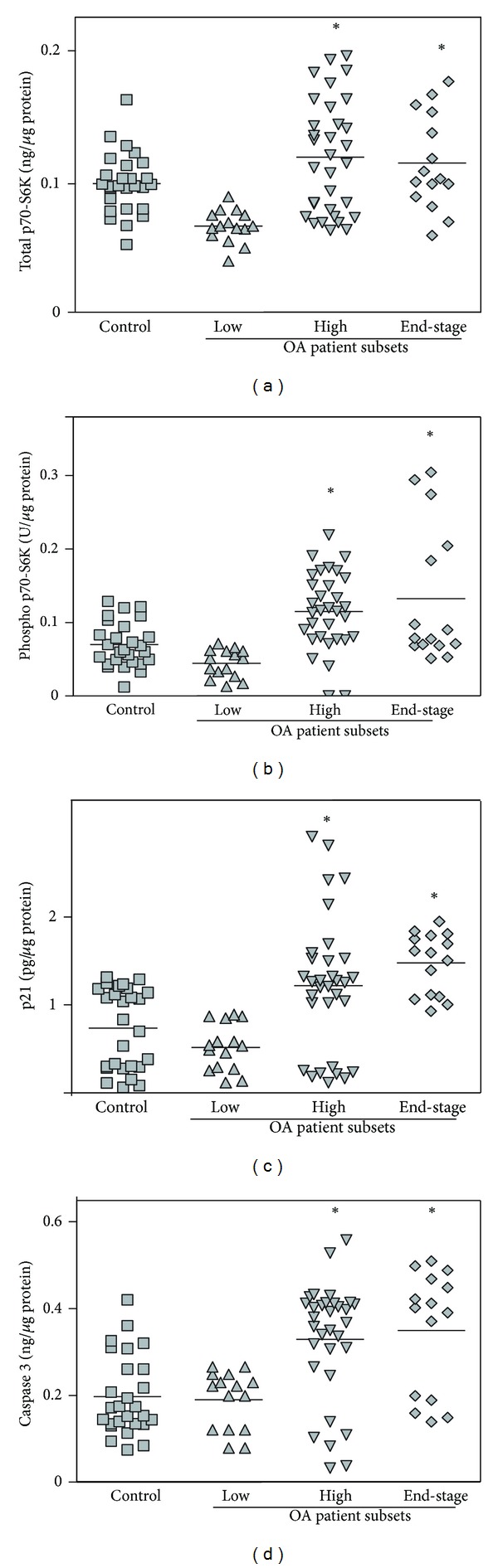
Protein concentrations of total p70-S6K (a), phospho-p70-S6K (b), p21 (c), and active caspase 3 (d) measured by ELISA in PBMCs from the “Low mTOR subset” (n = 15), the “High mTOR subset” (n = 32), and end-stage (n = 14) OA patients compared with control subjects (n = 27) (set 1). Asterisks indicate significant differences from the healthy control patients (Student's unpaired t-test).
3.5. Clinical Characteristics of “Low mTOR” and “High mTOR” Subsets of OA Outpatients
The outpatients in the designated subsets exhibited important differences in the manifestation of clinical traits (Table 1). The “Low mTOR” subset outpatients experienced significantly more pain upon walking and standing and increased total joint stiffness compared to the “High mTOR” OA subjects. They also were more often diagnosed Heberden's nodes, although not to a level of statistical significance.
In contrast, in the “High mTOR” subset of OA outpatients, we observed a significantly higher incidence of synovitis and reduced total femur BMD compared to the “Low mTOR” OA subjects, as well as an increased severity of night pain and lower BMD at the femoral neck, although these differences were not statistically significant.
The analysis of K&L radiological stage distribution among the OA outpatients revealed the higher relative numbers of stage II patients in the “High mTOR” subset compared to the “Low mTOR” subset. In contrast, a higher relative number of stage III patients belonged to the “Low mTOR” subset versus the “High mTOR” OA outpatients. However, these differences were not statistically significant.
The examination of the medication used for treatment revealed that the majority of OA outpatients (31 out of 47) in both subsets were treated by meloxicam, while 11 out of 47 outpatients received nimesulide. Only two patients from the subset “High mTOR” were treated with aceclofenac, while 3 patients from the subset “Low mTOR” did not require any anti-inflammatory medication. Chondroitin sulfate with or without glucosamine was used as a chondroprotective agent for OA outpatients of both subsets (Table 1). However, significantly higher number of the “High mTOR” subset outpatients were treated with chondroitin sulfate plus glucosamine compared to the “Low mTOR” OA outpatients.
The analysis of bivariate correlations using Pearson's correlation coefficients on expression of the examined genes showed that they were positively correlated to each other in the designated subsets of OA patients (Table 2). In contrast, healthy subjects showed a negative correlation between mTOR and TNFα gene expression, while ULK1 and caspase 3 expression was positively correlated with TNFα expression.
Table 2.
Correlation coefficients (Pearson's) and their significance (P; Student's unpaired t-test) are shown for the expression of mTOR, ULK1, p21, caspase 3, and TNFα genes in OA patients and healthy subjects in relation to each other and clinical traits.
| Set 1 patients | mTOR | ULK1 | Caspase 3 | TNFα |
|---|---|---|---|---|
|
“Low mTOR” subset OA patients (n = 15) |
||||
| Caspase 3 | 0.645 P = 0.009 |
|||
| Pain on walking | −0.568 P = 0.02 |
|||
| Pain on standing | −0.701 P = 0.004 |
|||
| Total physical function | −0.674 P = 0.007 |
|||
| Total WOMAC |
−0.633 P = 0.007 |
|||
| BMD L1–L4 |
0.544 P = 0.03 |
|||
| BMD total femur | 0.580 P = 0.02 |
|||
|
| ||||
| “High mTOR” subset OA patients (n = 32) |
||||
| mTOR | 0.369 P = 0.03 |
0.408 P = 0.02 |
0.691 P < 0.001 |
|
| P21 | 0.360 P = 0.04 |
0.683 P < 0.001 |
0.354 P = 0.04 |
|
| Caspase 3 | 0.502 P = 0.003 |
|||
| BMI |
0.432 P = 0.01 |
|||
| BMD L1–L4 |
0.454 P = 0.009 |
|||
| BMD femoral neck | 0.439 P = 0.01 |
|||
| ESR | 0.442 P = 0.01 |
|||
|
| ||||
| End-stage OA patients (n = 14) |
||||
| mTOR | 0.549 P = 0.04 |
|||
| ULK1 | 0.813 P < 0.001 |
|||
| P21 | 0.770 P = 0.001 |
0.756 P = 0.02 |
||
|
| ||||
| Healthy subjects | ||||
| TNFα | −0.560 P = 0.03 |
0.639 P = 0.01 |
0.556 P = 0.03 |
|
Only significant data are presented. ESR: erythrocyte sedimentation rate; WOMAC: Western Ontario and McMaster Universities osteoarthritis index; BMD: bone mineral density; BMI: body mass index.
The expression of the examined genes also correlated with clinical traits. Thus, the “Low mTOR subset” outpatients showed a positive correlation between caspase 3 gene expression and spine and femur BMD, while a negative correlation was observed between the expression of mTOR and caspase 3 genes and WOMAC indices (Table 2).
The “High mTOR” subset outpatients also demonstrated a positive correlation between spine and femur BMD and ULK1 and mTOR expression. TNFα gene expression was positively correlated with ESR while mTOR expression was positively correlated with BMI (Table 2).
3.6. Examination of Gene Expression in the Articular Cartilage of End-Stage OA Patients
Articular cartilage degradation in the 15 end-stage OA patients (set 1) at arthroplasty was associated with significantly higher levels of matrix metalloproteinase MMP-13 and MMP-9, as well as type X collagen (COL10A1) gene expression in cartilage compared to the corresponding levels in healthy subjects (Figure 4). This was accompanied by a significant upregulation of mTOR expression and downregulation of ULK1 and p21 in comparison to healthy controls (Figure 4). At the same time, no significant changes in caspase 3 gene expression in these OA patients versus healthy subjects were observed.
Figure 4.
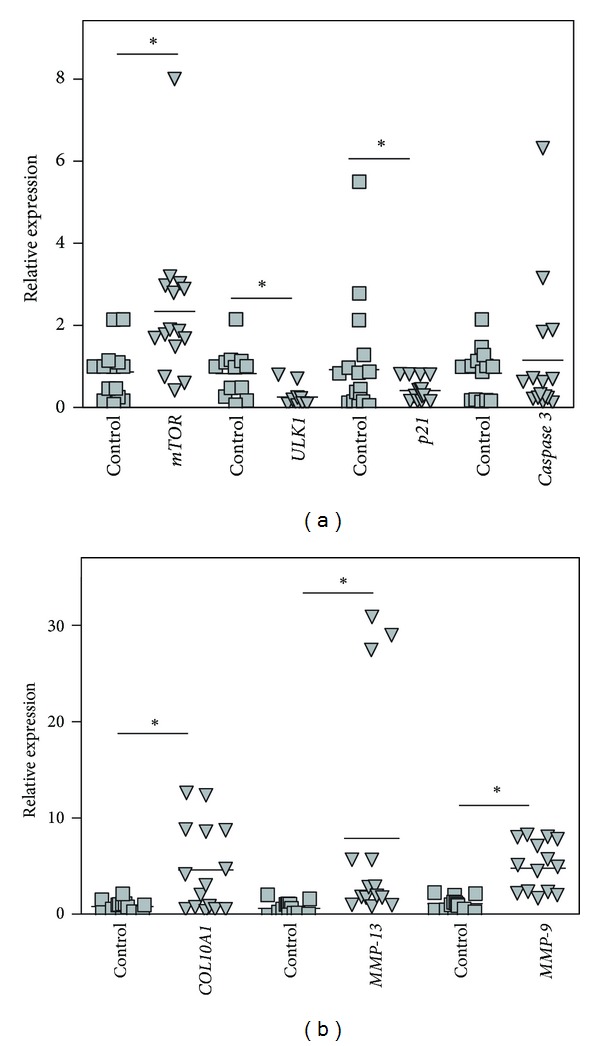
Relative expression of mTOR, ULK1, p21, caspase 3 (a), COL10A1, MMP-13, and MMP-9 (b) genes compared between healthy cartilages (n = 14, set 1) and osteoarthritic cartilages (n = 15, set 1) as determined by real-time PCR analyses with reference to β-actin. Controls are shown as 1.0 as required for relative quantification with the real-time PCR protocol. Asterisks (∗) indicate significant differences from the control (Student's unpaired t-test).
3.7. Correlation of Peripheral Blood Mononuclear Cell (PBMC) and Articular Cartilage Chondrocyte Gene Expression
Plotting of mTOR gene expression in PBMC versus that in the articular cartilage of the same 13 end-stage OA patients (set 2) revealed a significant correlation (Pearson's correlation coefficient r = 0.687; P = 0.01) (Figure 1(f)). Without the outliers Pearson's correlation coefficient came to r = 0.93; P = 0.0001 (Supplementary Figure 5).
3.8. Reproducibility of Data
To determine whether these results were reproducible, the expressions of the same genes were reexamined in other patients. Thus we analyzed the blood of another group of 18 OA outpatients (set 2) and another 13 end-stage OA patients (set 2); in addition we examined the articular cartilage of another 13 end-stage OA patients (set 2) compared to another set of healthy controls (set 2 controls, n = 12). Essentially similar results were obtained (Supplementary, Results, Figures 6 and 7).
4. Discussion
As repair potential of adult articular cartilage is very limited [1], informative indicators of OA disease activity are of importance, although OA is not classified as active or inactive. However, the rate of the disease progression is variable between individuals. Moreover, progression may be inconsistent in the same individual over time and joint degradation may occur intermittently [48–51]. Therefore, the distinction between disease and non-disease is not evident in OA [52]. The clinical definition of OA is based on a combination of symptoms and radiological findings, in which the correlation is weak, as patients with radiological OA may have no symptoms, while classical symptoms of OA may be accompanied by the absence of structural changes [53]. An approach involving registration of gene expression, which we applied in the present paper, offers the potential to better characterize disease activity and to distinguish between active versus inactive disease.
In this study, we demonstrate the value of an assessment of non-tissue-specific regulatory gene expression in the whole blood of OA patients for evaluating disease activity. We show that elevated mTOR gene expression in the PBMCs of a subset of OA patients with less-advanced disease might point to articular cartilage degradation because increased expression of this gene is seen in both peripheral blood and articular cartilage in end-stage OA patients requiring knee joint replacement. In addition, a positive correlation between mTOR gene expression in the blood and articular cartilage in the same end-stage OA patients suggests that upregulation of mTOR gene expression in the PBMCs might occur concomitantly with increased articular cartilage destruction.
Additionally, higher expression of the proinflammatory cytokine TNFα and the significantly higher incidence of synovitis, which was observed in the “High mTOR expression subset” of OA outpatients, as well as a positive correlation between mTOR and TNFα gene expression in all OA outpatient subsets but not in healthy subjects, would suggest an important contribution of inflammation to disease activity. This has also been suggested previously [4, 54–61]. The lower activity of synovitis in the “Low mTOR” subset OA outpatients is supported by the lower requirement for anti-inflammatory therapy as some patients could do without such medication.
At the same time examination of mTOR expression in the blood of postmenopausal OA women should be accompanied by a careful assessment of BMD indices. This became apparent in our other studies of a significant downregulation of mTOR gene expression in the blood of the postmenopausal osteoporotic patients ([62], Tchetina et al., paper in preparation).
In some studies, a positive association of knee pain with articular cartilage destruction has been observed in OA patients [63, 64]; however, we noticed that, although suffering significantly more pain, OA outpatients with low gene expression of mTOR were not observed among the end-stage OA patients undergoing joint replacement. Therefore, pain might be associated in these patients with periarticular tissue inflammation [65]. This is also supported by the observation that the subset “Low mTOR” of OA outpatients experienced increased pain upon joint function (walking or standing) but not at rest or at night. The limited value of knee pain in determining disease activity and progression has also been noted previously [66–68].
However, grade IV OA outpatients could become candidates for joint replacement and be considered end-stage patients if their pain was to exceed 70 cm according to the VAS, as severe pain usually leads to total joint replacement [69]. This could be accompanied by an increase in mTOR and proinflammatory cytokine gene expression. Therefore, the progression in disease in OA, which might be associated more with inflammation/synovitis than pain, could be monitored in the peripheral blood by mTOR gene expression level.
As the end-stage OA patients exhibited increased mTOR gene expression both in the PBMC and the articular cartilage, upregulation of this gene might designate those OA patients, which are more prone to joint replacement. Therefore, upregulation of mTOR gene expression might indicate the type of the OA disease activity associated with advanced cartilage destruction and synovitis. In contrast, mTOR downregulation might represent disease activity associated with reduced cartilage degeneration but with joint dysfunction resulting in increased joint pain and stiffness. The association of synovitis with night pain in the “High mTOR” group and pain on walking and standing in the “Low mTOR”set requires much studying to better understand these differences.
Although mTOR gene expression level might be indicative of OA activity, the assessment of gene expression alterations in markers for autophagy (ULK1), apoptosis (caspase 3), regulation of cell cycle progression (p21), and inflammation (TNFα) is also important. As these genes are responsible for global cell survival and function, they could collectively represent a “metabolic signature” of the OA patient, indicating overall gene expression disturbances associated with and reflective of the disease activity.
Essentially, the same pattern of mTOR, ULK1, p21, and caspase 3 gene expression was observed in the blood of the end-stage OA patients and the “High mTOR” subset of OA outpatients with less-advanced disease. However, the pattern of expression of the examined in our study genes was not always similar in the cartilage and blood of end-stage OA patients. This might result from differences in responses of chondrocytes versus white blood cells associated with the disease. mTOR reciprocally downregulates autophagy [70], as seen by the decrease of ULK 1 expression in the cartilage of the end-stage OA patients in this and other studies [20]. The chondrocyte hypertrophy associated with OA and evidenced by the upregulation of COL10A1, MMP-13, and MMP-9 gene expression in the examined OA cartilage has also been observed previously [71–75].
The results of our exploratory, correlative study suggest that upregulation of mTOR gene expression in the whole blood of OA outpatients is accompanied by increased synovial inflammation and might be associated with increased and possibly accelerated joint destruction later in the disease. Moreover, the assessment of mTOR, ULK1, p21, caspase 3, and TNFα gene expression in the blood of OA patients could provide a patient's “metabolic signature” associated with the disease, which might be of use in elucidating the clinical efficacy of OA treatment and contribute to our understanding of the mechanisms underlying OA therapy.
There are limitations to the present study. Because of the relatively small size of the cohorts that have been studied, and consequently underpowering of the study, there is clearly a need for this investigation to be repeated in much larger cohorts to avoid the influence of possible confounders. Yet the fact that these findings were confirmed in a second, albeit even smaller population, holds promise for the value of these initial observations: these also require examination in a longitudinal investigation to examine their potential prognostic value.
5. Conclusions
Increased expression of mTOR in PBMCs of OA patients is related to the presence of synovitis and is seen in all patients requiring joint replacement. Those patients with low expression of mTOR experienced more pain on walking and standing and increased joint stiffness but were not among those with end-stage disease requiring arthroplasty. These analyses may be of value in better characterizing disease activity and cartilage degeneration in patients with knee OA.
Supplementary Material
Supplementary Material section contains details on mTOR gene expression in the examined subjects as well as a description of gene and protein expression analyses in another set of 18 OA outpatients and 13 end-stage OA patients aiming to examine reproducibility of the data presented in the Results section.
Conflict of Interests
The authors declare that they have no competing interests.
Acknowledgments
This study was supported by the Russian Foundation for Basic Research (Projects nos. 09-04-01158-a and 12-04-00038a to Elena V. Tchetina) and the Russian Academy of Medical Sciences. The sponsor had no role in the study design or execution, data analysis, writing of the paper, or the decision to submit the paper for publication.
References
- 1.Poole AR, Guilak F, Abramson SB. Etiopathogenesis of osteoarthritis. In: Moskowitz RW, Altman RD, Hochberg MC, Buckwalter JA, Goldberg VM, editors. Osteoarthritis: Diagnosis and Medical/Surgical Management. 4th edition. Lippincott, Pa, USA: Williams & Wilkins; 2007. pp. 27–49. [Google Scholar]
- 2.Mahr S, Burmester GR, Hilke D, et al. Cis- and trans-acting gene regulation is associated with osteoarthritis. American Journal of Human Genetics. 2006;78(5):793–803. doi: 10.1086/503849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall KW, Zhang H, Yager TD, et al. Blood-based biomarkers for detecting mild osteoarthritis in the human knee. Osteoarthritis and Cartilage. 2005;13(10):861–871. doi: 10.1016/j.joca.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Attur M, Belitskaya-Lévy I, Oh C, et al. Increased interleukin-1β gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis and Rheumatism. 2011;63(7):1908–1917. doi: 10.1002/art.30360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tchetina EV. Developmental mechanisms in articular cartilage degradation in osteoarthritis. Arthritis. 2011;2011:16 pages. doi: 10.1155/2011/683970.683970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tchetina EV, Squires G, Poole AR. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. Journal of Rheumatology. 2005;32(5):876–886. [PubMed] [Google Scholar]
- 7.Tchetina EV, Kobayashi M, Yasuda T, Meijers T, Pidoux I, Poole AR. Chondrocyte hypertrophy can be induced by a cryptic sequence of type II collagen and is accompanied by the induction of MMP-13 and collagenase activity: implications for development and arthritis. Matrix Biology. 2007;26(4):247–258. doi: 10.1016/j.matbio.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Tchetina EV, Antoniou J, Tanzer M, Zukor DJ, Poole AR. Transforming growth factor-β2 suppresses collagen cleavage in cultured human osteoarthritic cartilage, reduces expression of genes associated with chondrocyte hypertrophy and degradation, and increases prostaglandin E 2 production. American Journal of Pathology. 2006;168(1):131–140. doi: 10.2353/ajpath.2006.050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rousseau JC, Delmas PD. Biological markers in osteoarthritis. Nature Clinical Practice Rheumatology. 2007;3(6):346–356. doi: 10.1038/ncprheum0508. [DOI] [PubMed] [Google Scholar]
- 10.Grcevic D, Jajic Z, Kovacic N, et al. Peripheral blood expression profiles of bone morphogenetic proteins, tumor necrosis factor-superfamily molecules, and transcription factor Runx2 could be used as markers of the form of arthritis, disease activity, and therapeutic responsiveness. Journal of Rheumatology. 2010;37(2):246–256. doi: 10.3899/jrheum.090167. [DOI] [PubMed] [Google Scholar]
- 11.Chang BD, Watanabe K, Broude EV, et al. Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(8):4291–4296. doi: 10.1073/pnas.97.8.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuda K. Progress of research in osteoarthritis. Involvement of reactive oxygen species in the pathogenesis of osteoarthritis. Clinical calcium. 2009;19(11):1602–1606. [PubMed] [Google Scholar]
- 13.Dai SM, Shan ZZ, Nakamura H, et al. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis and Rheumatism. 2006;54(3):818–831. doi: 10.1002/art.21639. [DOI] [PubMed] [Google Scholar]
- 14.Sesselmann S, Söder S, Voigt R, Haag J, Grogan SP, Aigner T. DNA methylation is not responsible for p21WAF1/CIP1 down-regulation in osteoarthritic chondrocytes. Osteoarthritis and Cartilage. 2009;17(4):507–512. doi: 10.1016/j.joca.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Hay N, Sonnenberg N. Upstream and downstream of mTOR . Genes & Development. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 16.Bohensky J, Leshinsky S, Srinivas V, Shapiro IM. Chondrocyte autophagy is stimulated by HIF-1 dependent AMPK activation and mTOR suppression. Pediatric Nephrology. 2010;25(4):633–642. doi: 10.1007/s00467-009-1310-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MS, Ke YW, Auyeung V, Chen Q, Gruppuso PA, Phornphutkul C. Leucine restriction inhibits chondrocyte proliferation and differentiation through mechanisms both dependent and independent of mTOR signaling. American Journal of Physiology. 2009;296(6):E1374–E1382. doi: 10.1152/ajpendo.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez CP, He YZ. Bone growth during rapamycin therapy in young rats. BMC Pediatrics. 2009;9, article 3 doi: 10.1186/1471-2431-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cejka D, Hayer S, Niederreiter B, et al. Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis and Rheumatism. 2010;62(8):2294–2302. doi: 10.1002/art.27504. [DOI] [PubMed] [Google Scholar]
- 20.Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis and Rheumatism. 2010;62(3):791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carames B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Annals of Rheumatic Diseases. 2012;71(4):575–581. doi: 10.1136/annrheumdis-2011-200557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laragione T, Gulko PS. mTOR regulates the invasive properties of synovial fibroblasts in rheumatoid arthritis. Molecular Medicine. 2010;16(9-10):352–358. doi: 10.2119/molmed.2010.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(13):7037–7044. doi: 10.1073/pnas.121145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu B, Capan E, Li C. Autophagy induction and autophagic cell death in effector T cells. Autophagy. 2007;3(2):158–159. doi: 10.4161/auto.3637. [DOI] [PubMed] [Google Scholar]
- 25.Wei Y, Sinha S, Levine B. Dual role of JNK1-mediated phosphorylation of Bcl-2 in autophagy and apoptosis regulation. Autophagy. 2008;4(7):949–951. doi: 10.4161/auto.6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis & Rheumatism. 1998;41(2):284–289. doi: 10.1002/1529-0131(199802)41:2<284::AID-ART12>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn K, D’Lima DD, Hashimoto S, Lotz M. Cell death in cartilage. Osteoarthritis & Cartilage. 2004;12(1):1–16. doi: 10.1016/j.joca.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 28.Yatsugi N, Tsukazaki T, Osaki M, Koji T, Yamashita S, Shindo H. Apoptosis of articular chondrocytes in rheumatoid arthritis and osteoarthritis: correlation of apoptosis with degree of cartilage destruction and expression of apoptosis-related proteins of p53 and c-myc. Journal of Orthopaedic Science. 2000;5(2):150–156. doi: 10.1007/s007760050142. [DOI] [PubMed] [Google Scholar]
- 29.Zhu M, Chen M, Zuscik M, et al. Inhibition of β-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis and Rheumatism. 2008;58(7):2053–2064. doi: 10.1002/art.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aigner T, Hemmel M, Neureiter D, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis & Rheumatism. 2001;44(6):1304–1312. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 31.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis & Rheumatism. 1999;39(9):1535–1544. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clinical Orthopaedics and Related Research. 2004;(427):S27–S36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 33.Fraenkel L, Roubenoff R, LaValley M, et al. The association of peripheral monocyte derived interleukin 1β (IL-1β), IL-1 receptor antagonist, and tumor necrosis factor-α with osteoarthritis in the elderly. Journal of Rheumatology. 1998;25(9):1820–1826. [PubMed] [Google Scholar]
- 34.Patel IR, Attur MG, Patel RN, et al. TNF-α convertase enzyme from human arthritis-affected cartilage: isolation of cDNA by differential display, expression of the active enzyme, and regulation of TNF-α . Journal of Immunology. 1998;160(9):4570–4579. [PubMed] [Google Scholar]
- 35.Altman R, Asch E, Bloch D. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis and Rheumatism. 1986;29(8):1039–1052. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 36.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. Journal of Bone and Joint Surgery A. 1971;53(3):523–537. [PubMed] [Google Scholar]
- 37.Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, Poole AR. Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. Journal of Clinical Investigation. 1995;96(6):2859–2869. doi: 10.1172/JCI118357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Annals of the Rheumatic Diseases. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellamy N. WOMAC Osteoarthritis Index: A User’s Guide. London, UK: University of Western Ontario; 1995. [Google Scholar]
- 40.Backhaus M, Burmester GR, Gerber T, et al. Guidelines for musculoskeletal ultrasound in rheumatology. Annals of the Rheumatic Diseases. 2001;60(7):641–649. doi: 10.1136/ard.60.7.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization Study Group: Assessment of Fracture Risk and is Application for Screening for Postmenopausal Osteoporosis. Geneva, Switzerland: WHO; 1994. [PubMed] [Google Scholar]
- 42.Son BK, Roberts RL, Ank BJ, Stiehm ER. Effects of anticoagulant, serum, and temperature on the natural killer activity of human peripheral blood mononuclear cells stored overnight. Clinical and Diagnostic Laboratory Immunology. 1996;3(3):260–264. doi: 10.1128/cdli.3.3.260-264.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isotani S, Hara K, Tokunaga C, Inoue H, Avruch J, Yonezawa K. Immunopurified mammalian target of rapamycin phosphorylates and activates p70 S6 kinase a in vitro . Journal of Biological Chemistry. 1999;274(48):34493–34498. doi: 10.1074/jbc.274.48.34493. [DOI] [PubMed] [Google Scholar]
- 44.Proud CG. p70 S6 kinase: an enigma with variations. Trends in Biochemical Sciences. 1996;21(5):181–185. [PubMed] [Google Scholar]
- 45.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes and Development. 2002;16(12):1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Analytical Biochemistry. 1976;72(1-2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 47.Livak KJ. User Bulletin No. 2. Foster City. Foster City, Calif, USA: PE Applied Biosystems; 1997. Comparative Ct method. ABI Prism 7700 sequence detection system. [Google Scholar]
- 48.Spector TD, Dacre JE, Harris PA, Huskisson EC. Radiological progression of osteoarthritis: an 11 year follow up study of the knee. Annals of the Rheumatic Diseases. 1992;51(10):1107–1110. doi: 10.1136/ard.51.10.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dieppe PA, Cushnaghan J, Shepstone L. The Bristol “OA500” Study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis and Cartilage. 1997;5(2):87–97. doi: 10.1016/s1063-4584(97)80002-7. [DOI] [PubMed] [Google Scholar]
- 50.Dougados M, Gueguen A, Nguyen M, et al. Longitudinal radiologic evaluation of osteoarthritis of the knee. Journal of Rheumatology. 1992;19(3):378–384. [PubMed] [Google Scholar]
- 51.Dougados M, Gueguen A, Nguyen M, et al. Radiological progression of hip osteoarthritis: definition, risk factors and correlations with clinical status. Annals of the Rheumatic Diseases. 1996;55(6):356–362. doi: 10.1136/ard.55.6.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lohmander LS. What can we do about osteoarthritis? Arthritis Research. 2000;2(2):95–100. doi: 10.1186/ar74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Reily S, Doherty M. Signs, symptoms, and laboratory tests. In: Brandt KD, Doherty M, Lohmander LS, editors. Osteoarthritis. Oxford, UK: Oxford University Press; 1998. pp. 74–84. [Google Scholar]
- 54.Ayral X, Pickering EH, Woodworth TG, Mackillop N, Dougados M. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis—results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis and Cartilage. 2005;13(5):361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 55.D’Agostino MA, Conaghan P, Le Bars M, et al. EULAR report on the use of ultrasonography in painful knee osteoarthritis. Part 1: prevalence of inflammation in osteoarthritis. Annals of the Rheumatic Diseases. 2005;64(12):1703–1709. doi: 10.1136/ard.2005.037994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conaghan PG, D’Agostino MA, Le Bars M, et al. Clinical and ultrasonographic predictors of joint replacement for knee osteoarthritis: results from a large, 3-year, prospective EULAR study. Annals of the Rheumatic Diseases. 2010;69(4):644–647. doi: 10.1136/ard.2008.099564. [DOI] [PubMed] [Google Scholar]
- 57.Cheung PP, Gossec L, Dougados M. What are the best markers for disease progression in osteoarthritis (OA)? Best Practice and Research. 2010;24(1):81–92. doi: 10.1016/j.berh.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Shibakawa A, Aoki H, Masuko-Hongo K, et al. Presence of pannus-like tissue on osteoarthritic cartilage and its histological character. Osteoarthritis and Cartilage. 2003;11(2):133–140. doi: 10.1053/joca.2002.0871. [DOI] [PubMed] [Google Scholar]
- 59.Kristoffersen H, Torp-Pedersen S, Terslev L, et al. Indications of inflammation visualized by ultrasound in osteoarthritis of the knee. Acta Radiologica. 2006;47(3):281–286. doi: 10.1080/02841850600551508. [DOI] [PubMed] [Google Scholar]
- 60.Chevalier X, Conrozier T, Richette P. Desperately looking for the right target in osteoarthritis: the anti-IL-1 strategy. Arthritis Research & Therapy. 2011;13(4):p. 124. doi: 10.1186/ar3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scanzello CR, McKeon B, Swaim BH, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis and Rheumatism. 2011;63(2):391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tchetina EV, Maslova K, Demin N, Myakotkin VA. Association of bone loss with upregulation of survival-related genes and concomitant downregulation of mammalian target of rapamycin (mTOR) and osteoblast differentiation-related genes in peripheral blood of osteoporotic postmenopausal women. Annals of Rheumatic Diseases. 2009;68(supplement 3):p. 491. doi: 10.1155/2015/802694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eckstein F, Cotofana S, Wirth W, et al. Greater rates of cartilage loss in painful knees than in pain-free knees after adjustment for radiographic disease stage: data from the osteoarthritis initiative. Arthritis and Rheumatism. 2011;63(8):2257–2267. doi: 10.1002/art.30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishijima M, Watari T, Naito K, et al. Relationships between biomarkers of cartilage, bone, synovial metabolism and knee pain provide insights into the origins of pain in early knee osteoarthritis. Arthritis Research and Therapy. 2011;13(1, article R22) doi: 10.1186/ar3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Levinger P, Caldow MK, Feller JA, et al. Association between skeletal muscle inflammatory markers and walking pattern in people with knee osteoarthritis. Arthritis Care & Research. 2011;63(12):1715–1721. doi: 10.1002/acr.20625. [DOI] [PubMed] [Google Scholar]
- 66.Belo JN, Berger MY, Reijman M, Koes BW, Bierma-Zeinstra SMA. Prognostic factors of progression of osteoarthritis of the knee: a systematic review of observational studies. Arthritis Care and Research. 2007;57(1):13–26. doi: 10.1002/art.22475. [DOI] [PubMed] [Google Scholar]
- 67.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskeletal Disorders. 2008;9, article 116 doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gossec L, Paternotte S, Maillefert JF, et al. The role of pain and functional impairment in the decision to recommend total joint replacement in hip and knee osteoarthritis: an international cross-sectional study of 1909 patients. Report of the OARSI-OMERACT Task Force on total joint replacement. Osteoarthritis and Cartilage. 2011;19(2):147–154. doi: 10.1016/j.joca.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moretti B, Notarnicola A, Moretti L, et al. I-ONE therapy in patients undergoing total knee arthroplasty: a prospective, randomized and controlled study. BMC Musculoskeletal Disorders. 2012;13, article 88 doi: 10.1186/1471-2474-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lum JJ, DeBerardinis RJ, Thompson CB. Autophagy in metazoans: cell survival in the land of plenty. Nature Reviews Molecular Cell Biology. 2005;6(6):439–448. doi: 10.1038/nrm1660. [DOI] [PubMed] [Google Scholar]
- 71.von der Mark K, Kirsch T, Nerlich A, et al. Type X collagen synthesis in human osteoarthritic cartilage: indication of chondrocyte hypertrophy. Arthritis and Rheumatism. 1992;35(7):806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]
- 72.Girkontaite I, Frischholz S, Lammi P, et al. Immunolocalization of type X collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies. Matrix Biology. 1996;15(4):231–238. doi: 10.1016/s0945-053x(96)90114-6. [DOI] [PubMed] [Google Scholar]
- 73.Dreier R. Hypertrophic differentiation of chondrocytes in osteoarthritis: the developmental aspect of degenerative joint disorders. Arthritis Research and Therapy. 2010;12(5, article 216) doi: 10.1186/ar3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Phander D, Swoboda B, Kirsch T. Expression of early and late differentiation markers (proliferating cell nuclear antigen, syndecan-3, annexin VI, and alkaline phosphatase) by human osteoarthritic chondrocytes. American Journal of Pathology. 2001;159(5):1777–1783. doi: 10.1016/S0002-9440(10)63024-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drissi H, Zuscik M, Rosier R, O’Keefe R. Transcriptional regulation of chondrocyte maturation: potential involvement of transcription factors in OA pathogenesis. Molecular Aspects of Medicine. 2005;26(3):169–179. doi: 10.1016/j.mam.2005.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material section contains details on mTOR gene expression in the examined subjects as well as a description of gene and protein expression analyses in another set of 18 OA outpatients and 13 end-stage OA patients aiming to examine reproducibility of the data presented in the Results section.


