Abstract
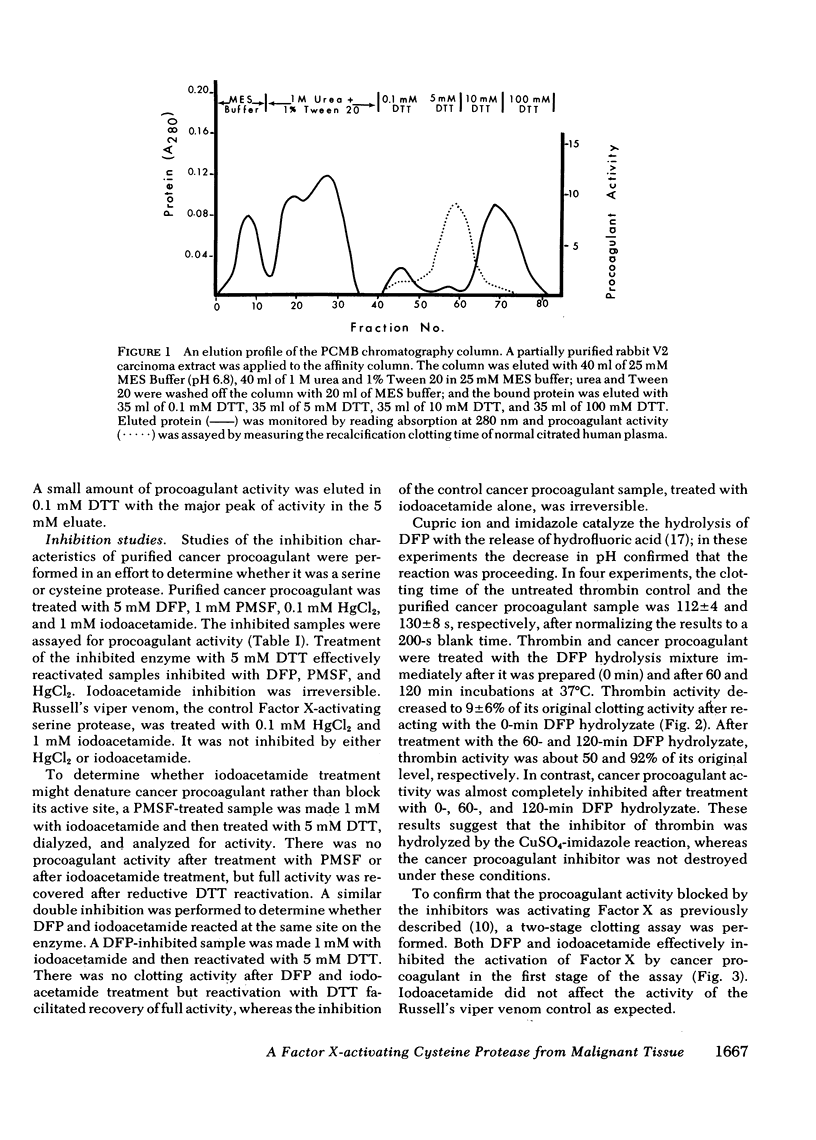
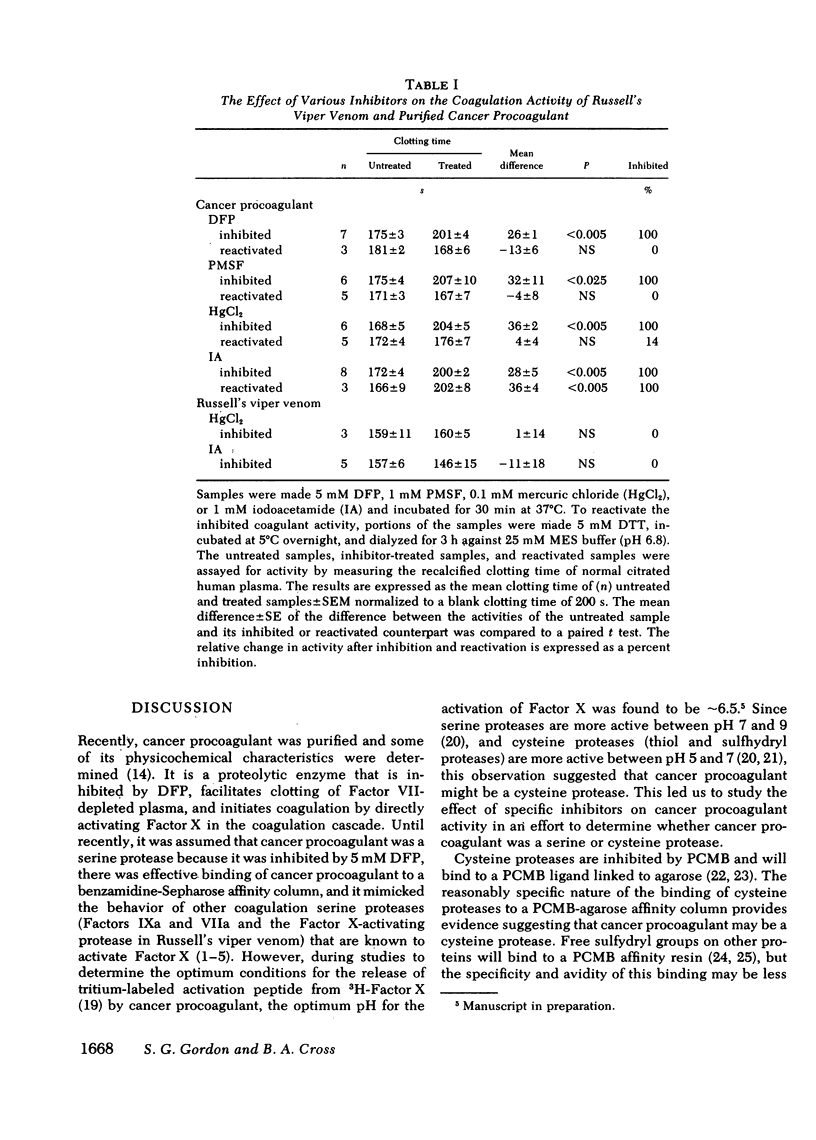
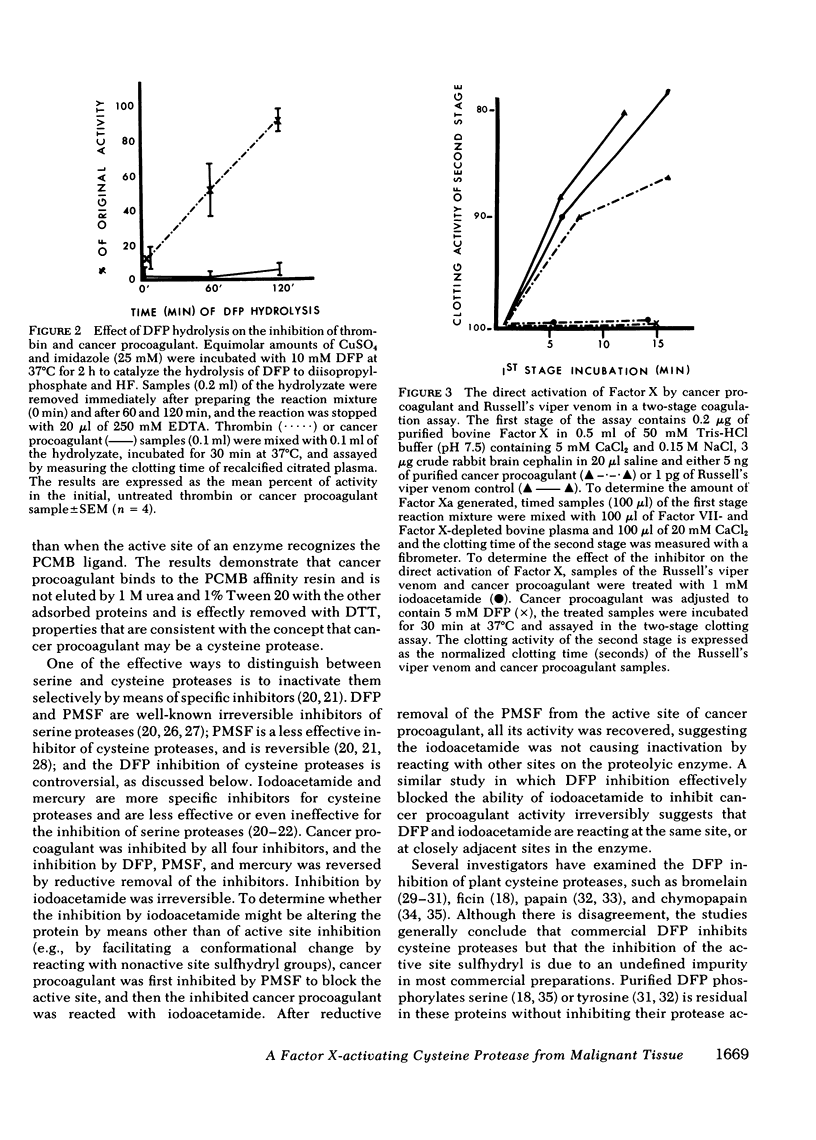
A proteolytic procoagulant has been identified in extracts of human and animal tumors and in cultured malignant cells. It directly activated Factor X but its similarity to other Factor S-activating serine proteases was not clear. This study describes work done to determine whether this enzyme, cancer procoagulant, is a serine or cysteine protease. Purified cancer procoagulant from rabbit V2 carcinoma was bound to a p-chloromercurialbenzoate-agarose affinity column and was eluted with dithiothreitol. The initiation of recalcified, citrated plasma coagulation activity by cancer procoagulant was inhibited by 5 mM diisopropylfluorophosphate, 1 mM phenylmethylsulfonylfluoride, 0.1 mM HgCl2, and 1 mM iodoacetamide. Activity was restored in the diisopropylfluorophosphate-, phenylmethylsulfonylfluoride-, and HgCl2-inhibited samples by 5 mM dithiothreitol; iodoacetamide inhibition was irreversible. Russell's viper venom, a control Factor X-activating serine protease, was not inhibited by either 0.1 mM HgCl2 or 1 mM iodoacetamide. The direct activation of Factor X by cancer procoagulant in a two-stage assay was inhibited by diisopropylfluorophosphate and iodoacetamide. Diisopropylfluorophosphate inhibits serine proteases, and an undefined impurity in most commercial preparations inhibits cysteine proteases. Hydrolysis of diisopropylfluorophosphate with CuSO4 and imidazole virtually eliminated inhibition of thrombin, but cancer procoagulant inhibition remained complete, suggesting that cancer procoagulant was inhibited by the undefined impurity. These results suggest that cancer procoagulant is a cysteine endopeptidase, which distinguishes it from other coagulation factors including tissue factor. This and other data suggest that neoplastic cells produce this unique cysteine protease which may initiate blood coagulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER B., PECHET L., KLIMAN A. Proteolysis, fibrinolysis, and coagulation. Significance in thrombolytic therapy. Circulation. 1962 Oct;26:596–611. doi: 10.1161/01.cir.26.4.596. [DOI] [PubMed] [Google Scholar]
- Azanza J. L., Raymond J., Robin J. M., Cottin P., Ducastaing A. Purification and some physico-chemical and enzymic properties of a calcium ion-activated neutral proteinase from rabbit skeletal muscle. Biochem J. 1979 Nov 1;183(2):339–347. doi: 10.1042/bj1830339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajaj S. P., Mann K. G. Simultaneous purification of bovine prothrombin and factor X. Activation of prothrombin by trypsin-activated factor X. J Biol Chem. 1973 Nov 25;248(22):7729–7741. [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Chaiken I. M., Smith E. L. Reaction of a specific tyrosine residue of papain with diisopropylfluorophosphate. J Biol Chem. 1969 Aug 10;244(15):4247–4250. [PubMed] [Google Scholar]
- Colucci M., Curatolo L., Donati M. B., Semeraro N. Cancer cell procoagulant activity: evaluation by an amidolytic assay. Thromb Res. 1980 May 1;18(3-4):589–595. doi: 10.1016/0049-3848(80)90359-x. [DOI] [PubMed] [Google Scholar]
- Curatolo L., Colucci M., Cambini A. L., Poggi A., Morasca L., Donati M. B., Semeraro N. Evidence that cells from experimental tumours can activate coagulation factor X. Br J Cancer. 1979 Aug;40(2):228–233. doi: 10.1038/bjc.1979.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scipio R. G., Hermodson M. A., Davie E. W. Activation of human factor X (Stuart factor) by a protease from Russell's viper venom. Biochemistry. 1977 Nov 29;16(24):5253–5260. doi: 10.1021/bi00643a015. [DOI] [PubMed] [Google Scholar]
- Ebata M., Tsunoda J., Asunobu K. T. Changes in the conformation of chymopapain during the activation process. Biochem Biophys Res Commun. 1966 Mar 8;22(5):455–458. doi: 10.1016/0006-291x(66)90294-4. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Coan M. H., Legaz M. E., Davie E. W. The mechanism of activation of bovine factor X (Stuart factor) by intrinsic and extrinsic pathways. Biochemistry. 1974 Dec 17;13(26):5290–5299. doi: 10.1021/bi00723a006. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., Legaz M. E., Davie E. W. Bovine factor X 1 (Stuart factor). Mechanism of activation by protein from Russell's viper venom. Biochemistry. 1972 Dec 19;11(26):4892–4899. doi: 10.1021/bi00776a003. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Matsunaga Y., Hayashi K. Purification and characterization of a coagulant protein from the venom of Russell's viper. Biochim Biophys Acta. 1976 Nov 26;453(1):48–61. doi: 10.1016/0005-2795(76)90249-x. [DOI] [PubMed] [Google Scholar]
- GOULD N. R., LIENER I. E. REACTION OF FICIN WITH DIISOPROPYLPHOSPHOROFLUORIDATE. EVIDENCE FOR A CONTAMINATING INHIBITOR. Biochemistry. 1965 Jan;4:90–98. doi: 10.1021/bi00877a016. [DOI] [PubMed] [Google Scholar]
- Gordon S. G., Franks J. J., Lewis B. J. Comparison of procoagulant activities in extracts of normal and malignant human tissue. J Natl Cancer Inst. 1979 Apr;62(4):773–776. [PubMed] [Google Scholar]
- Gordon S. G., Franks J. J., Lewis B. Cancer procoagulant A: a factor X activating procoagulant from malignant tissue. Thromb Res. 1975 Feb;6(2):127–137. doi: 10.1016/0049-3848(75)90018-3. [DOI] [PubMed] [Google Scholar]
- Gordon S. G., Lewis B. J. Comparison of procoagulant activity in tissue culture medium from normal and transformed fibroblasts. Cancer Res. 1978 Aug;38(8):2467–2472. [PubMed] [Google Scholar]
- HEINICKE R. M., MORI R. Effect of diisopropylfluorophosphate on sulfhydryl proteases. Science. 1959 Jun 19;129(3364):1678–1678. doi: 10.1126/science.129.3364.1678. [DOI] [PubMed] [Google Scholar]
- Harington J. S. The sulfhydryl group and carcinogenesis. Adv Cancer Res. 1967;10:247–309. doi: 10.1016/s0065-230x(08)60080-9. [DOI] [PubMed] [Google Scholar]
- Hilgard P., Whur P. Factor X-activating activity from Lewis lung carcinoma. Br J Cancer. 1980 Apr;41(4):642–643. doi: 10.1038/bjc.1980.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holyoke E. D., Frank A. L., Weiss L. Tumor thromboplastic activity in vitro. Int J Cancer. 1972 Mar 15;9(2):258–263. doi: 10.1002/ijc.2910090203. [DOI] [PubMed] [Google Scholar]
- Jesty J., Spencer A. K., Nemerson Y. The mechanism of activation of factor X. Kinetic control of alternative pathways leading to the formation of activated factor X. J Biol Chem. 1974 Sep 10;249(17):5614–5622. [PubMed] [Google Scholar]
- Khato J., Suzuki M., Sato H. Quantitative study on thromboplastin in various strains of Yoshida ascites hepatoma cells of rat. Gan. 1974 Aug;65(4):289–294. [PubMed] [Google Scholar]
- Kisiel W., Hermodson M. A., Davie E. W. Factor X activating enzyme from Russell's viper venom: isolation and characterization. Biochemistry. 1976 Nov 2;15(22):4901–4906. doi: 10.1021/bi00667a023. [DOI] [PubMed] [Google Scholar]
- Mcdonagh J., Waggoner W. G., Hamilton E. G., Hindenbach B., Mcdonagh R. P. Affinity chromatography of human plasma and platelet factor XIII on organomercurial agarose. Biochim Biophys Acta. 1976 Oct 28;446(2):345–357. doi: 10.1016/0005-2795(76)90002-7. [DOI] [PubMed] [Google Scholar]
- Mertens K., Bertina R. M. Pathways in the activation of human coagulation factor X. Biochem J. 1980 Mar 1;185(3):647–658. doi: 10.1042/bj1850647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murachi T., Inagami T., Yasui M. Evidence for alkylphosphorylation of tyrosyl residues of stem bromelain by diisopropylphosphorofluoridate. Biochemistry. 1965 Dec;4(12):2815–2825. doi: 10.1021/bi00888a036. [DOI] [PubMed] [Google Scholar]
- Nemerson Y., Clyne L. P. An assay for coagulation factor VII using factor VII-depleted bovine plasma. J Lab Clin Med. 1974 Feb;83(2):301–303. [PubMed] [Google Scholar]
- Ono M., Kawakami M. Separation of newly-synthesized RNA by organomercurial agarose affinity chromatography. J Biochem. 1977 May;81(5):1247–1252. [PubMed] [Google Scholar]
- PURCELL G. M., BARNHART M. I. PROTHROMBIN ACTIVATION WITH CATHEPSIN C. Biochim Biophys Acta. 1963 Dec 13;78:800–802. doi: 10.1016/0006-3002(63)91067-9. [DOI] [PubMed] [Google Scholar]
- Philipp M., Tsai I. H., Bender M. L. Comparison of the kinetic specificity of subtilisin and thiolsubtilisin toward n-alkyl p-nitrophenyl esters. Biochemistry. 1979 Aug 21;18(17):3769–3773. doi: 10.1021/bi00584a020. [DOI] [PubMed] [Google Scholar]
- Radcliffe R. D., Barton P. G. Comparisons of the molecular forms of activated bovine factor X. Products of activation with Russell's viper venom, insoluble trypsin, sodium citrate, tissue factor, and the intrinsic system. J Biol Chem. 1973 Oct 10;248(19):6788–6795. [PubMed] [Google Scholar]
- Recklies A. D., Tiltman K. J., Stoker T. A., Poole A. R. Secretion of proteinases from malignant and nonmalignant human breast tissue. Cancer Res. 1980 Mar;40(3):550–556. [PubMed] [Google Scholar]
- Silverberg S. A., Nemerson Y., Zur M. Kinetics of the activation of bovine coagulation factor X by components of the extrinsic pathway. Kinetic behavior of two-chain factor VII in the presence and absence of tissue factor. J Biol Chem. 1977 Dec 10;252(23):8481–8488. [PubMed] [Google Scholar]
- Whitaker J. R., Perez-Villase ñor J. Chemical modification of papain. I. Reaction with the chloromethyl ketones of phenylalanine and lysine and with phenylmethyl-sulfonyl fluoride. Arch Biochem Biophys. 1968 Mar 20;124(1):70–78. doi: 10.1016/0003-9861(68)90304-4. [DOI] [PubMed] [Google Scholar]


