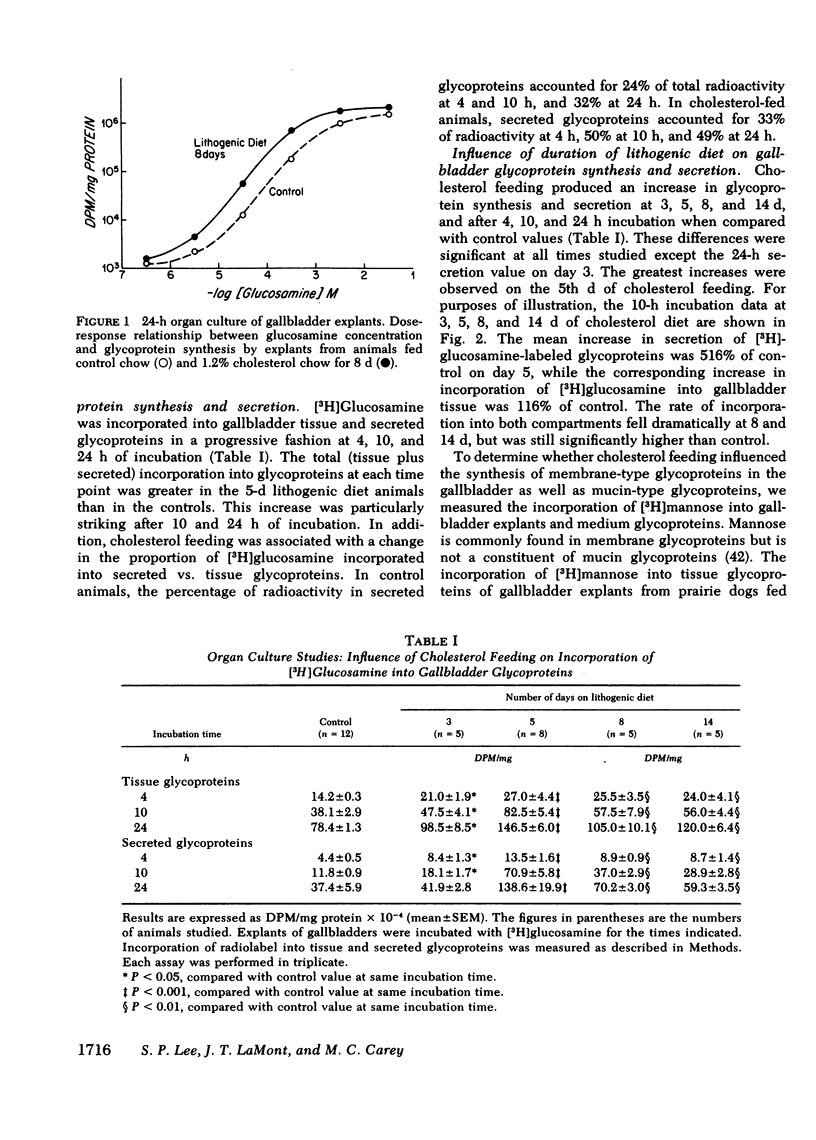
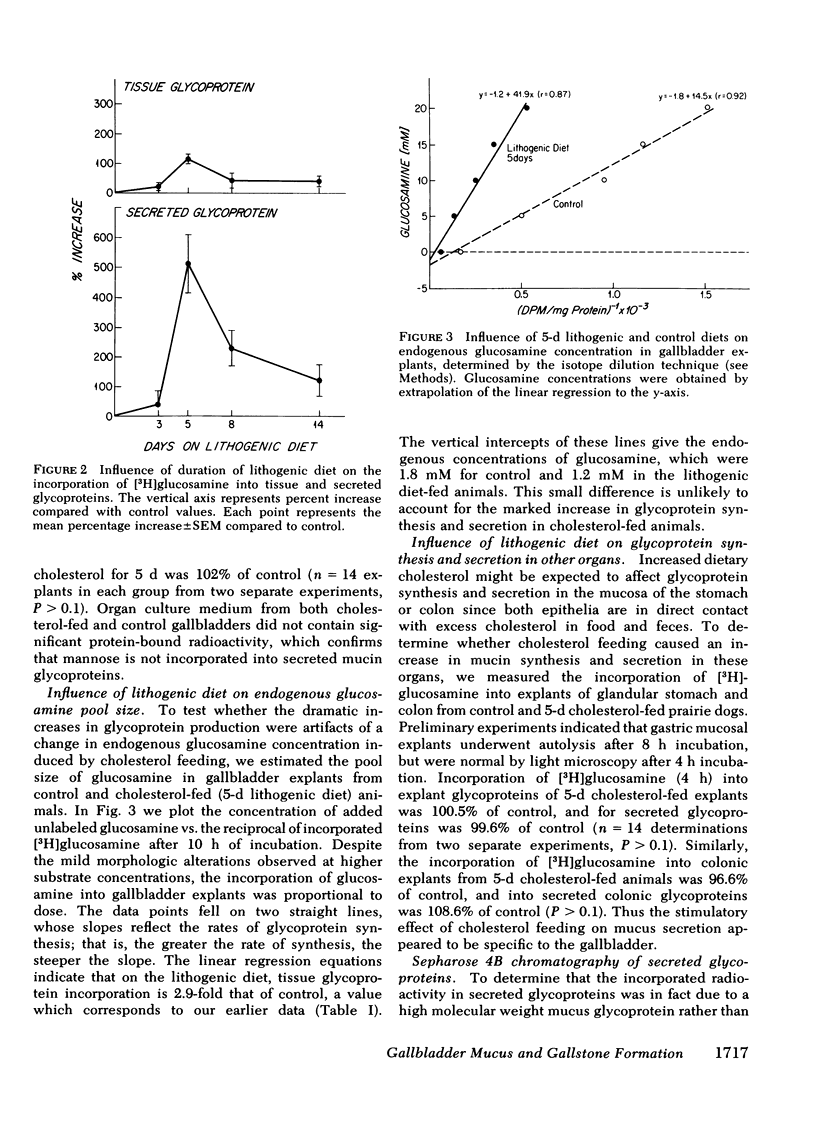
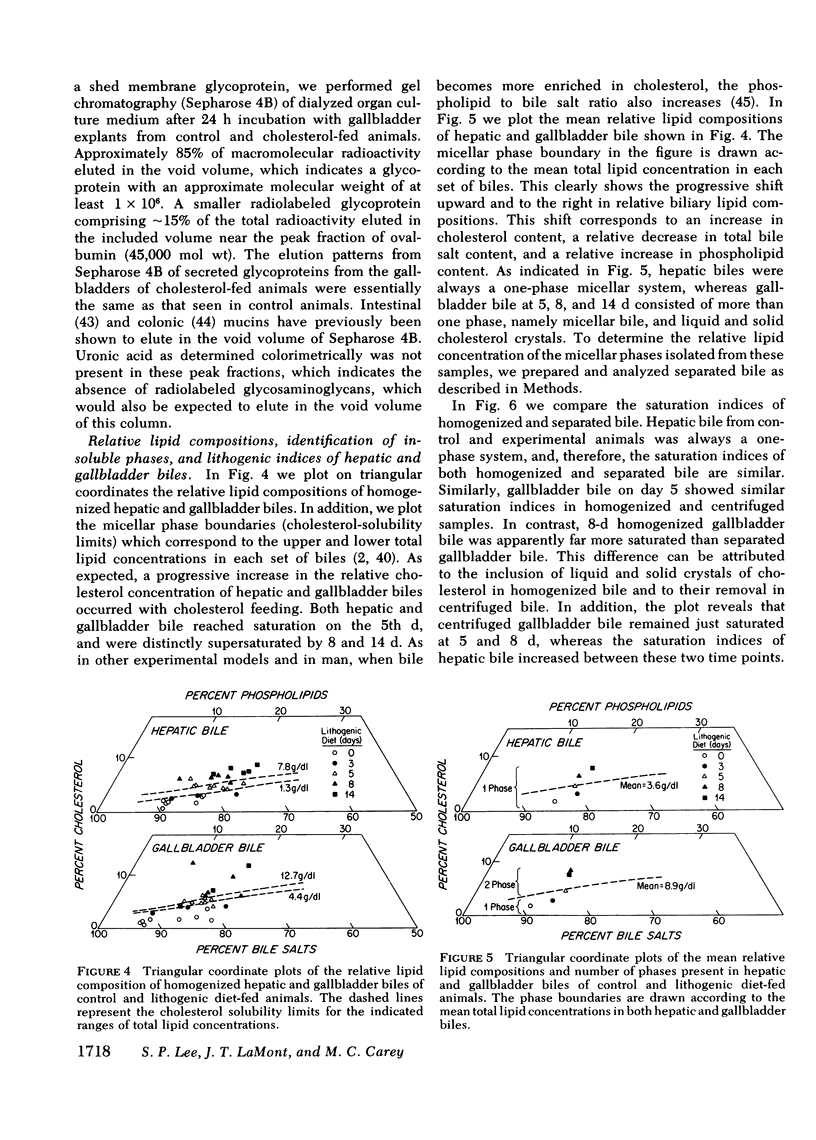
Abstract
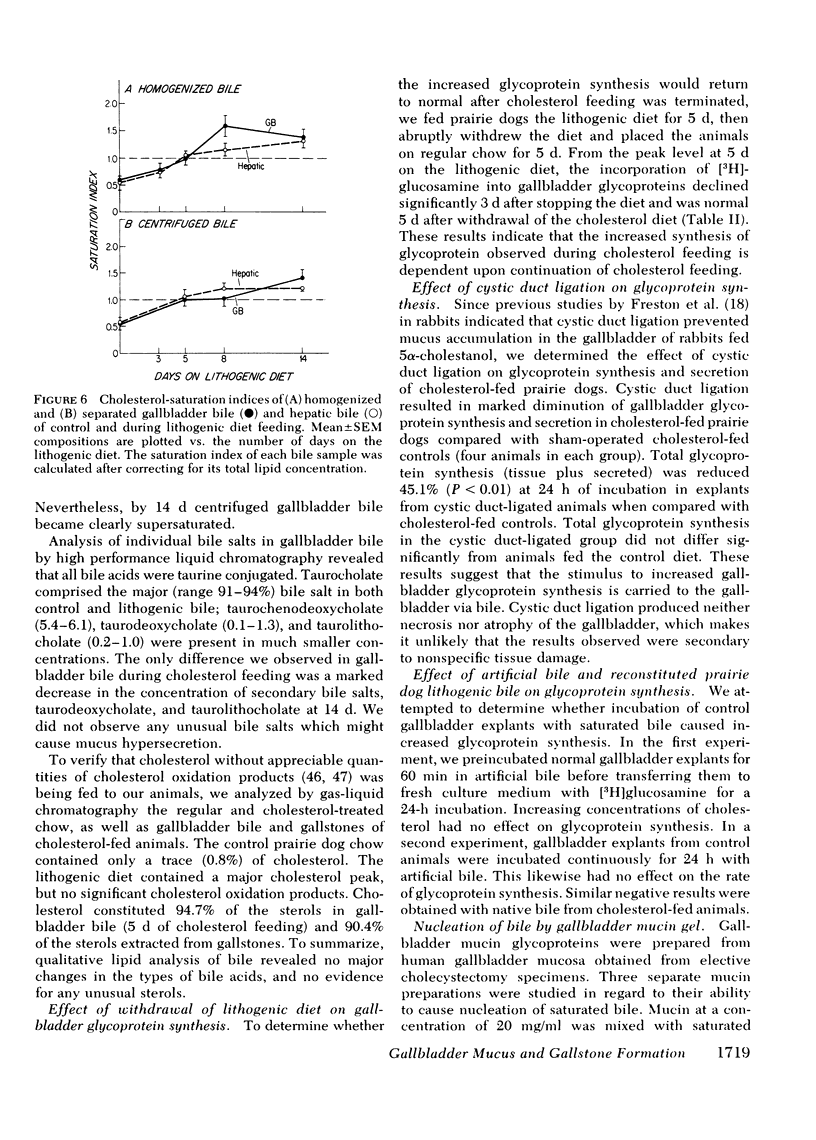
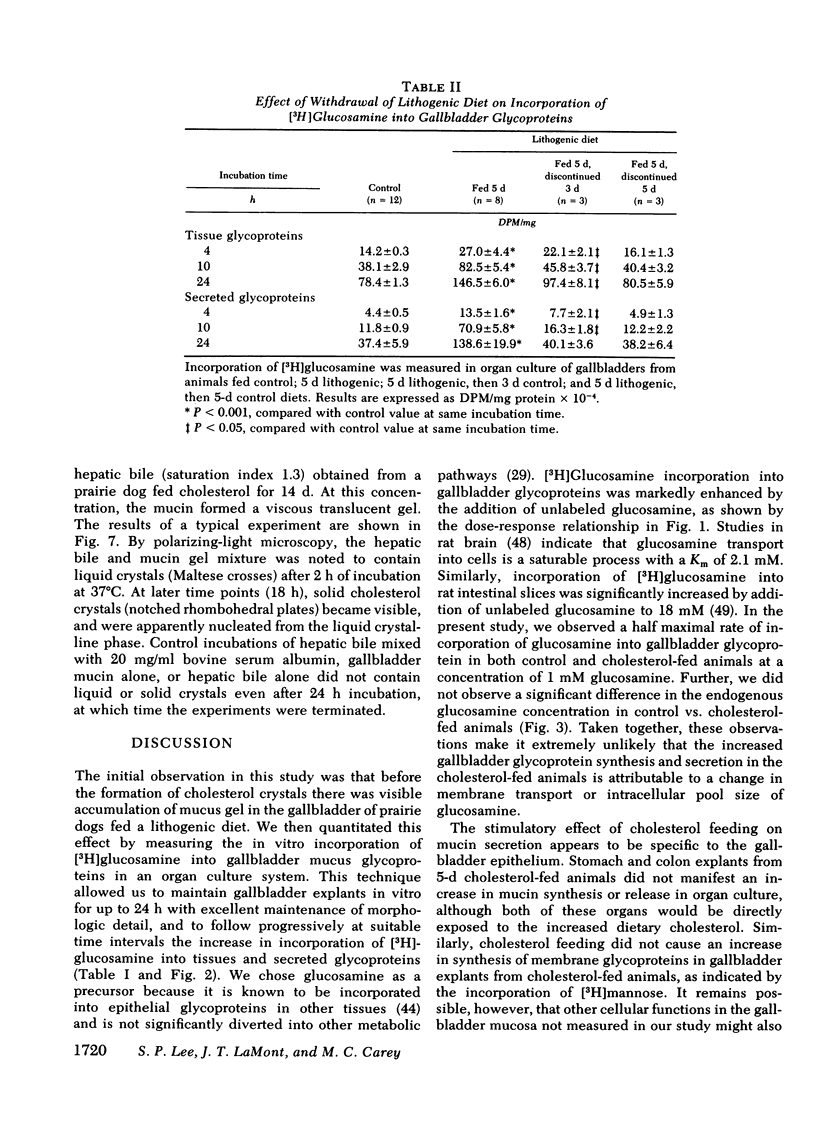
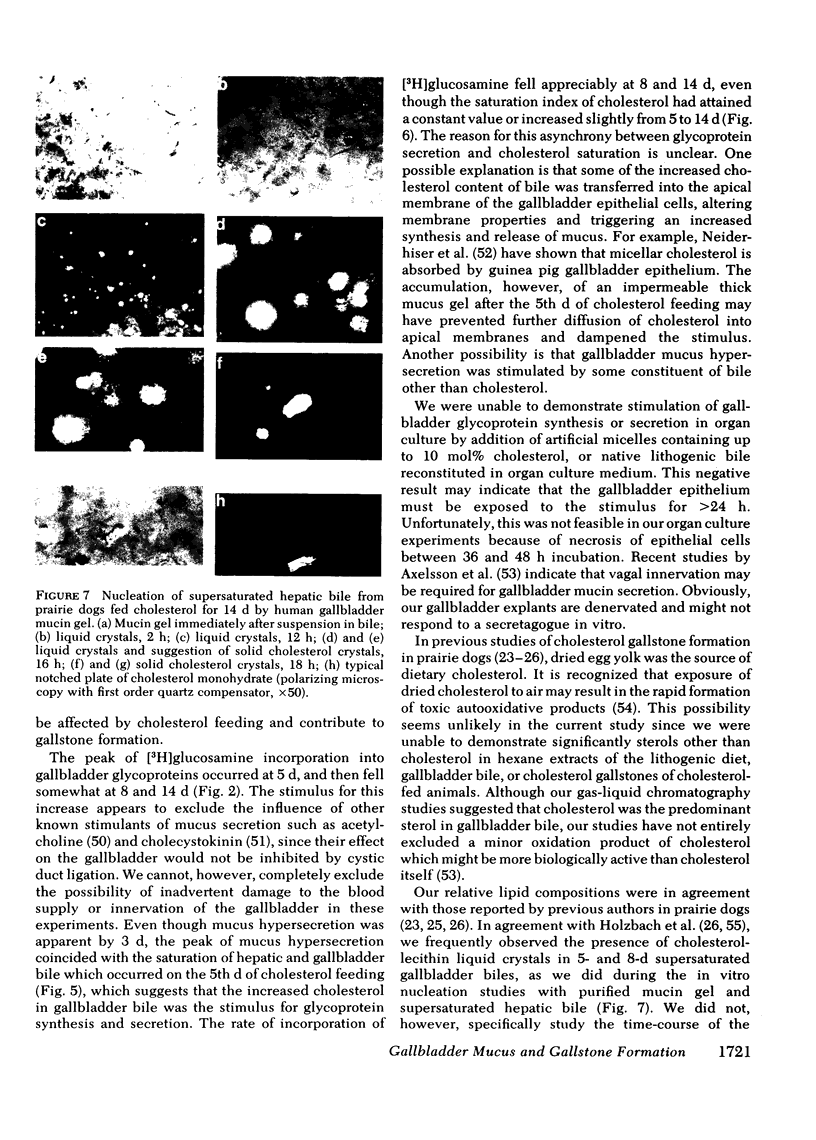
Because mucin glycoproteins may be important in the pathophysiology of gallstones, we studied the relationship among biliary lipids, gallbladder mucin secretion, and gallstone formation in cholesterol-fed prairie dogs. Organ culture studies of gallbladder explants revealed that the incorporation of [3H]glucosamine into tissue and secretory gallbladder glycoproteins was significantly increased at 3, 5, 8, and 14 d of feeding. Peak secretion of labeled mucin occurred at 5 d, when total tissue and secreted glycoprotein production was fivefold greater than control. Gel filtration of the secreted glycoprotein on Sepharose 4B indicated that the majority of radioactivity was present in a macromolecule of > 1 million molecular weight. The increased secretion of gallbladder mucin was organ specific, in that [3H]glucosamine incorporation into glycoproteins of stomach and colon was unaffected by cholesterol feeding. Similarly, the incorporation of [3H]mannose into gallbladder membrane glycoproteins was not altered by cholesterol feeding. The rate of glycoprotein synthesis and secretion returned to normal upon withdrawal of the cholesterol diet, and ligation of the cystic duct before cholesterol feeding prevented gallbladder mucin hypersecretion. Both results indicate that the stimulus to mucin secretion was a constituent of bile. Gallbladder bile after 5 d contained cholesterol in micelles, liquid crystals, and crystals, whereas hepatic bile remained a single micellar phase throughout cholesterol feeding. For this reason the cholesterol-saturation indices of gallbladder bile were compared in both homogenized and centrifuged samples. The micellar phase of gallbladder bile was appreciably less saturated than homogenized bile at 5 and 8 d, which reflects the continuous nucleation of cholesterol in the gallbladder. Purified human gallbladder mucin gels were shown to induce nucleation of lecithin-cholesterol liquid crystals from supersaturated hepatic bile. These in turn gave rise to cholesterol monohydrate crystals within 18 h. Control supersaturated hepatic bile could not be nucleated by the addition of other proteins, and was stable for days upon standing. These results suggest that the increase in cholesterol content of bile in cholesterolfed prairie dogs stimulates gallbladder mucus hypersecretion, and that gallbladder mucus gel is a nucleating agent for biliary cholesterol.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL L. L., LEVY B. B., BRODIE B. B., KENDALL F. E. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952 Mar;195(1):357–366. [PubMed] [Google Scholar]
- Adler R. D., Metzger A. L., Grundy S. M. Biliary lipid secretion before and after cholecystectomy in American Indians with cholesterol gallstones. Gastroenterology. 1974 Jun;66(6):1212–1217. [PubMed] [Google Scholar]
- Admirand W. H., Small D. M. The physicochemical basis of cholesterol gallstone formation in man. J Clin Invest. 1968 May;47(5):1043–1052. doi: 10.1172/JCI105794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers D. H., Philpott G. W. Control of deoxyribonucleic acid synthesis in normal rabbit colonic mucosa. Gastroenterology. 1975 Oct;69(4):951–959. [PubMed] [Google Scholar]
- Axelsson H., Danielsson A., Henriksson R., Wahlin T. Secretory behavior and ultrastructural changes in mouse gallbladder principal cells after stimulation with cholinergic and adrenergic drugs. A morphometric study. Gastroenterology. 1979 Feb;76(2):335–340. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bekesi J. G., Bekesi E., Winzler R. J. Inhibitory effect of D-glucosamine and other sugars on the biosynthesis of protein, ribonucleic acid, and deoxyribonucleic acid in normal and neoplastic tissues. J Biol Chem. 1969 Jul 25;244(14):3766–3772. [PubMed] [Google Scholar]
- Bennion L. J., Grundy S. M. Effects of obesity and caloric intake on biliary lipid metabolism in man. J Clin Invest. 1975 Oct;56(4):996–1011. doi: 10.1172/JCI108180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchier I. A., Cooperband S. R., el-Kodsi B. M. Mucous substances and viscosity of normal and pathological human bile. Gastroenterology. 1965 Oct;49(4):343–353. [PubMed] [Google Scholar]
- Brenneman D. E., Connor W. E., Forker E. L., DenBesten L. The formation of abnormal bile and cholesterol gallstones from dietary cholesterol in the prairie dog. J Clin Invest. 1972 Jun;51(6):1495–1503. doi: 10.1172/JCI106946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning T. H., Trier J. S. Organ culture of mucosal biopsies of human small intestine. J Clin Invest. 1969 Aug;48(8):1423–1432. doi: 10.1172/JCI106108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARR J. J., DREKTER I. J. Simplified rapid technic for the extraction and determination of serum cholesterol without saponification. Clin Chem. 1956 Oct;2(5):353–368. [PubMed] [Google Scholar]
- Carey M. C. Critical tables for calculating the cholesterol saturation of native bile. J Lipid Res. 1978 Nov;19(8):945–955. [PubMed] [Google Scholar]
- Carey M. C., Small D. M. The physical chemistry of cholesterol solubility in bile. Relationship to gallstone formation and dissolution in man. J Clin Invest. 1978 Apr;61(4):998–1026. doi: 10.1172/JCI109025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrin L. W., Baker A. P., Christian P., Wardell J. R., Jr Effect of cholinergic stimulation on the release of macromolecules by canine trachea in vitro. Am Rev Respir Dis. 1973 Jul;108(1):69–76. doi: 10.1164/arrd.1973.108.1.69. [DOI] [PubMed] [Google Scholar]
- Chang S. H., Ho K. J., Taylor C. B. Cholesterol gallstone formation and its regression in prairie dogs. Arch Pathol. 1973 Dec;96(6):417–426. [PubMed] [Google Scholar]
- DenBesten L., Safaie-Shirazi S., Connor W. E., Bell S. Early changes in bile composition and gallstone formation induced by a high cholesterol diet in prairie dogs. Gastroenterology. 1974 May;66(5):1036–1045. [PubMed] [Google Scholar]
- Dowling R. H., Bell G. D., White J. Lithogenic bile in patients with ileal dysfunction. Gut. 1972 Jun;13(6):415–420. doi: 10.1136/gut.13.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englert E., Jr, Harman C. G., Freston J. W., Straight R. C., Wales E. E., Jr Studies on the pathogenesis of diet-induced dog gallstones. Am J Dig Dis. 1977 Apr;22(4):305–314. doi: 10.1007/BF01072187. [DOI] [PubMed] [Google Scholar]
- Forsdyke D. R. Application of the isotope-dilution principle to the analysis of factors affecting the incorporation of (3H) uridine and (3H) cytidine into cultured lymphocytes. Evaluation of pools in serum and culture media. Biochem J. 1971 Dec;125(3):721–732. doi: 10.1042/bj1250721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freston J. W., Bouchier I. A., Newman J. Biliary mucous substances in dihydrocholesterol-induced cholelithiasis. Gastroenterology. 1969 Dec;57(6):670–678. [PubMed] [Google Scholar]
- HOFMANN A. F., MOSBACH E. H. IDENTIFICATION OF ALLODEOXYCHOLIC ACID AS THE MAJOR COMPONENT OF GALLSTONES INDUCED IN THE RABBIT BY 5-ALPHA-CHOLESTAN-3-BETA-OL. J Biol Chem. 1964 Sep;239:2813–2821. [PubMed] [Google Scholar]
- Holan K. R., Holzbach R. T., Hermann R. E., Cooperman A. M., Claffey W. J. Nucleation time: a key factor in the pathogenesis of cholesterol gallstone disease. Gastroenterology. 1979 Oct;77(4 Pt 1):611–617. [PubMed] [Google Scholar]
- Holzbach R. T., Corbusier C. Liquid crystals and cholesterol nucleation during equilibration in supersaturated bile analogs. Biochim Biophys Acta. 1978 Mar 30;528(3):436–444. doi: 10.1016/0005-2760(78)90033-4. [DOI] [PubMed] [Google Scholar]
- Holzbach R. T., Corbusier C., Marsh M., Naito H. K. The process of cholesterol cholelithiasis induced by diet in the prairie dog: a physicochemical characterization. J Lab Clin Med. 1976 Jun;87(6):987–998. [PubMed] [Google Scholar]
- Holzbach R. T., Marsh M., Olszewski M., Holan K. Cholesterol solubility in bile. Evidence that supersaturated bile is frequent in healthy man. J Clin Invest. 1973 Jun;52(6):1467–1479. doi: 10.1172/JCI107321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultén O. Formation of gallstones. II. Acta Chir Scand. 1968;134(7):557–561. [PubMed] [Google Scholar]
- JUNIPER K., Jr, BURSON E. N., Jr Biliary tract studies. II. The significance of biliary crystals. Gastroenterology. 1957 Feb;32(2):175-208; discussion, 208-11. [PubMed] [Google Scholar]
- LaMont J. T., Ventola A. Stimulation of colonic glycoprotein synthesis by dibutyryl cyclic AMP and theophylline. Gastroenterology. 1977 Jan;72(1):82–86. [PubMed] [Google Scholar]
- Lamont J. T., Ventola A. Synthesis and secretion of colonic glycoproteins: evidence for shedding in vivo of low molecular weight membrane components. Biochim Biophys Acta. 1980 May 22;629(3):553–565. [PubMed] [Google Scholar]
- Lee S. P., Carey M. C., LaMont J. T. Aspirin prevention of cholesterol gallstone formation in prairie dogs. Science. 1981 Mar 27;211(4489):1429–1431. doi: 10.1126/science.7466399. [DOI] [PubMed] [Google Scholar]
- Lee S. P., Lim T. H., Scott A. J. Carbohydrate moieties of glycoproteins in human hepatic and gall-bladder bile, gall-bladder mucosa and gall stones. Clin Sci (Lond) 1979 Jan;56(6):533–538. doi: 10.1042/cs0560533. [DOI] [PubMed] [Google Scholar]
- Loomis C. R., Janiak M. J., Small D. M., Shipley G. G. The binary phase diagram of lecithin and cholesteryl linolenate. J Mol Biol. 1974 Jun 25;86(2):309–324. doi: 10.1016/0022-2836(74)90021-7. [DOI] [PubMed] [Google Scholar]
- Loomis C. R., Shipley G. G., Small D. M. The phase behavior of hydrated cholesterol. J Lipid Res. 1979 May;20(4):525–535. [PubMed] [Google Scholar]
- Lukie B. E., Forstner G. G. Synthesis of intestinal glycoprotein. Incorporation of (I- 14 C) glucosamine in vitro. Biochim Biophys Acta. 1971 Feb 28;261(2):353–364. [PubMed] [Google Scholar]
- MIETTINEN T. A., AHRENS E. H., Jr, GRUNDY S. M. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL DIETARY AND FECAL NEUTRAL STEROIDS. J Lipid Res. 1965 Jul;6:411–424. [PubMed] [Google Scholar]
- Neiderhiser D. H., Harmon C. K., Roth H. P. Absorption of cholesterol by the gallbladder. J Lipid Res. 1976 Mar;17(2):117–124. [PubMed] [Google Scholar]
- Northfield T. C., Hofmann A. F. Biliary lipid output during three meals and an overnight fast. I. Relationship to bile acid pool size and cholesterol saturation of bile in gallstone and control subjects. Gut. 1975 Jan;16(1):1–11. doi: 10.1136/gut.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S. K., Tham P., Taylor C. B., Mikkelson B. Cytotoxicity of oxidation derivatives of cholesterol on cultured aortic smooth muscle cells and their effect on cholesterol biosynthesis. Am J Clin Nutr. 1979 May;32(5):1033–1042. doi: 10.1093/ajcn/32.5.1033. [DOI] [PubMed] [Google Scholar]
- Qureshi R., Forstner G. G., Forstner J. F. Radioimmunoassay of human intestinal goblet cell mucin. Investigation of mucus from different organs and species. J Clin Invest. 1979 Nov;64(5):1149–1156. doi: 10.1172/JCI109568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedaghat A., Grundy S. M. Cholesterol crystals and the formation of cholesterol gallstones. N Engl J Med. 1980 Jun 5;302(23):1274–1277. doi: 10.1056/NEJM198006053022302. [DOI] [PubMed] [Google Scholar]
- Small D. M. Cholesterol nucleation and growth in gallstone formation. N Engl J Med. 1980 Jun 5;302(23):1305–1307. doi: 10.1056/NEJM198006053022309. [DOI] [PubMed] [Google Scholar]
- TALALAY P. Enzymic analysis of steroid hormones. Methods Biochem Anal. 1960;8:119–143. doi: 10.1002/9780470110249.ch3. [DOI] [PubMed] [Google Scholar]
- Tan C. H., Peterson N. A., Raghupathy E. D-Glucosamine uptake by rat brain synaptosomes. Biochim Biophys Acta. 1977 Jan 21;464(2):459–462. doi: 10.1016/0005-2736(77)90020-7. [DOI] [PubMed] [Google Scholar]
- WOMACK N. A., ZEPPA R., IRVIN G. L., 3rd The anatomy of gallstones. Ann Surg. 1963 May;157:670–686. doi: 10.1097/00000658-196305000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlin T., Bloom G. D., Danielsson A. Effect of cholecystokinin-pancreozymin (CCK-PZ) on glycoprotein secretion from mouse gallbladder epithelium: an ultrastructural and cytochemical study. Cell Tissue Res. 1976 Sep 1;171(4):425–435. doi: 10.1007/BF00220235. [DOI] [PubMed] [Google Scholar]
- Womack N. A. The development of gallstones. Surg Gynecol Obstet. 1971 Dec;133(6):937–945. [PubMed] [Google Scholar]