A specific H+ antiport system mediates the vacuolar uptake of terpenoid indole alkaloids in Catharanthus roseus.
Abstract
Catharanthus roseus is one of the most studied medicinal plants due to the interest in their dimeric terpenoid indole alkaloids (TIAs) vinblastine and vincristine, which are used in cancer chemotherapy. These TIAs are produced in very low levels in the leaves of the plant from the monomeric precursors vindoline and catharanthine and, although TIA biosynthesis is reasonably well understood, much less is known about TIA membrane transport mechanisms. However, such knowledge is extremely important to understand TIA metabolic fluxes and to develop strategies aimed at increasing TIA production. In this study, the vacuolar transport mechanism of the main TIAs accumulated in C. roseus leaves, vindoline, catharanthine, and α-3′,4′-anhydrovinblastine, was characterized using a tonoplast vesicle system. Vindoline uptake was ATP dependent, and this transport activity was strongly inhibited by NH4+ and carbonyl cyanide m-chlorophenyl hydrazine and was insensitive to the ATP-binding cassette (ABC) transporter inhibitor vanadate. Spectrofluorimetry assays with a pH-sensitive fluorescent probe showed that vindoline and other TIAs indeed were able to dissipate an H+ gradient preestablished across the tonoplast by either vacuolar H+-ATPase or vacuolar H+-pyrophosphatase. The initial rates of H+ gradient dissipation followed Michaelis-Menten kinetics, suggesting the involvement of mediated transport, and this activity was species and alkaloid specific. Altogether, our results strongly support that TIAs are actively taken up by C. roseus mesophyll vacuoles through a specific H+ antiport system and not by an ion-trap mechanism or ABC transporters.
Alkaloids form a very diverse and prominent family of plant natural products, including many compounds with important pharmaceutical applications. Paramount examples are taxol and vinblastine (VLB), used as anticancer drugs, and morphine, used as a pain killer. In plants, alkaloids play a key role in defense against pathogens and herbivores, with plant-herbivore coevolution possibly having determined the strong physiological activity of these compounds in animals. The metabolism of medically important alkaloids has been thoroughly investigated, and much is known about their biosynthetic pathways. However, little is known about the transport and accumulation mechanisms of alkaloids, in spite of their importance for the final output of metabolic fluxes.
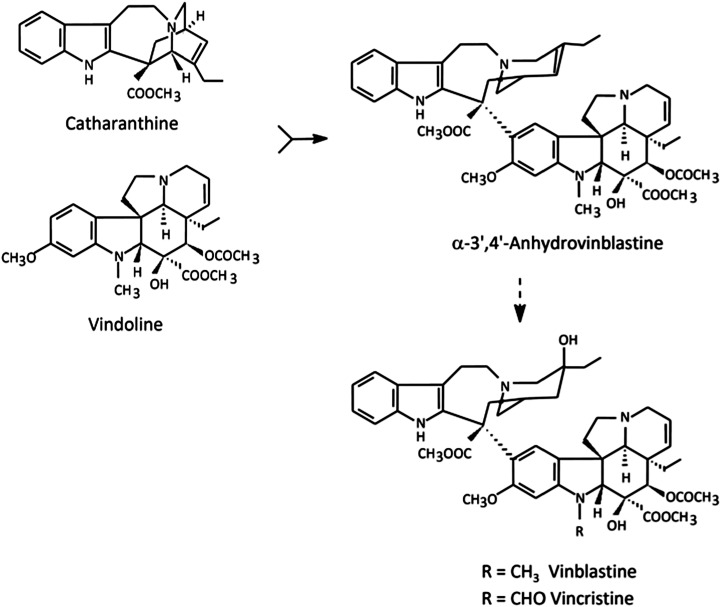
A paradigmatic example of highly valuable alkaloids produced in very low levels in the plant are the anticancer terpenoid indole alkaloids (TIAs) of Catharanthus roseus, VLB and vincristine (Fig. 1; Verpoorte et al., 2007). The great pharmacological importance of TIAs, associated with their low abundance in the plant (approximately 0.0005% dry weight), stimulated intense research on the TIA pathway, and C. roseus has become one of the most studied medicinal plants (van der Heijden et al., 2004; Verpoorte et al., 2007; Costa et al., 2008). TIA biosynthesis shows multicellular compartmentation in C. roseus leaves, with early steps occurring in epidermal cells and late steps occurring in laticifer and mesophyll idioblast cells, predicting intercellular translocation of TIA intermediates (St-Pierre et al., 1999; Murata et al. 2008; Guirimand et al., 2011). Moreover, the TIA pathway also shows a complex subcellular organization, with different parts of the pathway being localized in the plastids, the vacuole, the cytosol, and the endoplasmic reticulum (ER), predicting further transport events (Mahroug et al., 2007; Guirimand et al., 2011). In spite of the importance of all those TIA transmembrane transport steps as putative rate-limiting steps of the alkaloid metabolic fluxes, knowledge about the transport mechanisms involved is still very poor.
Figure 1.
Biosynthesis of VLB and vincristine from the monomeric precursors catharanthine and vindoline. AVLB is the product of the dimerization reaction and the direct precursor of the anticancer drugs.
Early work suggested that vacuolar accumulation of TIAs and other alkaloids was mediated either by highly specific carriers (Deus-Neumann and Zenk, 1984, 1986; Wink, 1993) or by an unspecific ion-trap mechanism in which the alkaloids, being weak bases, accumulate by diffusion in the acidic vacuole (Guern et al., 1987; Renaudin, 1989; Blom et al., 1991; Wink, 1993). However, recent work on alkaloid transmembrane transport has consistently supported the H+/alkaloid antiport mechanism for the alkaloids berberine in Coptis japonica and nicotine in tobacco (Nicotiana tabacum; Otani et al., 2005; Morita et al., 2009; Shoji et al., 2009). A plasma membrane influx permease functioning as a proton symporter of nicotine has also been characterized in tobacco, and two ATP-binding cassette (ABC) transporters, CjMDR1 and CjABCB2, were implicated in plasma membrane influx in C. japonica (Shitan et al., 2003, 2012; Hildreth et al., 2011). In C. roseus, nothing is known about TIA plasma membrane transport, and vacuolar transport remains poorly characterized.
Here, the vacuolar accumulation in C. roseus mesophyll cells of the main leaf TIAs vindoline, catharanthine, and α-3′,4′-anhydrovinblastine (AVLB) was characterized in highly pure tonoplast vesicles. Uptake of vindoline was dependent on ATP, with accumulation being strongly inhibited by H+ gradient dissipators and unaffected by the ABC transporter inhibitor vanadate. Likewise, vindoline and other TIAs induced dissipation of a preestablished pH gradient across the tonoplast, with proton movements following Michaelis-Menten kinetics, suggesting mediated transport. Overall, our results indicate that TIAs are accumulated in the vacuole of C. roseus mesophyll cells through a specific proton antiport system.
RESULTS
TIAs Are Accumulated Inside the Vacuoles of C. roseus Mesophyll Cells
Vacuoles are considered the final accumulation target of alkaloids, where they are thought to function as a toxic defense and do not interfere with basic plant cell metabolism (Wink, 1993). Therefore, the transtonoplast transport mechanism is particularly important for alkaloid metabolic fluxes. In order to confirm the general assumption that alkaloids are accumulated in the vacuoles for C. roseus mesophyll cells, the alkaloid profiles of leaves, isolated protoplasts, and vacuoles were investigated.
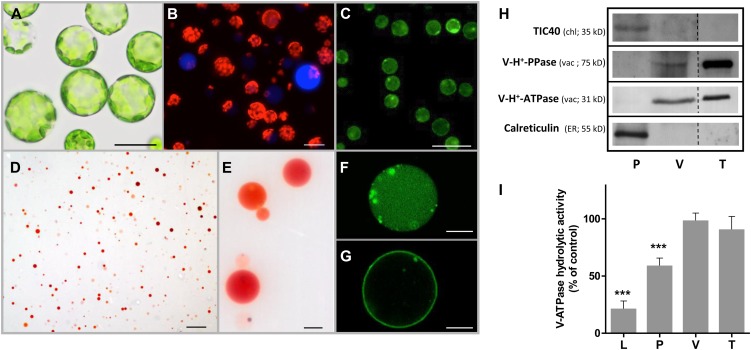
The protoplast suspension isolated from C. roseus leaves was highly pure, and the naked cells showed no apparent membrane damage or disintegration of the internal structure (Fig. 2A). The presence of protoplast idioblasts was confirmed by observation of their conspicuous blue fluorescence under the fluorescence microscope (Fig. 2B). Protoplast viability was estimated by staining with fluorescein diacetate (FDA) as being 95% to 98% (Fig. 2C). Under the light microscope, the final vacuole fraction showed no noticeable protoplast or chloroplast contamination, and the accumulation of neutral red inside the vacuoles indicated that a transmembrane pH gradient was maintained inside the organelles, confirming the integrity of the tonoplast (Fig. 2, D and E). Purified vacuoles exhibited intense fluorescence with Fluo 4-AM and FM1-43, indicating that the capacity to store high amounts of calcium and membrane integrity were preserved (Fig. 2, F and G). The vacuole preparation was free of markers defining other endomembrane compartments, including the ER and chloroplasts, and was highly enriched in vacuolar markers (Fig. 2H). Furthermore, ATP hydrolytic activity was insensitive to the plasma membrane H+-ATPase (P-H+-ATPase) inhibitor vanadate and the mitochondria H+-ATPase (F-H+-ATPase) inhibitor azide, indicating respectively the absence of contamination with plasma membrane or mitochondrial membranes and confirming the high purity of the vacuole fraction (Fig. 2I).
Figure 2.
Characterization of the purity of protoplasts, vacuoles, and tonoplast vesicles isolated from C. roseus leaves. A to G, Optical microscopy images of protoplast and vacuole populations. A, Bright-field image of protoplasts. B, Fluorescence image of protoplasts with merging of the red and blue channels. Red corresponds to chloroplast autofluorescence, and blue fluorescence reveals alkaloid-accumulating idioblast cells. C, Fluorescence image of protoplasts labeled with FDA. D and E, Bright-field images of intact vacuoles stained with neutral red. F and G, Confocal images of intact vacuoles labeled with Fluo 4-AM (F) and FM1-43 (G). Bars = 20 µm (A and B), 100 µm (C and D), and 10 µm (E to G). H, Western blots of protein extracts from protoplasts (P), vacuoles (V), and tonoplast vesicles (T), using specific antibodies raised against the chloroplast inner envelope protein TIC40, the vacuole-specific V-H+-PPase and V-H+-ATPase (subunit ε), and the ER-resident protein calreticulin. I, V-H+-ATPase hydrolytic activity of protein extracts from leaves (L), protoplasts (P), vacuoles (V), and tonoplast vesicles (T). For each fraction, ATP hydrolytic activity was measured in the presence of 0.5 mm azide (a mitochondrial ATPase inhibitor) and 100 µm vanadate (a plasma membrane ATPase inhibitor) and expressed as the percentage of the total ATP hydrolytic activity in the absence of inhibitors (142, 304, 451, and 1,839 nmol min−1 mg−1 protein for L, P, V, and T, respectively), revealing the residual percentage of activity corresponding to the V-H+-ATPase present in each fraction. Statistical significance was evaluated using Student’s t test for pairwise comparison (***P < 0.001). Data significantly different from the control are indicated. Error bars indicate sd from three biological replicates with two biochemical replicates each.
HPLC-diode array detector (DAD) analysis of alkaloid extracts from leaves showed that the main TIAs accumulated in C. roseus mesophyll cells are by far vindoline, catharanthine, and AVLB (Supplemental Fig. S1), respectively the two monomeric and the first dimeric precursors of the anticancer TIAs (Fig. 1). Analysis of protoplast and vacuole fractions showed the presence of high amounts of vindoline and catharanthine in both fractions, confirming the accumulation of these TIAs inside the vacuoles. AVLB was not found in isolated vacuoles but was present in the protoplasts (Table I).
Table I. TIA content estimated for leaves, protoplasts, and vacuoles.
n.d. Not detected.
Uptake of Vindoline by Tonoplast Vesicles Is Dependent on ATP
In order to investigate the mechanism of vacuolar accumulation of TIAs, preliminary uptake experiments were performed with intact vacuoles. However, these organelles were highly unstable in the presence of the TIAs; therefore, tonoplast vesicles were isolated from C. roseus leaves and evaluated as a potential system for the study of TIA transport. As shown before for intact vacuoles, the tonoplast fraction was highly pure, with strong enrichment of vacuolar markers and the absence of ER and chloroplast markers (Fig. 2H). In agreement, ATP hydrolytic activity was not significantly affected by plasma membrane and mitochondria ATPase inhibitors (Fig. 2I).
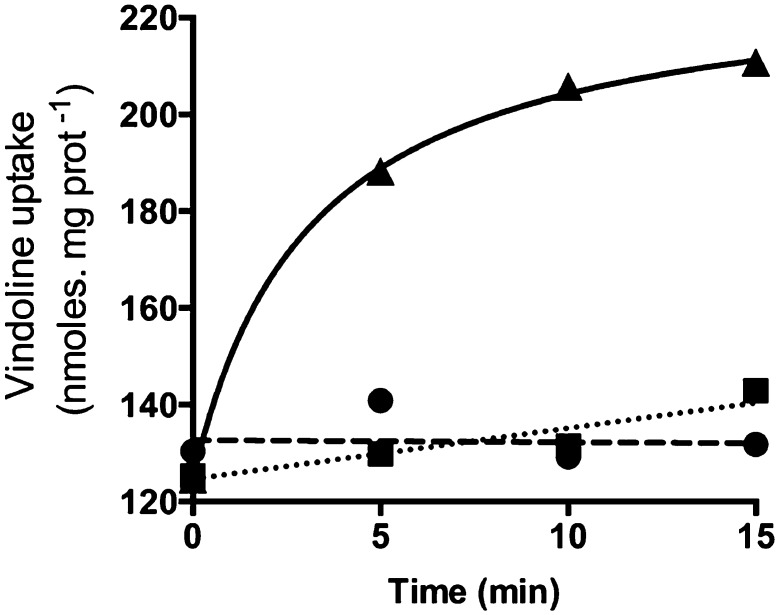
In order to measure the uptake of vindoline by tonoplast vesicles, the transport reaction proceeded in the presence of an ATP-regenerating system, which enabled a continuous energization of the vesicles for periods up to 30 min (Supplemental Fig. S2). After incubation, tonoplast vesicles were immediately recovered at ice-cold temperature by ultracentrifugation and were submitted to TIA extraction and analysis by HPLC-DAD. A time-course experiment revealed a rapid uptake of vindoline in the presence of ATP, whereas no uptake was observed in its absence, indicating that ATP is required for vindoline uptake (Fig. 3). In order to investigate if this was primary transport mediated by an ABC transporter, uptake was assayed in the presence of the specific inhibitor vanadate (Table II). This compound did not significantly affect vindoline uptake, ruling out direct transport by an ATP-dependent pump. On the other hand, the proton gradient dissipators NH4Cl and carbonyl cyanide m-chlorophenyl hydrazine (CCCP) significantly compromised ATP-dependent vindoline uptake, indicating the involvement of an ATP-dependent transtonoplast pH gradient. To further clarify the importance of the proton gradient, the time-course experiment was also performed in the presence of NH4Cl (Fig. 3), showing that dissipation of the proton gradient abolished the ATP-dependent vindoline uptake at all time points.
Figure 3.
Time course of vindoline uptake into tonoplast vesicles from C. roseus leaves. Tonoplast vesicles were incubated in the presence of 1 mm vindoline (circles), 1 mm vindoline, 1 mm ATP, 10 mm creatine phosphate, and 10 µg mL−1 creatine kinase (triangles), or 1 mm vindoline, 1 mm ATP, 10 mm creatine phosphate, 10 µg mL−1 creatine kinase, and 1.5 mm NH4Cl (squares). Values are means of two independent experiments.
Table II. Characterization of ATP-dependent vindoline uptake.
Tonoplast vesicles were incubated with 1 mm vindoline for 15 min under the different conditions listed. SD values are from at least three biological replicates (*P < 0.05, **P < 0.01).
| Condition | Uptake Vindoline |
|---|---|
|
% |
|
| +3 mm ATP |
100 |
| +3 mm ATP + NH4Cl (1.5 mm) |
2.8 ± 1.6** |
| +3 mm ATP + CCCP (50 μm) |
32.6 ± 0.07* |
| +3 mm ATP + vanadate (1 mm) | 113.8 ± 14.9 |
TIA Accumulation by C. roseus Tonoplast Vesicles Is H+ Dependent
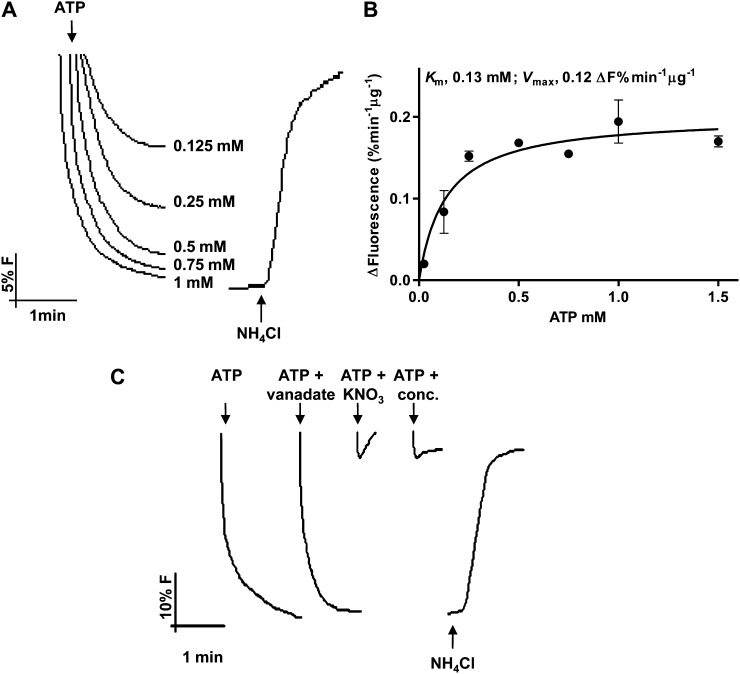
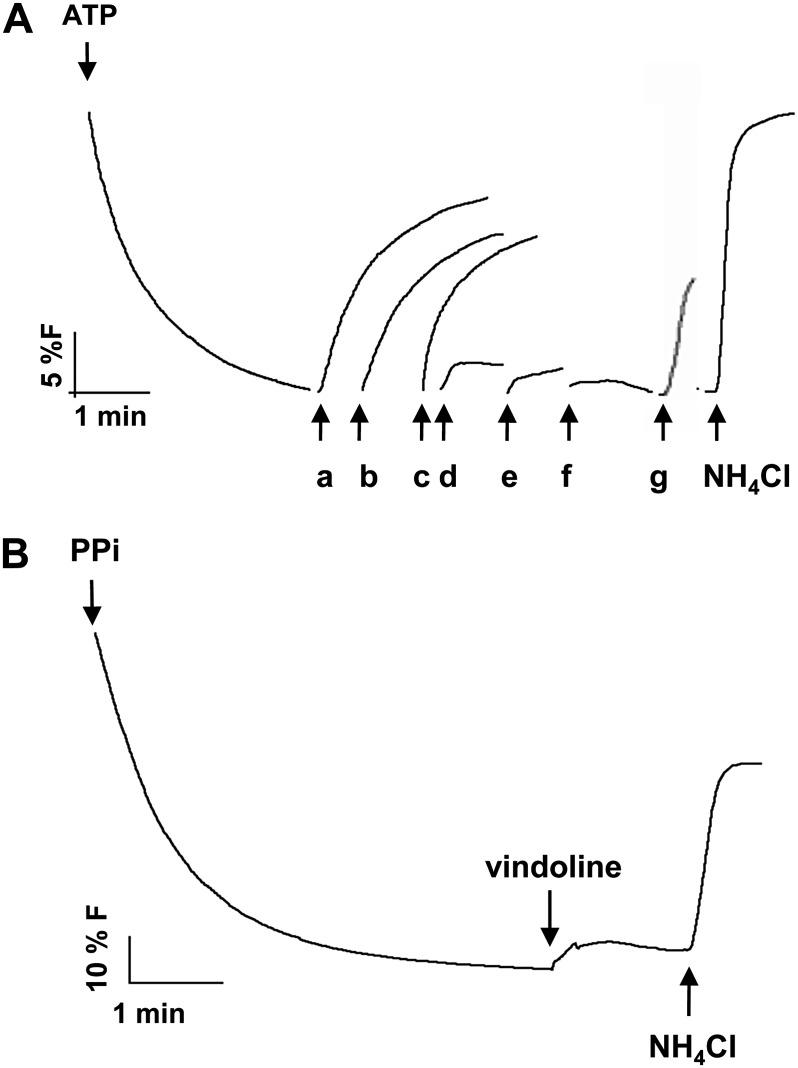
Following the indication that vindoline uptake could be dependent on a transtonoplast pH gradient, the capacity of ATP to generate a ΔpH across the membrane of the tonoplast vesicles was further studied using the pH-sensitive fluorescent probe 9-amino-6-chloro-2-methoxyacridine (ACMA; Fig. 4). Immediate fluorescence quenching signals were observed after the addition of ATP, following Michaelis-Menten kinetics, and with fluorescence promptly recovering upon addition of NH4Cl, all this demonstrating the generation of a transtonoplast pH gradient by an active vacuolar (V)-H+-ATPase (Fig. 4, A and B). The tonoplast-specific V-H+-pyrophosphatase (PPase) was also active in the isolated membranes (Supplemental Fig. S3, A and B), which were also shown to present high ATP and pyrophosphate (PPi) hydrolytic activities (Supplemental Fig. S3, C and D). The behavior of both H+ pumps in tonoplast vesicles was comparable to that measured in isolated vacuoles (Supplemental Fig. S4). The generation of the transtonoplast pH gradient by ATP was insensitive to the P-H+-ATPase inhibitor vanadate and was completely blocked by the tonoplast V-H+-ATPase-specific inhibitors concanamycin and KNO3, further confirming the purity of the membrane fraction (Fig. 4C).
Figure 4.
Pumping activities of V-H+-ATPase in tonoplast vesicles isolated from C. roseus leaves measured by the fluorescence quenching of the pH-sensitive probe ACMA. A, H+-pumping activity in tonoplast vesicles upon the addition of ATP. B, Michaelis-Menten plot of the initial rates of proton pumping in A. Values are means of three biological replicates. C, H+-pumping activity in the presence of the P-H+-ATPase inhibitor vanadate (100 µm) and the V-H+-ATPase inhibitors KNO3 (100 µm) and concanamycin (0.1 µm). Error bars indicate sd (n = 3).
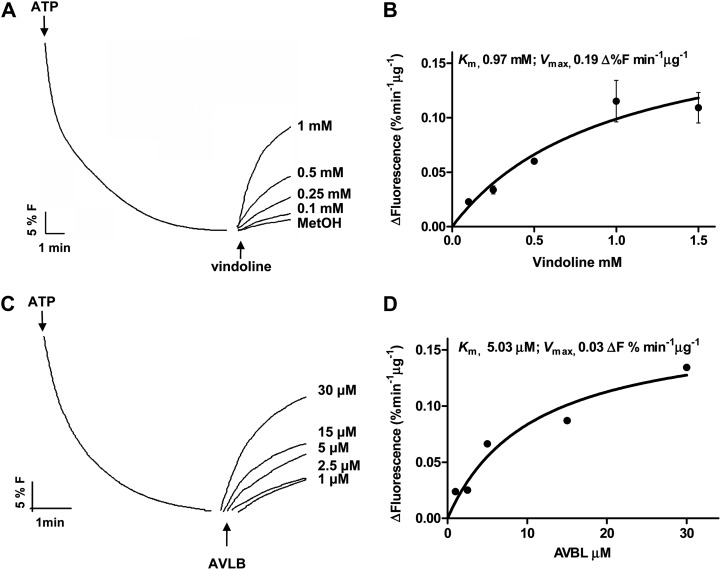
Vindoline, catharanthine, and AVLB, the main TIAs present in C. roseus mesophyll (Table I), promptly dissipated the proton gradient, indicating that catharanthine and AVLB are also incorporated into the vacuole by an H+-dependent mechanism (Fig. 5A). A positive signal was also observed after the addition of Ca2+ (Fig. 5A), in agreement with the known and observed capacity of the vacuoles to accumulate this cation (Fig. 2F; Hirschi, 2001). On the contrary, the alkaloids atropine, ajmaline, and papaverine, which are not produced by C. roseus, did not dissipate the H+ gradient (Fig. 5A). Significantly, vindoline did not affect the transtonoplast pH gradient of energized tonoplast vesicles isolated from grape (Vitis vinifera), a species that does not produce TIAs (Fig. 5B). The initial velocities of intravesicular alkalinization upon addition of vindoline and AVLB followed Michaelis-Menten kinetics, supporting the involvement of mediated transport for both substrates (Fig. 6). Vindoline was shown to also induce fluorescence recovery in tonoplast vesicles energized with PPi (data not shown).
Figure 5.
Effects of the addition of different solutes to a preestablished H+ gradient measured by the fluorescence quenching of the pH-sensitive probe ACMA in tonoplast vesicles isolated from C. roseus leaves and grape cell cultures. A, Addition of different solutes to energized C. roseus tonoplast vesicles: 0.03 mm AVLB (a), 1 mm vindoline (b), and 0.075 mm catharanthine (c) from C. roseus; 1 mm atropine (d) from Atropa belladonna; 0.05 mm ajmaline (e) from Rauwolfia serpentina; 0.1 mm papaverine (f) from Papaver somniferum; and 0.15 mm CaCl2 (g). B, Addition of 1 mm vindoline to energized grape tonoplast vesicles.
Figure 6.
Proton-dependent transport of vindoline and AVLB in tonoplast vesicles isolated from C. roseus leaves. A, Dissipation of a preestablished H+ gradient by different concentrations of vindoline, measured by the fluorescence recovery of the pH-sensitive probe ACMA. MetOH, Methanol. B, Michaelis-Menten plot of the initial rates of proton dissipation by vindoline in A. Error bars indicate sd (n = 3). C, Dissipation of a preestablished H+ gradient by different concentrations of AVLB, measured by the fluorescence recovery of the pH-sensitive probe ACMA. D, Michaelis-Menten plot of the initial rates of proton dissipation by AVLB in C. Results in D are from a single experiment.
The hydrolytic activity of the V-H+-ATPase in the presence of vindoline, catharanthine, or AVLB was also studied, to discard the possibility that the alkaloid-induced fluorescence recovery could be due to an alkaloid inhibition effect on the proton pump. The results showed that after a long incubation period (30 min) in the presence of high TIA concentrations (1 mm vindoline, 0.09 mm catharanthine, and 0.05 mm AVLB), there was a slight inhibition effect (11%, 28%, and 6%, respectively), by far not sufficient to explain the prompt and high fluorescence recovery observed (Figs. 5A and 6). Moreover, in proton transport assays, TIA addition prior to ATP did not significantly compromise the buildup of a pH gradient across the tonoplast (data not shown), and the alkaloids atropine, ajmaline, and papaverine did not cause any dissipation of the H+ gradient, further indicating that alkaloids were not inhibitory under the conditions used in the spectrofluorimetry assays.
Altogether, these results strongly indicated that TIAs dissipate the pH gradient in C. roseus vesicles due to the presence of a specific H+/TIA antiport system.
DISCUSSION
The membrane transport of plant secondary metabolites is still poorly characterized, but it is emerging as a newly developing research area due to their importance to understand metabolite fluxes and to implement metabolic engineering strategies aimed at increasing the levels of valuable secondary metabolites. In the case of alkaloids, their complex pathways involve several cell types and organelles, with a number of transmembrane steps being inferred (Shitan and Yazaki, 2007; Facchini and De Luca, 2008). Moreover, the final intracellular destination of most alkaloids is thought to be the vacuole, with the transtonoplast transport step putatively playing a key role for the final output of alkaloid metabolic fluxes. Here, a number of tools for the characterization of the membrane transport of alkaloids in C. roseus were established, the vacuolar localization of the main TIAs accumulated in mesophyll cells was determined, and the vacuolar accumulation mechanism of those TIAs was elucidated.
In this study, protocols for the isolation of highly pure, functional vacuoles and tonoplast vesicles from C. roseus leaves were established. These methods may now be used for many studies concerning vacuolar functions in C. roseus. Both the vacuoles and the tonoplast vesicle samples isolated were shown to be highly pure, meeting the purity standards used in previous studies (Carter et al., 2004; Jaquinod et al., 2007). On the other hand, both isolated vacuoles and tonoplast vesicles were shown to be functionally active in what concerns transport activities, indicating the preservation of active membrane protein complexes and membrane integrity.
HPLC analysis of the alkaloids extracted from C. roseus leaves clearly shows that the main TIAs accumulated in C. roseus mesophyll cells are by far vindoline, catharanthine, and AVLB (Supplemental Fig. S1). These are the direct precursors of the anticancer agents VLB and vincristine (Fig. 1); therefore, their cellular accumulation mechanisms may be particularly important for the final levels of the anticancer TIAs. In C. roseus, TIAs have been shown to accumulate in the vacuoles by histochemical and cytological techniques (Yoder and Mahlberg, 1976; Neumann et al., 1983), the TIA serpentine was shown to accumulate mostly in the vacuoles of cell cultures (Deus-Neumann and Zenk, 1984), and immunocytochemical localization of vindoline indicated its major presence in the central vacuole and in small vesicles of mesophyll cells (Brisson et al., 1992). However, a thorough characterization of the subcellular accumulation of TIAs in the leaves of C. roseus, the only organ where the anticancer TIAs are accumulated, has not been performed. Our results here indicate that vindoline and catharanthine, two of the three main TIAs present in C. roseus leaves, are localized mostly in the vacuoles (Table I). The third major TIA, AVLB, was not detected in isolated vacuoles but it is present in protoplasts, also suggesting vacuolar accumulation, since TIAs present in these naked cells can only be in the vacuole, sequestered away from the cytosol. This may suggest that, during vacuole isolation, AVLB is either lost by efflux or undergoes further metabolism or degradation, namely by the action of the vacuolar Catharanthus roseus peroxidase1 (CrPrx1), which was shown to oxidate AVLB (Costa et al., 2008). Specific accumulation of AVLB in vesicles/peripheral small vacuoles, rather than in the central vacuole, cannot be discarded as well. The TIA contents observed for leaf isolated protoplasts and vacuoles are 5- to 10-fold higher than the content of the TIA serpentine quantified for isolated vacuoles of C. roseus cell cultures (Deus-Neumann and Zenk, 1984) and 13- to 17-fold lower than the nicotine content determined for tobacco mesophyll vacuoles (Saunders, 1979).
The primary localization of catharanthine in the vacuoles observed here contrasts with a previous study showing that catharanthine accumulates “exclusively” in leaf wax exudates (Roepke et al., 2010). According to these authors, this delocalization of catharanthine relative to intracellular vindoline accounts for the low levels of the dimeric anticancer TIAs in C. roseus leaves. This is clearly not the case in the leaves of the C. roseus plants used in this study, since they accumulate high levels of AVLB (dimer), which can only result from colocalization of its monomeric precursors, catharanthine and vindoline. The differences observed in the two studies may result from the use of different cultivars, developmental stages, and growth conditions, all factors that may likely affect the compartmentalization expression of the TIA pathway.
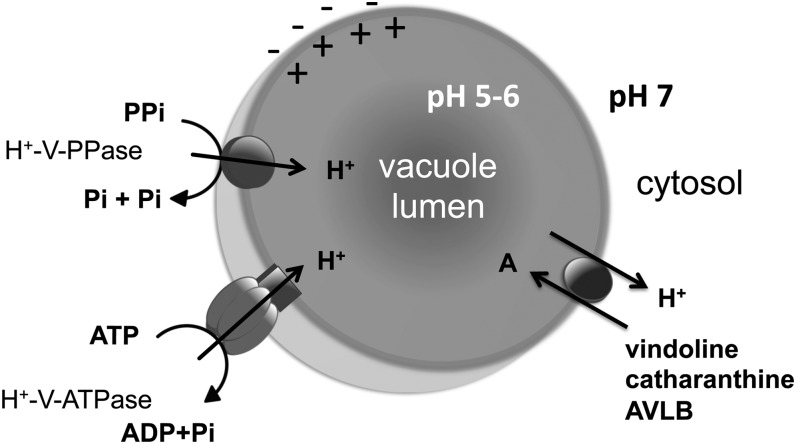
The observed vacuolar accumulation of vindoline, together with previous localization studies indicating that the last biosynthetic step of vindoline occurs in the cytosol (Vazquez-Flota et al., 1997), suggest an intensive transport of vindoline across the tonoplast, which is most likely true for the other TIAs. The results obtained here strongly indicate that TIAs are accumulated in the vacuoles of C. roseus mesophyll cells by a specific proton antiport system dependent on a transtonoplast pH gradient generated either by the V-H+-ATPase or the V-H+-PPase (Fig. 7). In fact, vindoline uptake was shown to be dependent on ATP (Fig. 3) and on the transtonoplast ΔpH, as supported by the observed inhibition with the H+ gradient dissipators NH4Cl and CCCP (Table II). In agreement, assays with the pH probe ACMA clearly indicated that several TIAs were capable of dissipating an ATP-dependent H+ gradient, and proton movements followed Michaelis-Menten kinetics (Figs. 5A and 6). Previously, TIAs have been suggested to accumulate in the vacuoles by an ion-trap mechanism, in which the cytosolic neutral form of the basic alkaloid is capable of diffusing freely across the tonoplast to form a cation at the acidic vacuolar pH, becoming trapped inside this organelle (Guern et al., 1987; Renaudin, 1989; Blom et al., 1991). Here, alkaloid uptake not only showed saturation (Fig. 6, B and D), incompatible with this mechanism, but was also alkaloid and species specific (Fig. 5), excluding the action of any unspecific mechanism such as ion trapping. The transmembrane transport of alkaloids may also be mediated by primary transport through ABC transporters, as in the case of berberine uptake across the plasma membrane of rhizome cells of C. japonica, where this alkaloid is accumulated upon translocation from the biosynthetic organ, the root (Shitan et al., 2003, 2012). Vacuolar accumulation of other secondary metabolites, such as anthocyanins, was also shown to involve ABC transporters (Goodman et al., 2004). However, in this study, vindoline accumulation by ATP-energized tonoplast vesicles was clearly not affected by the ABC transporter inhibitor vanadate, and accumulation was also sustained by PPi energization, excluding the involvement of an ABC transporter as the principal tonoplast transporter for vindoline vacuolar accumulation.
Figure 7.
Model proposed for TIA vacuolar accumulation in C. roseus mesophyll cells. Cytosolic TIAs are actively transported into the vacuole by a proton antiport system dependent on the transtonoplast pH gradient generated by either of the two tonoplast vacuolar pumps, V-H+-ATPase or V-H+-PPase. Pi, Inorganic phosphate.
The TIA transport assays indicate that the alkaloid transport system exhibits a higher affinity for AVLB and catharanthine than for vindoline (Figs. 5A and 6). Previous work from Deus Neumann and Zenk (1984) has actually estimated a Km for vindoline in the range of that determined here for AVLB. However, the two studies are most likely not comparable, since those authors used vacuoles from cell suspension cultures, which do not produce vindoline, and seem to have determined reaction velocities at 90 min, possibly affecting Km estimation. The concentration of vindoline in planta, estimated using the volume of protoplasts and vacuoles, is within the 1 mm range (Table I; r = 1.4 and 0.95 µm, respectively). Yet, we have to take into consideration that only a fraction of cells should accumulate vindoline (the idioblasts); thus, its concentration must achieve much higher values, well above the vindoline Km observed in this work. The dissimilar results for the different TIAs may be due to the involvement of different transport proteins or of one protein type with different affinities for each substrate. On the other hand, the behavior of the transporters in isolated tonoplast vesicles may differ substantially from in vivo, where many factors may influence or actually regulate transporter activity. It must be stressed that the vacuolar transport activity determined here is not necessarily the single transport mode present in the diversity of C. roseus leaf cells.
Overall, the results gathered in this study are consistent with the early work from Deus Neumann and Zenk (1984) suggesting the involvement of an active, energy-requiring process with high specificity for the accumulation of ajmalicine and vindoline in the vacuoles of C. roseus cell cultures. Moreover, these results are consistent with the observed H+-dependent vacuolar accumulation of the alkaloids berberine from C. japonica and nicotine from tobacco (Otani et al., 2005; Morita et al., 2009; Shoji et al., 2009). In the case of nicotine, vacuolar transport was shown to be mediated by one of three multidrug and toxic compound extrusion (MATE) transporters functioning as proton antiporters, which are differentially expressed in the root and leaves of the plant (Morita et al., 2009; Shoji et al., 2009). Altogether, these results raise the question of whether MATE transporters may be generally responsible for alkaloid vacuolar accumulation in plants. The plant MATE transporter subfamily is characterized by a high number of gene orthologs per species (58 in Arabidopsis), contrasting with bacterial and animal subfamilies (two in human), suggesting an association with the highly diverse secondary metabolism found in plants (Omote et al., 2006).
CONCLUSION
In conclusion, this study strongly indicates that the important alkaloids from the medicinal plant C. roseus, namely vindoline, catharanthine, and AVLB, are accumulated in the vacuoles of mesophyll cells by a specific proton antiport system, dependent on the transtonoplast pH gradient generated by V-H+-ATPase and V-H+-PPase (Fig. 7). This is in agreement with previous observations for the alkaloids berberine in C. japonica and nicotine in tobacco and further supports an H+ antiport mechanism as a general system for vacuolar accumulation of alkaloids in plants.
The approaches and results of this study open new perspectives to the search for vacuolar TIA transporter candidate genes, with MATE transporters being obvious targets. The question remains whether there is one vacuolar transporter with different affinities toward different TIAs, or there may exist an array of different vacuolar transporters with cell/organ differential localizations.
MATERIALS AND METHODS
Plant Material
Catharanthus roseus ‘Little Bright Eye’ plants were grown at 25°C in a growth chamber, under a 16-h photoperiod, using white fluorescent light with a maximum intensity of 70 μmol m−2 s−1. Seeds were acquired from AustraHort, and voucher specimens are deposited at the Herbarium of the Department of Biology of the Faculty of Sciences, University of Porto (PO 61912). Plants used for protoplast and vacuole isolation were 6 to 8 months old.
Isolation of C. roseus Mesophyll Protoplasts
C. roseus mesophyll protoplasts were obtained using a protocol adapted from Zheng et al. (1997) and Sottomayor et al. (1996). Approximately eight to 10 leaves (approximately 1.5–2 g) of adult plants (usually second and third pairs from the tip) were cut into approximately 1-mm strips and transferred to a petri dish with 10 mL of digestion medium composed of 2% (w/v) cellulase (Onozuka R-10; Duchefa), 0.3% (w/v) macerozyme (Onozuka R-10; Serva), and 0.1% (v/v) pectinase (Sigma) dissolved in MM buffer (0.4 m mannitol in 20 mm MES, pH 5.6–5.8). The medium was vacuum infiltrated for 15 min, and leaf strips were incubated for 3 h at 25°C in the dark. The suspension was then filtered through a 100-µm nylon mesh, and the filtrate was transferred into 15-mL Falcon tubes. The protoplast suspension was centrifuged at 65g for 5 min at 20°C, the supernatant was removed, and the protoplasts were washed four times in MM buffer. After the final wash, protoplasts were counted using a hemocytometer. The integrity of the isolated protoplasts was checked by observation with an optical microscope (Olympus), and images were acquired by a coupled Olympus DP 25 digital camera and respective software (Cell B; Olympus). To check the viability of the isolated protoplasts, these were stained with 5 µg mL−1 FDA and observed with the epifluorescence microscope (Leica Microsystems; DM-5000B) using excitation and emission wavelengths of 494 and 530 nm, respectively (Jones and Senft, 1985).
Isolation of C. roseus Mesophyll Vacuoles
The isolation of C. roseus mesophyll vacuoles was performed according to Fontes et al. (2010) with minor modifications. The isolated mesophyll protoplasts were centrifuged at 65g for 5 min at 20°C, and the protoplast pellets were chilled for at least 30 min on ice. Vacuoles were released following protoplast osmotic lysis at 42°C for 10 min in 9 mL of lysis buffer consisting of 150 µg mL−1 bovine serum albumin (BSA), 2 mm dithiothreitol (DTT), 0.2 m mannitol, 10% (w/v) Ficoll 400, and 15 mm EDTA in 10 mm MOPS, pH 8. The mixture was overlaid with 3 mL of 3% Ficoll in a 1:2 mixture of lysis and vacuole buffers and with 1 mL of vacuole buffer consisting of 2 mm DTT and 0.5 m mannitol in 10 mm MOPS, pH 7.5. Isolated vacuoles were recovered in the vacuole buffer layer after centrifuging at 1,000g for 17 min at room temperature. For visualization with the optical microscope, vacuoles were stained with neutral red and were counted using a hemocytometer. Vacuole integrity was tested using 10 µm FM1-43 (a membrane marker; Betz et al., 1996) and 3 μm Fluo 4-AM (an intracellular Ca2+ indicator). Labeling with the fluorescent probes was performed for 30 min at room temperature in the dark. FM1-43 and Fluo 4-AM fluorescence was examined using an SP2 AOBS SE confocal microscope (Leica) equipped with a scan head with an argon laser. Visualization of the fluorescent probes was performed using an excitation wavelength of 488 nm and an emission wavelength window from 500 to 650 nm.
Isolation of Tonoplast Vesicles
Tonoplast vesicles from C. roseus leaves were isolated according to Façanha and de Meis (1998) and Queirós et al. (2009) with minor modifications. Approximately 15 g of leaves was homogenized using an Ultra-Turrax T25 apparatus (IKA, Janke & Kunkel), at 13,000 rpm for 5 min at 4°C, in extraction buffer consisting of 1 mm phenylmethylsulfonyl fluoride, 0.1% (w/v) BSA, 2 mm DTT, 250 mm Suc, 3 mm MgCl2, 100 mm KCl, and 2 mm EDTA in 70 mm Tris-HCl, pH 8. The homogenate was filtered through four layers of cheesecloth and centrifuged for 10 min at 4°C at 10,000g. The supernatant was centrifuged at 100,000g for 1 h, and the pellet was resuspended in 8 mL of resuspension buffer consisting of 1 mm DTT, 1 mm phenylmethylsulfonyl fluoride, 15% (v/v) glycerol, and 1 mm EDTA in 20 mm Tris-HCl, pH 7.5, homogenized with a glass potter homogenizer (40-mL vessel; tight pestle; Wheaton), overlaid on a 32%/46% Suc step gradient in resuspension buffer, and centrifuged at 80,000g for 3 h at 4°C. The tonoplast fraction was obtained at the 0%/32% interface, diluted in resuspension buffer, and centrifuged at 100,000g for 45 min at 4°C. The membrane pellet was resuspended in 0.3 to 1 mL of resuspension buffer, frozen with liquid nitrogen, and kept at −80°C until use. Tonoplast vesicles from grape (Vitus vinifera) cell cultures were isolated according to Queirós et al. (2009) with the modifications from Martins et al. (2012). Protein quantification was performed using the method described by Lowry et al. (1951).
Protein Extraction and Quantification
For protein extraction, protoplasts, vacuoles, and tonoplast vesicles in the respective isolation buffers were frozen at −80°C and lyophilized for 2 d in a freeze-dry Edwards apparatus. Lyophilized samples were reconstituted in 50 mm Tris, pH 7.5, dialyzed against the same buffer to remove mannitol, and stored at −20°C until use. Protein extraction from C. roseus leaves was described previously by Ferreres et al. (2011). Protein concentration of the dialyzed samples was determined according to the method described by Bradford (1976).
Western-Blot Analysis
Protein samples obtained as described above were separated on 10% acrylamide gels as described by Laemmli (1970) and transferred to a nitrocellulose membrane. For the immunodetection of soluble proteins, samples were boiled for 2 min prior to gel loading, while for the immunodetection of membrane proteins, protein samples were heated at 70°C for 10 min and immediately loaded onto the gel. The amount of protein loaded was 10 µg, except for the extract of tonoplast vesicles used for V-H+-ATPase and V-H+-PPase detection, where 1 µg was used. The following primary antibodies were used: an ER marker, rabbit antiserum raised against calreticulin (a gift from J. Denecke, University of Leeds), at a 1:10,000 dilution; a chloroplast marker, rabbit antiserum raised against the chloroplast inner envelope TIC40 protein (AS 10709-10; Agrisera), at a 1:2,500 dilution; and two tonoplast markers, rabbit antisera raised against a V-H+-ATPase (AS 07213; Agrisera) and a V-H+-PPase (Maeshima and Yoshida, 1989), both at a 1:2,000 dilution. The secondary antibody used was a peroxidase-conjugated goat anti-rabbit antibody (Santa Cruz Biotechnology) at a 1:7,500 dilution, and detection was performed with the chemiluminescent substrate ECL (GE Healthcare, Lifesciences).
Determination of ATP and PPi Hydrolytic Activities
Rates of ATP and PPi hydrolysis were determined by measuring the release of inorganic phosphate, according to Vera-Estrella et al. (1994), with some modifications. For protoplasts, vacuoles, and tonoplast vesicles, ATP and PPi hydrolytic activities were assayed in fractions after freezing and thawing. For leaves, total protein extracts obtained as described by Ferreres et al. (2011) were used. Fifteen micrograms of total protein (in a maximum volume of 15 µL) was mixed with 300 µL of 3 mm ATP, 0.02% Triton X-100, 50 mm KCl, 1 mm sodium molybdate, and 6 mm MgSO4 in 30 mm Tris-MES, pH 8, and incubated for 30 min at 37°C with slow agitation. The reaction was stopped by the addition of 500 µL of cold 10% TCA and 4% perchloric acid, samples were kept 2 min on ice and centrifuged for 3 min at 2,400g, and 500 µL of the supernatant was mixed with 1.3 mL of Ames solution composed of 1 volume of 10% ascorbic acid mixed with 6 volumes of 4.2 g of ammonium molybdate and 28.6 mL of H2SO4 in 1 L of water (Ames, 1966). After 15 min at room temperature in the dark, absorbance was read at 820 nm using a blank control performed without protein and NaH2PO4 as a standard to build a calibration curve. For the determination of V-H+-ATPase hydrolytic activity, two different inhibitors were used, the F-H+-ATPase inhibitor sodium azide at 0.5 mm and the P-H+-ATPase inhibitor vanadate at 0.1 mm. Thus, the V-H+-ATPase hydrolytic activity presented in Supplemental Figure S3 was estimated as the difference between the total hydrolytic activity and the activity in the presence of both inhibitors.
Proton Transport Assays
ATP and PPi proton-dependent transport across the tonoplast was measured as the initial rate of fluorescence quenching of ACMA, according to Façanha and de Meis (1998), with minor modifications. For intact vacuoles, 104 vacuoles were used in each reaction; for tonoplast vesicles, a volume corresponding to 50 μg of tonoplast proteins was used in each reaction. The reaction mixture (1 mL) was composed of 5 mm MgCl2 and 2 µm ACMA in reaction buffer (30 mm KCl and 50 mm NaCl in 20 mm HEPES, pH 7.2). When using intact vacuoles, the mixture was supplemented with 0.1% (w/v) BSA. For the V-H+-ATPase activity assay, the reaction started with the addition of 0.05 to 1.5 mm ATP. For the V-H+-PPase activity assay, the reaction started with the addition of 0.00125 to 0.1 mm PPi. The protonophore 1.5 mm NH4Cl was added to confirm the establishment of an H+ gradient. Fluorescence quenching was registered by an LS-5B fluorescence spectrophotometer (Perkin-Elmer) at excitation and emission wavelengths of 415 and 485 nm, respectively. Reactions were performed at room temperature, and the results were expressed as Δ fluorescence in % min−1 µg−1 protein. Experimental data were analyzed with GraphPad Prism software. For the determination of V-H+-ATPase proton pump activity, three different inhibitors were used: the P-H+-ATPase inhibitor vanadate (100 µm), the V-H+-ATPase inhibitor nitrate (KNO3; 50 mm), and the V-H+-ATPase inhibitor concanamycin A (0.1 µm).
Assay of Proton Antiport Activities
For C. roseus tonoplast vesicles, the occurrence of transmembrane proton-exchange activities with several ions and compounds was inferred by the dissipation of a preformed pH gradient established by the addition of 1 mm ATP, using the conditions described for the proton transport assays above. For grape tonoplast vesicles, a volume corresponding to 30 μg of tonoplast proteins was used, the reaction buffer was composed of 2 μm ACMA, 100 mm KCl, 2 mm MgCl2, and 0.1% BSA (w/v) in 10 mm MOPS-Tris, pH 7.2, and energization was performed by the addition of 50 µm PPi. After the fluorescence quenching of ACMA reached a steady state, different ions and compounds were added with the specified concentrations, and uptake was inferred by measuring the fluorescence recovery of the ACMA probe during the first 15 s of reaction. The results were expressed as Δ fluorescence in % min−1 µg−1 protein. Experimental data were analyzed with GraphPad Prism software.
Uptake Assay
To assay the uptake of vindoline into tonoplast vesicles, membranes corresponding to 100 µg of proteins were incubated in reaction buffer in the presence of an ATP regenerator system (Marinova et al., 2007) consisting of 1 mm DTT, 5 mm MgCl2, 10 mm creatine phosphate, and 10 µg mL−1 creatine kinase (C9983-100UG; Sigma) in a final volume of 1.2 mL. Energization of the tonoplast vesicles was initiated by the addition of 3 mm ATP, 1 mm vindoline was added after 1 min, and the reaction proceeded for 15 min at room temperature with gentle shaking. To stop the reaction, the assay was transferred to ice, and the vesicles were immediately isolated from the medium by centrifugation at 100,000g for 30 min at 4°C. The supernatant was discarded, and the pellet was superficially washed with ice-cold reaction buffer and dried with nitrogen. The ABC inhibitor vanadate (1 mm) was added 5 min prior to ATP (Frangne et al., 2002), and the H+ gradient dissipators NH4Cl (1.5 mm) and CCCP (50 µm) were added to the reaction mixture after ATP and before vindoline addition. Control reactions were performed in the absence of ATP, inhibitors, and creatine kinase and subtracted from all other assays.
Alkaloid Extraction and HPLC-DAD Analysis
Alkaloid extraction and HPLC-DAD analysis were performed as described by Sottomayor et al. (1996), except for alkaloid extraction from tonoplast vesicles used in uptake assays, where alkaloids were directly extracted with HPLC-grade methanol. In all cases, the biological material was always lyophilized prior to extraction.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. HPLC chromatogram of an alkaloid extract from C. roseus leaves.
Supplemental Figure S2. H+-pumping activity in tonoplast vesicles upon the addition of 1 mm ATP in the presence or absence of an ATP-regenerating system (10 mm creatine phosphate plus 10 µg mL−1 creatine kinase).
Supplemental Figure S3. Pumping activities of V-H+-PPase in tonoplast vesicles, and ATP and PPi hydrolytic activities in vacuoles and tonoplast vesicles isolated from C. roseus leaves.
Supplemental Figure S4. Pumping activities of V-H+-PPase and V-H+-ATPase in intact vacuoles from C. roseus leaves.
Supplementary Material
Acknowledgments
We thank Dr. Jürgen Denecke (University of Leeds) for the gift of the anti-calreticulin, Dr. Masayoshi Maeshima (Nagoya University) for the gift of the anti-V-H+-PPase, Richard Gonçalves (University of Minho) for technical assistance with the HPLC device, and Dr. João Cabral and Dr. Jorge Azevedo (Instituto de Biologia Molecular e Celular) for reviewing the manuscript.
Glossary
- TIA
terpenoid indole alkaloid
- VLB
vinblastine
- ER
endoplasmic reticulum
- ABC
ATP-binding cassette
- AVLB
α-3′,4′-anhydrovinblastine
- FDA
fluorescein diacetate
- ACMA
9-amino-6-chloro-2-methoxyacridine
- V
vacuolar
- PPi
pyrophosphate
- MATE
multidrug and toxic compound extrusion
- BSA
bovine serum albumin
- DTT
dithiothreitol
References
- Ames BN. (1966) Assay of inorganic phosphate, total phosphate and phosphatases. Methods Enzymol 8: 115–118 [Google Scholar]
- Betz WJ, Mao F, Smith CB. (1996) Imaging exocytosis and endocytosis. Curr Opin Neurobiol 6: 365–371 [DOI] [PubMed] [Google Scholar]
- Blom TJM, Sierra M, Van Vliet TB, Franke-Van Dijk MEI, De Koning P, Van Iren F, Verpoorte R, Libbenga KR. (1991) Uptake and accumulation of ajmalicine into isolated vacuoles of cultured cells of Catharanthus roseus (L.) G. Don. and its conversion into serpentine. Planta 183: 170–177 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brisson L, Charest PM, De Luca V, Ibrahim RK. (1992) Immunocytochemical localization of vindoline in mesophyll protoplasts of Catharanthus roseus. Phytochemistry 31: 465–470 [Google Scholar]
- Carter C, Pan SQ, Zouhar J, Avila EL, Girke T, Raikhel NV. (2004) The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 16: 3285–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa MMR, Hilliou F, Duarte P, Pereira LG, Almeida I, Leech M, Memelink J, Barceló AR, Sottomayor M. (2008) Molecular cloning and characterization of a vacuolar class III peroxidase involved in the metabolism of anticancer alkaloids in Catharanthus roseus. Plant Physiol 146: 403–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deus-Neumann B, Zenk MH. (1984) A highly selective alkaloid uptake system in vacuoles of higher plants. Planta 162: 250–260 [DOI] [PubMed] [Google Scholar]
- Deus-Neumann B, Zenk MH. (1986) Accumulation of alkaloids in plant vacuoles does not involve an ion-trap mechanism. Planta 167: 44–53 [DOI] [PubMed] [Google Scholar]
- Façanha AR, de Meis L. (1998) Reversibility of H+-ATPase and H+-pyrophosphatase in tonoplast vesicles from maize coleoptiles and seeds. Plant Physiol 116: 1487–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facchini PJ, De Luca V. (2008) Opium poppy and Madagascar periwinkle: model non-model systems to investigate alkaloid biosynthesis in plants. Plant J 54: 763–784 [DOI] [PubMed] [Google Scholar]
- Ferreres F, Figueiredo R, Bettencourt S, Carqueijeiro I, Oliveira J, Gil-Izquierdo A, Pereira DM, Valentão P, Andrade PB, Duarte P, et al (2011) Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: an H2O2 affair? J Exp Bot 62: 2841–2854 [DOI] [PubMed] [Google Scholar]
- Fontes N, Silva R, Vignault C, Lecourieux F, Gerós H, Delrot S. (2010) Purification and functional characterization of protoplasts and intact vacuoles from grape cells. BMC Res Notes 3: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangne N, Eggmann T, Koblischke C, Weissenböck G, Martinoia E, Klein M. (2002) Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles: energization occurs by H+-antiport and ATP-binding cassette-type mechanisms. Plant Physiol 128: 726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CD, Casati P, Walbot V. (2004) A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays. Plant Cell 16: 1812–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guern J, Renaudin JP, Brown SC (1987) The compartmentation of secondary metabolites in plant cell cultures. In F Constabel, IK Vasil, eds, Cell Culture and Somatic Cell Genetics of Plants, Vol 4. Academic Press, San Diego, pp 43–76 [Google Scholar]
- Guirimand G, Guihur A, Poutrain P, Héricourt F, Mahroug S, St-Pierre B, Burlat V, Courdavault V. (2011) Spatial organization of the vindoline biosynthetic pathway in Catharanthus roseus. J Plant Physiol 168: 549–557 [DOI] [PubMed] [Google Scholar]
- Hildreth SB, Gehman EA, Yang HB, Lu RH, Ritesh KC, Harich KC, Yu S, Lin JS, Sandoe JL, Okumoto S, et al (2011) Tobacco nicotine uptake permease (NUP1) affects alkaloid metabolism. Proc Natl Acad Sci USA 108: 18179–18184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschi KD. (2001) Vacuolar H+/Ca2+ transport: who’s directing the traffic? Trends Plant Sci 6: 100–104 [DOI] [PubMed] [Google Scholar]
- Jaquinod M, Villiers F, Kieffer-Jaquinod S, Hugouvieux V, Bruley C, Garin J, Bourguignon J. (2007) A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Mol Cell Proteomics 6: 394–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KH, Senft JA. (1985) An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem 33: 77–79 [DOI] [PubMed] [Google Scholar]
- Laemmli UK. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275 [PubMed] [Google Scholar]
- Maeshima M, Yoshida S. (1989) Purification and properties of vacuolar membrane proton-translocating inorganic pyrophosphatase from mung bean. J Biol Chem 264: 20068–20073 [PubMed] [Google Scholar]
- Mahroug S, Burlat V, St-Pierre B. (2007) Cellular and sub-cellular organisation of the monoterpenoid indole alkaloid pathway in Catharanthus roseus. Phytochem Rev 6: 363–381 [Google Scholar]
- Marinova K, Pourcel L, Weder B, Schwarz M, Barron D, Routaboul JM, Debeaujon I, Klein M. (2007) The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 19: 2023–2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins V, Hanana M, Blumwald E, Gerós H (2012) Copper transport and compartmentation in grape cells. Plant Cell Physiol 53: 1866–1880 [DOI] [PubMed] [Google Scholar]
- Morita M, Shitan N, Sawada K, Van Montagu MCE, Inzé D, Rischer H, Goossens A, Oksman-Caldentey KM, Moriyama Y, Yazaki K. (2009) Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc Natl Acad Sci USA 106: 2447–2452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata J, Roepke J, Gordon H, De Luca V. (2008) The leaf epidermome of Catharanthus roseus reveals its biochemical specialization. Plant Cell 20: 524–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D, Krauss G, Hieke M, Gröger D. (1983) Indole alkaloid formation and storage in cell suspension cultures of Catharanthus roseus. Planta Med 48: 20–23 [DOI] [PubMed] [Google Scholar]
- Omote H, Hiasa M, Matsumoto T, Otsuka M, Moriyama Y. (2006) The MATE proteins as fundamental transporters of metabolic and xenobiotic organic cations. Trends Pharmacol Sci 27: 587–593 [DOI] [PubMed] [Google Scholar]
- Otani M, Shitan N, Sakai K, Martinoia E, Sato F, Yazaki K. (2005) Characterization of vacuolar transport of the endogenous alkaloid berberine in Coptis japonica. Plant Physiol 138: 1939–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queirós F, Fontes N, Silva P, Almeida D, Maeshima M, Gerós H, Fidalgo F. (2009) Activity of tonoplast proton pumps and Na+/H+ exchange in potato cell cultures is modulated by salt. J Exp Bot 60: 1363–1374 [DOI] [PubMed] [Google Scholar]
- Renaudin JP. (1989) Different mechanisms control the vacuolar compartmentation of ajmalicine in Catharanthus roseus cell cultures. Plant Physiol Biochem 27: 613–621 [Google Scholar]
- Roepke J, Salim V, Wu M, Thamm AMK, Murata J, Ploss K, Boland W, De Luca V. (2010) Vinca drug components accumulate exclusively in leaf exudates of Madagascar periwinkle. Proc Natl Acad Sci USA 107: 15287–15292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JA. (1979) Investigations of vacuoles isolated from tobacco. I. Quantitation of nicotine. Plant Physiol 64: 74–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitan N, Bazin I, Dan K, Obata K, Kigawa K, Ueda K, Sato F, Forestier C, Yazaki K. (2003) Involvement of CjMDR1, a plant multidrug-resistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica. Proc Natl Acad Sci USA 100: 751–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shitan N, Dalmas F, Dan K, Kato N, Ueda K, Sato F, Forestier C, Yazaki K. (2012) Characterization of Coptis japonica CjABCB2, an ATP-binding cassette protein involved in alkaloid transport. Phytochemistry (in press) [DOI] [PubMed] [Google Scholar]
- Shitan N, Yazaki K. (2007) Accumulation and membrane transport of plant alkaloids. Curr Pharm Biotechnol 8: 244–252 [DOI] [PubMed] [Google Scholar]
- Shoji T, Inai K, Yazaki Y, Sato Y, Takase H, Shitan N, Yazaki K, Goto Y, Toyooka K, Matsuoka K, et al (2009) Multidrug and toxic compound extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots. Plant Physiol 149: 708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottomayor M, dePinto MC, Salema R, DiCosmo F, Pedreno MA, Barcelo AR. (1996) The vacuolar localization of a basic peroxidase isoenzyme responsible for the synthesis of alpha-3′,4′-anhydrovinblastine in Catharanthus roseus (L) G. Don leaves. Plant Cell Environ 19: 761–767 [Google Scholar]
- St-Pierre B, Vazquez-Flota FA, De Luca V. (1999) Multicellular compartmentation of Catharanthus roseus alkaloid biosynthesis predicts intercellular translocation of a pathway intermediate. Plant Cell 11: 887–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden R, Jacobs DI, Snoeijer W, Hallard D, Verpoorte R. (2004) The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem 11: 607–628 [DOI] [PubMed] [Google Scholar]
- Vazquez-Flota F, De Carolis E, Alarco AM, De Luca V. (1997) Molecular cloning and characterization of desacetoxyvindoline-4-hydroxylase, a 2-oxoglutarate dependent-dioxygenase involved in the biosynthesis of vindoline in Catharanthus roseus (L.) G. Don. Plant Mol Biol 34: 935–948 [DOI] [PubMed] [Google Scholar]
- Vera-Estrella R, Barkla BJ, Higgins VJ, Blumwald E. (1994) Plant defense response to fungal pathogens: activation of host-plasma membrane H+-ATPase by elicitor-induced enzyme dephosphorylation. Plant Physiol 104: 209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpoorte R, Lata B, Sadowska A, editors (2007) Biology and Biochemistry of Catharanthus roseus (L.) G. Don. Phytochemistry Reviews, Vol 6, Issue 2-3. Springer-Verlag, Dordrecht, The Netherlands [Google Scholar]
- Wink M. (1993) The plant vacuole: a multifunctional compartment. J Exp Bot 44: 231–246 [Google Scholar]
- Yoder LR, Mahlberg PG. (1976) Reactions of alkaloid and histochemical indicators in laticifers and specialized parenchyma cells of Catharanthus roseus (Apocynaceae). Am J Bot 63: 1167–1173 [Google Scholar]
- Zheng HQ, Wang GL, Zhang L. (1997) Alfalfa mosaic virus movement protein induces tubules in plant protoplasts. Mol Plant Microbe Interact 10: 1010–1014 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.