Abstract
High levels of beta receptor agonist have previously been shown to down-regulate beta receptor density on circulating leukocytes in man; however, the factors controlling receptor density under physiological conditions have not previously been defined. To determine whether beta receptor density is normally down-regulated by circulating, physiological levels of catecholamines we have examined the relationship between receptor density and catecholamine levels. Urinary epinephrine and norepinephrine were significantly reciprocally correlated to lymphocyte receptor density. A similar relationship existed between beta receptor density and supine plasma epinephrine, norepinephrine, upright epinephrine, and norepinephrine levels. Change in sodium intake from 10 to 400 meq/d caused a 52% increase in lymphocyte and a 48% increase in polymorphonuclear beta receptor density. The changes in receptor density were accompanied by an increase in the sensitivity to isoproterenol measured as a fall in the dose of isoproterenol required to raise the heart rate by 25 beats per minute. Beta receptor density on both lymphocyte and polymorphonuclear cells was significantly correlated to the cardiac sensitivity to isoproterenol. Propranolol administration resulted in an increase in the density of beta receptors on lymphocyte and polymorphonuclear cells that correlated with the subject's pretreatment catecholamine levels.
These findings, therefore, suggest that physiological levels of catecholamines normally down-regulate beta receptors in man and that blockade of this down-regulation by propranolol allows receptor density to increase.
Full text
PDF

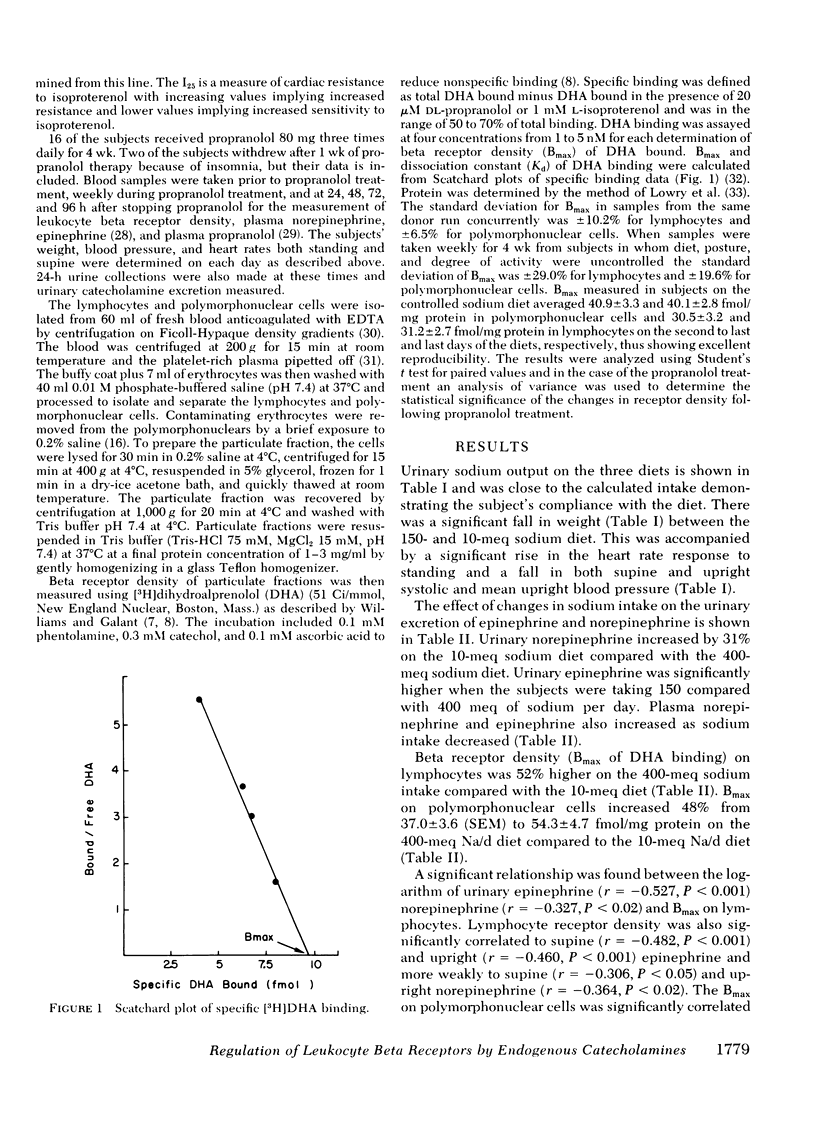


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarons R. D., Nies A. S., Gal J., Hegstrand L. R., Molinoff P. B. Elevation of beta-adrenergic receptor density in human lymphocytes after propranolol administration. J Clin Invest. 1980 May;65(5):949–957. doi: 10.1172/JCI109781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurbach G. D., Fedak S. A., Woodard C. J., Palmer J. S., Hauser D., Troxler F. Beta-adrenergic receptor: stereospecific interaction of iodinated beta-blocking agent with high affinity site. Science. 1974 Dec 27;186(4170):1223–1224. doi: 10.1126/science.186.4170.1223. [DOI] [PubMed] [Google Scholar]
- Boudoulas H., Lewis R. P., Kates R. E., Dalamangas G. Hypersensitivity to adrenergic stimulation after propranolol withdrawal in normal subjects. Ann Intern Med. 1977 Oct;87(4):433–436. doi: 10.7326/0003-4819-87-4-433. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Conolly M. E., Greenacre J. K. The beta-adrenoceptor of the human lymphocyte and human lung parenchyma. Br J Pharmacol. 1977 Jan;59(1):17–23. doi: 10.1111/j.1476-5381.1977.tb06971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conolly M. E., Greenacre J. K. The lymphocyte beta-adrenoceptor in normal subjects and patients with bronchial asthma: the effect of different forms of treatment on receptor function. J Clin Invest. 1976 Dec;58(6):1307–1316. doi: 10.1172/JCI108586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Galant S. P., Duriseti L., Underwood S., Insel P. A. Decreased beta-adrenergic receptors on polymorphonuclear leukocytes after adrenergic therapy. N Engl J Med. 1978 Oct 26;299(17):933–936. doi: 10.1056/NEJM197810262991707. [DOI] [PubMed] [Google Scholar]
- Galant S. P., Underwood S., Duriseti L., Insel P. A. Characterization of high-affinity beta2-adrenergic receptor binding of (-)-[3H]-dihydroalprenolol to human polymorphonuclear cell particulates. J Lab Clin Med. 1978 Oct;92(4):613–618. [PubMed] [Google Scholar]
- Glaubiger G., Lefkowitz R. J. Elevated beta-adrenergic receptor number after chronic propranolol treatment. Biochem Biophys Res Commun. 1977 Sep 23;78(2):720–725. doi: 10.1016/0006-291x(77)90238-8. [DOI] [PubMed] [Google Scholar]
- Greenacre J. K., Conolly M. E. Desensitization of the beta-adrenoceptor of lymphocytes from normal subjects and patients with phaeochromocytoma: studies in vivo. Br J Clin Pharmacol. 1978 Mar;5(3):191–197. doi: 10.1111/j.1365-2125.1978.tb01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenacre J. K., Schofield P., Conolly M. E. Desensitization of the beta-adrenoceptor of lymphocytes from normal subjects and asthmatic patients in vitro. Br J Clin Pharmacol. 1978 Mar;5(3):199–206. doi: 10.1111/j.1365-2125.1978.tb01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. L., Wolfe B. B., Harden T. K., Molinoff P. B., Perkins J. P. Role of beta-adrenergic receptors in catecholamine-induced desensitization of adenylate cyclase in human astrocytoma cells. J Biol Chem. 1978 Mar 10;253(5):1472–1480. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lefkowitz R. J. Direct binding studies of adrenergic receptors: biochemical, physiologic, and clinical implications. Ann Intern Med. 1979 Sep;91(3):450–458. doi: 10.7326/0003-4819-91-3-450. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Mukherjee C., Coverstone M., Caron M. G. Stereospecific (3H)(minus)-alprenolol binding sites, beta-adrenergic receptors and adenylate cyclase. Biochem Biophys Res Commun. 1974 Sep 23;60(2):703–709. doi: 10.1016/0006-291x(74)90297-6. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Roth J., Pricer W., Pastan I. ACTH receptors in the adrenal: specific binding of ACTH-125I and its relation to adenyl cyclase. Proc Natl Acad Sci U S A. 1970 Mar;65(3):745–752. doi: 10.1073/pnas.65.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Williams L. T. Catecholamine binding to the beta-adrenergic receptor. Proc Natl Acad Sci U S A. 1977 Feb;74(2):515–519. doi: 10.1073/pnas.74.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft F. C., Rankin L. I., Henry D. P., Bloch R., Grim C. E., Weyman A. E., Murray R. H., Weinberger M. H. Plasma and urinary norepinephrine values at extremes of sodium intake in normal man. Hypertension. 1979 May-Jun;1(3):261–266. doi: 10.1161/01.hyp.1.3.261. [DOI] [PubMed] [Google Scholar]
- Morris H. G., Rusnak S. A., Selner J. C., Barzens K., Barnes J. Adrenergic desensitization in leukocytes of normal and asthmatic subjects. J Cyclic Nucleotide Res. 1977 Dec;3(6):439–446. [PubMed] [Google Scholar]
- Mukherjee C., Caron M. G., Lefkowitz R. J. Catecholamine-induced subsensitivity of adenylate cyclase associated with loss of beta-adrenergic receptor binding sites. Proc Natl Acad Sci U S A. 1975 May;72(5):1945–1949. doi: 10.1073/pnas.72.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattel S., Rangno R. E., Van Loon G. Mechanism of propranolol withdrawal phenomena. Circulation. 1979 Jun;59(6):1158–1164. doi: 10.1161/01.cir.59.6.1158. [DOI] [PubMed] [Google Scholar]
- Newman K. D., Williams L. T., Bishopric N. H., Lefkowitz R. J. Identification of alpha-adrenergic receptors in human platelets by [3H]dihydroergocryptine binding. J Clin Invest. 1978 Feb;61(2):395–402. doi: 10.1172/JCI108950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls M. G., Kiowski W., Zweifler A. J., Julius S., Schork M. A., Greenhouse J. Plasma norepinephrine variations with dietary sodium intake. Hypertension. 1980 Jan-Feb;2(1):29–32. doi: 10.1161/01.hyp.2.1.29. [DOI] [PubMed] [Google Scholar]
- Passon P. G., Peuler J. D. A simplified radiometric assay for plasma norepinephrine and epinephrine. Anal Biochem. 1973 Feb;51(2):618–631. doi: 10.1016/0003-2697(73)90517-4. [DOI] [PubMed] [Google Scholar]
- Robertson D., Johnson G. A., Robertson R. M., Nies A. S., Shand D. G., Oates J. A. Comparative assessment of stimuli that release neuronal and adrenomedullary catecholamines in man. Circulation. 1979 Apr;59(4):637–643. doi: 10.1161/01.cir.59.4.637. [DOI] [PubMed] [Google Scholar]
- Schocken D. D., Roth G. S. Reduced beta-adrenergic receptor concentrations in ageing man. Nature. 1977 Jun 30;267(5614):856–858. doi: 10.1038/267856a0. [DOI] [PubMed] [Google Scholar]
- Shand D. G., Wood A. J. Propranolol withdrawal syndrome - why? Circulation. 1978 Aug;58(2):202–203. doi: 10.1161/01.cir.58.2.202. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Khorana J., Galgoci B. Quantitation and characterization of human platelet alpha-adrenergic receptors using [3H]phentolamine. Mol Pharmacol. 1979 Nov;16(3):719–728. [PubMed] [Google Scholar]
- Vestal R. E., Wood A. J., Shand D. G. Reduced beta-adrenoceptor sensitivity in the elderly. Clin Pharmacol Ther. 1979 Aug;26(2):181–186. doi: 10.1002/cpt1979262181. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Lefkowitz R. J. Identification of beta-adrenergic receptors in human lymphocytes by (-) (3H) alprenolol binding. J Clin Invest. 1976 Jan;57(1):149–155. doi: 10.1172/JCI108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood A. J., Carr K., Vestal R. E., Belcher S., Wilkinson G. R., Shand D. G. Direct measurement of propranolol bioavailability during accumulation to steady-state. Br J Clin Pharmacol. 1978 Oct;6(4):345–350. doi: 10.1111/j.1365-2125.1978.tb00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


