Linkages of leaf and mesophyll cell traits to CO2 diffusion, photosynthesis, transpiration, and water use efficiency were identified across accessions of the genus Oryza.
Abstract
The genus Oryza, which includes rice (Oryza sativa and Oryza glaberrima) and wild relatives, is a useful genus to study leaf properties in order to identify structural features that control CO2 access to chloroplasts, photosynthesis, water use efficiency, and drought tolerance. Traits, 26 structural and 17 functional, associated with photosynthesis and transpiration were quantified on 24 accessions (representatives of 17 species and eight genomes). Hypotheses of associations within, and between, structure, photosynthesis, and transpiration were tested. Two main clusters of positively interrelated leaf traits were identified: in the first cluster were structural features, leaf thickness (Thickleaf), mesophyll (M) cell surface area exposed to intercellular air space per unit of leaf surface area (Smes), and M cell size; a second group included functional traits, net photosynthetic rate, transpiration rate, M conductance to CO2 diffusion (gm), stomatal conductance to gas diffusion (gs), and the gm/gs ratio. While net photosynthetic rate was positively correlated with gm, neither was significantly linked with any individual structural traits. The results suggest that changes in gm depend on covariations of multiple leaf (Smes) and M cell (including cell wall thickness) structural traits. There was an inverse relationship between Thickleaf and transpiration rate and a significant positive association between Thickleaf and leaf transpiration efficiency. Interestingly, high gm together with high gm/gs and a low Smes/gm ratio (M resistance to CO2 diffusion per unit of cell surface area exposed to intercellular air space) appear to be ideal for supporting leaf photosynthesis while preserving water; in addition, thick M cell walls may be beneficial for plant drought tolerance.
Leaves have evolved in different environments into a multitude of sizes and shapes, showing great variation in morphology and anatomy (Evans et al., 2004). However, all leaf typologies share common functions associated with chloroplasts, namely to intercept sunlight, take up CO2 and inorganic nitrogen, and perform photosynthesis as a primary process for growth and reproduction.
Investigating relationships between leaf anatomy and photosynthetic features (CO2 fixation, which involves physical and biochemical processes and loss of water by transpiration) could lead to the identification of structural features for enhancing crop productivity and improve our understanding of plant evolution and adaptation (Evans et al., 2004).
Stomata, through which CO2 and water vapor diffuse into and out of the leaf, are involved in the regulation and control of photosynthetic and transpiration responses (Jarvis and Morison, 1981; Farquhar and Sharkey, 1982). Besides stomata distribution patterns between the abaxial and adaxial lamina surfaces (Foster and Smith, 1986), stomatal density and size are leaf anatomical traits contributing to build the leaf stomatal conductance to gas diffusion (gs). This is calculated as the reciprocal of the stomatal resistances to gas diffusion; stomatal control results in a lower concentration of CO2 in the leaf mesophyll (M) intercellular air space (Ci) than in the atmosphere (Ca; Nobel, 2009).
Leaf M architecture greatly contributes to the pattern of light attenuation profiles within the lamina (Terashima and Saeki, 1983; Woolley, 1983; Vogelmann et al., 1989; Evans, 1999; Terashima et al., 2011) and affects CO2 diffusion from the intercellular air space (IAS) to the chloroplast stroma. Therefore, it influences photosynthetic activity (Flexas et al., 2007, 2008) and can have effects on leaf hydrology and transpiration (Sack et al., 2003; Brodribb et al., 2010; Ocheltree et al., 2012). In addition, M architecture sets boundaries for leaf photosynthetic responses to changing environmental conditions (Nobel et al., 1975).
Fortunately, several methodologies are currently available (Flexas et al., 2008; Pons et al., 2009) to determine M conductance to CO2 diffusion (gm), expressed per unit of leaf surface area. It is calculated as the reciprocal of the cumulated partial resistances exerted by leaf structural traits and biochemical processes from the substomatal cavities to photosynthetic sites (Evans et al., 2009; Nobel, 2009). The resistance to CO2 diffusion in the liquid phase is 4 orders of magnitude higher than in the gaseous phase (Nobel, 2009); therefore, the changes in CO2 concentration in the leaf gas phase are small in comparison with the changes in the liquid phase (Niinemets, 1999; Aalto and Juurola, 2002; Nobel, 2009). In the liquid phase, the resistance to CO2 transfer is built from contributions by the cell walls, the plasmalemma, cytoplasm, chloroplast membranes, and stroma (Tholen and Zhu, 2011; Tholen et al., 2012); in addition, it involves factors associated with the carboxylation reaction (Kiirats et al., 2002; Evans et al., 2009). Thus, the concentration of CO2 in the chloroplasts (Cc) is lower than Ci and can limit photosynthesis.
At steady state, the relationships between the leaf net photosynthetic rate (A), the concentrations of CO2, and the stomatal conductance to CO2 diffusion (gs_CO2) and gm are modeled based on Fick’s first law of diffusion (Nobel, 2009) as:
| (1) |
where Ca, Ci, and Cc are as defined above (Flexas et al., 2008).
The magnitude of gm has been found to correlate with certain leaf structural traits in some species, in particular with the M cell surface area exposed to IAS per (one side) unit of leaf surface area (Smes) and its extent covered by chloroplasts (Schl; Evans and Loreto, 2000; Slaton and Smith, 2002; Tholen et al., 2012). From a physical modeling perspective, increasing Smes provides more pathways acting in parallel for CO2 diffusion (to and from the chloroplasts) per unit of leaf surface area; thus, it tends to reduce the resistance to CO2 movement into the M cells and to increase gm (Evans et al., 2009; Nobel, 2009). A number of leaf structural traits affect Smes, including leaf thickness, cell density, cell volume and shape, and the fraction of the M cell walls in contact with the IAS (Terashima et al., 2001, 2011), and the degree they are linked to Smes can vary between species (Slaton and Smith, 2002; Terashima et al., 2006). In particular, the presence of lobes on M cells, which are prominent in some Oryza species, may contribute to gm through increasing Smes (Sage and Sage, 2009; Terashima et al., 2011; Tosens et al., 2012). The M cell wall can provide resistance in series for M CO2 diffusion (Nobel, 2009); thicker cell walls may increase resistance to CO2 movement into the M cells and decrease gm (Terashima et al., 2006, 2011; Evans et al., 2009).
Other leaf traits, such as M porosity (the fraction of M volume occupied by air spaces [VolIAS]), has been shown to have a positive correlation with gm in some species (Peña-Rojas et al., 2005), but the association may be mediated by light availability (Slaton and Smith, 2002). Leaf thickness (Thickleaf) tends to be negatively linked to gm, and it may set an upper limit for the maximum gm, according to Terashima et al. (2006), Flexas et al. (2008), and Niinemets et al. (2009).
With respect to leaf structural traits and water relations, Thickleaf may increase the apoplast path length (resistances in series; Nobel, 2009) in the extra-xylem M (Sack and Holbrook, 2006; Brodribb et al., 2007) for water to reach the evaporation sites, which could decrease the conductance of water through the M and lower the transpiration rate. Interestingly, while thicker M cell walls may reduce gm, they can enable the development of higher water potential gradients between the soil and leaves, which can be decisive for plant survival and longevity under drought conditions (Steppe et al., 2011).
The purpose of this study was to provide insight into how the diversity of leaf structure relates to photosynthesis and transpiration among representative cultivated species and wild relatives in the genus Oryza. This includes, in particular, identifying leaf structural features associated with the diffusion of CO2 from the atmosphere to the chloroplasts, photosynthesis, transpiration efficiency (A/E), and drought tolerance. The genus consists of 10 genomic groups and is composed of approximately 24 species (the number depending on taxonomic preferences; Kellogg, 2009; Brar and Singh, 2011), including the cultivated species Oryza sativa and Oryza glaberrima. Oryza species are distributed around the world, and they exhibit a wide range of phenotypes, with annual versus perennial life cycles and sun- versus shade-adapted species (Vaughan, 1994; Vaughan et al., 2008; Brar and Singh, 2011; Jagadish et al., 2011). This diversity in the genus is an important resource, which is being studied to improve rice yield, especially under unfavorable environmental conditions. In particular, O. glaberrima, Oryza australiensis, and Oryza meridionalis are of interest as drought-tolerant species (Henry et al., 2010; Ndjiondjop et al., 2010; Scafaro et al., 2011, 2012), while Oryza coarctata is salt tolerant (Sengupta and Majumder, 2010). In this study, a total of 43 leaf functional and structural parameters were collected on 24 accessions corresponding to 17 species within eight genomes (Table I) to represent the spectrum of the leaf diversity in the genus Oryza.
Table I. Oryza accessions used in this study and identified by the assigned numbers.
Leaf functional measurements were performed on all accessions; structural measurements were made on accessions labeled with asterisks (for O. sativa cv IR72 and O. australiensis 22, structural measurements were only made on stomata but not other traits). Genomes for Oryza species are according to Brar and Singh (2011). Life cycle is as follows: A = annual; B = biennial; P = poliennial. Habitat is as follows: S = shade; S-Sh = sun-shade.
| Genome | Species | Life Cycle | Habitat | Accession | No. |
|---|---|---|---|---|---|
| AA | O. barthii | A | S | PI 590400* | 1 |
| AA | O. glaberrima | A | S | PI 450430* | 2 |
| AA | O. glumaepatula | P | S | PI 527362* | 3 |
| AA | O. longistaminata | P | S | IRGC 101207* | 4 |
| AA | O. longistaminata | P | S | IRGC 101754 | 5 |
| AA | O. meridionalis | A/P | S | IRGC 93265* | 6 |
| AA | O. nivara | A/B | S | PI 590405* | 7 |
| AA | O. rufipogon | P | S | PI 104640 | 8 |
| AA | O. rufipogon | S | PI 590421* | 9 | |
| AA | O. sativa | A | S | IR64* | 10 |
| AA | O. sativa | A | S | IR72 | 11 |
| BB | O. punctata | A | S-Sh | IRGC 105690* | 12 |
| BBCC | O. minuta | P | S-Sh | IRGC 101141* | 13 |
| CC | O. officinalis | P | S-Sh | PI 59412* | 14 |
| CC | O. rhizomatis | P | S | IRGC 101609 | 15 |
| CC | O. rhizomatis | P | S | IRGC 105950* | 16 |
| CCDD | O. alta | P | S-Sh | PI 590398* | 17 |
| CCDD | O. latifolia | P | S-Sh | IRGC 100959* | 18 |
| CCDD | O. latifolia | P | S-Sh | IRGC 105173 | 19 |
| EE | O. australiensis | P | S | IRGC 101397* | 20 |
| EE | O. australiensis | P | S | IRGC 105277* | 21 |
| EE | O. australiensis | P | S | IRGC 86527 | 22 |
| FF | O. brachyantha | B | S | IRGC 101232* | 23 |
| HHKK | O. coarctata | P | S | IRGC 104502* | 24 |
For evaluating aspects of photosynthesis, the model in Equation 1 was considered, and all the listed functional variables, A, gs_CO2, (Ca − Ci), gm, and (Ci − Cc), were determined. In addition, among the leaf functional traits, the M resistance to CO2 diffusion per unit of cell surface area exposed to IAS (reciprocal of gm/Smes) was calculated as described by Evans et al. (2009): it represents the resistance to CO2 diffusion from IAS to chloroplasts in a liquid solution through cell wall and membranes (Nobel, 2009). Leaf transpiration rate (E), A/E, the intrinsic A/E (ratio between A and stomatal conductance to water vapor diffusion [gs_H2O]), gm/gs_CO2 (representing the coordination between gm and gs), and the carbon isotope composition of leaf biomass (δ13C; calculated as 13C/12C) were determined. The value of δ13C has been recognized as a potential indicator of leaf A/E: increased limitations on photosynthesis by decreased gs can lead to higher A/gs_H2O ratios and less discrimination against assimilation of 13CO2 (for review, see Farquhar et al., 1989); the leaf A/E may also be positively linked to the gm/gs ratio (Flexas et al., 2008, 2013; Barbour et al., 2010). With respect to leaf structure, the stomatal density, stomatal pore length, and indices of stomatal pore area on both lamina sides (according to Sack et al., 2003), the Thickleaf, VolIAS, Smes, Schl, area of M cell section (acell) in leaf cross sections, cell wall thickness (Thickcw), and M cell surface lobing (Lobcell) were the principal traits estimated. A statistical multivariate analysis (Child, 2006) was employed to identify clusters of highly interrelated leaf traits; trait-to-trait correlation analysis was carried out to further examine leaf structural, functional, and structural-functional relationships.
The following are the main hypotheses examined in this study. (1) Leaf thickness will be associated with certain M structural features. (2) gm will be coordinated with M structural traits. (3) A will be correlated with gs, gm, and E. (4) Leaf structural traits will be involved in the relationship between A and E, which will affect leaf A/E. (5) The gm/gs ratio will be positively correlated with leaf A/E; associations with high Thickcw could have implications for plant drought tolerance.
RESULTS
Significance of ANOVA for Structural and Functional Leaf Traits
The accessions analyzed in this study, and the corresponding species and genomes, are listed in Table I; and, the list of leaf traits determined are shown in Table II. The overall significance for differences among Oryza genomes, species nested within genomes, and accessions nested within species and genomes, for each leaf structural and functional trait, are reported in Table III. Among genomes, there were significant differences in most structural and functional traits. Among species nested within genomes, there were significant differences in about half of the leaf traits, including stomatal pore area index in the abaxial lamina surface (Istab), mean Thickleaf, total Smes, Thickcw, maximum carboxylation efficiency (CE), gm, and A. Among accessions nested within species and genomes, there were significant differences in only a few leaf traits, notably Istab, Thickcw, gm, A, E, and A/E.
Table II. List of leaf traits estimated in this study, and symbols and units adopted.
| Trait Category/Trait | Symbol | Unit |
|---|---|---|
| Leaf structural traits | ||
| Stomatal density in the abaxial lamina surface | Dst ab | mm−2 |
| Stomatal density in the adaxial lamina surface | Dst ad | mm−2 |
| Stomatal pore length in the abaxial lamina surface | Lst ab | μm |
| Stomatal pore length in the adaxial lamina surface | Lst ad | μm |
| Stomatal pore area index in the abaxial lamina surface | Ist ab | mm2 mm−2 |
| Stomatal pore area index in the adaxial lamina surface | Ist ad | mm2 mm−2 |
| Stomatal pore area index per unit (one side) of lamina surface area | Ist | mm2 mm−2 |
| Leaf thickness | Thickleaf | μm |
| Fraction of leaf mesophyll volume occupied by intercellular air space | VolIAS | % |
| Total mesophyll cell surface area exposed to intercellular air space per unit (one side) of leaf surface area (M index) | Smes | μm2 μm−2 |
| Total mesophyll cell surface area occupied by chloroplasts exposed to intercellular air space per unit (one side) of leaf surface area (chloroplast index) | Schl | μm2 μm−2 |
| M cell structural traits | ||
| Area of cell section (in a leaf cross section) | acell | μm2 |
| Ratio between perimeter and area of a cell section (in a leaf cross section) | P/acell | μm μm−2 |
| Cell surface lobing (corresponding to cell perimeter tortuosity) | Lobcell | μm μm−1 |
| Cell wall thickness | Thickcw | μm |
| Length of a single cell wall exposed to intercellular air space | Lcw_IAS | μm |
| Fraction of cell wall exposed to intercellular air space | CWIAS | % |
| Length of a single cell wall exposed to intercellular air space covered by chloroplasts | Lcw_IAS_chl | μm |
| Fraction of cell wall exposed to intercellular air space covered by chloroplasts | CWIAS_chl | % |
| Length of a single cell wall adjacent to other cells | LACW | μm |
| Length of a single cell wall adjacent to other cells covered by chloroplasts | LACW_chl | μm |
| Fraction of cell wall adjacent to other cells covered by chloroplasts | ACWchl | % |
| Fraction of cell wall covered by chloroplasts | CWchl | % |
| Fraction of cell volume occupied by chloroplasts | Volchl | % |
| Fraction of cell volume occupied by vacuole | Volvac | % |
| Mitochondria minor axis | dmit | μm |
| Leaf functional traits | ||
| 13C/12C biomass isotopic signature | δ13C | ‰ |
| Maximum net photosynthetic rate per unit (one side) of leaf surface area | Amax | μmol CO2 m−2 s−1 |
| Maximum carboxylation efficiency | CE | mol CO2 m−2 s−1 bar−1 CO2 |
| Mesophyll conductance to CO2 diffusion | gma | mol CO2 m−2 s−1 bar−1 CO2 |
| Mesophyll resistance to CO2 diffusion per unit of cell surface area exposed to intercellular air space | Smes/gm | m2 s bar CO2 mol−1 CO2 |
| CO2 compensation point at 20% oxygen | Γ | μbar |
| Intercellular partial pressure of CO2 | Cia | μbar |
| Difference between atmospheric and intercellular partial pressure of CO2 | Ca − Cia | μbar |
| Chloroplastic partial pressure of CO2 | Cca | μbar |
| Difference between intercellular and chloroplastic partial pressure of CO2 | Ci − Cca | μbar |
| Stomatal conductance to water vapor diffusion | gs_H2Oa | mol water m−2 s−1 |
| Stomatal conductance to CO2 diffusion | gs_CO2a | mol CO2 m−2 s−1 |
| Ratio between mesophyll and stomatal conductance to CO2 diffusion | gm/gs_CO2 | bar−1 |
| Net photosynthetic rate per unit (one side) of leaf surface area | Aa | μmol CO2 m−2 s−1 |
| Transpiration rate per unit (one side) of leaf surface area | Ea | mmol water m−2 s−1 |
| Transpiration efficiency | A/E | μmol CO2 mmol−1 water |
| Intrinsic transpiration efficiency | A/gs_ H2O | μmol CO2 mol−1 water |
Determined at 350 μbar air partial pressure of CO2.
Table III. Overall significance of three-stage (genomes, species, accessions) nested ANOVA for all considered leaf structural and functional traits in the genus Oryza.
Trait symbols correspond to the descriptions in Table II. Asterisks represent the significance for differences among genomes, species nested within genomes, and accessions nested within species and genomes. *P < 0.05, **0.01 ≥ P ≥ 0.001, ***P < 0.001. Min and Max correspond to the minimum and maximum means. F = F test statistic; Num df and Den df = numerator and denominator degrees of freedom, respectively.
| Trait Category/Trait | Unit | Min | Max | Genomes |
Species |
Accessions |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | Num df | Den df | P | Min | Max | F | Num df | Den df | P | Min | Max | F | Num df | Den df | P | |||||
| Leaf and cell structural | ||||||||||||||||||||
| Dst ab | mm−2 | 156.8 | 391.2 | 8.63 | 7 | 39 | *** | 156.8 | 450.0 | 4.25 | 9 | 39 | *** | 156.8 | 450.0 | 1.10 | 3 | 39 | ||
| Dst ad | mm−2 | 167.3 | 501.0 | 11.61 | 7 | 38 | *** | 147.3 | 501.0 | 2.09 | 9 | 38 | 147.3 | 501.0 | 0.67 | 3 | 38 | |||
| Lst ab | μm | 9.67 | 17.32 | 14.67 | 7 | 39 | *** | 8.87 | 18.07 | 9.54 | 9 | 39 | *** | 8.87 | 19.03 | 0.91 | 3 | 39 | ** | |
| Lst ad | μm | 10.50 | 17.67 | 6.42 | 7 | 39 | *** | 10.1 | 17.67 | 2.11 | 9 | 39 | 10.1 | 18.57 | 0.99 | 3 | 39 | |||
| Ist ab | mm2 mm−2 | 0.028 | 0.062 | 5.47 | 7 | 39 | *** | 0.017 | 0.091 | 4.82 | 9 | 39 | *** | 0.017 | 0.099 | 5.87 | 3 | 39 | ** | |
| Ist ad | mm2 mm−2 | 0.009 | 0.012 | 8.89 | 7 | 38 | *** | 0.026 | 0.140 | 1.49 | 9 | 38 | 0.026 | 0.140 | 0.75 | 3 | 38 | |||
| Ist | mm2 mm−2 | 0.183 | 0.062 | 7.03 | 7 | 38 | *** | 0.043 | 0.183 | 3.53 | 9 | 38 | ** | 0.043 | 0.183 | 3.10 | 3 | 38 | * | |
| Thickleaf | μm | 57.8 | 120.4 | 26.02 | 7 | 28 | *** | 57.8 | 120.4 | 6.98 | 9 | 28 | *** | 57.8 | 125.3 | 1.44 | 1 | 28 | ||
| VolIAS | % | 15.75 | 25.70 | 4.59 | 7 | 28 | ** | 15.75 | 25.97 | 2.20 | 9 | 28 | 15.75 | 27.95 | 2.13 | 1 | 28 | |||
| Smes | μm μm−2 | 10.17 | 24.43 | 15.71 | 7 | 28 | *** | 8.77 | 24.43 | 4.25 | 9 | 28 | ** | 8.77 | 24.43 | 0.04 | 1 | 28 | ||
| Schl | μm μm−2 | 9.05 | 23.40 | 22.98 | 7 | 28 | *** | 6.27 | 23.40 | 6.15 | 9 | 28 | *** | 7.90 | 23.40 | 3.42 | 1 | 28 | ||
| acell | μm2 | 58.8 | 208.2 | 7.29 | 7 | 19 | *** | 58.8 | 242.6 | 2.14 | 9 | 19 | 58.8 | 242.6 | 0.11 | 1 | 19 | |||
| P/acell | μm μm−2 | 0.380 | 0.580 | 4.62 | 7 | 19 | ** | 0.380 | 0.590 | 1.58 | 9 | 19 | 0.370 | 0.590 | 0.12 | 1 | 19 | |||
| Lobcell | μm μm−1 | 1.218 | 1.403 | 8.83 | 7 | 19 | *** | 1.060 | 1.445 | 1.32 | 9 | 19 | 1.060 | 1.445 | 0.01 | 1 | 19 | |||
| Thickcw | μm | 0.150 | 0.190 | 3.43 | 7 | 20 | * | 0.125 | 0.190 | 5.55 | 9 | 20 | *** | 0.125 | 0.190 | 6.87 | 1 | 20 | * | |
| Lcw_IAS | μm | 14.60 | 40.78 | 7.41 | 7 | 20 | *** | 14.60 | 41.20 | 2.24 | 9 | 20 | 14.60 | 41.20 | 0.00 | 1 | 20 | |||
| CWIAS | % | 38.0 | 54.8 | 8.21 | 7 | 20 | *** | 35.0 | 54.8 | 2.50 | 9 | 20 | * | 35.0 | 55.5 | 0.16 | 1 | 20 | ||
| Lcw_IAS_chl | μm | 13.15 | 39.58 | 9.53 | 7 | 20 | *** | 13.15 | 39.58 | 2.00 | 9 | 20 | 13.15 | 39.80 | 0.01 | 1 | 20 | |||
| CWIAS_chl | % | 86.55 | 97.13 | 1.69 | 7 | 20 | 63.75 | 97.95 | 3.63 | 9 | 20 | ** | 63.75 | 97.95 | 0.01 | 1 | 20 | |||
| LACW | μm | 16.60 | 50.63 | 5.95 | 7 | 20 | *** | 16.60 | 52.30 | 1.24 | 9 | 20 | 16.60 | 52.30 | 0.09 | 1 | 20 | |||
| LACW_chl | μm | 14.15 | 36.10 | 3.82 | 7 | 20 | ** | 14.15 | 38.20 | 0.95 | 9 | 20 | 14.15 | 38.20 | 0.01 | 1 | 20 | |||
| ACWchl | % | 72.23 | 88.56 | 4.54 | 7 | 20 | ** | 68.85 | 93.05 | 1.95 | 9 | 20 | 68.85 | 93.05 | 0.60 | 1 | 20 | |||
| CWchl | % | 78.37 | 91.56 | 3.07 | 7 | 20 | * | 73.95 | 94.30 | 2.53 | 9 | 20 | * | 73.95 | 94.30 | 0.05 | 1 | 20 | ||
| Volchl | % | 49.85 | 61.68 | 2.09 | 7 | 20 | 39.00 | 64.45 | 3.84 | 9 | 20 | 39.00 | 64.45 | 0.28 | 1 | 20 | ||||
| Volvac | % | 15.30 | 27.35 | 2.23 | 7 | 20 | 8.20 | 43.25 | 6.63 | 9 | 20 | *** | 8.20 | 43.25 | 0.11 | 1 | 20 | |||
| dmit | μm | 0.475 | 0.745 | 6.49 | 7 | 20 | *** | 0.385 | 0.745 | 4.38 | 9 | 20 | ** | 0.385 | 0.755 | 0.10 | 1 | 20 | ||
| Leaf functional | ||||||||||||||||||||
| δ13C | ‰ | −26.86 | −24.03 | 13.18 | 7 | 43 | *** | −27.37 | −24.03 | 4.92 | 9 | 43 | *** | −27.42 | −24.03 | 2.38 | 6 | 43 | * | |
| Amax | μmol CO2 m−2 s−1 | 29.40 | 45.27 | 24.47 | 45.27 | 24.22 | 46.56 | |||||||||||||
| CE | mol CO2 m−2 s−1 bar−1 CO2 | 0.044 | 0.189 | 11.64 | 7 | 47 | *** | 0.044 | 0.189 | 5.61 | 9 | 46 | *** | 0.044 | 0.202 | 3.00 | 7 | 46 | ** | |
| gm | mol CO2 m−2 s−1 bar−1 CO2 | 0.051 | 0.467 | 19.94 | 7 | 46 | *** | 0.051 | 0.467 | 5.05 | 9 | 46 | *** | 0.051 | 0.555 | 9.38 | 7 | 46 | *** | |
| Smes/gm | m2 s bar CO2 mol−1 CO2 | 21.9 | 337.2 | 20.08 | 7 | 27 | *** | 21.9 | 337.2 | 1.52 | 9 | 27 | 21.9 | 337.2 | 0.55 | 1 | 27 | |||
| Γ | μbar | 40.55 | 52.92 | 11.58 | 7 | 48 | *** | 37.17 | 52.92 | 2.38 | 9 | 48 | * | 37.17 | 52.92 | 2.58 | 7 | 48 | * | |
| Ci | μbar | 219.7 | 278.4 | 4.65 | 7 | 47 | ** | 219.7 | 278.4 | 1.78 | 9 | 47 | 208.7 | 278.4 | 0.71 | 7 | 47 | |||
| Ca − Ci | μbar | 75.6 | 134.3 | 4.65 | 7 | 47 | ** | 75.6 | 134.3 | 1.75 | 9 | 47 | 75.6 | 145.3 | 0.79 | 7 | 47 | |||
| Cc | μbar | 89.1 | 178.4 | 7.32 | 7 | 41 | *** | 89.1 | 185.4 | 2.28 | 9 | 41 | * | 89.1 | 188.0 | 1.91 | 7 | 41 | ||
| Ci − Cc | μbar | 45.5 | 145.4 | 13.74 | 7 | 41 | *** | 45.5 | 145.4 | 3.37 | 9 | 41 | * | 40.5 | 145.4 | 2.05 | 7 | 41 | ||
| gs_H2O | mol water m−2 s−1 | 0.13 | 0.417 | 4.39 | 7 | 45 | ** | 0.13 | 0.520 | 1.38 | 9 | 45 | 0.13 | 0.572 | 1.67 | 7 | 45 | |||
| gm/gs_CO2 | bar−1 | 0.619 | 2.566 | 5.87 | 7 | 43 | *** | 0.619 | 2.566 | 0.93 | 9 | 43 | 0.619 | 3.369 | 2.84 | 7 | 43 | |||
| A | μmol CO2 m−2 s−1 | 9.65 | 22.87 | 11.67 | 7 | 47 | *** | 9.65 | 27.09 | 3.39 | 9 | 47 | ** | 9.65 | 27.09 | 2.76 | 7 | 47 | * | |
| E | mmol water m−2 s−1 | 2.40 | 6.08 | 4.00 | 7 | 45 | ** | 2.40 | 7.74 | 1.88 | 9 | 45 | 2.40 | 7.81 | 3.06 | 7 | 45 | * | ||
| A/E | μmol CO2 mmol−1 water | 2.85 | 4.49 | 1.42 | 7 | 45 | 2.85 | 4.69 | 1.97 | 9 | 45 | 2.85 | 5.40 | 4.05 | 7 | 45 | ** | |||
| A/gs_ H2O | μmol CO2 mol−1 water | 51.45 | 80.64 | 2.68 | 7 | 45 | * | 41.10 | 80.64 | 2.69 | 9 | 45 | * | 41.10 | 80.64 | 0.85 | 7 | 45 | ||
For each leaf trait, mean values (±se) per Oryza genome, species, and accession are reported in Supplemental Tables S1, S2, and S3, respectively. In addition, for each trait, corresponding letters of statistical significance (P < 0.05) between genomes, between Oryza species nested within genomes, and between Oryza accessions nested within species and genomes are reported in Supplemental Tables S1, S2, and S3, respectively.
Differences in Leaf and Cell Structural Traits across Accessions
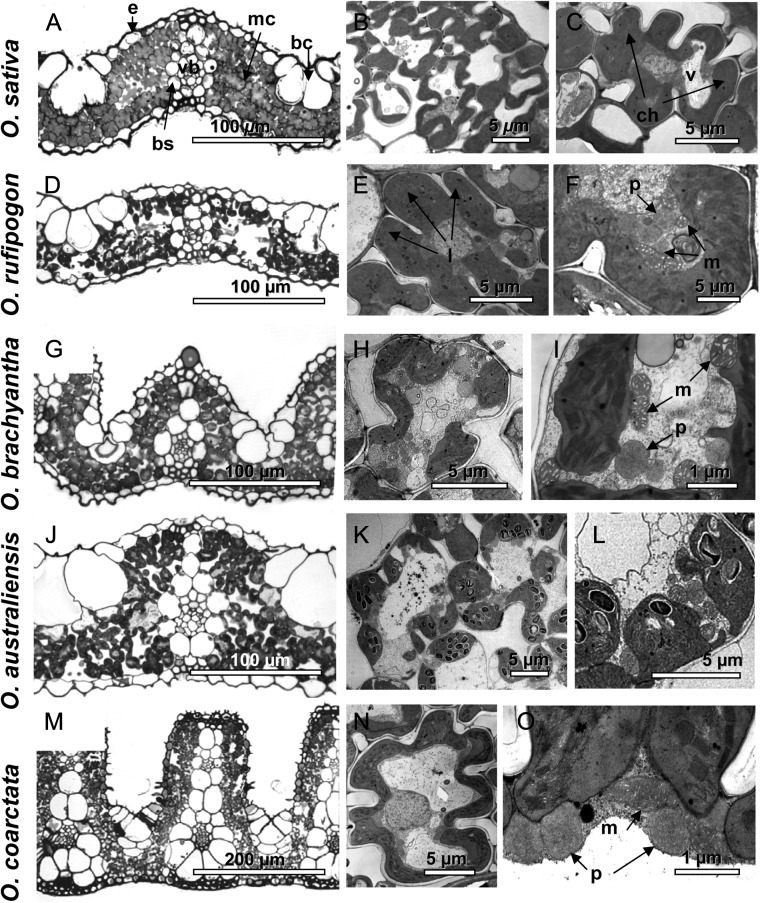
Light and electron micrographs of leaf cross sections for five representative species of Oryza are shown in Figure 1. These images illustrate variation in leaf thickness and shape, the structure of M cells including lobe development, the positions of organelles, and cell area occupied by the vacuole. The adaxial surface of leaves ranges from being relatively flat (Fig. 1, A, D, and J) to having deep invaginations with protrusions above the veins (by extension of bundle sheath and adjacent M cells), as observed, in particular, in Oryza brachyantha (Fig. 1G) and O. coarctata (Fig. 1M). In all species studied, the mitochondria and peroxisomes are preferentially located internal to the chloroplasts, with the latter being appressed to the plasma membrane (Fig. 1, F, I, L, and O).
Figure 1.
Light (left panels) and electron (middle and right panels) microscopy images of leaf anatomy in representative Oryza species (A–C, O. sativa cv IR64; D–F, O. rufipogon 9; G–I, O. brachyantha; J–l, O. australiensis 21; M–O, O. coarctata). A, D, G, J, and M, Leaf cross-sections. B, C, E, H, K, and N, M cell shape, vacuole development, distribution of chloroplasts, and development of lobes. F, I, L, and O, Positions of mitochondria and peroxisomes internal to the chloroplasts. bc, Bulliform cells; bs, bundle sheath; ch, chloroplast; e, epiderm; l, lobes; m, mitochondria; mc, mesophyll cell; n, nucleus; p, peroxisomes; v, vacuole; vb, vascular bundle.
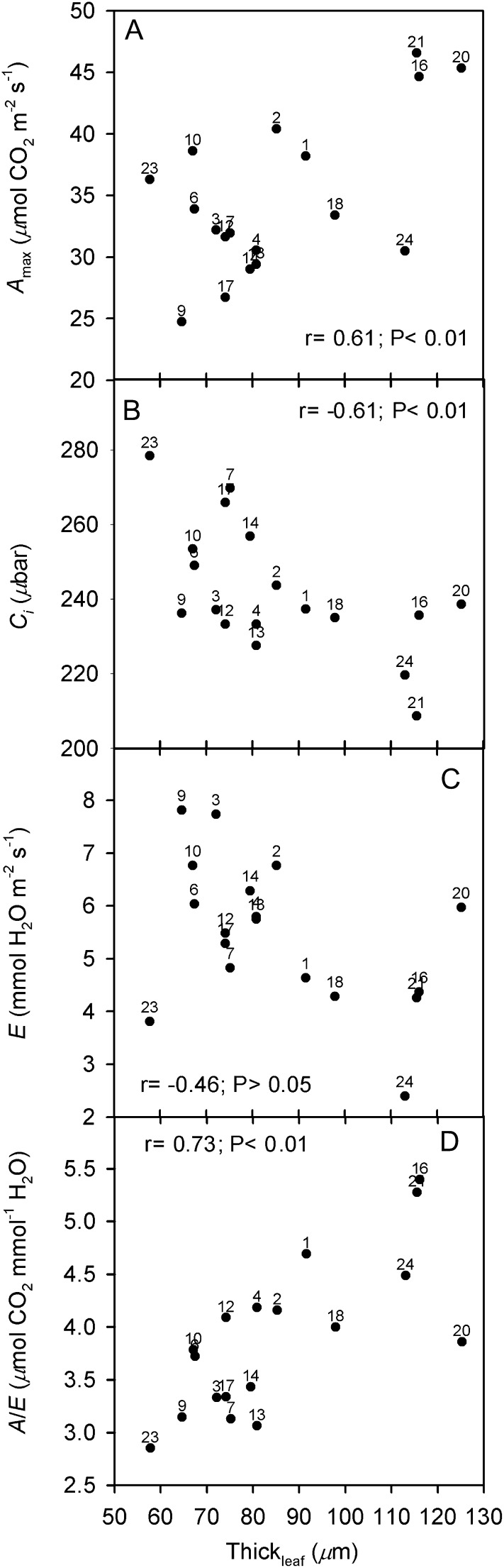
The minimum and maximum means within Oryza accessions for each leaf trait quantified by microscopy, are shown in Table III (see mean trait values for each accession in Supplemental Table S3). There is an approximately 2-fold difference in Thickleaf across accessions (from approximately 58 to 125 μm). Oryza rufipogon 9 (Fig. 1D) and O. brachyantha (Fig. 1G) have the thinnest leaves; O. australiensis 21 (Fig. 1J), O. australiensis 20, Oryza rhizomatis 16, and O. coarctata (Fig. 1M) have the thickest leaves. The VolIAS is lowest in O. brachyantha and highest (approximately 1.8 times) in O. australiensis 20.
Across accessions, 89% (overall mean) of Smes is covered by chloroplasts; the lowest values were 63% for O. glaberrima and 67% for O. meridionalis; all other values are above 83% (calculations are based on Smes and Schl data in Supplemental Table S3). There is an approximately 3-fold difference across accessions in Smes (and Schl); Oryza glumaepatula 3 had the lowest (8.8 μm2 μm−2) and O. coarctata had the highest (24.4 μm2 μm−2) Smes values. The acell varied approximately 4-fold across accessions (lowest in O. brachyantha to highest in Oryza latifolia 18). There is a 1.5-fold difference in the extent of Lobcell, which was lowest in O. brachyantha (1.06 μm μm−1), high in O. rufipogon 9 and O. sativa cv IR64, and highest in Oryza barthii (1.45 μm μm−1). Thickcw varied approximately 1.5-fold (from 0.125 μm in O. rufipogon 9, to 0.190 μm in Oryza minuta).
The fraction of M cell wall covered by chloroplasts (CWchl) was lowest in O. glaberrima (approximately 74%) and highest in Oryza nivara 7 (approximately 94%); most accessions have CWchl greater than 85% (Supplemental Table S3). Among accessions, the fraction of M cell wall exposed to the IAS (CWIAS) ranged from 35% to 56%, and the fraction of M cell wall exposed to IAS covered by chloroplasts (CWIAS_chl) ranged from 64% to 98%. The fraction of cell wall adjacent to other cells (ACW) and ACW covered by chloroplasts (ACWchl) varied from 69% in O. latifolia 18, to 93% in O. nivara. There was a 5.3-fold difference in the fraction of cell volume occupied by vacuoles; it was lowest in O. rufipogon 9 (8.2%; Fig. 1, E and F), medium high in O. coarctata (Fig. 1, N and O), and highest in O. glaberrima (43.3%).
There is an approximately 4-fold difference in the leaf cumulative stomatal pore area index (Ist), which is lowest in Oryza alta (having low stomatal density and size) and highest in O. barthii (having high stomatal density and size). In general, except for O. coarctata and O. brachyantha, the stomatal density is higher on the abaxial than on the adaxial lamina surface.
Differences in Leaf Functional Traits across Accessions
The minimum and maximum means within Oryza accessions for each leaf functional trait, are shown in Table III (for mean trait values for each accession, see Supplemental Table S3). The difference in A was approximately 2.9-fold (minimum of 9.7 μmol CO2 m−2 s−1 in O. brachyantha and maximum of 27.1 μmol CO2 m−2 s−1 in O. glaberrima), that in E was approximately 3-fold, and that in A/E and intrinsic transpiration efficiency (A/gs_H2O ratio) was approximately 2-fold. The difference in CE was approximately 4.6 fold (minimum in O. brachyantha and maximum in O. australiensis 22), in gm was approximately 10.9 fold (maximum in O. australiensis 21, minimum in O. coarctata), and in M resistance to CO2 per unit of cell surface area exposed to IAS (Smes/gm) was approximately 15-fold. In addition, across accessions, there was a range of approximately 1.4-fold in CO2 compensation point (Γ), approximately 1.3-fold in Ci, and approximately 2-fold in Cc. There was an approximately 4.4-fold difference in gs_H2O and an approximately 5.4-fold difference in the ratio between gm and gs_CO2.
Leaf Structural-Functional Principal Factor Analysis
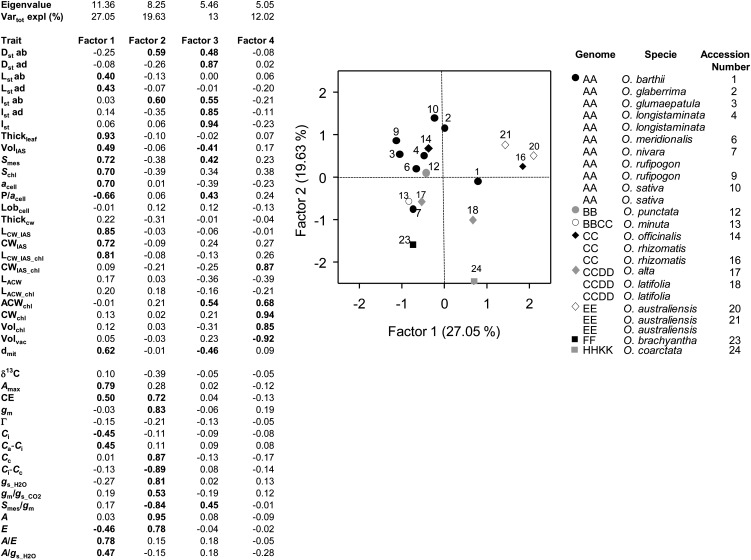
From principal factor analysis (PFA) performed on the leaf structural and functional traits of Oryza accessions four main axes of covariation (factors) were considered (for trait loadings on the factors, see Fig. 2, left side). The cluster of interrelated leaf traits with high positive association with factor 1 included Thickleaf (along with other structural features, VolIAS, Smes, Schl, and acell), maximum net photosynthetic rate (Amax), and A/E; A/gs_H2O had a lower positive association. In contrast, E and the ratio between M cell perimeter and cell section area in a leaf cross section (P/acell) had negative associations. Factor 1 was interpreted to represent M architecture. The cluster of interrelated leaf traits with high positive associations with factor 2 included A, CE, gm, Cc, gs_H2O, and E, while gm/gs_CO2 had a lower positive linkage; conversely, the difference between intercellular and chloroplastic partial pressure of CO2 (Ci − Cc) and Smes/gm had negative associations with factor 2 (Fig. 2, left side). Factor 2 was interpreted to represent photosynthetic-transpiration activity. Factors 1 and 2 accounted for 27.1% and 19.6% of the total variability, respectively.
Figure 2.
PFA of leaf structural and functional traits. At left are factor loadings for all leaf structural and functional traits determined on 18 Oryza accessions. Vartot expl, Total variance explained. Factor loadings greater than 0.4 in absolute value are shown in boldface. The graph associated with the data are in Supplemental Table S4, left side. Trait symbols are as in Table II. At right are loadings of the Oryza accessions (identified by number as in Table I) on factor 1 (M architecture) and factor 2 (photosynthetic-transpiration activity). Genome symbols are associated with each accession.
A third cluster of interrelated leaf traits positively associated with factor 3 included stomatal density in both the abaxial (Dstab) and adaxial (Dstad) lamina surfaces, Istab and stomatal pore area index in the adaxial lamina surface (Istad), leaf cumulative Ist, and ACWchl, while VolIAS had a negative linkage. Factor 3 was interpreted to represent leaf stomatal composition; it accounted for 13.0% of total variability (Fig. 2, left side; data not shown). The fourth cluster of interrelated leaf traits positively associated with factor 4 included CWIAS_chl, ACWchl, CWchl, and the fraction of cell volume occupied by chloroplasts (Volchl), while the fraction of cell volume occupied by vacuole (Volvac) had a negative loading. Factor 4 was taken to represent M cell chloroplast display; it accounted for 12.0% of the total variability (Fig. 2, left side; data not shown). In total, the main four factors accounted for 71.7% of the total variability in the data set.
The Oryza accessions loaded on M architecture (factor 1) and photosynthetic-transpiration activity (factor 2) are shown in Figure 2, right side (the corresponding scores are displayed in Supplemental Table S4, right side). In general, the accessions tended to group in three clusters based on M architecture, while they showed an overall higher variability on photosynthetic-transpiration activity. In particular, O. australiensis 20 and 21 and O. rhizomatis 16 had high scores on factor 1, while O. rufipogon 9 and O. glumaepatula 3 had low scores on this factor. However, all these species had high scores on factor 2. The rice accessions O. sativa cv IR64 (10) and O. glaberrima 2 had the highest scores on factor 2; vice versa, O. brachyantha 23 and O. coarctata 24 had the lowest scores (but O. coarctata had high scores on factor 1 compared with O. brachyantha 23). The accession scores for factor 3 and 4 are not graphed but are displayed in Supplemental Table S4, right side.
Pearson Correlation Matrices for Structural, Functional, and Structural-Functional Leaf Traits
To complement the PFA, trait-to-trait correlations were further analyzed, based on the data set. The Pearson correlation matrix for leaf structural traits is shown in Supplemental Table S5. In particular, Thickleaf showed positive correlations with several leaf structural features, including acell, Smes, Schl (P < 0.01), and VolIAS (P < 0.05). Smes and Schl are highly correlated (r = 0.97; P < 0.01). Smes was also closely associated with Istad (r = 0.67; P < 0.01). Lobcell showed a positive correlation with the length of M cell walls adjacent to other cells covered by chloroplasts (LACW_chl; P < 0.01), and also with acell, although not significant (r = 0.30). There was a close negative association between Volchl and Volvac (P < 0.01).
The Pearson correlation matrix for leaf functional traits is shown in Supplemental Table S6. There is a positive correlation between CE and both A and Amax (P < 0.01). gm has a positive association with A and Cc (P < 0.01) and with gs_H2O (P < 0.05). gm was negatively correlated (P < 0.01) with Smes/gm, which is linked to the large difference in gm across accessions compared with Smes. Smes/gm exhibited a negative association with Cc, gs_H2O, A, and E (P < 0.01) and with CE (P < 0.05). Ci was negatively associated with A/gs_H2O, A/E, and gm/gs_CO2 (P < 0.01). There was a tight positive correlation between A and E (P < 0.01). In addition, gs_H2O was positively correlated with both A and E and negatively associated with Ci − Cc (P < 0.01).
The portion of the Pearson correlation matrix showing the leaf structural-functional trait-to-trait associations is in Supplemental Table S7. Thickleaf had a close negative correlation with Ci (P < 0.01) and a positive association with Amax (P < 0.01); it had a negative relationship with E (P > 0.05) and significant positive associations with A/E (P < 0.01) and A/gs_H2O (P < 0.05). In addition, Smes had a tight negative association with E (P < 0.01) and close positive associations with A/E (P < 0.01) and A/gs_H2O (P < 0.05).
With respect to stomatal traits on the abaxial leaf surface, Dstab has a significant positive association with gs_H2O (P < 0.05) and with A and E (P < 0.01); in addition, Istab has a positive association with CE (P < 0.05) and a negative strong relationship with δ13C (P < 0.01). In contrast, on the adaxial leaf surface, there is a significant positive correlation between Istad and Smes/gm (P < 0.01). In addition, Istad has a negative association with E (r = −0.41; P > 0.05) and a positive association with A/gs_H2O (P < 0.05). Lobcell has a negative correlation with Γ (P < 0.05). Although not significant (P > 0.05), the results suggest that Thickcw has a negative relationship with gm (r = −0.42) and a positive association with Smes/gm (r = 0.37).
Trait-to-Trait Correlations
Considering the clusters of interrelated leaf traits closely associated with PFA factors 1 and 2, and of parameters of most interest with respect to relationships between leaf structure, photosynthesis, and transpiration (see introduction), trait-to-trait correlations were analyzed in plots of mean values for each Oryza accession (Figs. 3–10; note that in most panels, the Pearson correlation coefficients were significant; Supplemental Tables S5–S7).
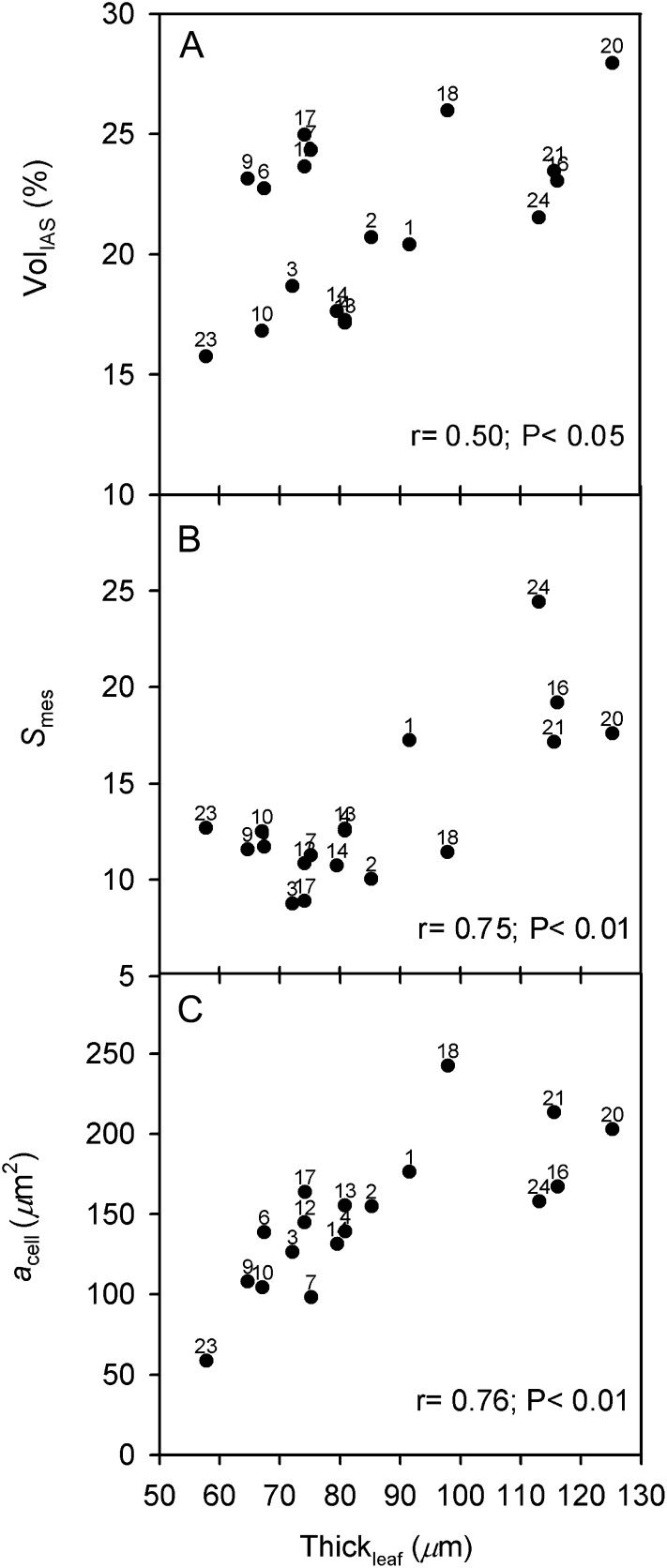
Figure 3.
Correlation of leaf thickness with other leaf structural traits in Oryza accessions. A, VolIAS. B, Smes. C, acell. Points are mean values per accession (se values are given in Supplemental Table S3). Numbers correspond to the accessions listed in Table I.
Figure 10.
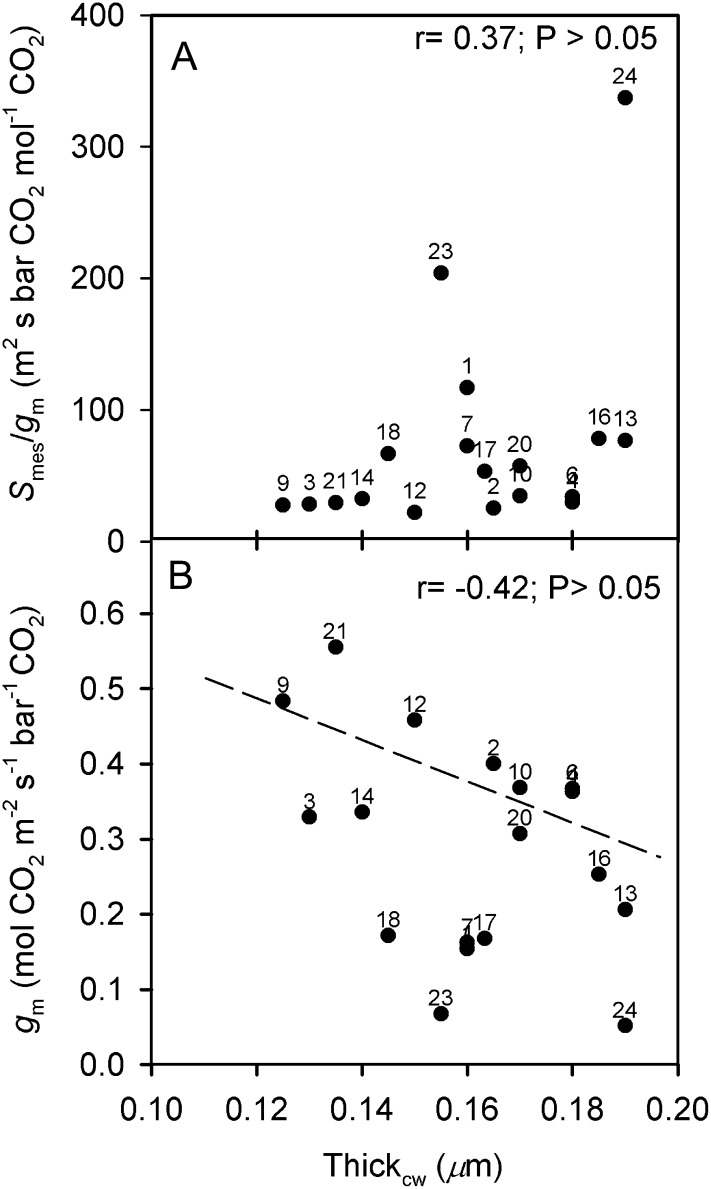
Correlations between Thickcw and functional traits in Oryza accessions. A, Smes/gm. B, gm. Accessions above the dashed line have gm > 0.35 mol CO2 m−2 s−1 bar−1 CO2 and show a negative relationship between gm and gs_CO2 (Fig. 5C). Points are mean values per accession (se values are given in Supplemental Table S3). Numbers correspond to the accessions listed in Table I.
Structural Traits
First, with respect to structural traits, the significant positive associations between mean values of VolIAS, Smes, and acell, and the corresponding mean values of Thickleaf, are shown in Figure 3. In particular, O. australiensis 20 and 21, O. rhizomatis 16, and O. coarctata 24 showed the highest values for these M and cell structural features. In contrast, O. brachyantha 23 and O. sativa cv IR64 (10) had the lowest values. There is also a close positive association between Lobcell and LACW_chl (Fig. 4), which is in part driven by O. brachyantha 23, which has the lowest LACW_chl value; O. barthii 1 has the highest LACW_chl value.
Figure 4.
Correlation, across Oryza accessions between Lobcell and LACW_chl. Points are mean values per accession (se values are given in Supplemental Table S3). Numbers correspond to the accessions listed in Table I.
Functional Traits
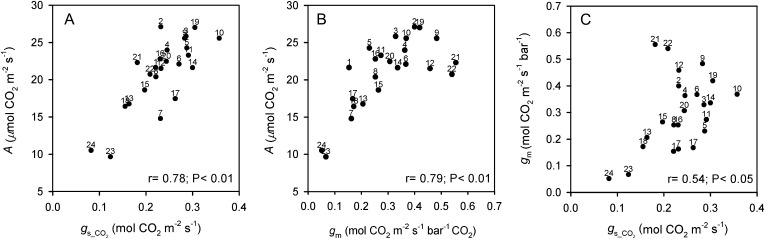
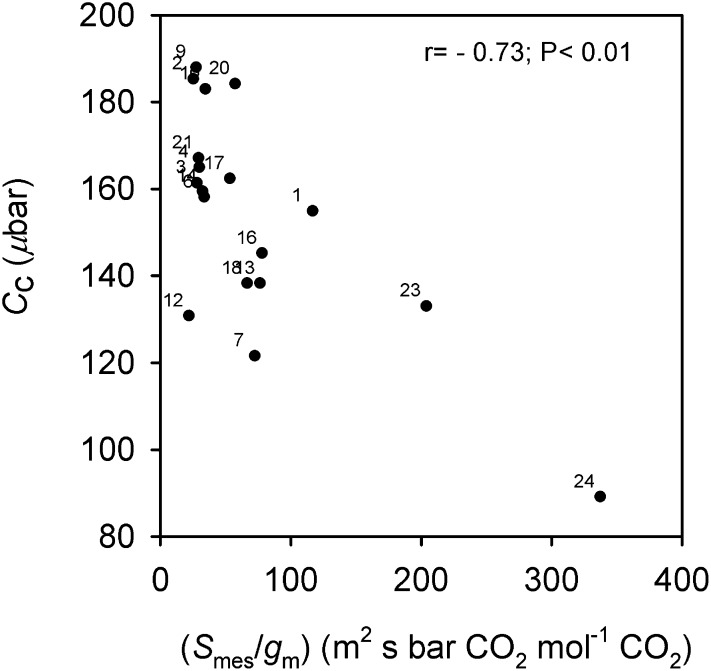
Significant relationships for leaf functional features associated with the photosynthetic process are shown in Figures 5 and 6. In the close positive association between A and gs_CO2 across accessions (Fig. 5A), O. coarctata 24 and O. brachyantha 23 have the lowest values for both traits; in contrast, O. sativa cv IR64 (10) and O. latifolia 19 have the highest A (above 25 μmol CO2 m−2 s−1) and gs_CO2 values. There is also a strong positive association between A and gm (Fig. 5B); however, there were deviations; O. australiensis 21 and 22 have the highest gm values (close to 0.55 mol CO2 m−2 s−1 bar−1 CO2) but not the highest A. Also, very high gm values (greater than 0.45 mol CO2 m−2 s−1 bar−1 CO2) were found in O. rufipogon 9, with A approximately 25 μmol CO2 m−2 s−1, and in Oryza punctata 12, with A approximately 22 μmol CO2 m−2 s−1. In the plot of gm versus gs_CO2 (Fig. 5C), there is no apparent linear relationship; rather, two pools of data are recognized. In particular, there is a close positive relationship between gm and gs_CO2 up to gm values of 0.35 mol CO2 m−2 s−1 bar−1 CO2 (r = 0.78; P < 0.01). At the higher gm values, from 0.35 up to 0.56 mol CO2 m−2 s−1 bar−1 CO2, there is a negative relationship between gm and gs_CO2 (r = −0.64; P > 0.05), notably in O. australiensis 21 and 22, O. rufipogon 9, O. punctata 12, O. latifolia 19, O. glaberrima 2, Oryza longistaminata 4, and O. meridionalis 6. Figure 6 shows the significant negative correlation between Cc and Smes/gm, which is partially driven by the high Smes/gm values of O. coarctata 24 and O. brachyantha 23 (above 180 m2 s bar CO2 mol−1 CO2); most accessions have mean Smes/gm values in the range of 25 to 80 m2 s bar CO2 mol−1 CO2.
Figure 5.
Correlations between leaf functional traits associated with the photosynthetic process in accessions of Oryza species. A, A versus stomatal conductance to CO2 diffusion. B, A versus M conductance to CO2 diffusion. C, gm versus stomatal conductance to CO2 diffusion. Points are mean values per accession (se values are given in Supplemental Table S3). Numbers correspond to the accessions listed in Table I.
Figure 6.
Correlation between Cc and Smes/gm across Oryza accessions. Points are mean values per accession (se values are given in Supplemental Table S3). Numbers correspond to the accessions listed in Table I.
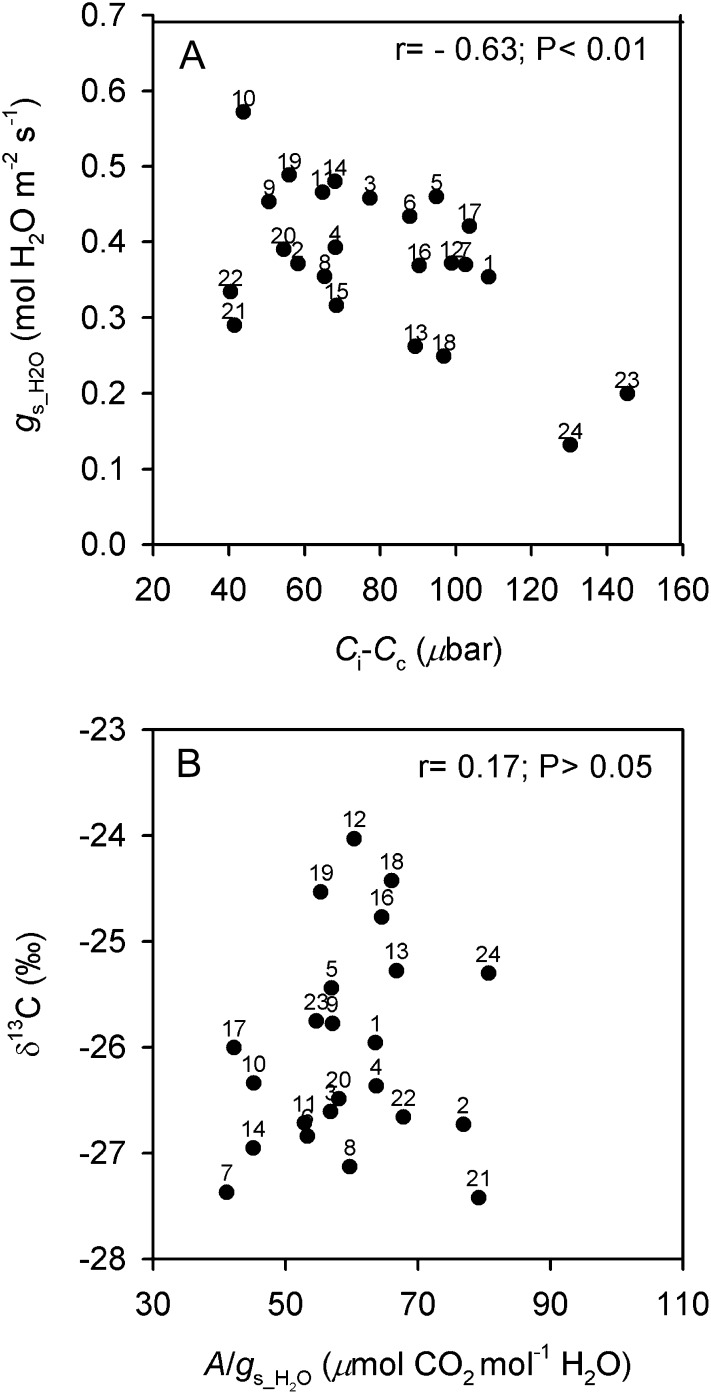
Plots of important relationships between leaf functional features associated with transpiration are shown in Figure 7. In Figure 7A, the significant negative correlation between gs_H2O and Ci − Cc (which is partially driven by the highest Ci − Cc and the lowest gs_H2O values of O. coarctata 24 and O. brachyantha 23) reflects a significant negative association between E and Ci − Cc (data not shown). O. australiensis 21 and 22, O. rufipogon 9, and O. sativa cv IR64 (10) had the lowest Ci − Cc values; among these accessions, O. australiensis 21 also had a medium-low gs_H2O. O. glaberrima 2, O. latifolia 19, and O. australiensis 20 also had relatively low Ci − Cc values. The tendency for a general positive association between δ13C and A/gs_H2O (i.e. less negative δ13C tends to correspond to high A/gs_H2O) is displayed in Figure 7B. In particular, O. nivara 7 has the lowest δ13C (the most negative value) and the lowest A/gs_H2O, while O. punctata 12 has the highest δ13C and a medium A/gs_H2O value. O. australiensis 21 and O. glaberrima 2 were outliers, as they have low δ13C values (more negative than −26.7‰) and the highest A/gs_H2O values.
Figure 7.
Correlations between leaf functional traits associated with the transpiration process in Oryza accessions. A, gs_H2O and Ci − Cc. B, δ13C and A/gs_H2O. Points are mean values per accession (se values are given in Supplemental Table S3). Numbers correspond to the accessions listed in Table I.
Structural Versus Functional Traits
Important relationships between leaf structural and functional traits related to both photosynthesis and transpiration are shown in Figures 8–10. There is a significant positive association between Thickleaf and Amax (Fig. 8A), with O. australiensis 20 and 21 and O. rhizomatis 16 having the highest values for both traits. There is a close negative association between Thickleaf and Ci (Fig. 8B), with O. australiensis 21 and O. coarctata 24 having the lowest Ci values. Results suggestive of a negative association between Thickleaf and E are displayed in Figure 8C; O. australiensis 20 is an outlier, having high Thickleaf and a relatively high E, while O. brachyantha 23 is an outlier that has the lowest Thickleaf along with low E. Figure 8D shows the close positive association between Thickleaf and A/E; among accessions having the thickest leaves, O. rhizomatis 16 and O. australiensis 21 have the highest A/E values, while O. australiensis 20 is an outlier with a medium A/E value. Also, there is a significant positive association (r = 0.59; P < 0.05) between Thickleaf and A/gs_H2O (data not shown).
Figure 8.
Correlations between Thickleaf and leaf functional traits in Oryza accessions. A, Amax. B, Ci. C, E. D, A/E. Points are mean values per accession (se values are given in Supplemental Table S3). Numbers correspond to the accessions listed in Table I.
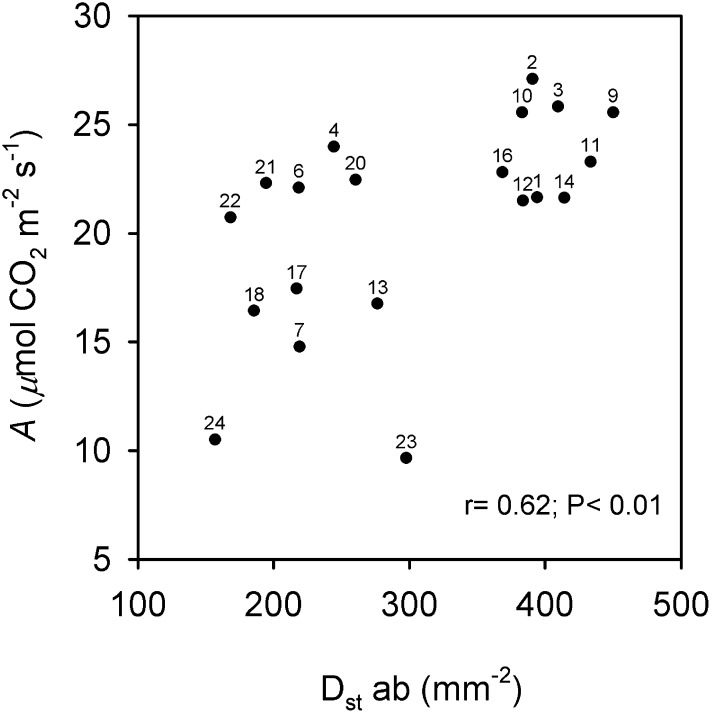
The significant positive relationship between A and Dstab is shown in Figure 9. O. coarctata 24 has the lowest Dstab, while O. rufipogon 9 has the highest value. O. sativa cv IR64 (10) and O. glaberrima 2 have medium to high Dstab and the highest A values.
Figure 9.
Correlation in Oryza accessions between leaf A and Dst ab. Points are mean values per accession (se values are given in Supplemental Table S3). Numbers correspond to the accessions listed in Table I.
The data suggestive of a positive association between Thickcw and M resistance to CO2 diffusion per unit of cell surface area exposed to IAS are shown in Figure 10A. O. coarctata 24 and O. brachyantha 23 are outliers, with high Smes/gm values. Results suggestive of a negative correlation between Thickcw and gm are shown in Figure 10B. In particular, accessions having gm values greater than 0.45 mol CO2 m−2 s−1 bar−1 CO2 (including O. rufipogon 9, O. punctata 12, and O. australiensis 21) had Thickcw of 0.150 μm or less. All the accessions above the dashed line in Figure 10B correspond to those in Figure 10A having the lowest Smes/gm values and to those in Figure 5C having a negative association between gm (for values above 0.35 mol CO2 m−2 s−1 bar−1 CO2) and gs_CO2. In contrast, the accessions below the dashed line have the highest Smes/gm (Fig. 10A) and a positive correlation between gm (below 0.35 mol CO2 m−2 s−1 bar−1 CO2) and gs_CO2 (Fig. 5C).
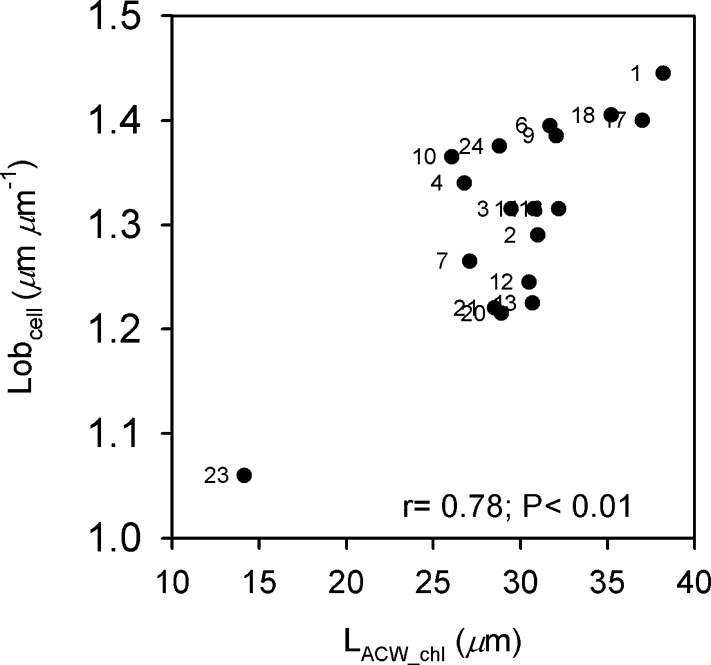
There is a negative association between Γ and Lobcell (data not shown; r = −0.53; P < 0.05); this is driven in part by O. brachyantha 23, which has the highest Γ (52.9 μbar) and the lowest Lobcell (1.060 μm μm−1), and by O. minuta 13, which also has a high Γ (51.7 μbar) and Lobcell of 1.225 μm μm−1 (Supplemental Table S3).
DISCUSSION
Interrelations among Leaf Thickness and Other Leaf Structural Traits
The hypothesis that in Oryza species some M structural traits will be associated with leaf thickness was confirmed by the PFA results for factor 1 (M architecture) and by trait-to trait correlation analysis; in particular, Thickleaf had significant positive correlations with Smes and Schl, VolIAS, and acell (Terashima et al., 2006).
The positive correlation between Thickleaf and Smes (and Schl) suggests that the higher number of chloroplasts in thicker leaves is coordinated with increases in Smes. In the absence of an association between Thickleaf and Smes, more chloroplasts would become located away from the cell surface area facing the IAS: this would increase the M diffusive resistance to CO2 (Nobel et al., 1975; Evans et al., 2009), and light availability for many chloroplasts could also become limited (Oguchi et al., 2003). In this regard, Hanba et al. (1999) found that the association between Smes and Thickleaf holds across different leaf types among evergreen species.
A positive association was found across Oryza accessions between Thickleaf and VolIAS, with the mean Thickleaf ranging from approximately 58 to 125 μm and VolIAS from approximately 16% to 28%. Niinemets (1999), in a study on woody shrubs and grass species, reported a huge variation in leaf anatomy, with Thickleaf ranging from approximately 55 to 1,960 μm and M porosity (corresponding to VolIAS in this study) ranging from 10% to 36%. It was suggested that the variation in Thickleaf and in the corresponding leaf structural profile affects the pattern of light absorption through the layers of M cells and the distribution of chlorophyll and Rubisco, while the consequences for the diffusion of CO2 in the IAS are considerably smaller.
In addition, in Oryza species, there was a tendency for a positive association between acell and Lobcell (r = 0.30; P > 0.05); this suggests that Lobcell may increase with cell volume, which could reduce the resistance to CO2 diffusion into the larger M cells. Also, among Oryza accessions there is a tendency toward a positive association between Thickcw and both Smes and Thickleaf (r = 0.42 and 0.24, respectively; P > 0.05); Thickcw, acting as resistance in series for M CO2 diffusion, may reduce gm, as observed by Terashima et al. (2006).
Coordination between gm and M Structural Traits
Another premise of this study was that gm in Oryza accessions would be correlated with leaf M structural traits. Based on trait-to-trait analysis, gm was not significantly correlated with any single M structural trait, including Smes, Lobcell, or Thickcw; however, there was a tendency toward a lower gm and higher Smes/gm with increasing M cell wall thickness (P > 0.05; Fig. 10). Lack of close correlations of gm with single traits might be expected if there are covariations of multiple leaf and M cell structural traits that tend to have both positive (e.g. Smes) and negative (e.g. Thickcw) effects on gm (Supplemental Materials and Methods S3; Terashima et al., 2011).
Based on the analysis conducted on leaf data from multiple species by Evans et al. (2009), there was an apparent positive correlation between gm and Schl, but with a low slope when Thickcw was greater than 0.140 µm. Since Thickcw in Oryza accessions is also relatively high (approximately 0.125–0.190 µm), this may account for the lack of a positive correlation between gm and Smes (or Schl). Evans et al. (2009) estimated that the cell wall may contribute from 25% to 30% or more of the total M resistance to CO2, even if changes in one component of the conduction pathway may be countered by pleiotropic compensatory changes; these findings are in agreement with Barbour et al. (2010).
Interestingly, lobes in M cells can increase the M cell surface to cell volume (Parker and Ford, 1982; Burundukova et al., 2003), which is convenient for CO2 diffusion into the cell (Nobel, 2009), and they may contribute to Smes (Nobel, 2009) and gm (Evans and Loreto, 2000; Evans et al., 2009). M cell lobes are prominent in some Oryza species, and most lobes of contiguous cells do not interlock. Approximately 50% of the M cell wall is adjacent to other cells, with a high percentage of this area also occupied by chloroplasts. In contrast, C3 species with high photosynthetic activity generally have few chloroplasts located where the cell wall is in contact with other cells (Evans et al., 2004). In Oryza accessions Lobcell was positively correlated with the length of the cell wall adjacent to another cell (LACW) and with LACW_chl. These tight associations suggest that cell lobing may allow a larger proportion of the cell wall to be adjacent to other cells, while allowing for CO2 and light access to the chloroplasts via the cell walls between the lobes. These M cell structural features may contribute to an increase in gm and enhance leaf photosynthetic activity, with a positive feedback on the refixation of photorespired CO2 (Nobel, 2009); this could lower Γ by decreasing the CO2 chloroplastic photocompensation point. In this study, in fact, there was a significant negative association between Lobcell and Γ (P < 0.05), and the results are also suggestive of a positive association between Lobcell and A (P > 0.05). M cell lobes may be particularly beneficial in hot environments, where rates of photorespiration are high (Peterhansel and Maurino, 2011). Also, the relative positions of mitochondria and peroxisomes compared with chloroplasts in lobed M cells (Fig. 1; Sage and Sage, 2009) may enhance CO2 refixation (Busch et al., 2013).
In addition to these leaf and M cell structural traits, the composition of cell walls, plasma membrane, and chloroplast envelope (Kogami et al., 2001; Nobel, 2009) as well as biochemical factors increasing membrane permeability to CO2 are recognized to affect the CO2 diffusion from IAS to chloroplast stroma (Bernacchi et al., 2002; Terashima and Ono, 2002; Terashima et al., 2006; Uehlein et al., 2008; Evans et al., 2009; Tosens et al., 2012). According to Flexas et al. (2007), the implication of active transport of CO2 by cooporins in the regulation of gm (Hanba et al., 2004; Flexas et al., 2006) may explain the weak relationship between gm and leaf M structure. In this study, intrinsic differences in biochemical factors might partially explain the variation in Smes/gm among Oryza accessions.
No correlation was found across Oryza accessions between VolIAS and gm, which might be explained by the fact that changes in the gaseous CO2 diffusion resistances to variation in VolIAS tend to be relatively small (Niinemets, 1999; Nobel, 2009). Both negative and positive correlations between M porosity (corresponding to VolIAS) and gm have been observed in previous studies (Flexas et al., 2008); this might depend on the covariation of some other structural trait that affects the resistance in the liquid phase and, consequently, gm (Evans et al., 2009; Nobel, 2009). Several studies have shown that leaf density (g m−3), leaf thickness, and their product leaf dry mass per unit (one side) of the leaf surface area (g m−2) tend to be negatively linked to gm and may set an upper limit for the maximum value of gm (Terashima et al., 2006; Flexas et al., 2008; Niinemets et al., 2009). In particular, high leaf dry mass may be associated with thick leaves having thick M cell walls, which could offset the positive effect of Smes on gm and could contribute to an increase in the M resistance to CO2 diffusion (Evans and von Caemmerer, 1996; Niinemets, 1999). Controversial results were found among evergreen, deciduous, and annual species in these relationships (Hanba et al., 1999).
Coordination between Functional Traits: A, gs and gm, and E
The hypothesis of correlations between A, gs and gm, and E in Oryza species was supported by the PFA results for factor 2 (photosynthesis-transpiration activity) and by trait-to-trait correlation analysis. The data indicate a coupling between photosynthesis and transpiration processes and are aligned with evidence that both gs and gm are important physical determinants of the CO2 supply from the atmosphere to the chloroplasts (Evans and von Caemmerer, 1996; Evans, 1999; Evans and Loreto, 2000; Barbour et al., 2010).
However, across Oryza accessions despite an overall positive correlation between gs_CO2 and gm (P < 0.05), a strong positive relationship (P < 0.01) between gs_CO2 and gm up to gm values of approximately 0.35 mol CO2 m−2 s−1 bar−1 CO2 was observed, corresponding to gm/gs_CO2 ratios from 0.62 to approximately 1.4 bar−1. Above this gm threshold, there was a negative correlation (P > 0.05) between gm and gs_CO2, with gm/gs_CO2 ratios between approximately 1.0 and 3.4 bar−1. The contrasting relationships between gm and gs in the two gm ranges have important implications for A/E in the different accessions. A meta-analysis of data from a multitude of grass, shrub, and tree species (Niinemets et al. (2009) revealed how photosynthesis tends to become more sensitive to fluctuations in stomatal conductance than in M conductance at high gm values (or under conditions where gm is higher than gs). Similarly, in Oryza accessions the low sensitivity of A to changes in gm higher than 0.35 mol CO2 m−2 s−1 bar−1 CO2 could be associated with a redistribution of overall CO2 diffusion limitation between stomata (gs) and M cells, which affects the coordination between photosynthetic and transpiration processes.
In Oryza species, there is a close negative relationship between leaf A and Ci − Cc and a strong positive correlation between A and Cc. A positive correlation between Smes (or Schl) and A, which was not found in this study, would have kept Ci − Cc independent of A (Evans, 1999). In addition, a tendency for a negative correlation between Ca − Ci and Ci − Cc was observed (P > 0.05), and based on Equation 1, gm together with gs should be involved in the tradeoff between Ca − Ci and Ci − Cc. In fact, there was a significant negative correlation between gm and Ci − Cc (P < 0.01); also, a tight positive association between Smes/gm and Ci − Cc was found (P < 0.01). In this regard, Niinemets et al. (2009) described how a drawdown of CO2 partial pressure from substomatal cavities to chloroplasts (Ci − Cc), rather than gm per se, characterizes the M diffusion limitation of photosynthesis.
Across Oryza accessions the data suggest that a negative correlation exists between gm and Ci (P > 0.05); a higher gm when Ci is low could prevent Cc from becoming too low with loss of efficiency of Rubisco activity, while a lower gm associated with a higher Ci tends to avoid excess Cc compared with Rubisco capacity. There is evidence for a strong inverse relationship between gm and Ci when photosynthesis is not limited by CO2 but by regeneration of Rubisco or triose phosphate utilization (Flexas et al., 2007). In addition, there is a weak negative association between gs and Ca − Ci and a strong negative correlation between gs and Ci − Cc (P < 0.01), which is partially driven by O. brachyantha and O. coarctata (data not shown). This is plausible, since Ci − Cc is affected by the coordination between carboxylation activity and gm, which can adjust faster than gs, so that changes in Ci − Cc may occur quicker than in Ca − Ci (Flexas et al., 2007).
Relationships between Leaf Structural Traits and A/E
The results are consistent with the hypothesis that leaf structural traits in Oryza accessions are involved in the relationship between A and E, which affects A/E. Based on PFA, A/E was among the interrelated leaf traits (besides Thickleaf and a number of structural factors) that were positively associated with M architecture; vice versa, E showed a negative association. Trait-to-trait analysis also suggested a negative relationship between Thickleaf and E (P > 0.05), which reflects the negative correlation found between Thickleaf and gs (P > 0.05). When excluding two outlier accessions (O. brachyantha 23 and O. australiensis 20), a significant negative association between Thickleaf and E was observed (P < 0.01; data not shown).
When considering features associated with photosynthesis, there was a close negative correlation (P < 0.01) between Thickleaf and Ci, which was mirrored by a corresponding positive correlation between Thickleaf and Ca − Ci. This suggests that Cc could potentially become more limiting for photosynthesis in thick leaves. With respect to leaf thickness, Parkhurst et al. (1988) reported that, in general, the activity of Rubisco varies through the M in response to a light gradient, making it possible to have a balance between local CO2 pressures and local enzyme activities in a way that may increase the overall assimilation rate of the leaf. However, in the Oryza accessions Thickleaf was not positively associated with A. This result, which is in agreement with Niinemets (1999), can be explained by the lack of correlation between Thickleaf and gm discussed earlier, by the tight negative relationship between Thickleaf and Ci (and a weak negative correlation between Thickleaf and gs), and by the weak negative correlation between Thickleaf and Ci − Cc. In contrast, there is a significant positive correlation between Thickleaf and Amax; this may occur considering that saturating CO2 will increase Ci and the Ci − Cc gradient, promoting CO2 diffusion to the chloroplasts.
With respect to stomata, there were positive associations between the stomatal density on the abaxial side of the lamina and features of both CO2 and water exchange, but not on the adaxial side. The results suggest that the stomata on the abaxial side of the lamina are mainly conducting CO2 and water vapor between the leaf and atmosphere; that is, the amphistomatous leaves of genus Oryza could functionally behave as hypostomatous, by closing or nearly closing the adaxial stomata (Reich, 1984). While a strong stomatal regulation on the adaxial side of leaves to limit water loss tends to occur naturally in leaves (Smith et al., 1997), it may have been intensified in this study by light exposure of the lamina in the cuvette.
The tendency for a negative correlation (P > 0.05) between Thickleaf and Dstab, and a significant positive linkage between Dstab and both gs and E, may explain the tendency for a negative association observed across Oryza accessions between Thickleaf and E. In addition, the leaf hydraulic architecture might contribute to the relationships between leaf structure and transpiration (Brodribb et al., 2007, 2010; Guyot et al., 2012). In this regard, bundle sheath extensions are known to facilitate leaf water conductivity through the M to the stomata (Sack and Holbrook, 2006). However, O. coarctata and O. brachyantha, which have prominent bundle sheath extensions toward the adaxial surface, have the majority of stomata located deep in the furrows between bundle sheaths. This leaf stomatal arrangement may provide an additional boundary layer resistance that limits E while maintaining a high leaf water potential. The structural design of O. coarctata appears to restrict the loss of water at the expense of photosynthesis (low A), by having the lowest gs and gm values and a low gm/gs_CO2 ratio, with a high A/E (among the highest A/E and the highest A/gs_H2O across Oryza accessions).
In summary, across Oryza accessions under current ambient CO2 partial pressures and at 30°C leaf temperature, there is a positive significant association between Thickleaf and leaf A/E (this occurs with Thickleaf having a negative correlation with E and without a correlation with A across accessions). Higher levels of CO2 can increase plant A/E by enhancing photosynthesis and decreasing water loss (due to a decrease in gs with increasing CO2; Long et al., 2004; Lammertsma et al., 2011). In Oryza accessions under CO2 saturating conditions, there is a positive correlation of Amax with Thickleaf; thus, accessions having thicker leaves could especially benefit from increased photosynthesis and A/E above ambient levels of CO2 (i.e. when CO2 is not limiting).
Correlation between gm/gs Ratio and Leaf A/E
A high gm/gs ratio is considered favorable for increasing A/E (Flexas et al., 2008, 2013; Barbour et al., 2010). The hypothesis that the gm/gs ratio would positively correlate with leaf A/E in Oryza species was supported. Several accessions with gm/gs_CO2 greater than 1.0 and high gm values had medium to high A and A/gs_H2O (and A/E) ratios. They were O. australiensis 21 and 22, O. glaberrima 2, O. longistaminata 4, O. rufipogon 9, O. punctata 12, and O. latifolia 19; in addition, they had relatively low Smes/gm. These accessions have leaf features that may be of value for maintaining carbon gain while conserving water. O. rufipogon (Zhao et al., 2008, 2010) and O. australiensis (Zhao et al., 2010) are two of the wild relative resources for crop improvement of O. sativa, and there is interest in O. australiensis and O. glaberrima for attributes associated with drought tolerance (see introduction).
The δ13C has been used as an indicator of leaf A/E in several species (Flexas et al., 2008; Barbour et al., 2010), including rice (Dingkuhn et al., 1991; Kondo et al., 2004; Centritto et al., 2009; Xu et al., 2009). In this study on Oryza accessions a weak positive association between δ13C and A/gs_H2O was observed (P > 0.05). Interestingly, O. glaberrima 2 and O. australiensis 21 were outliers, having high A/gs_H2O but among the most negative δ13C values. In these two accessions, the high gm with medium to high gs_H2O values could maintain high Cc, A, and A/gs_H2O with lower δ13C values.
Leaf Traits Contributing to Plant Drought Tolerance
Plants have diverse leaf morphoanatomical and architectural adaptations to face the challenge of coordinating structural requirements for strength and durability, controlling rates of water loss, and functioning photosynthetically (light capture, facilitating the diffusion of CO2 to the chloroplasts, and the development of metabolic capacity for carbon assimilation; Evans, 1999; Evans et al., 2004; Brodribb et al., 2010; Waite and Sack, 2010).
In this study, there was support for the hypothesis that a high gm/gs ratio is coordinated with M leaf traits to confer plant drought tolerance. In particular, the results indicate that a high gm and gm/gs ratio, a low Smes/gm, along with a high M Thickcw are features that could be beneficial in warm climates and limited water availability. O. glaberrima, O. meridionalis, and O. australiensis 20, which are recognized as species having tolerance to drought, have relatively high Thickcw (greater than 0.165 μm) compared with most Oryza species. In addition, they have relatively high gm and gm/gs ratio and low Smes/gm ratio despite having high Thickcw; also, O. longistaminata 4 has similar features. Further study of these species is needed to determine the contribution of nonstructural factors to the relationships between high gm, low Smes/gm ratio, and high cell wall thickness.
CONCLUSION
This study provides insight into the diversity of leaf structure and how it relates to photosynthesis and transpiration between representatives of cultivated rice (O. sativa and O. glaberrima) and a number of wild relatives in the genus Oryza. There are significant correlations between certain structural traits, between certain functional traits associated with photosynthesis and transpiration, and fewer correlations of function with individual structural traits. There is a close positive relationship between A and gm, but neither of these two fundamental leaf functional traits is significantly correlated, at ambient CO2 levels, with Thickleaf or any other single M structural feature. It is recognized that the value of gm in plants depends on multiple structural traits that can covary between species with Thick leaf (which may include leaf Smes and M cell features such as Thickcw, cell volume, and Lobcell); in addition, gm may be affected by cell wall composition, along with biochemical factors controlling the levels and transport of inorganic carbon in the liquid phase from IAS to chloroplast stroma. Large differences were observed in leaf A/E, which is suggested to be dependent on a tradeoff between gm and gs, and leaf structural features (e.g. a positive correlation with leaf thickness). Interestingly, species were identified having high A/E while sustaining photosynthesis. They have high gm values, along with high gm/gs ratios, low Smes/gm, and, in some species, relatively thick M cell walls, which could be beneficial traits for rice cultivated in warm climates and limiting availability of water.
MATERIALS AND METHODS
Plant Material
Accessions of Oryza species (listed in Table I) were grown in 3-L free-drainage pots in a controlled-environment growth chamber. The photoperiod was 12 h (8 am to 8 pm standard time) with air temperature of 28°C, and the dark period was with air temperature of 25°C. Light was emitted by 400-W metal halide and high-pressure sodium lamps and supplied to the canopy in a bell-shaped pattern (by incremental changes in photosynthetically active photon flux density [PPFD] every 2 h) with maximum PPFD of 1,100 µmol photons m−2 s−1 for 4 h. Air relative humidity was kept at approximately 70%, which corresponded to a maximum air vapor pressure deficit of approximately 1.8 kPa.
Three plants per accession were grown (one per pot) in a Sunshine Mix LC-1 soil (Sun Gro Horticulture) mixed with turface (ratio of 3:1 in volume). They were irrigated daily and fertilized twice per week to pot saturation with a nutrient solution including Sprint 330 iron chelate (1.3 g L−1), magnesium sulfate (0.6 g L−1), Scotts-Peters Professional 10-30-20 compound (2.8 g L−1), and Scott-Peters Soluble Trace Element Mix (8.0 mg L−1; Scotts).
Throughout, O. sativa accessions are written in full. For the wild relatives, when multiple accessions were analyzed per species, the species name is followed by the accession number (from Table I). Some figures identify accessions by the numbers in Table I (1–24); when referring to these figures, the accession number is always given.
Leaf and M Cell Structural Traits
Sample Preparation for Light and Electron Microscopy
The study of leaf anatomy was carried out on three 20- to 40-d-old plants per each Oryza accession marked with an asterisk in Table I (note that for O. sativa cv IR72 and O. australiensis 22, structural measurements were only made on stomata). On each plant, the midportion of fully expanded leaves (usually the third and fourth leaves from the apex on the central stem) was sampled. They were fixed at 4°C in 2% (v/v) paraformaldehyde and 2% (v/v) glutaraldehyde in 0.1 m phosphate buffer (pH 7.2), postfixed in 2% (w/v) OsO4, and, after a standard acetone dehydration procedure, embedded in Spurr’s epoxy resin. Leaf cross sections were made using a Reichert Ultracut R ultramicrotome (Reichert-Jung). For light microscopy, semithin leaf sections were stained with 1% (w/v) toluidine blue O in 1% (w/v) Na2B4O7 and observed at 100× magnification with the Olympus BH-2 light microscope (Olympus Optical) equipped with LM Digital Camera & Software (Jenoptik ProgRes Camera, C12plus). For transmission electron microscopy, ultrathin leaf cross sections were stained with 4% (w/v) uranyl acetate followed by 2% (w/v) lead citrate. Transmission electron microscopes, H-600 (Hitachi) and JEM-1200 EX (JEOL USA) equipped with a MegaView III Digital Camera, and Soft Imaging System software were used for observation and photography.
Analyses of Leaf Cross Sections
Leaf and cell structural traits, which were determined from light and electron microscopy on leaf cross sections, are listed in Table II. For each accession, the micrographs analyzed were on two to three leaves, depending on the technical difficulty, with each leaf taken from a different plant (n = 2 or n = 3). All measurements were performed on digital images using an image-analysis program (UTHSCSA Image Tool for Windows, version 3.00; University of Texas Health Science Center). For a consistent protocol, measurements on leaf M cells from light and electron microscopy were made on the first cell layer facing the adaxial side of leaves. The appearance of the size and structure of M cells throughout a leaf cross section was similar except for the few distinctive paraveinal M cells (which are very large in some species), which were excluded from analysis.
Light Microscopy
From light microscopy images of leaf cross sections, Thickleaf (μm) was measured between the bundles and bulliform cells (Fig. 1A). The lumen acell (μm2) was determined (and taken as a surrogate for M cell volume; see Supplemental Materials and Methods S1). From micrographs made on each leaf, 15 to 25 Thickleaf and acell measurements were taken, and the corresponding mean values per leaf were calculated.
The leaf M porosity (%), defined as VolIAS, was calculated as
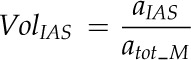 |
(2) |
where atot_M (μm2) is the total area between epidermal layers (including M, air space, and vascular tissue) from measurement of the leaf cross sections and aIAS (μm2) is the area of the IAS in the leaf cross-section (Supplemental Fig. S2A).
The total length of M cell wall exposed to IAS (μm) and the width of the leaf cross section analyzed (μm) were measured, and the corresponding total Smes (μm2 μm−2) was inferred based on Evans et al. (1994) as:
 |
(3) |
where F is the curvature correction factor. A general prolate spheroid shape with the major axis being twice the length of the other two minor axes was assumed, as in Sage and Sage (2009) and Scafaro et al. (2011), and accordingly, based on Thain (1983), an F value of 1.42 was used.
Electron Microscopy
From electron micrographs of leaf cross sections, the total M cell wall length exposed to IAS covered by chloroplasts (μm) was determined; this was used as the numerator in Equation 3 (above) to calculate Schl (μm2 μm−2). The extent of Lobcell was assumed to correspond to the cell perimeter tortuosity (μm μm−1), which was determined from measurements on individual M cells (Supplemental Fig. S2B). In the absence of lobes, cell perimeter tortuosity = 1.0. Other structural measurements on M cells included Thickcw (μm), Lcw_IAS (μm), CWIAS (%), length of a single cell wall exposed to intercellular air space covered by chloroplasts (Lcw_IAS_chl; μm), CWIAS_chl (%), LACW (μm), LACW_chl (μm), ACWchl (%), CWchl (%), Volchl (%), Volvac (%), and minor diameters of mitochondria (μm). A minimum of 25 measurements per each M cell trait were taken on each leaf, and a mean value (per leaf) was calculated.
In addition, the mean P/acell (μm μm−2) for each leaf analyzed was calculated, where P is the perimeter of the M cell section. P/acell was taken as a relative gauge of the ratio of M cell surface area/cell volume (Supplemental Materials and Methods S1).
Analysis of Leaf Surfaces
Images of the adaxial and abaxial epidermal surfaces were captured on three live leaves (each from different plants; n = 3) per Oryza accession under the low-vacuum mode with a Quanta 200F scanning electron microscope (FEI). For each leaf, stomatal pore length in the adaxial and abaxial lamina surface (Lstad and Lstab; μm) were calculated based on 10 to 25 measurements. The stomatal density on each leaf side was determined as Dstad and Dstab (mm−2). For each leaf, stomatal pore area index (Ist, mm2 mm−2) was calculated as an index of maximum stomatal pore area per lamina area based on Sack et al. (2003) as
| (4) |
where Dst is the cumulative (adaxial plus abaxial) number of stomata per unit (one side) of lamina surface area and Lst is the mean stomatal pore length. In addition, the Istad and Istab were calculated.
Leaf Functional Measurements
Gas-Exchange Measurements: Equipment Setup and Protocol
Leaf-atmosphere CO2 and H2O exchange measurements were performed in Pullman, WA (mean atmospheric pressure of 92.1 kPa) using a LI-6400XT portable photosynthesis system equipped with a 6400-40 Leaf Chamber Fluorometer (LI-COR Biosciences). The Leaf Chamber Fluorometer is designed with a uniform, integrated red/blue light-emitted diode light source and a pulse-amplitude modulated (PAM) fluorometer enabling simultaneous measurement of chlorophyll fluorescence and gas exchange over the same leaf area of 2.0 cm2. The fraction of blue light was set to 10% of the PPFD (μmol photons m−2 s−1) to maximize stomatal aperture. A CO2 cartridge was used, and a 6400-01 CO2 Injector System controlled the CO2 partial pressure entering the cuvette (Ca, μbar). The oxygen partial pressure entering the leaf cuvette was set by mixing different partial pressures of nitrogen and oxygen in a CO2-free air stream through two mass flow controllers (MKS Instruments).
For all accessions of Oryza species in Table II, measurements were made on one leaf on three different plants (n = 3). In general, the same leaves, or leaves in the same position as those sampled for leaf anatomical survey, were chosen. The mid to distal portion of each leaf blade was inserted in the leaf chamber for gas-exchange measurements. For almost all Oryza accessions (except for O. alta, O. latifolia 18, and O. longistaminata 5), two leaf blades were used to cover the cuvette luminal surface area.
The data were acquired between 9 am and 4 pm standard time in a room with air temperature around 30°C. Measurements were made after plants were acclimated for approximately 1 h. Leaf functional traits that were provided by leaf gas exchange are listed in Table II.
Leaf A-Ci Curves
A (µmol CO2 m−2 s−1) and Ci (μbar) were determined at 184.2 mbar ambient oxygen partial pressure (near current ambient levels at the study site, and corresponding to 200 mmol oxygen mol−1 air). PPFD was set at 1,500 µmol photons m−2 s−1, leaf temperature at 30°C, while leaf-to-air vapor pressure deficit was maintained between 1.0 and 1.5 kPa.
For each Oryza accession, an A-Ci response curve was acquired on three different plants at (decreasing) Ca from 350 to 18 µbar. In one of the replications, following measurements with decreasing CO2, Ca was increased from 350 to 1,380 µbar to determine the Amax (µmol CO2 m−2 s−1).
Kneading synthetic rubber (Terostat IX; Henkel Technologies) was put around the gaskets of the Leaf Chamber Fluorometer; this was to limit CO2 diffusion leaks into and from the chamber when imposing Ca in the cuvette lumen below and above the CO2 partial pressure in the outside air. In addition, the magnitude of the cuvette CO2 diffusion leaks was estimated, and a CO2 diffusion correction term was included in A and Ci computations (Supplemental Material and Methods S2).
For each A-Ci response curve, least-square regression analysis was applied to the initial slope (Ci ≤ 100 µbar) to determine the CE (mol CO2 m−2 s−1 bar−1 CO2) and the Γ (µbar). Additionally, A, Ci, Ca − Ci, E (mmol water m−2 s−1), and gs_H2O (mol water m−2 s−1) at 350 µbar Ca (near current ambient levels at the study site) were extracted from the data set, and the corresponding leaf A/E (μmol CO2 mmol−1 water) and A/gs_H2O (µmol CO2 mol−1 water) were calculated.
Also, gs_CO2 (mol CO2 m−2 s−1) was determined from gs_H20 as
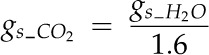 |
(5) |
where 1.6 is the ratio between the molecular diffusivities of water and CO2 in air (Massman, 1998).
The data set submitted to statistical analysis was composed of three repetitions of A, CE, Γ, Ci, Ca − Ci, E, gs_H2O, A/gs_H2O, and A/E (n = 3) and one value of Amax (n = 1) for each Oryza accession.
Leaf Chlorophyll Fluorescence Measurements
Leaf chlorophyll fluorescence was measured using the 6400-40 fluorometer (LI-COR Biosciences) simultaneously while acquiring the A-Ci response curves. Steady-state fluorescence (Fs) and maximum fluorescence (Fm′) were recorded after equilibration to a steady state. Maximum fluorescence was measured using a 0.8-s saturating pulse of light (approximately 8,000 µmol photons m−2 s−1 supplied by light-emitting diodes with peak emission at 630 nm).
Estimation of M Conductance to CO2 Diffusion Based on Leaf Gas-Exchange and Chlorophyll Fluorescence Measurements
The combined leaf gas-exchange and chlorophyll fluorescence data from the A-Ci response curves were used to calculate gm (mol CO2 m−2 s−1 bar−1 CO2) using the variable J method (Di Marco et al., 1990; Harley et al., 1992), applied as in Li et al. (2009). According to Genty et al. (1989), the total electron transport rate (JT; µmol e− m−2 s−1) was determined as
| (6) |
where αleaf is the leaf absorbance of photosynthetic quanta, β is the fraction of photons absorbed by PSII, PPFD (µmol photons m−2 s−1) is the PPFD incident on the leaf, and ϕPSII is the photochemical yield of PSII. The default value taken for αleaf was 0.85 (Li et al., 2009) and that for β was 0.45, assuming that approximately 45% of the photons are absorbed by PSII (Laisk et al., 2006). ϕPSII was computed as
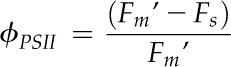 |
(7) |
Electron transport rates associated with carboxylation (Jc) and oxygenation (Jo) were calculated according to Epron et al. (1995) as
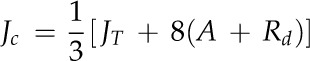 |
(8) |
and
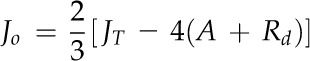 |
(9) |
where A (μmol CO2 m−2 s−1) is the net photosynthetic rate per unit of leaf surface area and Rd (μmol CO2 m−2 s−1) is the corresponding mitochondrial respiration rate in the light (assumed to be 0.7 μmol CO2 m−2 s−1 based on experimental measurements; data not shown). The Cc (µbar) was calculated according to Epron et al. (1995) as
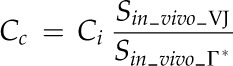 |
(10) |
where Sin_vivo_VJ is the in vivo apparent CO2/oxygen specificity factor for Rubisco determined from the initial slope of the linear regression forced to the origin from plots of Jc/Jo versus Ci/oxygen partial pressure (at decreasing Ca from 350 μbar) and Sin_vivo_Γ* is the in vivo apparent CO2/oxygen specificity factor for Rubisco associated with the photorespiratory compensation point (Γ*; μbar) estimated by gas-exchange measurements. In this study, Γ* was determined based on the method of Laisk (1977) at 184 mbar oxygen partial pressure and at 30°C leaf temperature. Four Oryza species were considered, two of them having among the lowest (O. sativa cv IR64 and O. rufipogon 8) and two among the highest (O. glumaepatula and O. minuta) Γ values; Γ* ranged from 42 to 48 μbar.
The equation developed by Laing et al. (1974) and Jordan and Ogren (1984) describes the relationship between S and Γ* as
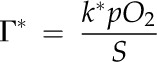 |
(11) |
where pO2 (μbar) is air partial pressure of oxygen, k is the stoichiometry factor between carboxylase and oxygenase activity at the photorespiratory CO2 compensation point, and S is the effective CO2/oxygen specificity factor for Rubisco based on kinetic properties determined in vitro (bar bar−1). k = 0.5 at the photorespiratory compensation point under the assumption that one CO2 is generated in the glycolate pathway for every two oxygens reacting with ribulose-1,5-biphosphate.
The equation can be used to calculate the apparent Rubisco specificity factor Sin_vivo_Γ* from the measurement of Γ* as
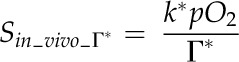 |
(12) |
gm (mol CO2 m−2 s−1 bar−1 CO2) was then calculated, based on Equation 1, as
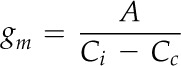 |
(13) |
where A, Ci, and Cc are as already defined. Per each leaf, the gm values at Ca = 350 µbar were used in data analysis.
The ratio between gm at Ca= 350 μbar and the corresponding gs_CO2 (gm/gs_CO2, bar−1 CO2), and the ratio between Smes and gm (Smes/gm, m2 s bar CO2 mol−1 CO2, which represents the M resistance to CO2 diffusion per unit Smes and is listed in Table II among the functional traits) were calculated. In particular, the terms included in gm and the physical meaning of Smes/gm, based on Fick’s laws, are described in Supplemental Materials and Methods S3.
Based on the protocol followed, three replications (n = 3) of Cc, Ci − Cc, gm, gm/gs_CO2, and Smes/gm for each Oryza accession were used in the statistical analysis.
For comparison with the chlorophyll fluorescence method, M conductance was estimated on a few Oryza accessions based on simultaneous measurements of leaf gas exchange and 13C isotope discrimination (Evans et al., 1986; Pons et al., 2009). Leaves of O. sativa cv IR64, O. glaberrima 2, O. glumaepatula 3, and O. rufipogon 9 were sampled according to the methodology described for leaf gas-exchange measurements. A LI-6400XT portable photosynthesis system equipped with a 6400-22L lighted opaque conifer chamber (LI-COR Biosciences) was used under the same environmental conditions described above for gas exchange measurements, except the Ca was 350 μbar. The LI-6400XT was coupled to a tunable-diode laser absorption spectroscope (TDLAS, model TGA 200A, Campbell Scientific) with a laser that measures CO2 and 13C (Bowling et al., 2003; Ubierna et al., 2013). M conductance calculations were performed based on the “single-point method” (Lloyd et al., 1992); they provided gm values similar to those estimated by chlorophyll and gas-exchange measurements. Precisely, O. sativa cv IR64, O. glaberrima 2, O. glumaepatula 3, and O. rufipogon 9 showed mean gm values of 0.342, 0.358, 0.411, and 0.457 mol CO2 m−2 s−1 bar−1, respectively (for comparison, see corresponding gm data in Supplemental Table S3).
Stable Carbon Isotope Analysis of Leaf Biomass
A portion of each leaf used for the A-Ci response curve was collected for analysis of δ13C (‰). Dried samples were combusted using a Eurovector elemental analyzer. The resulting nitrogen and CO2 gases were separated by gas chromatography and admitted into the inlet of a Micromass Isoprime isotope ratio mass spectrometer for determination of 13C/12C ratio. δ13C (‰) was used to represent the 13C/12C ratio of the leaf sample relative to the isotopic reference material Vienna Pee Dee Belemnite (Bender et al., 1973), and it was determined as
 |
(14) |
where Rsample and Rstandard are the 13C/12C ratios of the leaf samples and the Vienna Pee Dee Belemnite limestone, respectively. Three repetitions of δ13C (n = 3) were obtained for each Oryza accession.
Statistical Analyses
Statistical analyses were performed similar to Waite and Sack (2010) using SAS version 9.2 (SAS Institute). First, a three-stage (genomes, species, accessions) nested ANOVA was carried out to describe the variability of each structural and functional leaf trait, based on the entire data set for Oryza accessions. Significance for differences among genomes, species nested within genomes, and accessions nested within species and genomes was evaluated at P < 0.05, 0.01 ≥ P ≥ 0.001, and P < 0.001.
PFA (Child, 2006) was run to identify the most important axes of covariation based on a total of 42 structural plus functional leaf traits (Table II). The mean values of each trait for each of 18 accessions were used. Based on a Varimax orthogonal rotation, eight factors associated with eigenvalues greater than 1 were retained. For each trait, factor loadings greater than 0.4 in absolute value were considered important; based on the size and the sign of the important loadings, a meaningful name was suggested for each of the first four PFA factors. The eight factor scores for each Oryza accession were computed. Since the PFA was conducted on a large set of leaf traits in relation to the number of accessions examined, this can reduce the power to detect the underlying factors (Osborne and Costello, 2004).
Pearson correlation matrices were calculated based on mean values of each leaf structural and functional trait per each Oryza accession to evaluate the trait-to-trait associations. The significance of correlation coefficients was determined at P < 0.05 and P < 0.01.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Scanning electron microscope images illustrating leaf surfaces of two Oryza species having different stomatal density and size.
Supplemental Figure S2. Example of a light microscopy image of a leaf cross section, and the scheme followed to estimate cell surface lobing.
Supplemental Table S1. Mean values, se, and significance between Oryza genomes for each leaf structural and functional trait.
Supplemental Table S2. Mean values, se, and significance between Oryza species (nested within genomes) for each leaf structural and functional trait.
Supplemental Table S3. Mean values, se, and significance between Oryza accessions (nested within species and genomes) for each leaf structural and functional trait.
Supplemental Table S4. Leaf structural-functional PFA: factor loadings and factor scores on the first four main factors.
Supplemental Table S5. Pearson correlation matrix for leaf structural traits.
Supplemental Table S6. Pearson correlation matrix for leaf functional traits.
Supplemental Table S7. Portion of the Pearson correlation matrix for leaf structural to functional traits.
Supplemental Materials and Methods S1. Estimate of cell volume from values of acell.
Supplemental Materials and Methods S2. Magnitude of the CO2 diffusion leaks into and from the Leaf Chamber Fluorometer and its effect on A and Ci calculations.
Supplemental Materials and Methods S3. Fick’s laws applied to estimate gm.
Supplementary Material
Acknowledgments
We thank Berkley Walker for continuous advice and discussion on gas-exchange measurements, Dr. Ray W. Lee for performing the analysis of biomass carbon isotope composition, and Sandy Edwards for editing. We are grateful to the Franceschi Microscopy and Imaging Center at Washington State University for the use of its facilities and staff assistance and to Charles A. Cody for plant growth management.
Glossary
- gs
stomatal conductance to gas diffusion
- M
mesophyll
- Ci
CO2 concentration in the intercellular air space
- Ca
CO2 concentration in the atmosphere
- IAS
intercellular air space
- gm
mesophyll conductance to CO2 diffusion
- Cc
CO2 concentration in the chloroplasts
- A
net photosynthetic rate
- gs_CO2
stomatal conductance to CO2 diffusion
- SMES
mesophyll cell surface area exposed to intercellular air space per unit of leaf surface area
- Schl
total mesophyll cell surface area occupied by chloroplasts exposed to intercellular air space per unit of leaf surface area
- VolIAS
fraction of mesophyll volume occupied by air spaces
- Thickleaf
leaf thickness
- E
leaf transpiration rate
- A/E
transpiration efficiency
- δ13C
carbon isotope composition of leaf biomass
- gs_H2O
stomatal conductance to water vapor diffusion
- Thickcw
cell wall thickness
- acell
area of mesophyll cell section
- Lobcell
mesophyll cell surface lobing
- CE
carboxylation efficiency
- Ist ab
stomatal pore area index in the abaxial lamina surface
- Ist ad
stomatal pore area index in the adaxial lamina surface
- CWchl
fraction of mesophyll cell wall covered by chloroplasts
- CWIAS
fraction of mesophyll cell wall exposed to the intercellular air space
- CWIAS_chl
fraction of mesophyll cell wall exposed to intercellular air space covered by chloroplasts
- ACW
fraction of cell wall adjacent to other cells
- ACWchl
fraction of cell wall adjacent to other cells covered by chloroplasts
- Ist
stomatal pore area index
- Γ
CO2 compensation point
- PFA
principal factor analysis
- Amax
maximum net photosynthetic rate
- P/acell
ratio between mesophyll cell perimeter and cell section area in a leaf cross section
- Ci − Cc
difference between intercellular and chloroplastic partial pressure of CO2
- Ca − Ci
difference between atmospheric and intercellular partial pressure of CO2
- Dst ab
stomatal density in the abaxial lamina surface
- Dst ad
stomatal density in the adaxial lamina surface
- Volchl
fraction of cell volume occupied by chloroplasts
- LACW_chl
length of mesophyll cell walls adjacent to other cells covered by chloroplasts
- LACW
length of the cell wall adjacent to another cell
- SMES/gm
mesophyll resistance to CO2 diffusion per unit cell surface area exposed to intercellular air space
- A/gs_H2O
intrinsic transpiration efficiency
- PPFD
photosynthetically active photon flux density
- Lst ad
stomatal pore length in the adaxial lamina surface
- Lst ab
stomatal pore length in the abaxial lamina surface
References
- Aalto T, Juurola E. (2002) A three-dimensional model of CO2 transport in airspaces and mesophyll cells of a silver birch leaf. Plant Cell Environ 25: 1399–1409 [Google Scholar]
- Barbour MM, Warren CR, Farquhar GD, Forrester GUY, Brown H. (2010) Variability in mesophyll conductance between barley genotypes, and effects on transpiration efficiency and carbon isotope discrimination. Plant Cell Environ 33: 1176–1185 [DOI] [PubMed] [Google Scholar]
- Bender MM, Rouhani I, Vines HM, Black CC. Jr (1973) 13C/12C ratio changes in Crassulacean acid metabolism plants. Plant Physiol 52: 427–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP. (2002) Temperature response of mesophyll conductance: implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol 130: 1992–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling DR, Sargent SD, Tanner BD, Ehleringer JR. (2003) Tunable diode laser absorption spectroscopy for stable isotope studies of ecosystem-atmosphere CO2 exchange. Agric Meteorol 118: 1–19 [Google Scholar]
- Brar DS, Singh K (2011) Oryza In C Kole, ed, Wild Crop Relatives: Genomics and Breeding Resources, Cereals. Springer-Verlag, Berlin, pp 321–365 [Google Scholar]
- Brodribb TJ, Field TS, Jordan GJ. (2007) Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiol 144: 1890–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, Field TS, Sack L. (2010) Viewing leaf structure and evolution from a hydraulic perspective. Funct Plant Biol 37: 488–498 [Google Scholar]
- Burundukova OL, Zhuravlev Y-N, Solopov NV, Pyankov VI. (2003) A method for calculating the volume and surface area in rice mesophyll cells. Russ J Plant Physiol 50: 133–139 [Google Scholar]
- Busch FA, Sage TL, Cousins AB, Sage RF. (2013) C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant Cell Environ 36: 200–212 [DOI] [PubMed] [Google Scholar]
- Centritto M, Lauteri M, Monteverdi MC, Serraj R. (2009) Leaf gas exchange, carbon isotope discrimination, and grain yield in contrasting rice genotypes subjected to water deficits during the reproductive stage. J Exp Bot 60: 2325–2339 [DOI] [PubMed] [Google Scholar]
- Child D (2006) The Essentials of Factor Analysis, Ed 3. Continuum International Publishing Group, London [Google Scholar]
- Di Marco G, Manes F, Tricoli D, Vitale E. (1990) Fluorescence parameters measured concurrently with net photosynthesis to investigate chloroplastic CO2 concentration in leaves of Quercus ilex. J Plant Physiol 136: 538–543 [Google Scholar]
- Dingkuhn M, Farquhar GD, De Datta SK, O’Toole JC (1991) Discrimination of 13C among upland rices having different water use efficiencies. Aust J Agric Res 42: 1123–1131 [Google Scholar]
- Epron D, Godard D, Cornic G, Genty B. (1995) Limitation of net CO2 assimilation rate by internal resistances to CO2 transfer in the leaves of two tree species (Fagus sylvatica L. and Castanea sativa Mill.). Plant Cell Environ 18: 43–51 [Google Scholar]
- Evans JR. (1999) Leaf anatomy enables more equal access to light and CO2 between chloroplasts. New Phytol 143: 93–104 [Google Scholar]
- Evans JR, Kaldenhoff R, Genty B, Terashima I. (2009) Resistances along the CO2 diffusion pathway inside leaves. J Exp Bot 60: 2235–2248 [DOI] [PubMed] [Google Scholar]
- Evans JR, Loreto F (2000) Acquisition and diffusion of CO2 in higher plant leaves. In RC Leegood, TD Sharkey, S von Caemmerer, eds, Photosynthesis: Physiology and Metabolism. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 321–351 [Google Scholar]
- Evans JR, Sharkey TD, Berry JA, Farquhar GD. (1986) Carbon isotope discrimination measured concurrently with gas exchange to investigate CO2 diffusion in leaves of higher plants. Aust J Plant Physiol 13: 281–292 [Google Scholar]
- Evans JR, Terashima I, Hanba Y, Loreto F (2004) CO2 capture by the leaf. In WK Smith, TC Vogelmann, C Critchley, eds, Photosynthetic Adaptation: Chloroplast to the Landscape. Ecological Studies 178. Springer, New York, pp 107–132 [Google Scholar]
- Evans JR, von Caemmerer S. (1996) Carbon dioxide diffusion inside leaves. Plant Physiol 110: 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR, von Caemmerer S, Setchell BA, Hudson GS. (1994) The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of RuBiscCO. Funct Plant Biol 21: 475–495 [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. (1989) Carbon isotope discrimination and photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 40: 503–537 [Google Scholar]
- Farquhar GD, Sharkey TD. (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33: 317–345 [Google Scholar]
- Flexas J, Bota J, Galmés J, Medrano H, Ribas-Carbó M. (2006) Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiol Plant 127: 343–352 [Google Scholar]
- Flexas J, Diaz-Espejo A, Galmés J, Kaldenhoff R, Medrano H, Ribas-Carbo M. (2007) Rapid variations of mesophyll conductance in response to changes in CO2 concentration around leaves. Plant Cell Environ 30: 1284–1298 [DOI] [PubMed] [Google Scholar]
- Flexas J, Niinemets U, Gallé A, Barbour MM, Centritto M, Diaz-Espejo A, Douthe C, Galmés J, Ribas-Carbo M, Rodriguez PLet al. (2013) Diffusional conductances to CO2 as a target for increasing photosynthesis and photosynthetic water-use efficiency. Photosynth Res (in press) [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H. (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ 31: 602–621 [DOI] [PubMed] [Google Scholar]
- Foster JR, Smith WK. (1986) Influence of stomatal distribution on transpiration in low-wind environments. Plant Cell Environ 9: 751–759 [Google Scholar]
- Genty B, Briantais J-M, Baker NR. (1989) The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta 990: 87–92 [Google Scholar]
- Guyot G, Scoffoni C, Sack L. (2012) Combined impacts of irradiance and dehydration on leaf hydraulic conductance: insights into vulnerability and stomatal control. Plant Cell Environ 35: 857–871 [DOI] [PubMed] [Google Scholar]
- Hanba YT, Miyazawa SI, Terashima I. (1999) The influence of leaf thickness of the CO2 transfer conductance and leaf stable carbon isotope ratio for some evergreen tree species in Japanese warm-temperature forests. Funct Ecol 13: 632–639 [Google Scholar]
- Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M. (2004) Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol 45: 521–529 [DOI] [PubMed] [Google Scholar]
- Harley PC, Loreto F, Di Marco G, Sharkey TD. (1992) Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2. Plant Physiol 98: 1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R, Rice N, Waters D, Kasem S, Ishikawa R, Hao Y, Dillon S, Crayn D, Wing R, Vaughan D. (2010) Australian Oryza: utility and conservation. Rice 3: 235–241 [Google Scholar]
- Jagadish KSV, Cairns JE, Kumar A, Somayanda IM, Craufurd PQ. (2011) Does susceptibility to heat stress confound screening for drought tolerance in rice? Funct Plant Biol 38: 261–269 [DOI] [PubMed] [Google Scholar]
- Jarvis PG, Morison JIL (1981) The control of transpiration and photosynthesis by stomata. In PG Jarvis, TA Mansfield, eds, Stomatal Physiology. Cambridge University Press, Cambridge, UK, pp 247–279 [Google Scholar]
- Jordan DB, Ogren WL. (1984) The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase oxygenase-dependence on ribulose bisphosphate concentration, pH and temperature. Planta 161: 308–313 [DOI] [PubMed] [Google Scholar]
- Kellogg E. (2009) The evolutionary history of Ehrhartoideae, Oryzeae, and Oryza. Rice 2: 1–14 [Google Scholar]
- Kiirats O, Lea PJ, Franceschi VR, Edwards GE. (2002) Bundle sheath diffusive resistance to CO2 and effectiveness of C4 photosynthesis and refixation of photorespired CO2 in a C4 cycle mutant and wild-type Amaranthus edulis. Plant Physiol 130: 964–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogami H, Hanba YT, Kibe T, Terashima I, Masuzawa T. (2001) CO2 transfer conductance, leaf structure and carbon isotope composition of Polygonum cuspidatum leaves from low and high altitudes. Plant Cell Environ 24: 529–538 [Google Scholar]
- Kondo M, Pablico P, Aragones D, Agbisit R. (2004) Genotypic variations in carbon isotope discrimination, transpiration efficiency, and biomass production in rice as affected by soil water conditions and N. Plant Soil 267: 165–177 [Google Scholar]
- Laing WA, Ogren WL, Hageman RH. (1974) Regulation of soybean net photosynthetic CO2 fixation by the interaction of CO2, O2, and ribulose 1,5-diphosphate carboxylase. Plant Physiol 54: 678–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laisk AK (1977) Kinetics of Photosynthesis and Photorespiration in C3 Plants. Nauka, Moscow [Google Scholar]
- Laisk AK, Eichelmann H, Oja V, Rasulov B, Rämma H. (2006) Photosystem II cycle and alternative electron flow in leaves. Plant Cell Physiol 47: 972–983 [DOI] [PubMed] [Google Scholar]
- Lammertsma EI, de Boer HJ, Dekker SC, Dilcher DL, Lotter AF, Wagner-Cremer F. (2011) Global CO2 rise leads to reduced maximum stomatal conductance in Florida vegetation. Proc Natl Acad Sci USA 108: 4035–4040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao Y, Xu X, Shen Q, Guo S. (2009) Light-saturated photosynthetic rate in high-nitrogen rice (Oryza sativa L.) leaves is related to chloroplastic CO2 concentration. J Exp Bot 60: 2351–2360 [DOI] [PubMed] [Google Scholar]
- Lloyd J, Syvertsen JP, Kriedeman PE, Farquhar GD. (1992) Low conductances for CO2 diffusion from stomata to the sites of carboxylation in leaves of woody species. Plant Cell Environ 15: 873–899 [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR. (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55: 591–628 [DOI] [PubMed] [Google Scholar]
- Massman WJ. (1998) A review of the molecular diffusivities of H2O, CO2, CH4, CO, O3, SO2, NH3, N2O, NO and NO2 in air, O2 and N2 near STP. Atmos Environ 32: 1111–1127 [Google Scholar]
- Ndjiondjop MN, Cisse F, Girma G, Sow M, Bocco M, Djedatin G, Blandine F. (2010) Morpho-agronomic and molecular characterisation of Oryza glaberrima germplasm from Mali. Afr J Biotechnol 9: 7409–7417 [Google Scholar]
- Niinemets Ü. (1999) Components of leaf dry mass per area–thickness and density–alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol 144: 35–47 [Google Scholar]
- Niinemets Ü, Díaz-Espejo A, Flexas J, Galmés J, Warren CR. (2009) Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. J Exp Bot 60: 2249–2270 [DOI] [PubMed] [Google Scholar]
- Nobel PS (2009) Physicochemical and Environmental Plant Physiology, Ed 4. Academic Press, San Diego [Google Scholar]
- Nobel PS, Zaragoza LJ, Smith WK. (1975) Relation between mesophyll surface area, photosynthetic rate, and illumination level during development for leaves of Plectranthus parviflorus Henckel. Plant Physiol 55: 1067–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocheltree TW, Nippert JB, Prasad PV. (2012) Changes in stomatal conductance along grass blades reflect changes in leaf structure. Plant Cell Environ 35: 1040–1049 [DOI] [PubMed] [Google Scholar]
- Oguchi R, Hikosaka K, Hirose T. (2003) Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell Environ 26: 505–512 [Google Scholar]
- Osborne JW, Costello AB. (2004) Sample size and subject to item ratio in principal components analysis. Pract Assess Res Eval 9: http://pareonline.net/getvn.asp?v=9&n=11 [Google Scholar]
- Parker ML, Ford MA. (1982) The structure of the mesophyll of flag leaves in three Triticum species. Ann Bot (Lond) 49: 165–176 [Google Scholar]
- Parkhurst DF, Wong SC, Farquhar GD, Cowan IR. (1988) Gradients of intercellular CO2 levels across the leaf mesophyll. Plant Physiol 86: 1032–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña-Rojas K, Aranda X, Joffre R, Fleck I. (2005) Leaf morphology, photochemistry and water status changes in resprouting Quercus ilex during drought. Funct Plant Biol 32: 117–130 [DOI] [PubMed] [Google Scholar]
- Peterhansel C, Maurino VG. (2011) Photorespiration redesigned. Plant Physiol 155: 49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons TL, Flexas J, von Caemmerer S, Evans JR, Genty B, Ribas-Carbo M, Brugnoli E. (2009) Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. J Exp Bot 60: 2217–2234 [DOI] [PubMed] [Google Scholar]
- Reich PB. (1984) Leaf stomatal density and diffusive conductance in three amphistomatous hybrid poplar cultivars. New Phytol 98: 231–239 [Google Scholar]
- Sack L, Cowan PD, Prasad PVV. (2003) The ‘hydrology’ of leaves: co-ordination of structure and function in temperate woody species. Plant Cell Environ 26: 1343–1356 [Google Scholar]
- Sack L, Holbrook NM. (2006) Leaf hydraulics. Annu Rev Plant Biol 57: 361–381 [DOI] [PubMed] [Google Scholar]
- Sage TL, Sage RF. (2009) The functional anatomy of rice leaves: implications for refixation of photorespiratory CO2 and efforts to engineer C4 photosynthesis into rice. Plant Cell Physiol 50: 756–772 [DOI] [PubMed] [Google Scholar]
- Scafaro AP, Von Caemmerer S, Evans JR, Atwell BJ. (2011) Temperature response of mesophyll conductance in cultivated and wild Oryza species with contrasting mesophyll cell wall thickness. Plant Cell Environ 34: 1999–2008 [DOI] [PubMed] [Google Scholar]
- Scafaro AP, Yamori W, Carmo-Silva AE, Salvucci ME, von Caemmerer S, Atwell BJ. (2012) Rubisco activity is associated with photosynthetic thermotolerance in a wild rice (Oryza meridionalis). Physiol Plant 146: 99–109 [DOI] [PubMed] [Google Scholar]
- Sengupta S, Majumder AL. (2010) Porteresia coarctata (Roxb.) Tateoka, a wild rice: a potential model for studying salt-stress biology in rice. Plant Cell Environ 33: 526–542 [DOI] [PubMed] [Google Scholar]
- Slaton MR, Smith WK. (2002) Mesophyll architecture and cell exposure to intercellular air apace in alpine, desert, and forest species. Int J Plant Sci 163: 937–948 [Google Scholar]
- Smith WK, Vogelmann TC, DeLucia EH, Bell DT, Shepherd KA. (1997) Leaf form and photosynthesis. Bioscience 47: 785–793 [Google Scholar]
- Steppe K, Niinemets Ü, Teskey RO (2011) Tree size- and age-related changes in leaf physiology and their influence on carbon gain. In FC Meinzer, B Lachenbruch, TE Dawson, eds, Size- and Age-Related Changes in Tree Structure and Function. Springer, Dordrecht, The Netherlands, pp 235–253 [Google Scholar]
- Terashima I, Hanba YT, Tazoe Y, Vyas P, Yano S. (2006) Irradiance and phenotype: comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J Exp Bot 57: 343–354 [DOI] [PubMed] [Google Scholar]
- Terashima I, Hanba YT, Tholen D, Niinemets Ü. (2011) Leaf functional anatomy in relation to photosynthesis. Plant Physiol 155: 108–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima I, Miyazawa SI, Hanba YT. (2001) Why are sun leaves thicker than shade leaves? Consideration based on analyses of CO2 diffusion in the leaf. J Plant Res 114: 93–105 [Google Scholar]
- Terashima I, Ono K. (2002) Effects of HgCl2 on CO2 dependence of leaf photosynthesis: evidence indicating involvement of aquaporins in CO2 diffusion across the plasma membrane. Plant Cell Physiol 43: 70–78 [DOI] [PubMed] [Google Scholar]
- Terashima I, Saeki T. (1983) Light environment within a leaf. I. Optical properties of paradermal sections of Camellia leaves with special reference to differences in the optical properties of palisade and spongy tissues. Plant Cell Physiol 24: 1493–1501 [Google Scholar]
- Thain JF. (1983) Curvature correction factors in the measurements of cell surface areas in plant tissues. J Exp Bot 34: 87–94 [Google Scholar]
- Tholen D, Ethier G, Genty B, Pepin S, Zhu X-G. (2012) Variable mesophyll conductance revisited: theoretical background and experimental implications. Plant Cell Environ 35: 2087–2103 [DOI] [PubMed] [Google Scholar]
- Tholen D, Zhu X-G. (2011) The mechanistic basis of internal conductance: a theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiol 156: 90–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosens T, Niinemets Ü, Vislap V, Eichelmann H, Castro Díez P. (2012) Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant Cell Environ 35: 839–856 [DOI] [PubMed] [Google Scholar]
- Ubierna N, Sun W, Kramer DM, Cousins AB. (2013) The efficiency of C4 photosynthesis under low light conditions in Zea mays, Miscanthus × giganteus and Flaveria bidentis. Plant Cell Environ 36: 365–381 [DOI] [PubMed] [Google Scholar]
- Uehlein N, Otto B, Hanson DT, Fischer M, McDowell N, Kaldenhoff R. (2008) Function of Nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell 20: 648–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan DA (1994) The Wild Relatives of Rice: A Genetic Handbook. International Rice Research Institute, Manila, The Philippines [Google Scholar]
- Vaughan DA, Lu BR, Tomooka N. (2008) The evolving story of rice evolution. Plant Sci 174: 394–408 [Google Scholar]
- Vogelmann TC, Bornman JF, Josserand S. (1989) Photosynthetic light gradients and spectral regime within leaves of Medicago sativa Philos Trans R Soc Lond B 323: 411–421 [Google Scholar]
- Waite M, Sack L. (2010) How does moss photosynthesis relate to leaf and canopy structure? Trait relationships for 10 Hawaiian species of contrasting light habitats. New Phytol 185: 156–172 [DOI] [PubMed] [Google Scholar]
- Woolley JT. (1983) Maintenance of air in intercellular spaces of plants. Plant Physiol 72: 989–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, This D, Pausch RC, Vonhof WM, Coburn JR, Comstock JP, McCouch SR. (2009) Leaf-level water use efficiency determined by carbon isotope discrimination in rice seedlings: genetic variation associated with population structure and QTL mapping. Theor Appl Genet 118: 1065–1081 [DOI] [PubMed] [Google Scholar]
- Zhao M, Acuña TLB, Lafitte HR, Dimayuga G, Sacks E. (2008) Perennial hybrids of Oryza sativa × Oryza rufipogon. II. Carbon exchange and assimilate partitioning. Field Crops Res 106: 214–223 [Google Scholar]
- Zhao M, Ding Z, Lafitte R, Sacks E, Dimayuga G, Holt D. (2010) Photosynthetic characteristics in Oryza species. Photosynthetica 48: 234–240 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.