Abstract
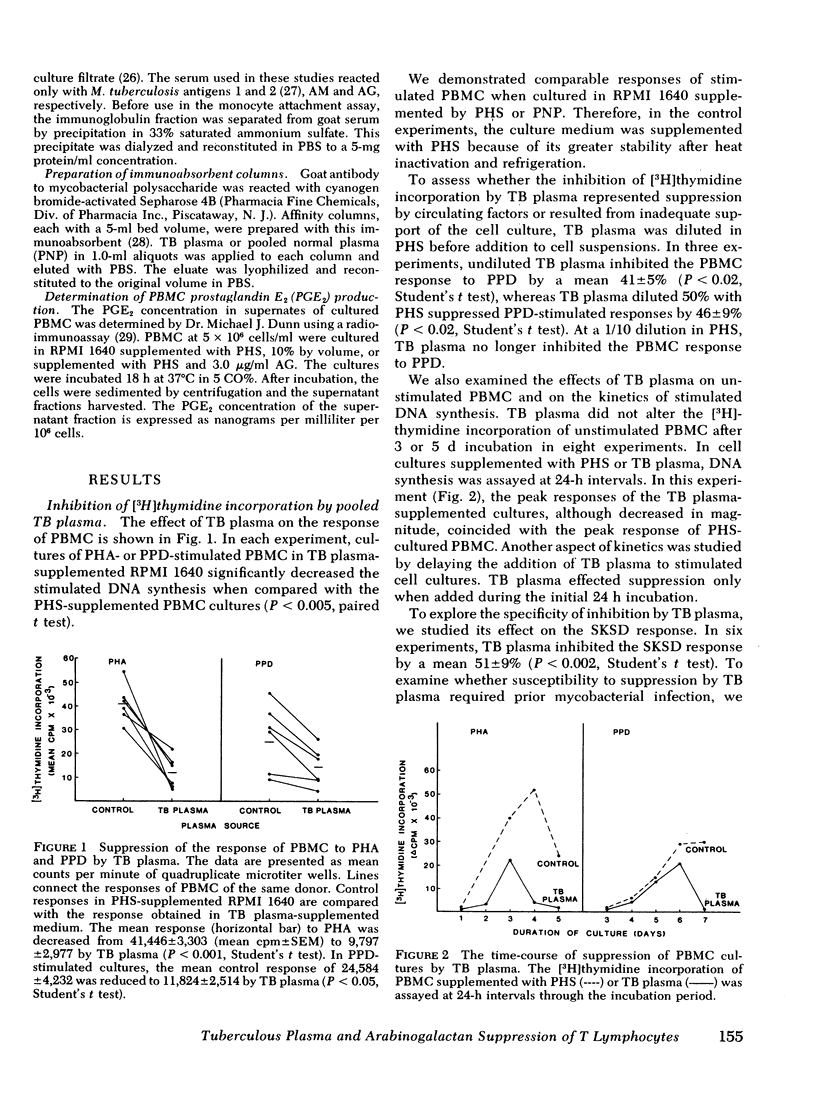
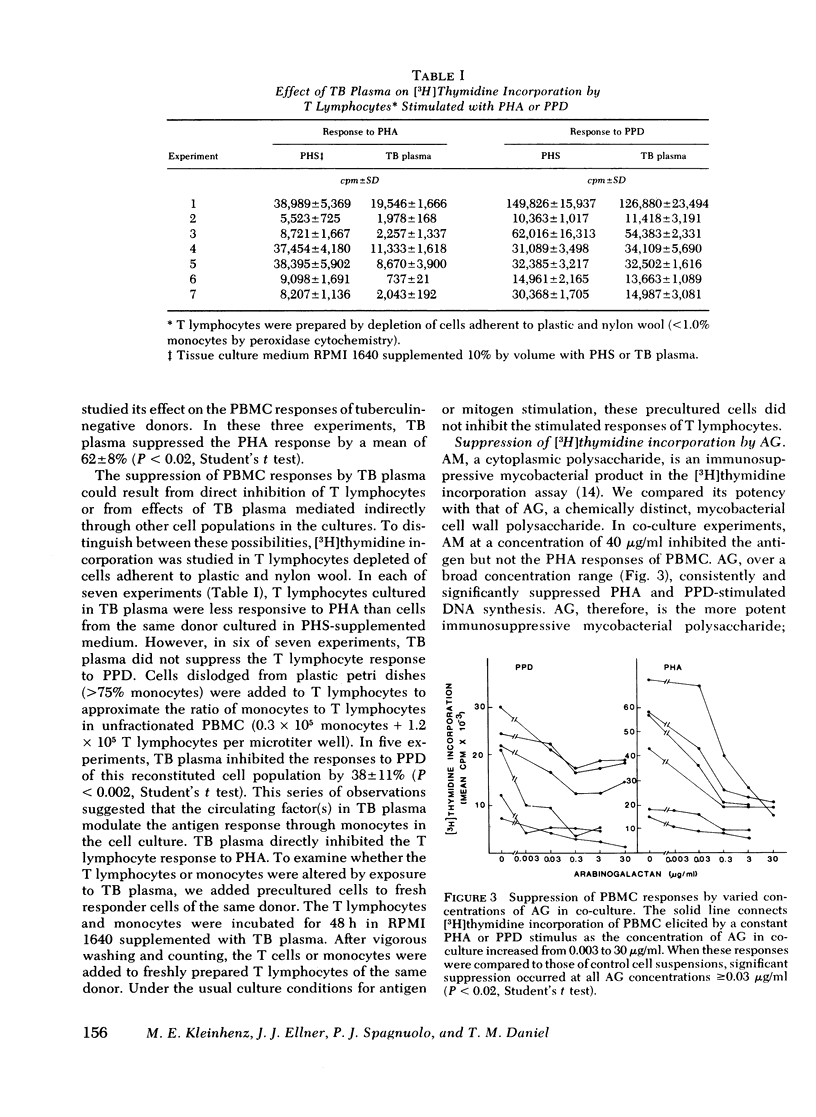
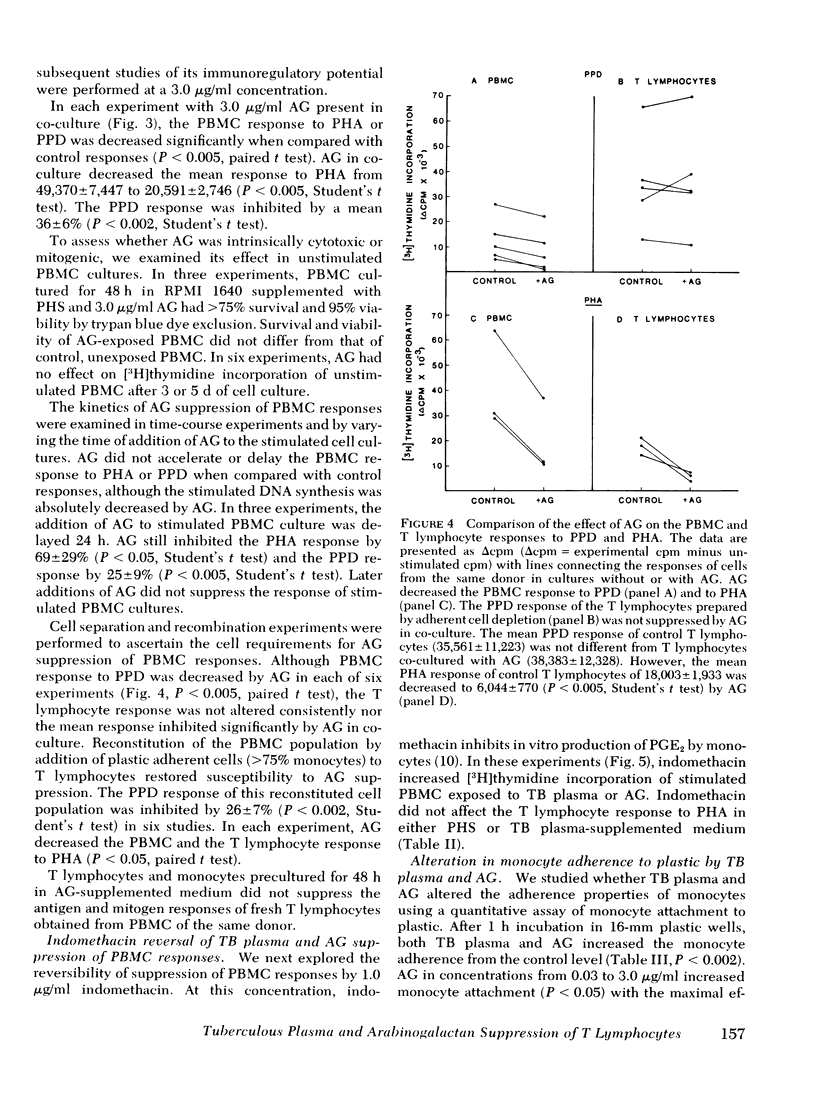
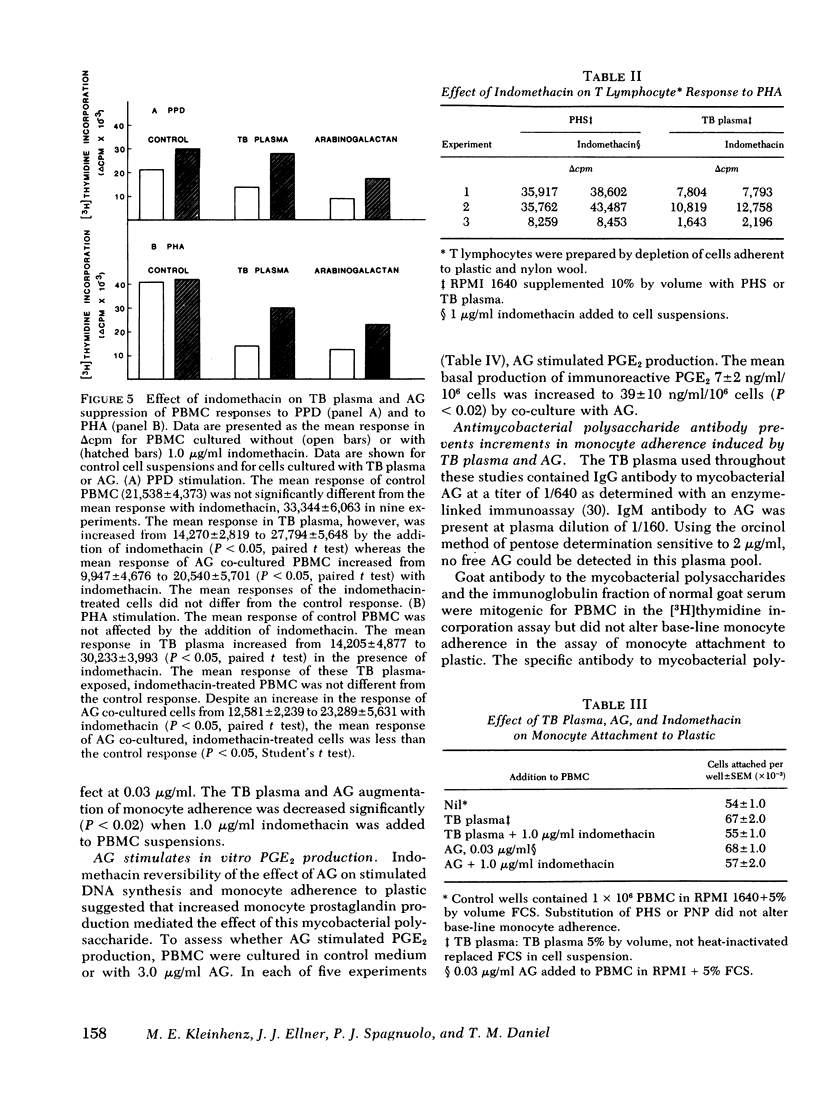
During tuberculosis, exposure of monocytes to circulating factors may induce the suppressor activity observed in some anergic patients. To explore this possibility, we examined the effects of plasma pooled from 28 untreated tuberculosis (TB) patients and the mycobacterial cell wall polysaccharide D-arabino-D-galactan (AG) on the in vitro function of peripheral blood mononuclear cells (PBMC) from healthy donors. In the [3H] thymidine incorporation assay, stimulated responses of PBMC incubated in culture medium supplemented with TB plasma or co-cultured with 3.0 microgram/ml AG were depressed significantly when compared with control responses. Cytotoxicity and altered kinetics of stimulated DNA synthesis did not contribute to the observed suppression. TB plasma and AG-induced suppression of the PBMC response to purified protein derivative was monocyte dependent and indomethacin reversible. In addition, TB plasma and AG directly inhibited the phytohemagglutinin-stimulated responses of T lymphocytes. In a quantitative assay of monocyte attachment to plastic, both TB plasma and AG significantly increased monocyte adherence from basal levels. These effects on monocyte adherence were reversed with indomethacin or antibody to mycobacterial polysaccharide. In addition, TB plasma passed over an immunoabsorbent column of Sepharose-linked antibody to mycobacterial polysaccharide was depleted of the suppressive and monocyte-adherence augmenting factors. 3.0 microgram/ml AG stimulated a fivefold increase in prostaglandin E2 production by cultured mononuclear cells. Our data suggest that AG circulating alone or bound in immune complexes may account for the observed effects of TB plasma. Similar in vivo exposure may contribute to the cell-mediated suppression of lymphocyte responses in tuberculosis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernhard M. I., Pace R. C., Unger S. W., Wanebo H. J. The influence of incubation time and mitogen concentration on lymphocyte blastogenic response: determination of conditions that maximize population differences. J Immunol. 1980 Feb;124(2):964–968. [PubMed] [Google Scholar]
- Bjune G. In vitro lymphocyte stimulation in leprosy; simultaneous stimulation with Mycobacterium leprae antigens and phytohaemagglutinin. Clin Exp Immunol. 1979 Jun;36(3):479–487. [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Colley D. G., Hieny S. E., Bartholomew R. K., Cook J. A. Immune responses during human schistosomiasis mansoni. III. Regulatory effect of patient sera on human lymphocyte blastogenic responses to schistosome antigen preparations. Am J Trop Med Hyg. 1977 Sep;26(5 Pt 1):917–925. [PubMed] [Google Scholar]
- Cox R. A. Immunologic studies of patients with histoplasmosis. Am Rev Respir Dis. 1979 Jul;120(1):143–149. doi: 10.1164/arrd.1979.120.1.143. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Anderson P. A. The use of immunoabsorbents for the purification of mycobacterial antigens. J Lab Clin Med. 1977 Aug;90(2):354–360. [PubMed] [Google Scholar]
- Daniel T. M., Good R. C., Janicki B. W. Immunoelectrophoresis of Mycobacterium tuberculosis antigens. Comparative analysis of cell extract and culture filtrate antigens. Am Rev Respir Dis. 1975 Nov;112(5):639–644. doi: 10.1164/arrd.1975.112.5.639. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Misaki A. Carbohydrate analysis of concanavalin A-reactive and concanavalin A-nonreactive mycobacterial polysaccharides. Am Rev Respir Dis. 1976 May;113(5):705–706. doi: 10.1164/arrd.1976.113.5.705. [DOI] [PubMed] [Google Scholar]
- Daniel T. M., Oxtoby M. J., Pinto E., Moreno E. The immune spectrum in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1981 May;123(5):556–559. doi: 10.1164/arrd.1981.123.5.556. [DOI] [PubMed] [Google Scholar]
- Daniel T. M. The antigenicity in guinea pigs and monkeys of three mycobacterial polysaccharides purified by affinity chromatography with concanavalin A. Am Rev Respir Dis. 1975 Jun;111(6):787–793. doi: 10.1164/arrd.1975.111.6.787. [DOI] [PubMed] [Google Scholar]
- Daniel T. M. The purification of mycobacterial polysaccharides with concanavalin A. Am Rev Respir Dis. 1974 Nov;110(5):634–640. doi: 10.1164/arrd.1974.110.5.634. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Daniel T. M. Immunosuppression by mycobacterial arabinomannan. Clin Exp Immunol. 1979 Feb;35(2):250–257. [PMC free article] [PubMed] [Google Scholar]
- Ellner J. J., Spagnuolo P. J. Suppression of antigen and mitogen induced human T lymphocyte DNA synthesis by bacterial lipopolysaccharide: mediation by monocyte activation and production of prostaglandins. J Immunol. 1979 Dec;123(6):2689–2695. [PubMed] [Google Scholar]
- Ellner J. J. Suppressor adherent cells in human tuberculosis. J Immunol. 1978 Dec;121(6):2573–2579. [PubMed] [Google Scholar]
- Fischer A., Ballet J. J., Griscelli C. Specific inhibition of in vitro Candida-induced lymphocyte proliferation by polysaccharidic antigens present in the serum of patients with chronic mucocutaneous candidiasis. J Clin Invest. 1978 Nov;62(5):1005–1013. doi: 10.1172/JCI109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Bankhurst A. D., Messner R. P. Suppression of human T-cell mitogenesis by prostaglandin. Existence of a prostaglandin-producing suppressor cell. J Exp Med. 1977 Dec 1;146(6):1719–1734. doi: 10.1084/jem.146.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Bankhurst A. D., Peake G. T., Saiki J. H., Williams R. C., Jr Prostaglandin-producing suppressor cells in Hodgkin's disease. N Engl J Med. 1977 Nov 3;297(18):963–968. doi: 10.1056/NEJM197711032971802. [DOI] [PubMed] [Google Scholar]
- Goodwin J. S., Messner R. P., Peake G. T. Prostaglandin suppression of mitogen-stimulated lymphocytes in vitro. Changes with mitogen dose and preincubation. J Clin Invest. 1978 Oct;62(4):753–760. doi: 10.1172/JCI109186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Purification of human T and B lymphocytes. J Immunol. 1974 Jan;112(1):420–423. [PubMed] [Google Scholar]
- Heilman D. H., McFarland W. Inhibition of tuberculin-induced mitogenesis in cultures of lymphocytes from tuberculous donors. Int Arch Allergy Appl Immunol. 1966;30(1):58–66. doi: 10.1159/000229793. [DOI] [PubMed] [Google Scholar]
- Humes J. L., Burger S., Galavage M., Kuehl F. A., Jr, Wightman P. D., Dahlgren M. E., Davies P., Bonney R. J. The diminished production of arachidonic acid oxygenation products by elicited mouse peritoneal macrophages: possible mechanisms. J Immunol. 1980 May;124(5):2110–2116. [PubMed] [Google Scholar]
- KAPLOW L. S. SIMPLIFIED MYELOPEROXIDASE STAIN USING BENZIDINE DIHYDROCHLORIDE. Blood. 1965 Aug;26:215–219. [PubMed] [Google Scholar]
- Katz P., Goldstein R. A., Fauci A. S. Immunoregulation in infection caused by Mycobacterium tuberculosis: the presence of suppressor monocytes and the alteration of subpopulations of T lymphocytes. J Infect Dis. 1979 Jul;140(1):12–21. doi: 10.1093/infdis/140.1.12. [DOI] [PubMed] [Google Scholar]
- McCarthy J. B., Wahl S. M., Rees J. C., Olsen C. E., Sandberg L., Wahl L. M. Mediation of macrophage collagenase production by 3'-5' cyclic adenosine monophosphate. J Immunol. 1980 May;124(5):2405–2409. [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Fields J. P., Bloom B. R. Lepromin-induced suppressor cells in patients with leprosy. J Immunol. 1979 Oct;123(4):1813–1817. [PubMed] [Google Scholar]
- Ottesen E. A. Modulation of the host response in human schistosomiasis. I. Adherent suppressor cells that inhibit lymphocyte proliferative responses to parasite antigens. J Immunol. 1979 Oct;123(4):1639–1644. [PubMed] [Google Scholar]
- Passwell J. H., Dayer J. M., Merler E. Increased prostaglandin production by human monocytes after membrane receptor activation. J Immunol. 1979 Jul;123(1):115–120. [PubMed] [Google Scholar]
- Piessens W. F., Ratiwayanto S., Tuti S., Palmieri J. H., Piessens P. W., Koiman I., Dennis D. T. Antigen-specific suppressor cells and suppressor factors in human filariasis with Brugia malayi. N Engl J Med. 1980 Apr 10;302(15):833–837. doi: 10.1056/NEJM198004103021503. [DOI] [PubMed] [Google Scholar]
- Spagnuolo P. J., Ellner J. J., Bouknight R., Tomford J. W., Kleinhenz M. E., Edmonds K. L. Interrelationships of immunoregulatory cells and serum factors in sarcoidosis. J Immunol. 1980 Sep;125(3):1071–1077. [PubMed] [Google Scholar]
- Stobo J. D. Immunosuppression in man: suppression by macrophages can be mediated by interactions with regulatory T cells. J Immunol. 1977 Sep;119(3):918–924. [PubMed] [Google Scholar]
- Stobo J. D., Paul S., Van Scoy R. E., Hermans P. E. Suppressor thymus-derived lymphocytes in fungal infection. J Clin Invest. 1976 Feb;57(2):319–328. doi: 10.1172/JCI108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd C. W., Goodgame R. W., Colley D. G. Immune responses during human schistosomiasis mansoni. V. Suppression of schistosome antigen-specific lymphocyte blastogenesis by adherent/phagocytic cells. J Immunol. 1979 Apr;122(4):1440–1446. [PubMed] [Google Scholar]
- Tsuyuguchi I., Shiratsuchi H., Teraoka O., Hirano T. Increase in T cells bearing IgG Fc receptors in peripheral blood of patients with tuberculosis by in vitro stimulation with purified protein derivative. Am Rev Respir Dis. 1980 Jun;121(6):951–957. doi: 10.1164/arrd.1980.121.6.951. [DOI] [PubMed] [Google Scholar]
- Weiner M. S., Bianco C., Nussenzweig V. Enhanced binding of neuraminidase-treated sheep erythrocytes to human T lymphocytes. Blood. 1973 Dec;42(6):939–946. [PubMed] [Google Scholar]


