Abstract
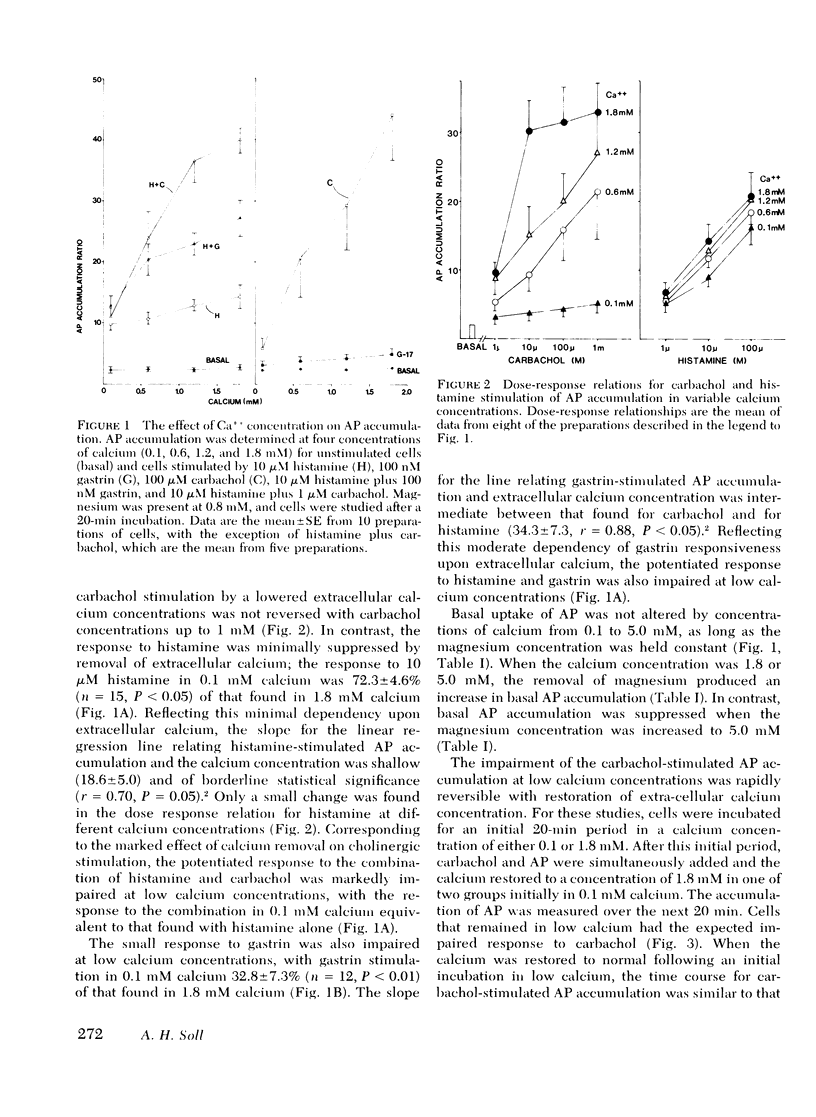
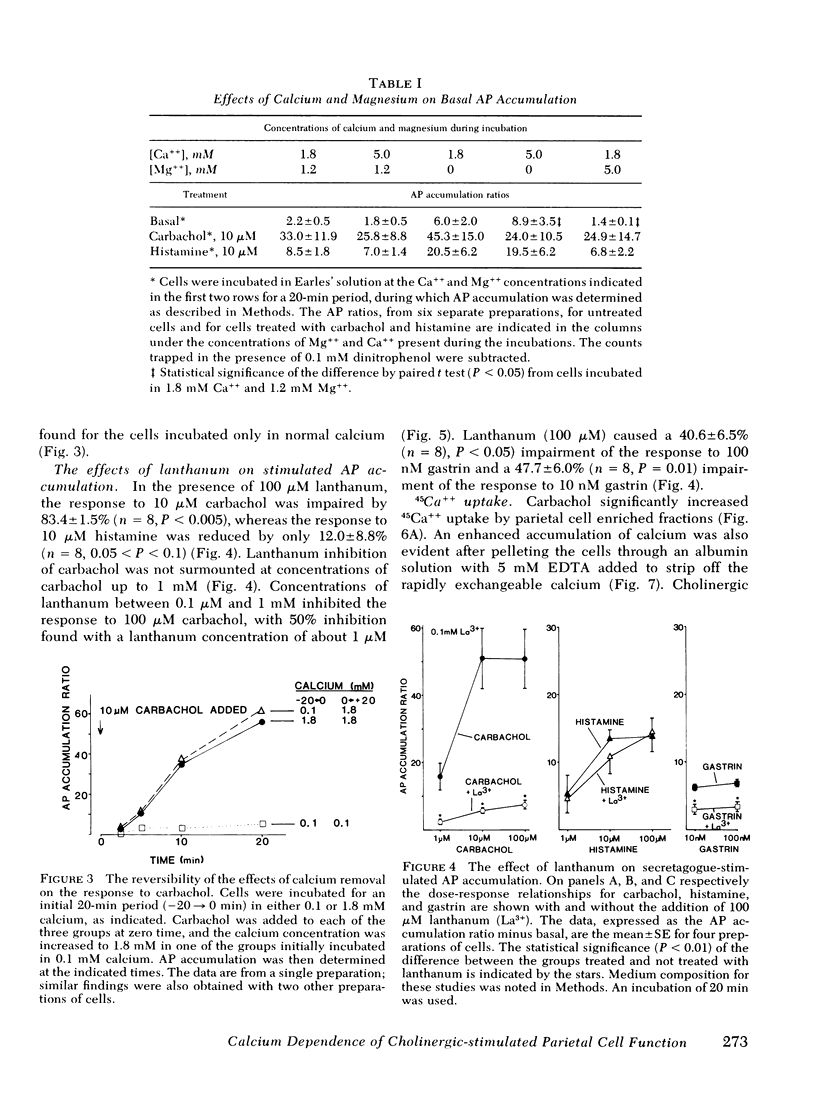
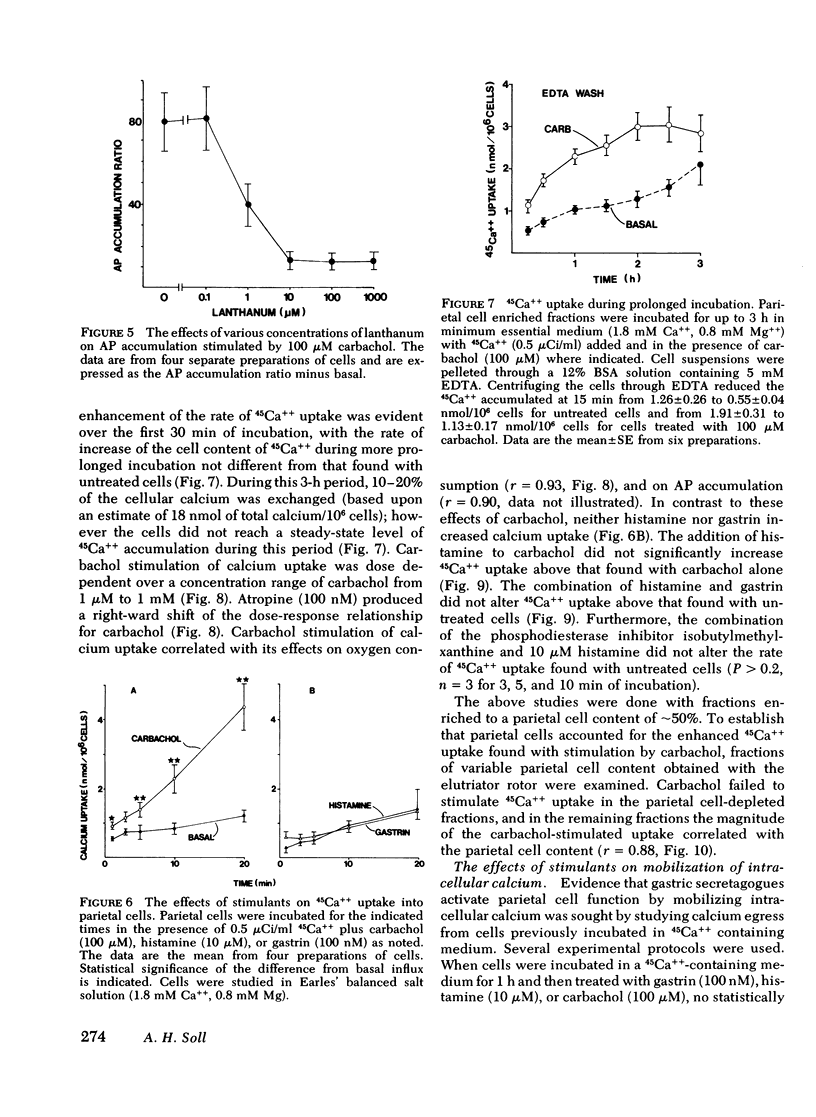
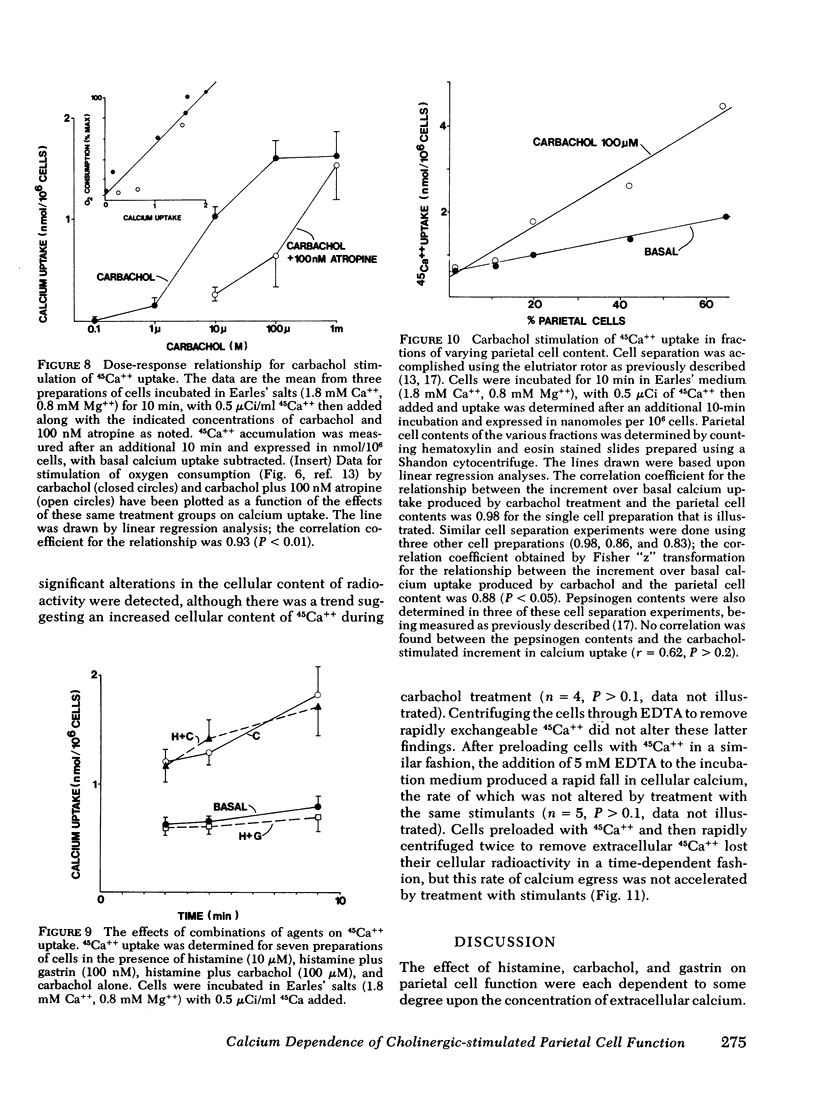
The role of calcium gating in cholinergic stimulation of the function of parietal cells was studied using cells isolated from canine fundic mucosa by treatment with collagenase and EDTA and enriched by velocity separation in an elutriator rotor. Monitoring the accumulation of [14C[ aminopyrine as an index of parietal cell response, stimulation by carbachol, but not by histamine, was highly dependent upon the concentration of extracellular calcium. Incubation of parietal cells in 0-.1 mM calcium, rather than the usual 1.8 mM concentration, reduced the response to 100 microM carbachol by 92 +/- 2%, whereas histamine stimulation was impaired by 28 +/- 5%. A similar reduction in extracellular calcium suppressed the response to gastrin (100 nM) by 67 +/- 7%. The impairment of cholinergic stimulation found at low extracellular calcium concentrations was rapidly reversed with the readdition of calcium. Lanthanum, which blocks calcium movement across membranes, caused a similar pattern of effects on secretagogue stimulation of aminopyrine accumulation, with 100 microM lanthanum suppressing carbachol stimulation by 83 +/- 2%. This concentration of lanthanum suppressed gastrin stimulation by 40 +/- 7% and histamine stimulation by only 12 +/- 9%. Carbachol, but not histamine nor gastrin, stimulated 45Ca++ uptake. The magnitude of carbachol-stimulated calcium uptake correlated with the parietal cell content of the fractions examined (r = 0.88), and was dose responsive over carbachol concentrations from 1 microM to 1 mM. Atropine (100 nM) caused surmountable inhibition, and these effects of carbachol and atropine on calcium uptake correlated with their effects on oxygen consumption (r = 0.93) and [14C]-aminopyrine accumulation (r = 0.90). Cells preloaded with 45Ca++ lost cellular calcium in a time-dependent fashion; however, this rate of egress was not accelerated by treatment with histamine, gastrin, or carbachol, thus failing to implicate mobilization of intracellular calcium as primary mechanism for activation of parietal cell function. These data indicate a close link between stimulation of parietal cell function and enhancement of calcium influx by cholinergic agents.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basso N., Passaro E., Jr Calcium-stimulated gastric secretion in the Zollinger-Ellison syndrome. Arch Surg. 1970 Sep;101(3):399–402. doi: 10.1001/archsurg.1970.01340270047013. [DOI] [PubMed] [Google Scholar]
- Basso N., Passaro E., Jr Effect of calcium on pentagastrin-, histamine-, bethanecol-, and insulin-stimulated gastric secretion in the ferret. J Surg Res. 1972 Jul;13(1):32–38. doi: 10.1016/0022-4804(72)90037-6. [DOI] [PubMed] [Google Scholar]
- Berglindh T., Dibona D. R., Ito S., Sachs G. Probes of parietal cell function. Am J Physiol. 1980 Mar;238(3):G165–G176. doi: 10.1152/ajpgi.1980.238.3.G165. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. The interaction of cyclic nucleotides and calcium in the control of cellular activity. Adv Cyclic Nucleotide Res. 1975;6:1–98. [PubMed] [Google Scholar]
- Borle A. B. Calcium metabolism at the cellular level. Fed Proc. 1973 Sep;32(9):1944–1950. [PubMed] [Google Scholar]
- Chandler D. E. Control of pancreatic enzyme secretion: a critique on the role of calcium. Life Sci. 1978 Jul 24;23(4):323–333. doi: 10.1016/0024-3205(78)90016-4. [DOI] [PubMed] [Google Scholar]
- Christiansen J., Rehfeld J. F., Kirkegaard P. Interaction of calcium, magnesium, and gastrin on gastric acid secretion. Gastroenterology. 1979 Jan;76(1):57–61. [PubMed] [Google Scholar]
- Christiansen J., Rehfeld J. F., Stadil F. The effect of calcium on gastric acid and gastrin secretion in antrectomized subjects. Gut. 1974 Aug;15(8):622–625. doi: 10.1136/gut.15.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry D. L., Bennett L. L., Grodsky G. M. Requirement for calcium ion in insulin secretion by the perfused rat pancreas. Am J Physiol. 1968 Jan;214(1):174–178. doi: 10.1152/ajplegacy.1968.214.1.174. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. On the mode of action of acetylcholine in evoking adrenal medullary secretion: increased uptake of calcium during the secretory response. J Physiol. 1962 Aug;162:385–392. doi: 10.1113/jphysiol.1962.sp006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The role of the alkaline earth ions in anaphylactic histamine secretion. J Physiol. 1972 Aug;224(3):753–769. doi: 10.1113/jphysiol.1972.sp009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Conlon T. P., Kleveman H. L., Adams T. D., Ondetti M. A. Action of cholecystokinin and cholinergic agents on calcium transport in isolated pancreatic acinar cells. J Clin Invest. 1975 Aug;56(2):366–375. doi: 10.1172/JCI108101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J. D., Jackson M. J. Regulation of amylase release from dispersed pancreatic acinar cells. J Physiol. 1977 Sep;270(2):439–454. doi: 10.1113/jphysiol.1977.sp011961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G. A. Events at the cardiac sarcolemma: localization and movement of contractile-dependent calcium. Fed Proc. 1976 May 1;35(6):1274–1278. [PubMed] [Google Scholar]
- Langer G. A., Frank J. S. Lanthanum in heart cell culture. Effect on calcium exchange correlated with its localization. J Cell Biol. 1972 Sep;54(3):441–455. doi: 10.1083/jcb.54.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaisse-Lagae F., Malaisse W. J. The stimulus-secretion coupling of glucose-induced insulin release. 3. Uptake of 45 calcium by isolated islets of Langerhans. Endocrinology. 1971 Jan;88(1):72–80. doi: 10.1210/endo-88-1-72. [DOI] [PubMed] [Google Scholar]
- Prince W. T., Berridge M. J., Rasmussen H. Role of calcium and adenosine-3':5'-cyclic monophosphate in controlling fly salivary gland secretion. Proc Natl Acad Sci U S A. 1972 Mar;69(3):553–557. doi: 10.1073/pnas.69.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Soll A. H. Secretagogue stimulation of [14C]aminopyrine accumulation by isolated canine parietal cells. Am J Physiol. 1980 Apr;238(4):G366–G375. doi: 10.1152/ajpgi.1980.238.4.G366. [DOI] [PubMed] [Google Scholar]
- Soll A. H. The actions of secretagogues on oxygen uptake by isolated mammalian parietal cells. J Clin Invest. 1978 Feb;61(2):370–380. doi: 10.1172/JCI108947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H. The interaction of histamine with gastrin and carbamylcholine on oxygen uptake by isolated mammalian parietal cells. J Clin Invest. 1978 Feb;61(2):381–389. doi: 10.1172/JCI108948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soll A. H., Wollin A. Histamine and cyclic AMP in isolated canine parietal cells. Am J Physiol. 1979 Nov;237(5):E444–E450. doi: 10.1152/ajpendo.1979.237.5.E444. [DOI] [PubMed] [Google Scholar]
- Williams J. A. Regulation of pancreatic acinar cell function by intracellular calcium. Am J Physiol. 1980 Apr;238(4):G269–G279. doi: 10.1152/ajpgi.1980.238.4.G269. [DOI] [PubMed] [Google Scholar]
- Wollin A., Soll A. H., Samloff I. M. Actions of histamine, secretin, and PGE2 on cyclic AMP production by isolated canine fundic mucosal cells. Am J Physiol. 1979 Nov;237(5):E437–E443. doi: 10.1152/ajpendo.1979.237.5.E437. [DOI] [PubMed] [Google Scholar]


