Abstract
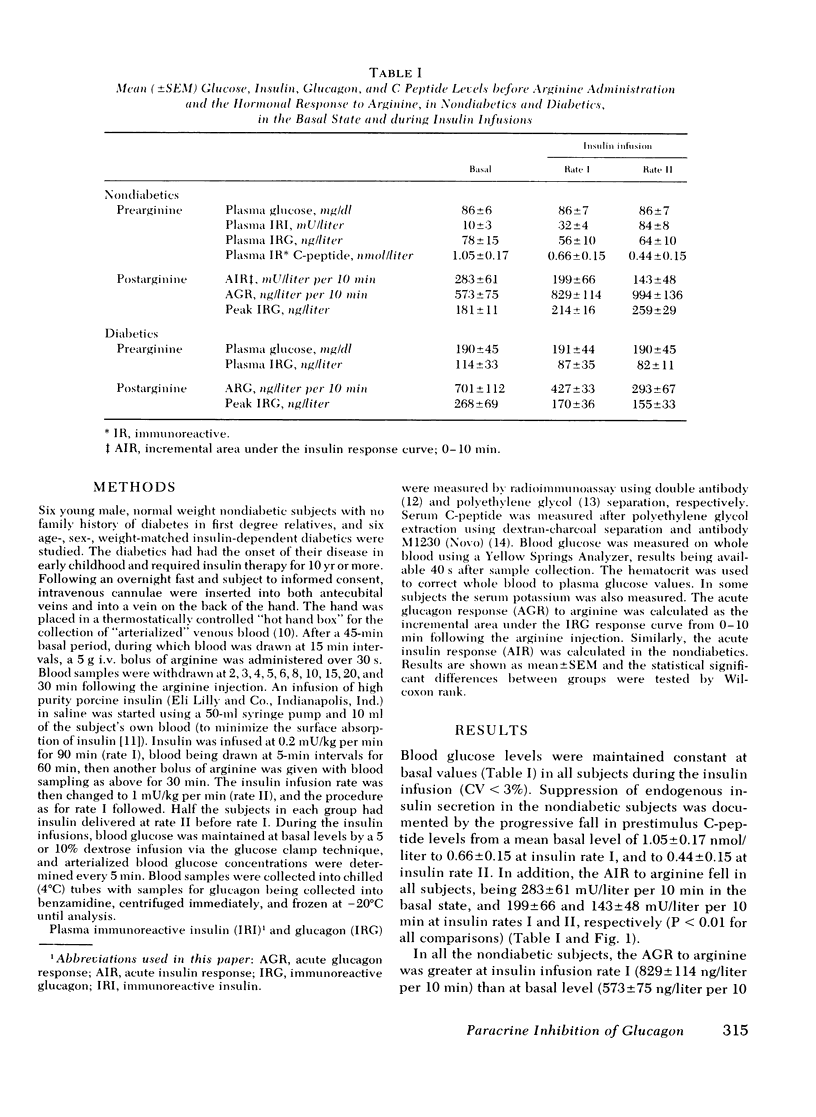
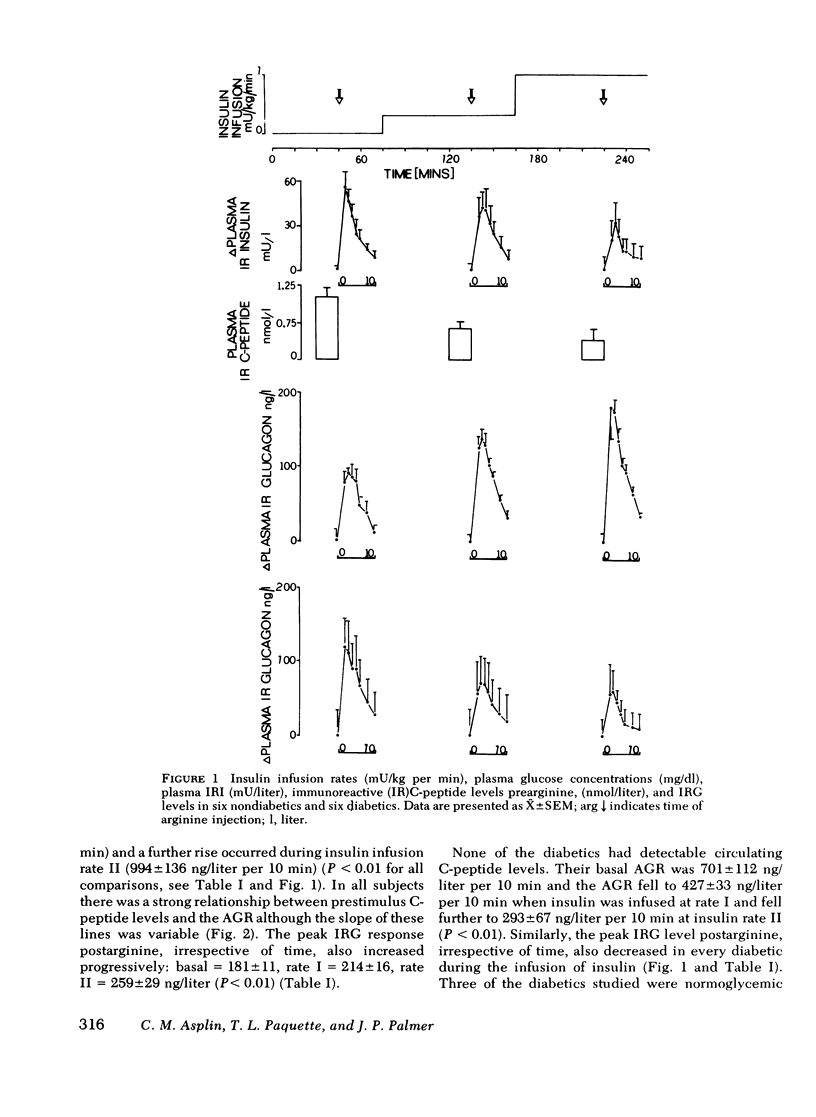
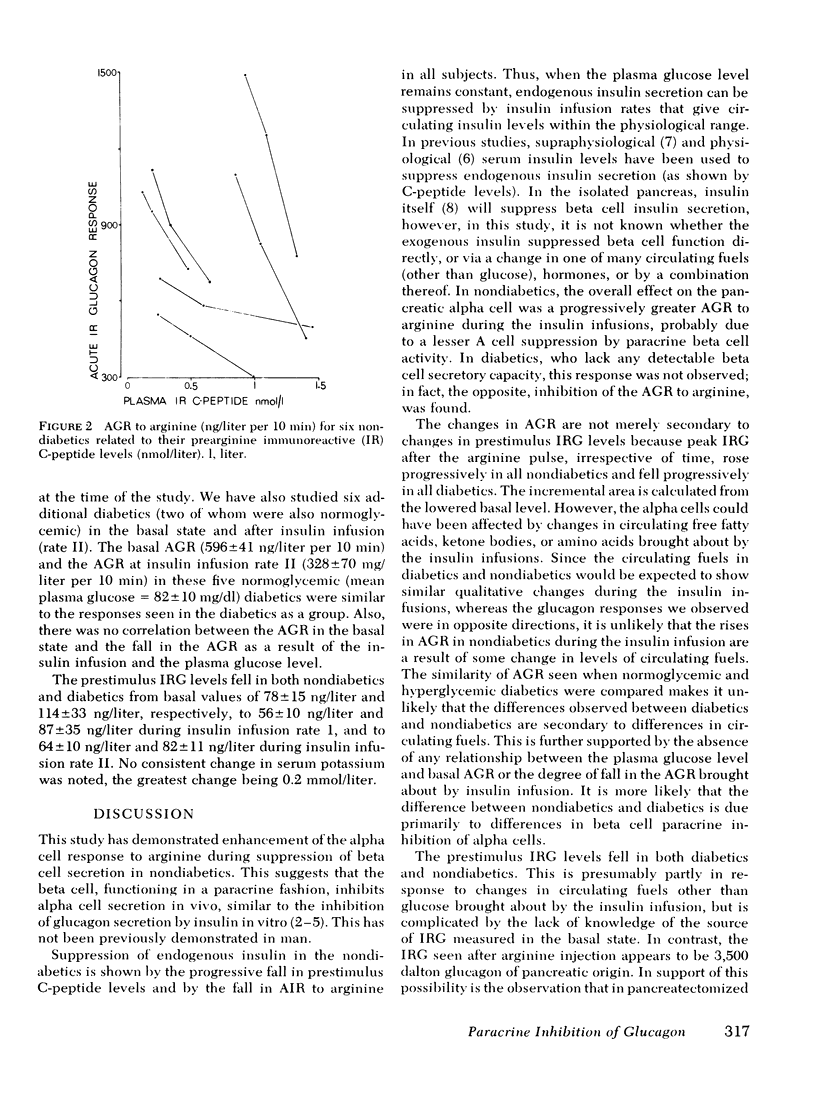
The close anatomical relationships betaeen pancreatic alpha and beta cells makes possible their interaction at a local (paracrine) level. To demonstrate this in vivo, we have compared the acute glucagon response to intravenous arginine in the basal state and after beta cell suppression by infusions of insulin. The plasma glucose concentration was maintained by the glucose clamp technique. In six normal weight nondiabetics, infusion of insulin at 0.2 mU/kg per min (rate 1) raised the mean +/- SEM plasma insulin levels from 10 +/- 3 to 32 +/- 4 mU/liter and at 1 mU/kg per min (rate 2) raised plasma insulin to 84 +/- 8 mU/liter. This resulted in beta cell suppression, as shown by a diminution in the acute insulin response (incremental area under the insulin response curve, 0-10 min): basal = 283 +/- 61, 199 +/- 66 (rate 1) and 143 +/- 48 mU/liter per 10 min (rate 2) and a fall in prestimulus C-peptide from 1.05 +/- 0.17 to 0.66 +/- 0.15 and to 0.44 +/- 0.15 mM/liter (all P less than 0.01). This beta cell suppression was associated with increased glucagon responses to arginine: 573 +/- 75 (basal), 829 +/- 114 (rate 1), and 994 +/- 136 ng/liter per 10 min (rate 2) and increased peak glucagon responses 181 +/- 11 (basal), 214 +/- 16 (rate 1), and 259 +/- 29 ng/liter (rate 2) (all P less than 0.01). In all subjects, there was a proportional change between the rise in he acute glucagon response to arginine and the fall in the prearginine C-peptide level. To demonstrate that augmented glucagon response was due to betw cell suppression, and not to the metabolic effect of infused insulin, similar studies were performed in C-peptide-negative-diabetics. Their acute glucagon response to arginine was inhibited by the insulin infusion: 701 +/- 112 (basal), 427 +/- 33 (rate 1), and 293 +/- 67 ng/liter per 10 min (rate 2) as was their peak glucagon response: 268 +/- 69, 170 +/- 36, and 115 +/- 33 ng/liter (all P less than 0.01). Thus, hyperinsulinemia, within the physiological range achieved by insulin infusion, inhibits beta cell secretion which, via a paracrine mechanism, potentiates glucagon secretion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beischer W., Schmid M., Kerner W., Keller L., Pfeiffer E. F. Does insulin play a role in the regulation of its own secretion? Horm Metab Res. 1978 Mar;10(2):168–169. doi: 10.1055/s-0028-1095819. [DOI] [PubMed] [Google Scholar]
- Benson J. W., Jr, Johnson D. G., Palmer J. P., Werner P. L., Ensinck J. W. Glucagon and catecholamine secretion during hypoglycemia in normal and diabetic man. J Clin Endocrinol Metab. 1977 Mar;44(3):459–464. doi: 10.1210/jcem-44-3-459. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Tobin J. D., Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979 Sep;237(3):E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Faber O. K., Markussen J., Naithani V. K., Binder C. Production of antisera to synthetic benzyloxycarbonyl-C-peptide of human proinsulin. Hoppe Seylers Z Physiol Chem. 1976 Jun;357(6):751–757. doi: 10.1515/bchm2.1976.357.1.751. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Langlois M., Noacco C., Karam J. H., Forsham P. H. Lack of glucagon response to hypoglycemia in diabetes: evidence for an intrinsic pancreatic alpha cell defect. Science. 1973 Oct 12;182(4108):171–173. doi: 10.1126/science.182.4108.171. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Malvaux P., Lambert A. E. Glucagon immunoassay using polyethylene glycol to precipitate antibody-bound hormone. Diabetologia. 1974 Feb;10(1):61–68. doi: 10.1007/BF00421415. [DOI] [PubMed] [Google Scholar]
- Honey R. N., Fallon M. B., Weir G. C. Effects of exogenous insulin, glucagon, and somatostatin on islet hormone secretion in the perfused chicken pancreas. Metabolism. 1980 Dec;29(12):1242–1246. doi: 10.1016/0026-0495(80)90152-3. [DOI] [PubMed] [Google Scholar]
- Lefèbvre P. J., Luyckx A. S. Factors controlling gastric-glucagon release. J Clin Invest. 1977 Apr;59(4):716–722. doi: 10.1172/JCI108690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljenquist J. E., Horwitz D. L., Jennings A. S., Chiasson J. L., Keller U., Rubenstein A. H. Inhibition of insulin secretion by exogenous insulin in normal man as demonstrated by C-peptide assay. Diabetes. 1978 May;27(5):563–570. doi: 10.2337/diab.27.5.563. [DOI] [PubMed] [Google Scholar]
- McGuire E. A., Helderman J. H., Tobin J. D., Andres R., Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976 Oct;41(4):565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- Orci L., Unger R. H. Functional subdivision of islets of Langerhans and possible role of D cells. Lancet. 1975 Dec 20;2(7947):1243–1244. doi: 10.1016/s0140-6736(75)92078-4. [DOI] [PubMed] [Google Scholar]
- Raskin P., Fujita Y., Unger R. H. Effect of insulin-glucose infusions on plasma glucagon levels in fasting diabetics and nondiabetics. J Clin Invest. 1975 Nov;56(5):1132–1138. doi: 10.1172/JCI108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin P., Unger R. H. Effect of insulin therapy on the profiles of plasma immunoreactive glucagon in juvenile-type and adult-type diabetics. Diabetes. 1978 Apr;27(4):411–419. doi: 10.2337/diab.27.4.411. [DOI] [PubMed] [Google Scholar]
- Unger R. H. Role of glucagon in the pathogenesis of diabetes: the status of the controversy. Metabolism. 1978 Nov;27(11):1691–1709. doi: 10.1016/0026-0495(78)90291-3. [DOI] [PubMed] [Google Scholar]
- Werner P. L., Palmer J. P. Immunoreactive glucagon responses to oral glucose, insulin infusion and deprivation, and somatostatin in pancreatectomized man. Diabetes. 1978 Oct;27(10):1005–1012. doi: 10.2337/diab.27.10.1005. [DOI] [PubMed] [Google Scholar]


