Abstract
Osmoregulation was studied in near term and age-matched Sprague-Dawley rats. Basal plasma osmolality (Posm) and plasma sodium (PNa) were 281±3 mosmol/kg and 134±3 meq/liter, respectively, on the 20th gestational day compared with 292±3 mosmol/kg and 140±1 meq/liter in virgin animals (P < 0.001), whereas Purea and plasma water content were similar in pregnant and control rats. These differences could not be reproduced in animals receiving progesterone, estrone, or a combination of progesterone and estradiol for 2 wk.
Pregnant and control rats were deprived of water for periods ranging from 0 to 48 h. Posm, always lower in gravidity, was 290±3 mosmol/kg after 2 d of water deprivation in pregnant animals compared with 300±2 mosmol/kg in controls (P < 0.001). Thus 48 h of dehydration were required before Posm in gravid rats was similar to basal values in the age-matched virgins.
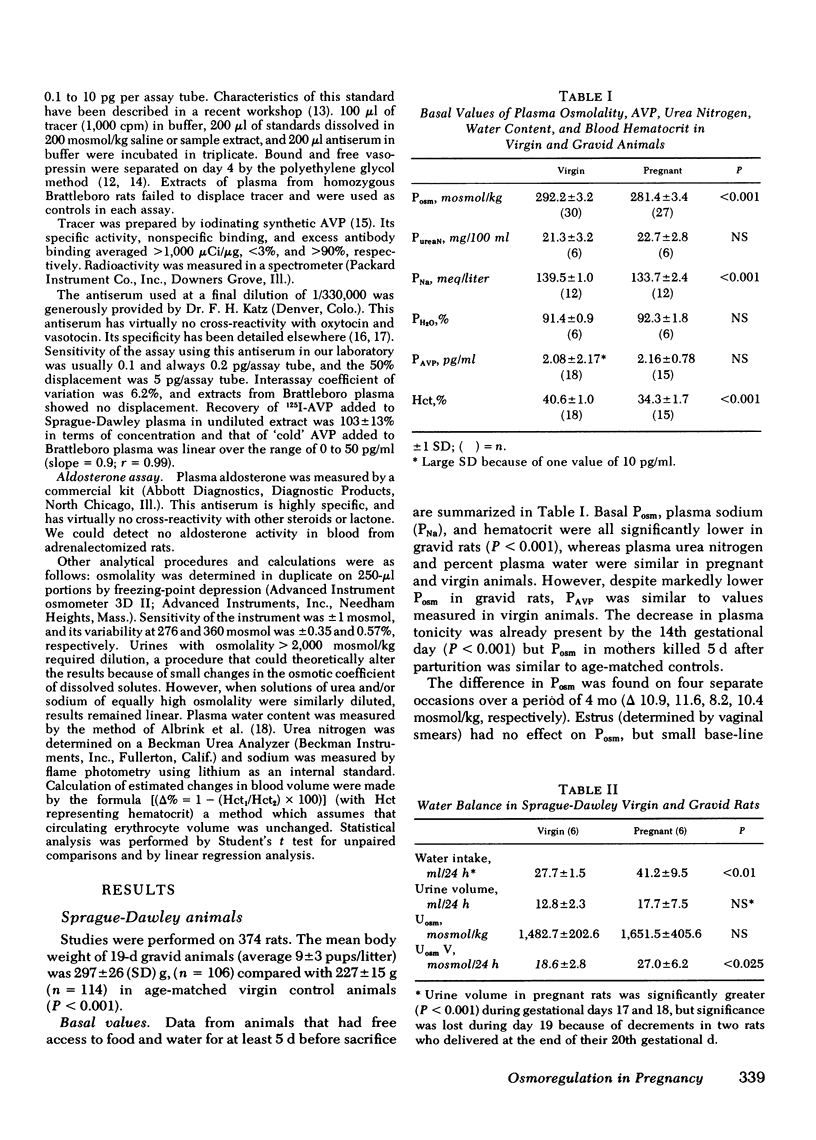
Despite strikingly lower Posm, plasma arginine vasopressin (PAVP) and urinary osmolality (Uosm) were similar in the basal state averaging 2.16±0.78 pg/ml and 1,652±406 mosmol/kg, respectively, during pregnancy compared with 2.08±2.17 pg/ml and 1,483±203 mosmol/kg in controls (NS). Water deprivation increased PAVP and Uosm similarly in pregnant and virgin rats: these values reached 22.7±3.3 pg/ml and 3,300±123 mosmol/kg at 48 h in gravid compared with 26.0±6.4 pg/ml and 3,342±141 mosmol/kg in the controls (NS). Regression equations for PAVPvs. Posm which were highly significant (P < 0.001) in both groups demonstrated an apparent threshold for AVP secretion approximately 11 mosmol lower in gravid animals.
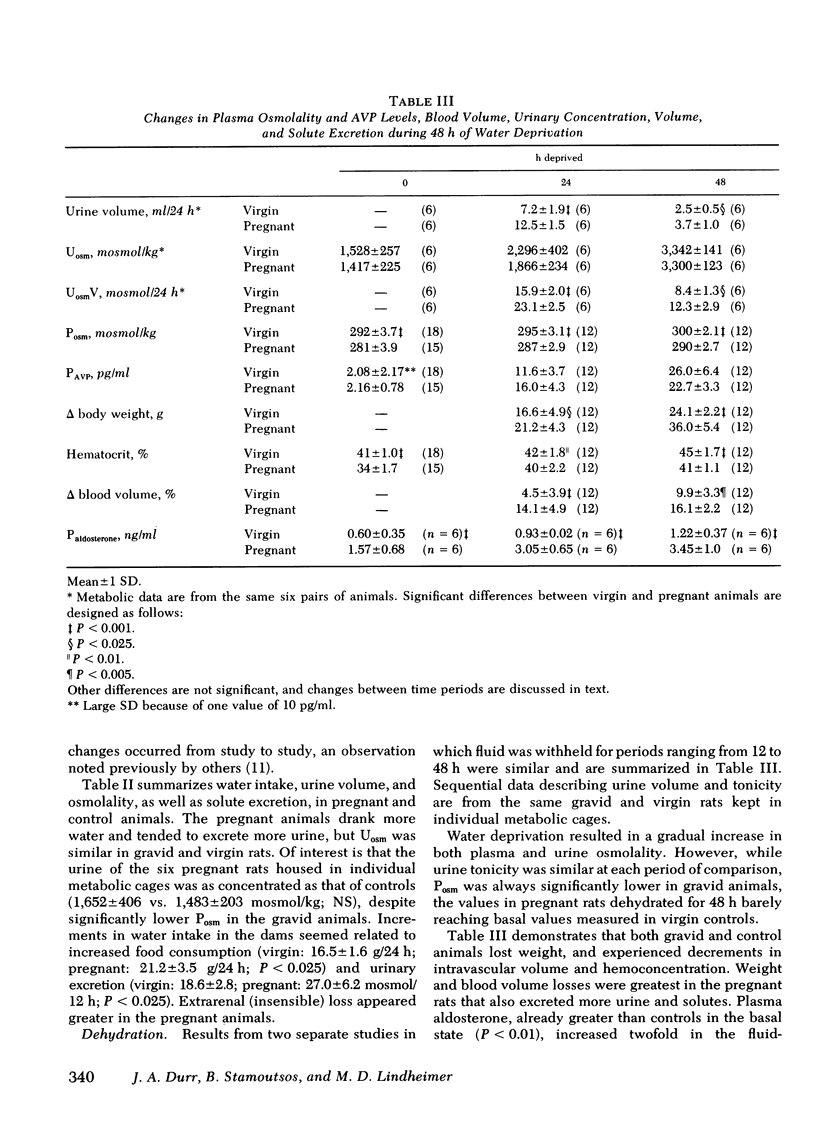
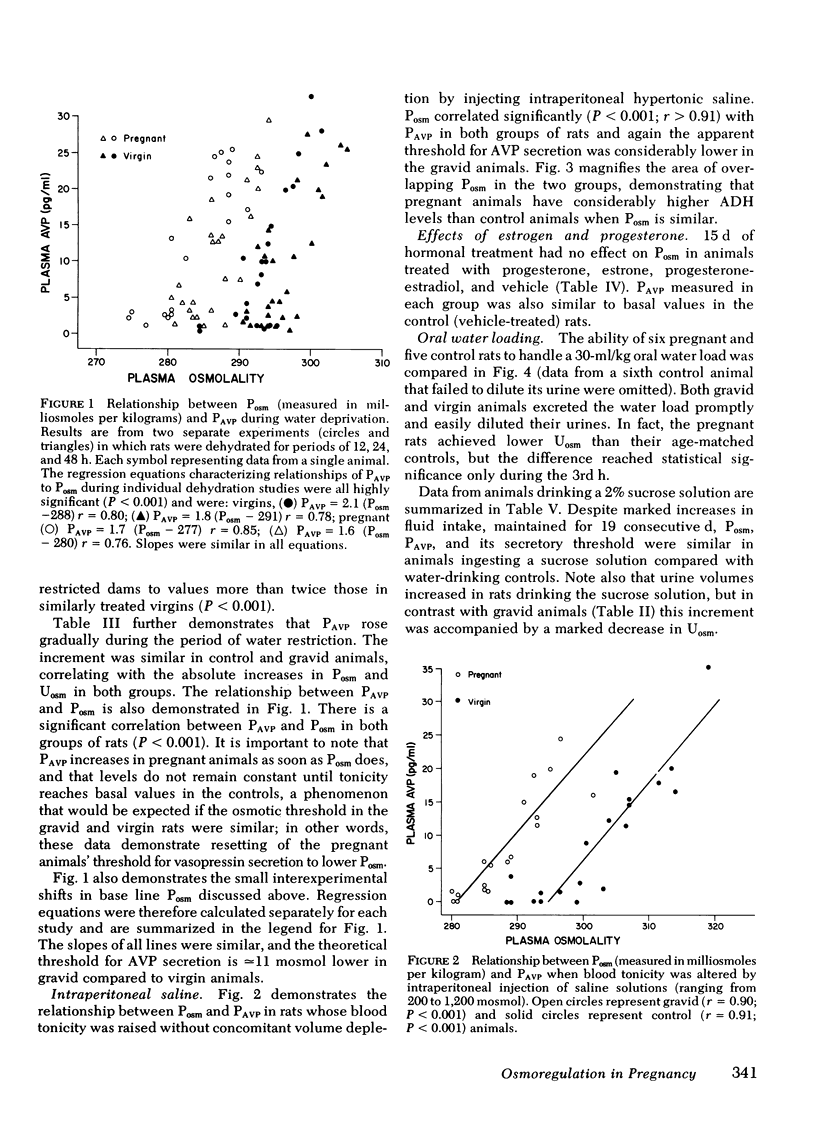
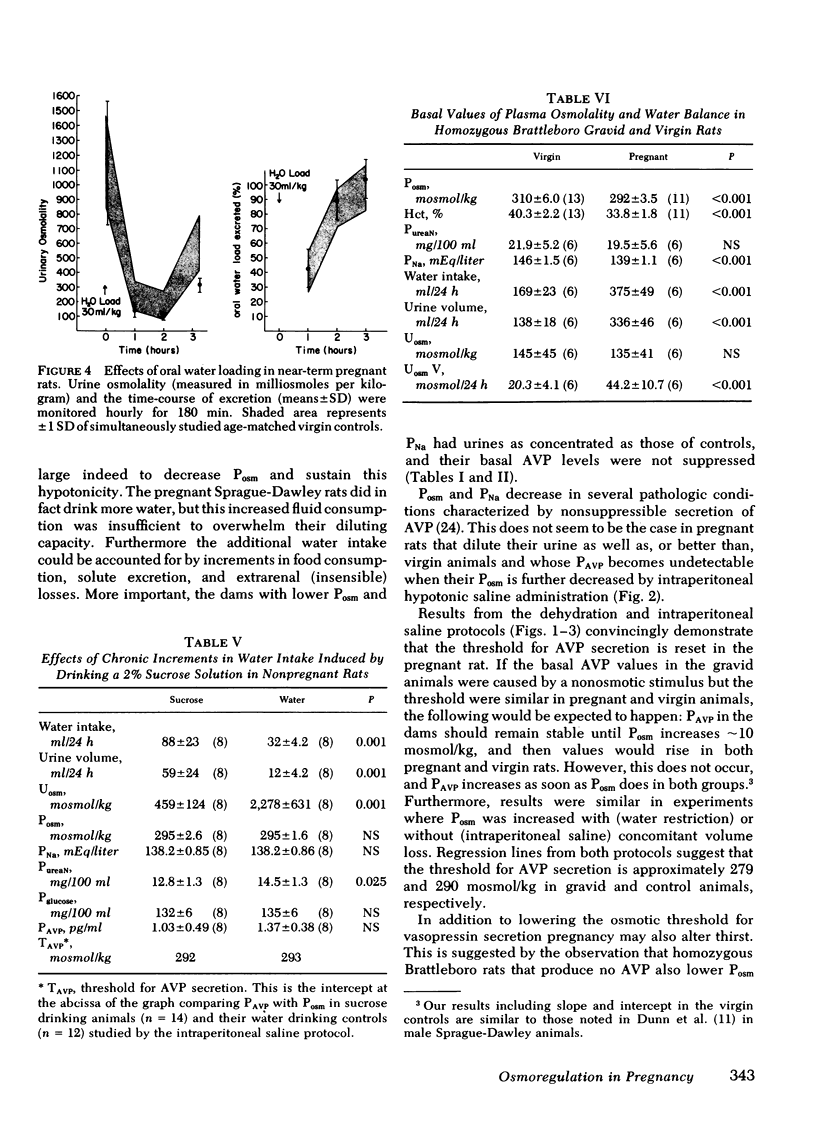
Intravascular volume decreased, and plasma aldosterone increased during water deprivation, and both changes (Δ%) were significantly greater in the gravid animals (P <0.01). Therefore, Posm was increased without concomitant volume depletion by intraperitoneal hypertonic saline. Again PAVPvs. Posm correlated significantly (r > 0.9; P < 0.001) in each group, and the apparent threshold was 14 mosmol lower in pregnant animals. Diluting ability, tested by oral water loading, was not impaired in the pregnant animals which excreted a 30 ml/kg load as well as controls. Also, chronically hydrated virgin animals whose fluid intake was more than twice that of pregnant rats (for 19 d) did not lower their Posm.
In separate studies homozygous Brattleboro rats, which produce no endogenous vasopressin, were also shown to have a decreased Posm (pregnant 292±4 mosmol/kg; virgin 310±6 mosmol/kg P < 0.001), but unchanged Uosm during pregnancy.
Data demonstrate a resetting of the osmostat in gravid Sprague-Dawley rats as Posm and the threshold for AVP secretion both decrease significantly during gestation in this species. Studies in homozygous Brattleboro animals with hereditary diabetes insipidus suggest that the osmotic threshold for thirst is reset as well.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. J., Pluss R. G., Berns A. S., Jackson J. T., Arnold P. E., Schrier R. W., McDonald K. E. Mechanism of effect of hypoxia on renal water excretion. J Clin Invest. 1978 Oct;62(4):769–777. doi: 10.1172/JCI109188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay W. H., Ferris T. F. Factors controlling plasma renin and aldosterone during pregnancy. Hypertension. 1979 Jul-Aug;1(4):410–415. doi: 10.1161/01.hyp.1.4.410. [DOI] [PubMed] [Google Scholar]
- Chesley L. C. Plasma and red cell volumes during pregnancy. Am J Obstet Gynecol. 1972 Feb 1;112(3):440–450. doi: 10.1016/0002-9378(72)90493-0. [DOI] [PubMed] [Google Scholar]
- Churchi-l S. E., Bengele H. H., Alexander E. A. Sodium balance during pregnancy in the rat. Am J Physiol. 1980 Jul;239(1):R143–R148. doi: 10.1152/ajpregu.1980.239.1.R143. [DOI] [PubMed] [Google Scholar]
- Csontos K., Rust M., Höllt V., Mahr W., Kromer W., Teschemacher H. J. Elevated plasma beta-endorphin levels in pregnant women and their neonates. Life Sci. 1979 Sep 3;25(10):835–844. doi: 10.1016/0024-3205(79)90541-1. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Dickmann Z. Hormonal requirements for the survival of blastocyts in the uterus of the rat. J Endocrinol. 1967 Apr;37(4):455–461. doi: 10.1677/joe.0.0370455. [DOI] [PubMed] [Google Scholar]
- Dunn F. L., Brennan T. J., Nelson A. E., Robertson G. L. The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest. 1973 Dec;52(12):3212–3219. doi: 10.1172/JCI107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gledhill N., Beirne G. J., Dempsey J. A. Renal response to short-term hypocapnia in man. Kidney Int. 1975 Dec;8(6):376–384. doi: 10.1038/ki.1975.130. [DOI] [PubMed] [Google Scholar]
- Herrera E., Knopp R. H., Freinkel N. Carbohydrate metabolism in pregnancy. VI. Plasma fuels, insulin, liver composition, gluconeogenesis, and nitrogen metabolism during late gestation in the fed and fasted rat. J Clin Invest. 1969 Dec;48(12):2260–2272. doi: 10.1172/JCI106192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M. K., Manger W. M., Rock T. W., Weiss R. J., Frantz A. G. Vasopressin release due to manual restraint in the rat: role of body compression and comparison with other stressful stimuli. Endocrinology. 1979 Mar;104(3):641–644. doi: 10.1210/endo-104-3-641. [DOI] [PubMed] [Google Scholar]
- Hytten F. E. Physiological changes in early pregnancy. J Obstet Gynaecol Br Commonw. 1968 Dec;75(12):1193–1197. doi: 10.1111/j.1471-0528.1968.tb02915.x. [DOI] [PubMed] [Google Scholar]
- KAITZ A. L. Urinary concentrating ability in pregnant women with asymptomatic bacteriuria. J Clin Invest. 1961 Jul;40:1331–1338. doi: 10.1172/JCI104363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRKSEY A., PIKE R. L., CALLAHAN J. A. Some effects of high and low sodium intakes during pregnancy in the rat. II. Electrolyte concentrations of maternal plasma, muscle, bone and brain and of placenta, amniotic fluid, fetal plasma and total fetus in normal pregnancy. J Nutr. 1962 May;77:43–51. doi: 10.1093/jn/77.1.43. [DOI] [PubMed] [Google Scholar]
- Katz F. H., Smith J. A., Lock J. P., Loeffel D. E. Plasma vasopressin variation and renin activity in normal active humans. Horm Res. 1979;10(6):289–302. doi: 10.1159/000179011. [DOI] [PubMed] [Google Scholar]
- LICHTON I. J. Salt saving in the pregnant rat. Am J Physiol. 1961 Nov;201:765–768. doi: 10.1152/ajplegacy.1961.201.5.765. [DOI] [PubMed] [Google Scholar]
- Lim V. S., Katz A. I., Lindheimer M. D. Acid-base regulation in pregnancy. Am J Physiol. 1976 Dec;231(6):1764–1769. doi: 10.1152/ajplegacy.1976.231.6.1764. [DOI] [PubMed] [Google Scholar]
- Lindheimer M. D., Katz A. I. Kidney function in the pregnant rat. J Lab Clin Med. 1971 Oct;78(4):633–641. [PubMed] [Google Scholar]
- Lindheimer M. D., Weston P. V. Effect of hypotonic expansion on sodium, water, and urea excretion in late pregnancy: the influence of posture on these results. J Clin Invest. 1969 May;48(5):947–956. doi: 10.1172/JCI106054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R., Torres C., Marchena C., Whittembury J., Monge C. C. Response to metabolic (ammonium chloride) acidosis at sea level and at high altitude. Nephron. 1969;6(2):102–109. doi: 10.1159/000179718. [DOI] [PubMed] [Google Scholar]
- MCCARTNEY C. P., SPARGO B., LORINCZ A. B., LEFEBVRE Y., NEWTON R. E. RENAL STRUCTURE AND FUNCTION IN PREGNANT PATIENTS WITH ACUTE HYPERTENSION; OSMOLAR CONCENTRATION. Am J Obstet Gynecol. 1964 Nov 1;90:579–592. doi: 10.1016/s0002-9378(16)34985-7. [DOI] [PubMed] [Google Scholar]
- MacGillivray I., Rose G. A., Rowe B. Blood pressure survey in pregnancy. Clin Sci. 1969 Oct;37(2):395–407. [PubMed] [Google Scholar]
- Nolten W. E., Ehrlich E. N. Sodium and mineralocorticoids in normal pregnancy. Kidney Int. 1980 Aug;18(2):162–172. doi: 10.1038/ki.1980.125. [DOI] [PubMed] [Google Scholar]
- Page E. W., Christianson R. The impact of mean arterial pressure in the middle trimester upon the outcome of pregnancy. Am J Obstet Gynecol. 1976 Jul 15;125(6):740–746. doi: 10.1016/0002-9378(76)90839-5. [DOI] [PubMed] [Google Scholar]
- Robertson E. G., Cheyne G. A. Plasma biochemistry in relation to oedema of pregnancy. J Obstet Gynaecol Br Commonw. 1972 Sep;79(9):769–776. doi: 10.1111/j.1471-0528.1972.tb12918.x. [DOI] [PubMed] [Google Scholar]
- Robertson G. L., Mahr E. A., Athar S., Sinha T. Development and clinical application of a new method for the radioimmunoassay of arginine vasopressin in human plasma. J Clin Invest. 1973 Sep;52(9):2340–2352. doi: 10.1172/JCI107423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson G. L., Shelton R. L., Athar S. The osmoregulation of vasopressin. Kidney Int. 1976 Jul;10(1):25–37. doi: 10.1038/ki.1976.76. [DOI] [PubMed] [Google Scholar]
- Rosenbloom A. A., Sack J., Fisher D. A. The circulating vasopressinase of pregnancy: species comparison with radioimmunoassay. Am J Obstet Gynecol. 1975 Feb 1;121(3):316–320. doi: 10.1016/0002-9378(75)90005-8. [DOI] [PubMed] [Google Scholar]
- SEVERINGHAUS J. W., MITCHELL R. A., RICHARDSON B. W., SINGER M. M. RESPIRATORY CONTROL AT HIGH ALTITUDE SUGGESTING ACTIVE TRANSPORT REGULATION OF CSF PH. J Appl Physiol. 1963 Nov;18:1155–1166. doi: 10.1152/jappl.1963.18.6.1155. [DOI] [PubMed] [Google Scholar]
- Skowsky W. R., Swan L., Smith P. Effects of sex steroid hormones on arginine vasopressin in intact and castrated male and female rats. Endocrinology. 1979 Jan;104(1):105–108. doi: 10.1210/endo-104-1-105. [DOI] [PubMed] [Google Scholar]
- Zeballos J., Galdos B., Quintanilla A. Plasma osmolality in subjects acclimatised at high altitude. Lancet. 1973 Feb 3;1(7797):230–231. doi: 10.1016/s0140-6736(73)90068-8. [DOI] [PubMed] [Google Scholar]


