Abstract
Mitochondrial Ca2+ uptake contributes important feedback controls to limit the time course of Ca2+signals. Mitochondria regulate cytosolic [Ca2+] over an exceptional breath of concentrations (∼200 nM to >10 μM) to provide a wide dynamic range in the control of Ca2+ signals. Ca2+ uptake is achieved by passing the ion down the electrochemical gradient, across the inner mitochondria membrane, which itself arises from the export of protons. The proton export process is efficient and on average there are less than three protons free within the mitochondrial matrix. To study mitochondrial function, the most common approaches are to alter the proton gradient and to measure the electrochemical gradient. However, drugs which alter the mitochondrial proton gradient may have substantial off target effects that necessitate careful consideration when interpreting their effect on Ca2+ signals. Measurement of the mitochondrial electrochemical gradient is most often performed using membrane potential sensitive fluorophores. However, the signals arising from these fluorophores have a complex relationship with the electrochemical gradient and are altered by changes in plasma membrane potential. Care is again needed in interpreting results. This review provides a brief description of some of the methods commonly used to alter and measure mitochondrial contribution to Ca2+ signaling in native smooth muscle.
Keywords: Smooth muscle, mitochondria, calcium signalling, imaging
Fine Control of Ca2+ Signaling and Biological Responses
Changes in the [Ca2+]c trigger numerous vascular smooth muscle cell activities, which include cell division, growth, metabolism, contraction, and death. To enable the ion to modulate such a diversity of activities, there are a wealth of different types of Ca2+ signals (the Ca2+ toolkit, 6,45) with various amplitudes, durations, and frequencies and the signal may be confined to particular parts of the cell. Each of these features (amplitude, frequency, duration, location) may selectively target Ca2+ signals to particular physiological responses. A central requirement for the existence of the various temporal and spatial signals are local feedback processes which shape the Ca2+ increase or restrict changes in the [Ca2+]c to small parts of the cell. Mitochondria are of acknowledged significance in the feedback control of Ca2+ signaling 15. The organelle's facility for rapid Ca2+ uptake controls the Ca2+ signal and the altered signal is transduced to a biological response by mitochondria themselves or by other parts of the cell.
The two main sources of Ca2+ are the extracellular fluid and the intracellular stores (the SR). Ca2+ enters the cell from the extracellular fluid via channels on the plasma membrane such as the voltage-dependent and store-operated Ca2+ channels. The other main Ca2+ source is the SR store from which release proceeds via two receptor-controlled channels—the IP3R and the RyR 8,44. Significantly, the sources of Ca2+ are not independent; Ca2+ influx regulates Ca2+ release and Ca2+ release regulates Ca2+ influx. For example, Ca2+ release from the SR may alter plasma membrane ion channel activity to regulate the membrane potential and Ca2+ entry, whereas depletion of the SR of Ca2+ activates influx via store-operated Ca2+ channels 5,47,53,80. Thus, a change in [Ca2+]c arising from the activity of channels in the plasma membrane or the SR will itself regulate ion channel activity to provide feedback control of Ca2+ signals.
The strategic positioning of channels, receptors, and organelles is important in facilitating the operation of feedback processes. Various channels, receptors, and organelles combine to become functional units and enable Ca2+ to act as either a highly localized signal or to evoke more widespread effects through the cell. For example, in sympathetic neurons, while muscarinic and bradykinin receptors each stimulate PLC to produce IP3, only bradykinin receptors co-immunoprecipitate with, and activate, IP3R to evoke Ca2+ release 19. The arrangement enables different responses to be evoked depending on whether PLC is activated by muscarinic or bradykinin receptors; muscarinic receptors play a key role in regulating neuronal excitability 10 and bradykinin receptors mediate inflammation and hyperalgesia 20.
Active IP3R are positioned near the plasma membrane providing another mechanism for agonists, acting via IP3, to target specific cellular responses by generating Ca2+ rises in specific regions of the cell 74. For example, in cerebral arteries endothelial membrane projections extend through the internal elastic lamina to adjacent smooth muscle membranes. In the projections, local IP3-mediated Ca2+ release events (referred to as “pulsars”) activate intermediate conductance, Ca2+-sensitive potassium channels, which co-localize to the same region, to hyperpolarize the endothelial membrane. The resultant membrane potential change is transmitted from the endothelium to the smooth muscle cells by coupling of the membrane via the projections. In this way localized IP3-mediated Ca2+ release in endothelial cells triggers relaxation in smooth muscle cells 40. RyR are also organized to contribute to feedback activity and may be coupled to channels on the plasma membrane to form functional units. Local Ca2+ release events from RyR (Ca2+ sparks), may activate either Ca2+-activated K+ channels or Ca2+-activated Cl− channels or both to generate spontaneous transient outward (hyperpolarizing) or inward (depolarizing) currents on the plasma membrane 4,63,80,81. This facility again permits local Ca2+ release via RyR to activate or inhibit smooth muscle function. Another structural element to the organization of Ca2+ signals lies in the clustering surface receptors in certain regions on the plasma membrane. The clustering of surface receptors provides areas with increased sensitivity to extracellular stimuli 77 that contribute to feedback control and achieves local specificity in Ca2+ signaling.
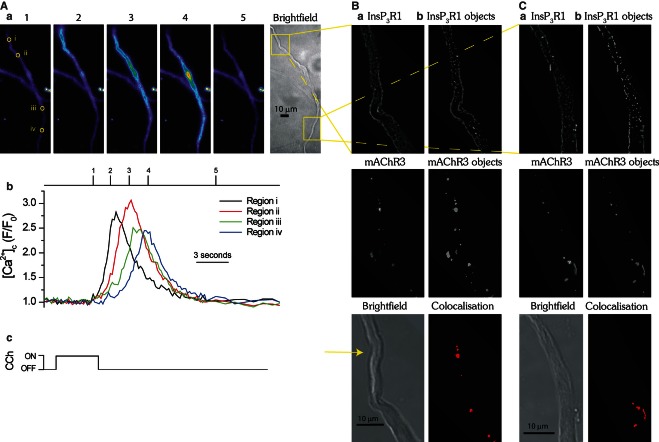
Regenerative propagation of the Ca2+ rise (a “Ca2+ wave”) occurs by positive feedback control of the Ca2+ rise and is another example of interaction among components, in this case to increase the reach of a local Ca2+ signal. In smooth muscle, local Ca2+ release from the SR may activate neighboring closely positioned ion channels on the SR to propagate the signal from site to site through the cell 9,32,42,46,50 in a way reminiscent of a “fire-beacon” relay network. An interesting feature of waves in smooth muscle is that the Ca2+ rise begins at precisely the same small site on each activation despite the cell being stimulated uniformly across the plasma membrane, indicating that is there is a preferred site of wave initiation 60. That local precision in wave initiation is also explained by receptor complexes, in this case which contain mAChR3 and IP3R1 that are structurally and functionally coupled (Figure 1) 60. mAChR3 and IP3R co-localize to lie within 40–100 nm of each other to generate junctions which facilitate a privileged delivery of IP3 to particular IP3R 60. This arrangement circumvents the diffuse global signaling that is normally associated with IP3 and the inositide acts as a targeted, highly localized signal. Specific associations between signaling proteins located at the plasma membrane (e.g., mAChR3) and the SR membrane (IP3R) is one element of feedback control in the Ca2+ toolkit enabling receptors to generate differences in the signaling pathways subsequently activated 28,79.
Figure 1.
The colocalization of InsP3R1 and mAChR3 at sites of Ca2+ wave initiation. Carbachol (CCh, 4 s; Ac) evoked a Ca2+ wave (Aa, Ab) which initiated from a single site in a single colonic myocyte (Aa; frame 1, region i) and propagated from there. The Ca2+ wave repeatedly initiated from the same site during subsequent carbachol applications (data not shown). The extent of IP3R1 and mAChR3 colocalization was next assessed in the same cell at the site of Ca2+ wave initiation and compared to another separate region of the cell (Aa; yellow boxes on bright field image). The cell was fixed, prepared for immunocytochemistry, labeled with IP3R1 monoclonal and mAChR3 polyclonal antibodies, and visualized by confocal microscopy using a fluorescently conjugated secondary antibody (Ba, Ca; top and middle panels). Colocalization was quantified using image analysis software ImageJ 65 and the plugin JACoP to examine object-based colocalization. Colocalization of the center of mass of three dimensional IP3R1 and mAChR3 objects created using the 3D object counter plugin (Bb, Cb; top and middle panels) were quantified by determining the number of centers from one image that were colocalized with objects from the other image (Bb, Cb; bottom panel). In the above experiment, 9.0% of the objects colocalized (9 out of 100 objects detected) at the initiation site and 4.6% colocalized (7 out of 152 objects detected) at the other site. The images shown are from plane 45 of 70 (B; initiation site) and plane 34 of 50 (C; same cell, other site) plane z stacks, each image taken at 150 nm intervals. Brightfield image (Ba, Ca; lower panel) of the same cell; initiation site indicated (Ba; yellow arrow). Note: Scale bar at bottom of bright field images. The images acquired using confocal microscopy (B,C) were rotated for clarity to match the orientation of the images acquired using epifluorescence microscopy (A). From Olson et al. 60 with permission.
Role of Mitochondria in Ca2+ Signaling
In addition to the feedback arising from the positioning of channels and receptors, intracellular organelles may also regulate Ca2+ signals to contribute to the Ca2+ toolkit. Mitochondria may take up and sequester a large amount of Ca2+ from the cytoplasm and modulate the time course and amplitude of Ca2+ signals and shape the resulting message. The mitochondrial capacity to sequester Ca2+ is enormous and the buffer power [100,000; 14] is three orders of magnitude greater than that of cytoplasm [∼100; 35]. The mitochondrial buffer power arises largely from the quantities of phosphate within the organelle (∼5 mM). After uptake, Ca2+ is slowly exported from mitochondria via a Na+-(or H+-) Ca2+ antiporter mechanism 13.
Mitochondria regulate numerous Ca2+ signals including those arising from Ca2+ release via IP3R or RyR or voltage-dependent Ca2+ entry across the outside membrane. Interestingly, mitochondrial Ca2+ uptake may decrease or increase the amplitude of Ca2+ signals. In some cells, mitochondrial Ca2+ uptake decreases the amplitude of IP3-evoked Ca2+ signals; preventing the organelle from taking up Ca2+ increases IP3-mediated Ca2+ release in cultured hepatocytes and HeLa cells and Ca2+ wave velocity in cultured astrocytes 1,7,26. In other cells (smooth muscle, astrocytes and HeLa cells) mitochondrial Ca2+ uptake facilitates IP3-evoked Ca2+ release so that preventing mitochondria from taking up Ca2+ reduces the amplitude of the Ca2+ signal 13,16,23,31,59,73 (see below).
Ca2+ signals from RyR activity may also be regulated by mitochondrial Ca2+ uptake. Preventing mitochondria from taking up Ca2+ prolonged caffeine-evoked [Ca2+]c increases in aortic and arterial myocytes 25,36. However, in other studies, preventing mitochondrial Ca2+ uptake did not alter caffeine-evoked SR Ca2+ release in cardiac or various smooth muscles 38,72,75,78. The Ca2+ transient arising from voltage-dependent Ca2+ entry has an accelerated rate of decline as a consequence of mitochondrial Ca2+ uptake and when the uptake is inhibited, the rate of Ca2+ decline is substantially slowed (Figure 2) 21,27,49,52.
Figure 2.
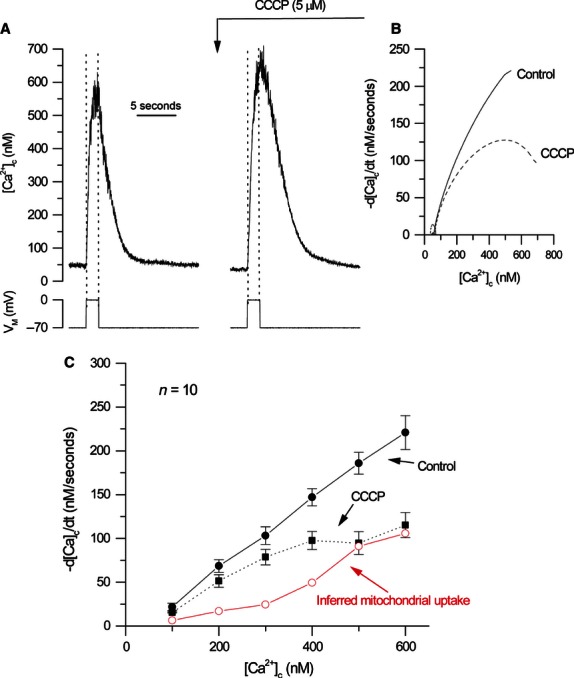
Mitochondria contribute to [Ca2+]c decline following voltage-dependent Ca2+ entry in smooth muscle. (A) Depolarization (−70 to 0 mV) activated a voltage-dependent Ca2+ current (data not shown) and increased [Ca2+]c in a single colonic myocyte. CCCP (5 μM) slowed the rate of decline of [Ca2+]c on repolarization compared with control. (B) The rate of decline (−d[Ca2+]c/dt), obtained from high order polynomial fits to the declining phase of the transients, shows a significant slowing when mitochondria were prevented from accumulating Ca2+. (C) A summary of the rates of decline for 10 cells in the presence and absence of CCCP. The inferred mitochondrial contribution to the decline of [Ca2+]c (red line) was obtained by subtracting control rates from those seen in CCCP and shows that mitochondrial Ca2+ uptake occurred above 200 nM [Ca2+]c (from McCarron & Muir 49 with permission).
In controlling Ca2+ signals, mitochondria operate over a very wide [Ca2+]c range (200 nM to >10 μM) 51,64,76. In the example shown in Figure 2, mitochondria modulate signals over the [Ca2+]c range 200–600 nM, which demonstrates that mitochondria have a high affinity for Ca2+ (in the sub-micromolar range; Figure 2; [see also 64,75]). Interestingly, mitochondria do not appear to alter the rate of rise of the Ca2+ transient suggesting that the organelle does not modulate the high local [Ca2+] (>10 μM) near active voltage-dependent Ca2+ channels (Figure 2) or the activity of the channels themselves. However, mitochondria do have the capacity to modulate Ca2+ signals that are ∼2 orders of magnitude larger than the global Ca2+ transient from voltage-dependent Ca2+ channels and in the tens of micromolar range 67. One notable example in smooth muscle is mitochondrial regulation of the Ca2+ signals which arise from the activity of a single IP3R cluster (“Ca2+ puffs”; Figure 3). When mitochondria are prevented from taking up Ca2+ (using uncouplers, complex I inhibitors or uniporter inhibitors), Ca2+ puffs are inhibited 59. This observation suggests the organelle may also have a very low affinity for Ca2+ (as Ca2+ puffs are >10 μM) and that Ca2+ uptake is fast enough to modulate concentrations near ion channels.
Figure 3.
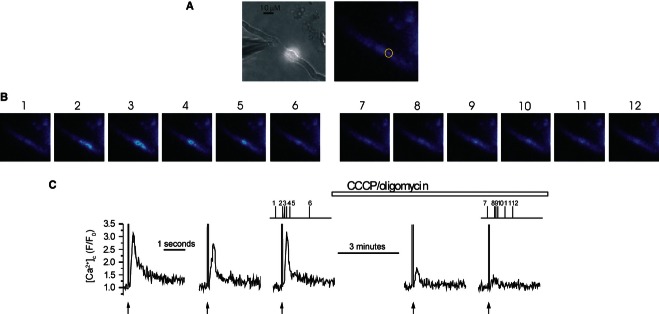
Depolarization of the mitochondrial membrane potential with CCCP inhibits Ca2+ release from an IP3R cluster (Ca2+ puffs). At −70 mV, locally photolyzed caged IP3 (25 μM) (↑, C) in a ∼20 μm diameter region (A; bright spot in left hand panel, see also whole cell electrode, left side) evoked Ca2+ puffs in a single colonic smooth muscle cell (B, C). There were two individual Ca2+ puff sites activated by photorelease of IP3. Flash photolysis of IP3 every ∼60 s generated approximately comparable [Ca2+]c increases (C). Superfusion of CCCP (applied with oligomycin; 1 and 6 μM, respectively) while continuing to photolyze IP3 at ∼60 intervals, decreased the amplitude of IP3-mediated Ca2+ puffs (B, C). The [Ca2+]c images (B) are derived from the time points indicated by the corresponding numbers in (C). [Ca2+]c changes in (B) are expressed by color; dark blue low and light blue high [Ca2+]c. Measurements were made from a 3 × 3 pixel box (A; right hand panel, white square). The large increase in fluorescence (C) at the time of photolysis (↑) is artifact from the flash lamp (from Olson et al. 59 with permission).
The question arises, how do mitochondria, by removing Ca2+ from the cytoplasm (i.e., lowering [Ca2+]), generate a larger [Ca2+]c rise? IP3R is regulated by Ca2+-dependent positive and negative feedback mechanisms. Mitochondrial Ca2+ uptake limits a negative feedback inhibition of Ca2+ on IP3R. There are at least two types of Ca2+-dependent negative feedback mechanisms, which may deactivate smooth muscle IP3R. In the first, a Ca2+-dependent deactivation of IP3R occurs at [Ca2+]c which exceed ∼300 nM 29. The onset is rapid and the deactivation persists for ∼5 s after the [Ca2+]c increase ends in permeabilized vascular smooth muscle 30. Another form of Ca2+-dependent deactivation of IP3R, once initiated by an increased [Ca2+]c, persisted long (tens of seconds) after [Ca2+]c had regained resting values, that is became, at least partially, refractory 48,58. Each of these processes would persistently inhibit Ca2+ release via IP3R. Mitochondrial Ca2+ uptake by buffering the Ca2+ rise at IP3R presumably prevents a persistent deactivation of IP3R to increase the overall release of Ca2+ (Figure 4).
Figure 4.
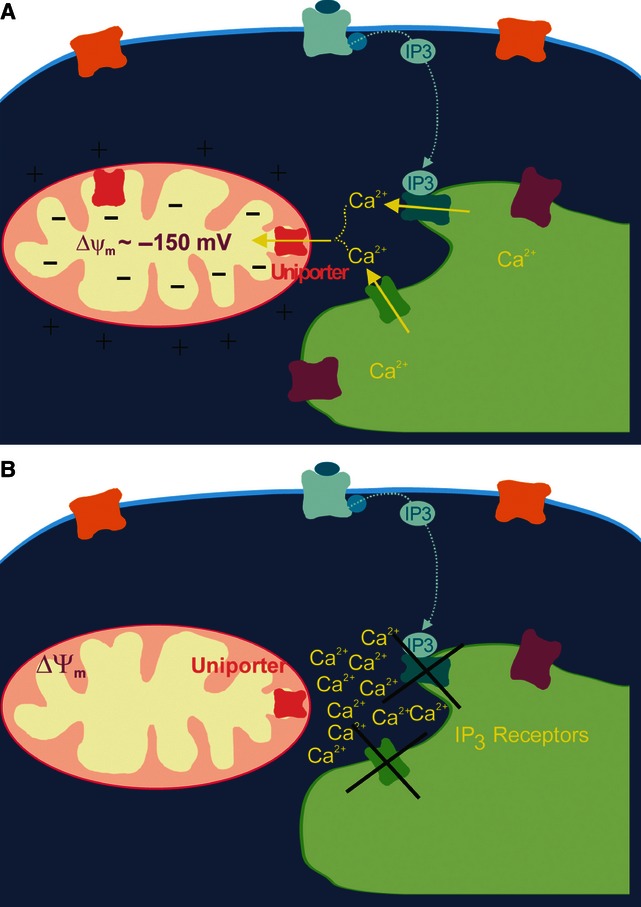
Depolarization of the mitochondrial membrane potential with CCCP inhibits IP3-evoked Ca2+ release. (A) Mitochondria, by buffering the Ca2+ rise at IP3R, prevents a Ca2+-dependent persistent deactivation of IP3R to maintain the overall release of Ca2+ from the SR. (B) When mitochondria are prevented from taking up Ca2+ (using uncouplers, complex I inhibitors or uniporter inhibitors) there is a local increase in [Ca2+] at IP3R promoting Ca2+-dependent negative feedback inhibition of IP3R activity and preventing Ca2+ release.
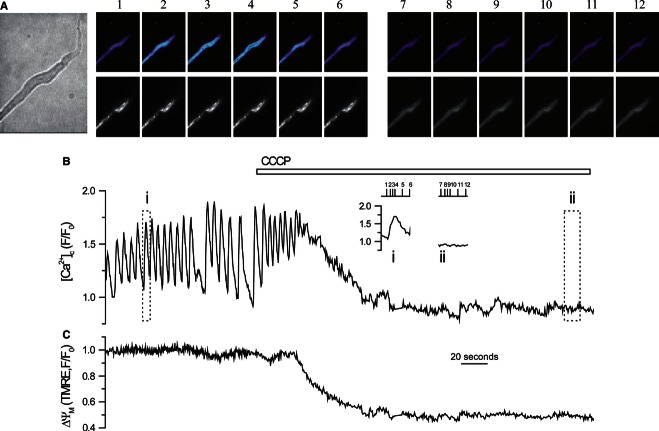
The control that mitochondria exert on Ca2+ puffs enables the organelle to exert particularly dramatic effects on Ca2+ waves and repetitive Ca2+ rises (oscillations). When mitochondria are prevented from taking up Ca2+, waves and oscillations halt (Figure 5), that is mitochondrial control extends beyond modulation of the time course of a Ca2+ rise and the organelle determines whether some signals (waves and oscillations) occur at all.
Figure 5.
Depolarization of the mitochondrial membrane potential with CCCP blocks Ca2+ oscillations. Ca2+ oscillations measured with fluo-4 (A) upper panel and (B) in a single portal vein smooth muscle cell (A left panel) were inhibited by CCCP (applied with oligomycin; 1 and 6 μM, respectively; B open bar). CCCP depolarized ΔΨM (A lower panel, C) as measured by TMRE fluorescence changes and shown as the decrease in fluorescence ratio (C). This suggests that mitochondrial Ca2+ uptake is required for Ca2+ oscillations to occur (see text). The [Ca2+]c images (A) are derived from the time points indicated by the corresponding numbers in (B). The insets (B i,ii) are Ca2+ responses in boxes i,ii shown on an expanded time base. [Ca2+]c changes in (A, upper panels) are expressed by color; dark blue low and light blue high [Ca2+]c. ΔΨM (A, lower panels) is shown as punctate white staining, which decreases with ΔΨM depolarization. Frames 1–6 (A) are controls (before CCCP/oligomycin) and frame 7–12 after CCCP/oligomycin.
In some cell types, mitochondria act as a “firewall” and prevent the Ca2+ signal from reaching certain regions of the cell. In pancreatic acinar cells, mitochondria are arranged as a “belt” and prevent Ca2+ signals from entering the basal part of the cell 62. However, in smooth muscle, mitochondria do not normally appear to prevent the Ca2+ wave from progressing through the cell, but rather the organelle performs a decision-making role to determine whether or not the signal is permitted to progress. If the ΔΨM is normal and polarized, the Ca2+ signal progresses. On the other hand, if ΔΨM is depolarized (i.e., has a reduced ATP producing capacity) the mitochondria exerts control by inhibiting the local IP3-mediated Ca2+ signal so that the wave does not progress. Thus, as the Ca2+ signal regeneratively propagates from site to site through the cell (like a “fire-beacon” network), mitochondria act as “beacon-wardens” and determine whether or not the Ca2+ signal is transmitted to the next point of the cell 3,59. Presumably mitochondria modulate IP3R, but not voltage-dependent Ca2+ channel activity because the organelle is held close to the former, but not to the latter.
Mechanisms and Driving Force for Mitochondrial Ca2+ Uptake
The large [Ca2+] range over which mitochondria are effective in regulating Ca2+ signaling prompted widespread interest into precisely how this is achieved. One mechanism is the MCU, a ruthenium red-sensitive, inwardly rectifying, and highly Ca2+ selective voltage-dependent channel of acknowledged importance in Ca2+ uptake by the organelle. In voltage-clamp experiments Ca2+ flux via MCU did not saturate until [Ca2+] exceeded 100 mM and the K1/2 was 19 mM 39. MCU may fulfill the low affinity role required of the organelle to modulate Ca2+ increases near Ca2+ channels (e.g., IP3R). Another proposed Ca2+ transporter present on the inner mitochondrial membrane is LetM1 33. LetM1, identified in genome-wide siRNA screen studies, is proposed to be a high affinity Ca2+/H+ exchanger that transports Ca2+ into mitochondria during low (<1 μM) global [Ca2+]c increases when the free mitochondrial matrix [Ca2+] is also low (∼5 μM) 33. LetM1 may fulfill the role required for high affinity Ca2+ uptake by mitochondria. However, LetM1 was suggested to be a K+/H+ exchanger 56 several years before being proposed as a Ca2+ transporter. The Ca2+ transport facility attributed to LetM1 is not universally accepted 18,57.
Mitochondrial Ca2+ uptake mechanisms pass Ca2+ down an electrochemical gradient generated across the inner mitochondrial membrane by an outward movement of H+ via complexes I, III and IV of the electron transport chain. This proton movement establishes both the electrical potential difference (ΔΨM) and the [H+] gradient. The [H+] gradient is significant; cytoplasmic pH is ∼7.2 while the pH within the mitochondrial matrix is ∼7.8. Indeed the effectiveness of complexes I, III, and IV in exporting H+ is emphasized by considering the average number of free protons within the matrix (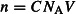 ; n, number of ions; C, concentration; NA, Avogadro's number; V, volume). A mitochondrial pH of 7.8 corresponds to a [H+] of ∼16 nM (1.58 E−8M). Taking the mitochondrion to be a prolate spheroid with dimensions of 2 μm (major axis) by 0.5 μm (minor axis) the volume (
; n, number of ions; C, concentration; NA, Avogadro's number; V, volume). A mitochondrial pH of 7.8 corresponds to a [H+] of ∼16 nM (1.58 E−8M). Taking the mitochondrion to be a prolate spheroid with dimensions of 2 μm (major axis) by 0.5 μm (minor axis) the volume (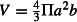 where a is the minor axis radius and b the major axis radius) is 0.26 fL. 1 g-H+/L = 6.023 E23 ions/L so that a [H+] concentration of 1.58 E−8 M = 9.5 E15 ions/L (1.58 E−8 × 6.023 E23) and the number of H+ per mitochondrion = 9.5 E15 × 0.26 E−15
= 2.5. Thus, on average there are only ∼2.5 H+ free within the mitochondrial matrix.
where a is the minor axis radius and b the major axis radius) is 0.26 fL. 1 g-H+/L = 6.023 E23 ions/L so that a [H+] concentration of 1.58 E−8 M = 9.5 E15 ions/L (1.58 E−8 × 6.023 E23) and the number of H+ per mitochondrion = 9.5 E15 × 0.26 E−15
= 2.5. Thus, on average there are only ∼2.5 H+ free within the mitochondrial matrix.
Altering Mitochondrial Function and Ca2+ Signaling
The low internal proton numbers and significant pH gradient are critical for the performance of mitochondria and mitochondrial control of cell function. Together the transmembrane [H+] gradient and ΔΨM provide the protomotive force (approximately −180 mV) to drive ADP phosphorylation (catalyzed by the ATP synthase). ATP production approximately doubles with each 10 mV increase in protomotive force 37. The uptake of Ca2+ ions is driven by ΔΨM. Unsurprisingly, a major method of determining the contribution of mitochondria to various cell activities (including Ca2+ signaling) is to collapse the proton gradient using drugs such as protonophores and electron transport chain inhibitors. Protonophores (e.g., CCCP and FCCP) are mildly acidic lipophilic compounds that are deprotonated in the mitochondrial matrix to form lipophilic anions. The deprotonated form crosses the inner mitochondrial membrane from the matrix, picks up a proton on the cytoplasmic side, and returns. In this way protonophores collapse the proton gradient and ΔΨM and, as a result, inhibit ATP synthesis and mitochondrial Ca2+ uptake. For example, protonophores slow the rate of [Ca2+]c decline in smooth muscle (Figure 2) following depolarization-evoked Ca2+ entry. This experiment (Figure 2) reveals the ability of mitochondria to accumulate Ca2+, highlights the significance of the proton gradient in mitochondrial Ca2+ uptake and demonstrates the ease of use of protonophores to study mitochondrial activity.
However, protonophores may have significant off target effects and care is required in interpreting data from these experiments. Protonophores incorporate into the plasma membrane as well as the inner mitochondrial membrane and by facilitating the flux of protons may substantially alter the cytoplasmic pH. The effect of protonophores may be substantial. Extracellular pH is ∼7.4 (i.e., a [H+] of ∼40 nM) while cytoplasmic pH is ∼7.2 (i.e., a [H+] of ∼63 nM). The [H+] is thus highest in cytoplasm and lower in the extracellular space. However, the resting plasma membrane potential (approximately −60 mV; established by K+ permeability) may remain unaltered in the presence of protonophores. Because of its magnitude, the plasma membrane potential will determine the net flux of H+ and the concentration of H+ in the cytoplasm will increase via protonophore activity (i.e., decrease in pH). A 60 mV (inside negative) membrane potential difference will result in ∼10-fold increase in cytoplasmic [H+] to 400 nM (i.e., 10 times the external [H+]). Therefore, cytoplasmic pH will decrease from 7.2 to 6.4 when a protonophore is applied. Such a substantial decrease in pH is likely to exert several physiological changes and could result in a false-positive misinterpretation of the effects of protonophores on mitochondrial activity. A way around the pH change is to control cytoplasmic pH (in patch clamp experiments) using high concentrations of H+ buffers for example, 30 mM HEPES 12,13,49 or to target the protonophore specifically to the mitochondria to ensure significant cytoplasmic pH changes do not occur 11.
Even when changes in pH are considered and controlled, drugs which alter mitochondrial function may also alter the extent of free radical generation or ATP levels in cells (Table 1). Collapse of the proton gradient does not just prevent the production of ATP but results in the ATP synthase running in reverse to become an ATPase and deplete the cell of ATP, an effect prevented by using oligomycin in combination with drugs that collapse the proton gradient.
Table 1.
Effect of drugs that alter mitochondrial function
| Drug | Primary target | Off target/unwanted effects | Other limitations |
|---|---|---|---|
| Uncouplers/protonophores: CCCP, FCCP, DNP, 1799 | ΔΨM | Cytosolic pH changes; ATP consumption due to ATPsynthase reversal | Increased H+ permeability of other cellular membranes |
| Antimycin A | Complex III blockade (at Qn/i site) | ROS production | |
| Rotenone | Complex I blockade | Can cause ROS production, depending on substrate utilized | |
| Myxothiazol | Complex III blockade (at Qp/0 site) | ||
| Ruthenium red | MCU | Ca2+ and K+ channels | Low cell permeability |
| Ru360 | MCU | Low cell permeability | |
| CGP37157 | mNCX | >10 μM also inhibits other Ca2+ channels | |
| Oligomycin | ATPsynthase | ||
| Cyclosporin A | Permeability Transition Pore (PTP) by binding cyclophilin D in matrix | Immunosuppresant that also inhibits calcineurin by binding to cytosolic cyclophilins | |
| Atractyloside/carboxyactractyloside | Adenine Nucleotide Translocator (ANT) competitive inhibitor, locks ANT into cytosolic-facing conformation & promotes PTP opening | ||
| Bongkrekic acid | Non-competitive inhibitor of ANT, locks ANT into matrix-facing conformation & inhibits PTP opening | ||
| Ionomycin | Ca2+>Mg2+/2H+ exchange | ΔΨM depolarization when Ca2+ is present | |
| A23187 | Ca2+/2H+ exchange | ΔΨM depolarization when Ca2+ is present | Fluorescence that interferes with fluorescent Ca2+ dyes (4-bromo-A23187 is non-fluorescent version) |
| Azide | ATP synthase inhibitor | ||
| Cyanide | Complex IV inhibitor |
When each of these issues is considered and controlled, drugs which alter mitochondrial function provide a powerful experimental tool to examine the role of mitochondria in Ca2+ signaling.
Measuring Mitochondrial Function
One of the most common methods of examining mitochondrial function is to optically measure the output of one of several fluorophores that are sensitive to ΔΨM. The most popular belong to various families of compounds, which include rhodamine fluorophores 54, carbocyanins such as JC-1 66 and DiOC6 69, merocyanines 34 or oxonols 17. The major indicators presently used are the rhodamine fluorophores and JC-1 which will be discussed further below.
Rhodamine fluorophores (e.g., TMRE; TMRM; rhod-123) are fluorescent lipophilic cations that pass readily through lipid bilayers because their charge is dispersed over a large surface area. The mitochondria's negative membrane potential drives the fluorescent lipophilic cations into the matrix 2,68 and fluorophore distribution is described approximately by an Nernstian relationship, that is, ∼10-fold accumulation for every 61.5 mV of membrane potential at 37°C. Because of the large mitochondrial surface area to volume ratio, the accumulation of the fluorophore in the organelle changes rapidly (<1 s) with variations in the mitochondrial membrane potential 12. The more hyperpolarized (increasingly negative) the mitochondria become, the more fluorophore accumulates; the more depolarized (increasingly positive), the less fluorophore accumulates. When these fluorophores are used at low concentrations, changes in fluorescence signal follow changes in dye accumulation in mitochondria. Depolarized mitochondria will have a lower fluorophore concentration and a smaller fluorescence signal. Hyperpolarized mitochondria will have a higher fluorophore concentration and greater fluorescence signal (Figure 6A).
Figure 6.
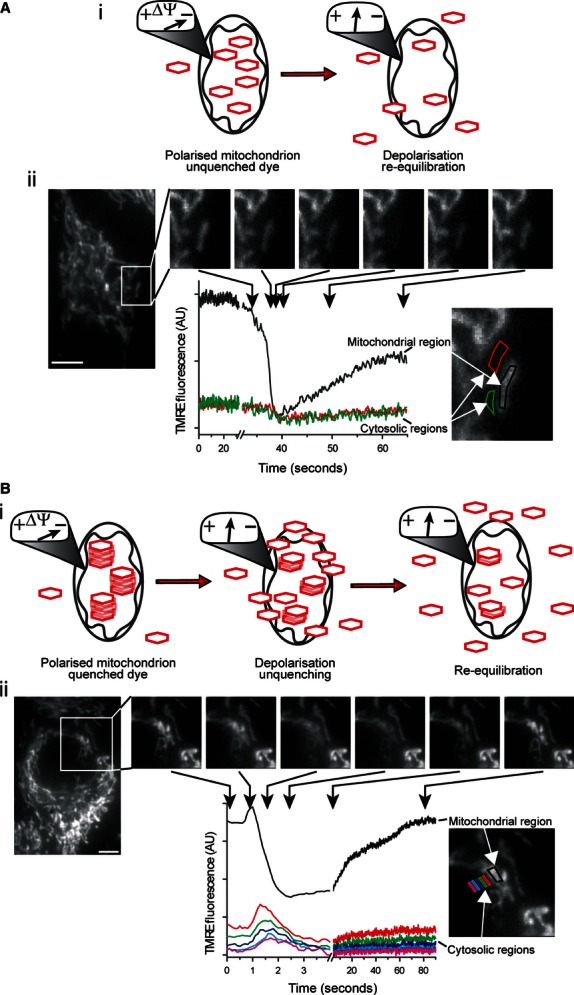
Membrane-permeant, lipophilic fluorescent cations can be used to monitor ΔΨM in “quenched” and “unquenched” modes. These fluorophores equilibrate across membranes in a Nernstian fashion (i.e., ∼10-fold per 60 mV). (A) at low fluorophore concentration the observed fluorescence is proportional to ΔΨM (i). A transient ΔΨM depolarization of an individual mitochondrion in a primary cerebral vascular smooth muscle cell loaded with TMRE (100 nM) resulted in a transient decrease in the fluorescence intensity of the mitochondrial region (ii, black line) with no measureable fluorescence change in the surrounding cytosolic regions (ii, red & green lines) (ii, scale bar = 5 μm), as visualized by epifluorescence microscopy; that is the change in [TMRE] outside the mitochondria, after ΔΨM depolarization, is presumably rapidly decreased in the larger volume of the cytoplasm so that a change in fluorescence is not measured. (B) At higher concentrations these fluorophores aggregate in mitochondria and self-quench, so that the observed fluorescence is no longer directly proportional to ΔΨM. ΔΨM depolarization relieves this quenching, resulting in a transient increase in fluorescence (Bi & Bii). In this case, a transient ΔΨM depolarization of an individual mitochondrion resulted in a transient increase in the fluorescence intensity of the mitochondrial region (Bii, black line) and an observable dissipation of the released fluorophore into the surrounding cytosolic regions (Bii, colored lines) (ii, scale bar = 5 μm) because of the larger amount of fluorophore being released from mitochondria to the cytoplasm. When the dye is fully unquenched, the fluorescent signal declines as the dye continues to leave the mitochondria.
However, these fluorophores are somewhat complicated in their action and at high concentration form non-fluorescent aggregates (i.e., “quench”) so that fluorescence emission is reduced. Under these conditions, when mitochondria are hyperpolarized more fluorophore will enter the organelle, but this will cause additional quenching to decrease the fluorescent signal. Conversely, when mitochondria are depolarized, fluorophore concentration will decrease and fluorescence emission will now increase because the lower fluorophore concentration results in unquenching (Figure 6B). Under continued depolarization, the fluorophore will continue to leave the mitochondria. Eventually, when the fluorophore is unquenched, the fluorescent signal will decline with depolarization (Figure 6B).
The response from the rhodamine fluorophores therefore depends on the concentration at which they are used, which are referred to as either “quenching” (Figure 6B) or “unquenching” (Figure 6A) modes. The precise concentrations for quenching or unquenching modes should be determined empirically for each cell type. Typically <10 nM will result produce an unquenched concentration within the mitochondrial while >100 nM will result in quenching. There are advantages to each mode of fluorophore behavior. In unquenching mode, there is considerable sensitivity; changes in ΔΨM of a few millivolts may be detected and imaging ΔΨM of single mitochondria is possible. The signal may also be quantified in unquench mode. The relationship between fluorescence and ΔΨM is described from the ratio of the free mitochondrial fluorophore concentration to free cytosolic fluorophore concentration and is an exponential function of ΔΨM 41,61. However, in practice several difficulties limit quantification. First, the fluorophores may exhibit significant binding to the inner mitochondrial membrane so that most fluorescence comes not from free fluorophore, but from bound fluorophore 61,71. Changes in binding of the bound fluorophore are non-Nernstian and there is a larger accumulation than is predicted by the Nernst equation alone. While some studies have carefully addressed these issues to quantify ΔΨM 24,61, the majority of studies measure relative changes in fluorescence as a measure of ΔΨM.
“Quench” mode has the advantage of producing larger signals (when compared with those in unquench mode) which are detected readily at a whole cell (rather than mitochondrial) level resulting in a more straightforward imaging procedure. The response of the fluorophore to membrane potential changes may be highly nonlinear in quench mode, however, and will show both increases and decreases in response to ΔΨM depolarization. Some consideration must also be given to the sampling frequency; transient changes in whole cell fluorescence in response to ΔΨM alterations may be missed with low frequency measurements.
As with all agents that are introduced into cells, fluorophores may have some toxicity to mitochondria and cells. Problems can arise from “phototoxicity” when fluorescent molecules react with molecular oxygen to produce free radicals that may damage subcellular components. The lowest possible excitation light intensity should be used. The fluorophores themselves at high concentrations may also inhibit the electron transport chain so the lowest possible fluorophore concentration should be also used.
Rhod-2 have also been used to measure mitochondrial [Ca2+] in native cells 22,23,49. However, rhod-2 is not straightforward to use and there is the potential for a significant cytosolic contribution to the signal. Rhod-2 AM ester is thought to be a suitable indicator for mitochondrial loading because it is the only cell-permeant Ca2+ indicator, which bears a net positive charge. This net positive charge is suggested to promote the uptake of rhod-2 AM into the mitochondrial matrix because of the strongly negative ΔΨM. Once inside the mitochondrial matrix, esterases hydrolyse the AM group leaving the Ca2+-sensitive membrane impermeable form of rhod-2 trapped. However, while rhod-2 bears one net positive charge in the AM form, the de-esterfied rhod-2 bears three net negative charges and (even a partially de-esterified indicator) is unlikely to accumulate in mitochondria. The balance of esterase activity in the cytoplasm and mitochondria will therefore determine the major source of the signal from rhod-2 and is likely to contribute to variations in results among studies.
The other major class of fluorophore used commonly to measure the mitochondrial membrane potential is represented by JC-1. JC-1 is fluorescent and, like the rhodamine type fluorophores, accumulates in the mitochondria in a membrane potential dependent way. JC-1 has the useful feature of being a ratiometric indicator. In the cytoplasm JC-1 is usually “non-aggregated” (monomeric) and shows green fluorescence emission. When the concentration increases (in mitochondria) JC-1 aggregates and the fluorescence emission shifts from green to red. It is this fluorescence shift that permits ratiometric imaging of ΔΨM. In some studies JC-1 has been used successfully to measure ΔΨM 43,70, but other investigations have been less successful and report that fluorescence from the aggregated (red) form of the fluorophore may change in a way that is independent of ΔΨM. Moreover, while the monomeric (green) form of JC-1 has been reported to equilibrate on a time scale similar to that of TMRM/TMRE, the aggregate (red) form of the dye takes six times longer to equilibrate 43. The aggregate form is required for JC-1 to act as a ratiometric probe and the differences in time required for equilibration of the two forms of the dye complicate interpretations of fluorescence changes. JC-1 may also report changes in ΔΨM when none exist, perhaps because of the dye's sensitivity to H2O2. Notwithstanding, JC-1 has been used successfully in some studies to image change in ΔΨM.
One additional issue that requires consideration, regardless of the ΔΨM indicator, is the contribution that the plasma membrane potential makes to the signal measured from mitochondria, that is the fluorescence signal in the mitochondria is not independent of changes in plasma membrane potential. This situation arises because the concentration of fluorophore in mitochondria results from an equilibrium established by the concentration in the cytoplasm and the driving force for dye entry to mitochondria provided by ΔΨM. Significantly, the concentration of fluorophore in the cytoplasm is itself an equilibrium involving the extracellular dye concentration and the plasma membrane potential. The contribution of the plasma membrane potential is significant and there is a 10-fold increase in cytoplasmic dye concentration for a 60 mV inside negative membrane potential. Drugs or physiological activators that change the plasma membrane potential will alter the concentration of fluorophore in the cytoplasm. As a result, there will be a re-equilibration of fluorophore concentration in mitochondria with the new cytoplasmic concentration and a change in mitochondrial fluorescence even in the absence of a ΔΨM change. This change in fluorescence could result in a misinterpretation of an effect of a drug or activator as altering ΔΨM when no such change has occurred. Solutions are either to clamp the plasma membrane potential or to correct the mitochondrial signals after measuring alterations in the plasma membrane potential 12,55,61.
Another significant consideration in the use of these drugs is an absence of spatial control of the organelles affected. One approach to overcome the lack of spatial control has been the development of photoactivatable forms of uncouplers 11. These photoactivatable uncouplers are membrane permeant, targeted to mitochondria, but are inactive until instructed otherwise by a locally directed pulse of light. Only those mitochondria exposed to the light will be affected by the drug. Individual cells or even single mitochondria can be targeted to generate precise spatial and temporal control. Photoactivateable drugs provide a significant advance in determining mitochondria's subcellular control of Ca2+ signals.
Perspective
The mitochondrial proton gradient and ΔΨM drive Ca2+ uptake into the organelle. In studying the role of mitochondria in Ca2+ signaling, the proton gradient and ΔΨM are both measured and manipulated experimentally. Measurement of the mitochondrial electrochemical gradient is most often performed using membrane potential sensitive fluorophores. However, the signals arising from these fluorophores have a complex relationship with the mitochondrial electrochemical gradient and are altered by changes in plasma membrane potential. The solution to the latter problem is either to clamp the plasma membrane voltage or to determine the contribution of plasma membrane voltage changes to the mitochondrial signal. In determining the contribution of mitochondria to the cytosolic Ca2+ signal a large number of drugs offer a potent mechanism to alter the organelle's ability to take up Ca2+. These drugs alter mitochondrial Ca2+ uptake by changing the proton gradient across the inner mitochondrial membrane but may have significant off target effects. The off target effects include cytosolic pH changes, ATP depletion and alterations in free radical production. However, each off target effect may be controlled experimentally so the drugs offer a powerful method to study the role of mitochondria in Ca2+ signaling.
Acknowledgments
This work was funded by the Wellcome Trust (092292/Z/10/Z) and British Heart Foundation (PG/11/70/29086) and EPSRC; their support is gratefully acknowledged.
Glossary
- [Ca2+]
cytoplasmic Ca2+ concentration
- AM
acetoxymethyl
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- CCh
carbachol
- DiOC6
3,3-dihexyloxacarbocyanine iodide
- FCCP
carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone
- IP3
inositol 1,4,5-trisphosphate
- IP3R
inositol 1,4,5-trisphosphate receptor
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide
- LetM1
leucine zippered-EF-hand containing transmembrane protein 1
- mAChR3
muscarinic type 3 receptor
- MCU
mitochondrial uniporter
- mNCX
mitochondrial Na+/Ca2+ exchanger
- PLC
phospholipase C
- PTP
permeability transition pore
- rhod-2
rhodamine-like fluorophores
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
- TMRE
tetramethylrhodamine ethyl ester
- TMRM
tetramethylrhodamine methyl ester
- ΔΨM
mitochondrial membrane potential
References
- 1.Arnaudeau S, Kelley WL, Walsh JV, Jr, Demaurex N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J Biol Chem. 2001;276:29430–29439. doi: 10.1074/jbc.M103274200. [DOI] [PubMed] [Google Scholar]
- 2.Azzone GF, Pietrobon D, Zoratti M. Determination of the proton electrochemical gradient across biological-membranes. Curr. Top. Bioenerg. 1984;13:1–77. [Google Scholar]
- 3.Balemba OB, Bartoo AC, Nelson MT, Mawe GM. Role of mitochondria in spontaneous rhythmic activity and intracellular calcium waves in the guinea pig gallbladder smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2008;294:G467–G476. doi: 10.1152/ajpgi.00415.2007. [DOI] [PubMed] [Google Scholar]
- 4.Bao R, Lifshitz LM, Tuft RA, Bellve K, Fogarty KE, ZhuGe R. A close association of RyRs with highly dense clusters of Ca2+-activated Cl− channels underlies the activation of STICs by Ca2+ sparks in mouse airway smooth muscle. J Gen Physiol. 2008;132:145–160. doi: 10.1085/jgp.200709933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benham CD, Bolton TB. Spontaneous transient outward currents in single visceral and vascular smooth muscle cells of the rabbit. J Physiol. 1986;381:385–406. doi: 10.1113/jphysiol.1986.sp016333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 7.Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol. 1999;145:795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bootman MD, Collins TJ, Peppiatt CM, Prothero LS, MacKenzie L, De Smet P, Travers M, Tovey SC, Seo JT, Berridge MJ, Ciccolini F, Lipp P. Calcium signalling–an overview. Semin Cell Dev Biol. 2001;12:3–10. doi: 10.1006/scdb.2000.0211. [DOI] [PubMed] [Google Scholar]
- 9.Bootman M, Niggli E, Berridge M, Lipp P. Imaging the hierarchical Ca2+ signalling system in HeLa cells. J Physiol. 1997;499:307–314. doi: 10.1113/jphysiol.1997.sp021928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caulfield MP. Muscarinic receptors–characterization, coupling and function. Pharmacol Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 11.Chalmers S, Caldwell ST, Quin C, Prime TA, James AM, Cairns AG, Murphy MP, McCarron JG, Hartley RC. Selective uncoupling of individual mitochondria within a cell using a mitochondria-targeted photoactivated protonophore. J Am Chem Soc. 2012;134:758–761. doi: 10.1021/ja2077922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chalmers S, McCarron JG. The mitochondrial membrane potential and Ca2+ oscillations in smooth muscle. J Cell Sci. 2008;121:75–85. doi: 10.1242/jcs.014522. [DOI] [PubMed] [Google Scholar]
- 13.Chalmers S, McCarron JG. Inhibition of mitochondrial calcium uptake rather than efflux impedes calcium release by inositol-1,4,5-trisphosphate-sensitive receptors. Cell Calcium. 2009;46:107–113. doi: 10.1016/j.ceca.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. J Biol Chem. 2003;278:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 15.Chalmers S, Olson ML, MacMillan D, Rainbow RD, McCarron JG. Ion channels in smooth muscle: regulation by the sarcoplasmic reticulum and mitochondria. Cell Calcium. 2007;42:447–466. doi: 10.1016/j.ceca.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Collins TJ, Lipp P, Berridge MJ, Li W, Bootman MD. Inositol 1,4,5-trisphosphate-induced Ca2+ release is inhibited by mitochondrial depolarization. Biochem J. 2000;347:593–600. doi: 10.1042/0264-6021:3470593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper CE, Bruce D, Nicholls P. Use of oxonol V as a probe of membrane potential in proteoliposomes containing cytochrome oxidase in the submitochondrial orientation. Biochemistry. 1990;29:3859–3865. doi: 10.1021/bi00468a009. [DOI] [PubMed] [Google Scholar]
- 18.Csordas G, Varnai P, Golenar T, Sheu SS, Hajnoczky G. Calcium transport across the inner mitochondrial membrane: molecular mechanisms and pharmacology. Mol Cell Endocrinol. 2012;353:109–113. doi: 10.1016/j.mce.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delmas P, Wanaverbecq N, Abogadie FC, Mistry M, Brown DA. Signaling microdomains define the specificity of receptor-mediated InsP3 pathways in neurons. Neuron. 2002;34:209–220. doi: 10.1016/s0896-6273(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 20.Dray A. Kinins and their receptors in hyperalgesia. Can J Physiol Pharmacol. 1997;75:704–712. [PubMed] [Google Scholar]
- 21.Drummond RM, Fay FS. Mitochondria contribute to Ca2+ removal in smooth muscle cells. Pflugers Arch. 1996;431:473–482. doi: 10.1007/BF02191893. [DOI] [PubMed] [Google Scholar]
- 22.Drummond RM, Mix TC, Tuft RA, Walsh JV, Jr, Fay FS. Mitochondrial Ca2+ homeostasis during Ca2+ influx and Ca2+ release in gastric myocytes from Bufo marinus. J Physiol. 2000;522:375–390. doi: 10.1111/j.1469-7793.2000.t01-2-00375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drummond RM, Tuft RA. Release of Ca2+ from the sarcoplasmic reticulum increases mitochondrial [Ca2+] in rat pulmonary artery smooth muscle cells. J Physiol. 1999;516((Pt 1)):139–147. doi: 10.1111/j.1469-7793.1999.139aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerencser AA, Chinopoulos C, Birket MJ, Jastroch M, Vitelli C, Nicholls DG, Brand MD. Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium-induced de- and hyperpolarization of neuronal mitochondria. J Physiol. 2012;590:2845–2871. doi: 10.1113/jphysiol.2012.228387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gurney AM, Drummond RM, Fay FS. Calcium signalling in sarcoplasmic reticulum, cytoplasm and mitochondria during activation of rabbit aorta myocytes. Cell Calcium. 2000;27:339–351. doi: 10.1054/ceca.2000.0124. [DOI] [PubMed] [Google Scholar]
- 26.Hajnoczky G, Hager R, Thomas AP. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+ J Biol Chem. 1999;274:14157–14162. doi: 10.1074/jbc.274.20.14157. [DOI] [PubMed] [Google Scholar]
- 27.Herrington J, Park YB, Babcock DF, Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996;16:219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- 28.Hur EM, Park YS, Huh YH, Yoo SH, Woo KC, Choi BH, Kim KT. Junctional membrane inositol 1,4,5-trisphosphate receptor complex coordinates sensitization of the silent EGF-induced Ca2+ signaling. J Cell Biol. 2005;169:657–667. doi: 10.1083/jcb.200411034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca2+ release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990;95:1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iino M, Tsukioka M. Feedback control of inositol trisphosphate signalling by calcium. Mol Cell Endocrinol. 1994;98:141–146. doi: 10.1016/0303-7207(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 31.Ishii K, Hirose K, Iino M. Ca2+ shuttling between endoplasmic reticulum and mitochondria underlying Ca2+ oscillations. EMBO Rep. 2006;7:390–396. doi: 10.1038/sj.embor.7400620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaggar JH, Nelson MT. Differential regulation of Ca2+ sparks and Ca2+ waves by UTP in rat cerebral artery smooth muscle cells. Am J Physiol Cell Physiol. 2000;279:C1528–C1539. doi: 10.1152/ajpcell.2000.279.5.C1528. [DOI] [PubMed] [Google Scholar]
- 33.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalenak A, McKenzie RJ, Conover TE. Response of the electrochromic dye, merocyanine 540, to membrane potential in rat liver mitochondria. J Membr Biol. 1991;123:23–31. doi: 10.1007/BF01993959. [DOI] [PubMed] [Google Scholar]
- 35.Kamishima T, McCarron JG. Depolarization-evoked increases in cytosolic calcium concentration in isolated smooth muscle cells of rat portal vein. J Physiol. 1996;492:1–74. doi: 10.1113/jphysiol.1996.sp021289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamishima T, Quayle JM. Mitochondrial Ca2+ uptake is important over low [Ca2+]i range in arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2002;283:H2431–H2439. doi: 10.1152/ajpheart.00865.2001. [DOI] [PubMed] [Google Scholar]
- 37.Kawamata H, Starkov AA, Manfredi G, Chinopoulos C. A kinetic assay of mitochondrial ADP-ATP exchange rate in permeabilized cells. Anal Biochem. 2010;407:52–57. doi: 10.1016/j.ab.2010.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kettlewell S, Cabrero P, Nicklin SA, Dow JA, Davies S, Smith GL. Changes of intra-mitochondrial Ca2+ in adult ventricular cardiomyocytes examined using a novel fluorescent Ca2+ indicator targeted to mitochondria. J Mol Cell Cardiol. 2009;46:891–901. doi: 10.1016/j.yjmcc.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 39.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 40.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci USA. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loew LM, Tuft RA, Carrington W, Fay FS. Imaging in five dimensions: time-dependent membrane potentials in individual mitochondria. Biophys J. 1993;65:2396–2407. doi: 10.1016/S0006-3495(93)81318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchant J, Callamaras N, Parker I. Initiation of IP3-mediated Ca2+ waves in Xenopus oocytes. EMBO J. 1999;18:5285–5299. doi: 10.1093/emboj/18.19.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mathur A, Hong Y, Kemp BK, Barrientos AA, Erusalimsky JD. Evaluation of fluorescent dyes for the detection of mitochondrial membrane potential changes in cultured cardiomyocytes. Cardiovasc Res. 2000;46:126–138. doi: 10.1016/s0008-6363(00)00002-x. [DOI] [PubMed] [Google Scholar]
- 44.McCarron JG, Bradley KN, MacMillan D, Chalmers S, Muir TC. The sarcoplasmic reticulum, Ca2+ trapping, and wave mechanisms in smooth muscle. News Physiol Sci. 2004;19:138–147. doi: 10.1152/nips.01518.2004. [DOI] [PubMed] [Google Scholar]
- 45.McCarron JG, Chalmers S, Bradley KN, Macmillan D, Muir TC. Ca2+ microdomains in smooth muscle cell. Calcium. 2006;40:461–493. doi: 10.1016/j.ceca.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 46.McCarron JG, Chalmers S, MacMillan D, Olson ML. Agonist-evoked Ca2+ wave progression requires Ca2+ and IP3. J Cell Physiol. 2010;224:334–344. doi: 10.1002/jcp.22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarron JG, Flynn ER, Bradley KN, Muir TC. Two Ca2+ entry pathways mediate InsP3-sensitive store refilling in guinea-pig colonic smooth muscle. J Physiol. 2000;525(11):3–24. doi: 10.1111/j.1469-7793.2000.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarron JG, MacMillan D, Bradley KN, Chalmers S, Muir TC. Origin and mechanisms of Ca2+ waves in smooth muscle as revealed by localized photolysis of caged inositol 1,4,5-trisphosphate. J Biol Chem. 2004;279:8417–8427. doi: 10.1074/jbc.M311797200. [DOI] [PubMed] [Google Scholar]
- 49.McCarron JG, Muir TC. Mitochondrial regulation of the cytosolic Ca2+ concentration and the InsP3-sensitive Ca2+ store in guinea-pig colonic smooth muscle. J Physiol. 1999;516:149–161. doi: 10.1111/j.1469-7793.1999.149aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McCarron JG, Olson ML. A single luminally continuous sarcoplasmic reticulum with apparently separate Ca2+ stores in smooth muscle. J Biol Chem. 2008;283:7206–7218. doi: 10.1074/jbc.M708923200. [DOI] [PubMed] [Google Scholar]
- 51.McCarron JG, Olson ML, Chalmers S. Mitochondrial regulation of cytosolic Ca2+ signals in smooth muscle. Pflugers Arch. 2012;464:51–62. doi: 10.1007/s00424-012-1108-9. [DOI] [PubMed] [Google Scholar]
- 52.McGeown JG, Drummond RM, McCarron JG, Fay FS. The temporal profile of calcium transients in voltage clamped gastric myocytes from Bufo marinus. J Physiol. 1996;497(Pt 2):321–336. doi: 10.1113/jphysiol.1996.sp021771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng LC, Gurney AM. Store-operated channels mediate Ca2+ influx and contraction in rat pulmonary artery. Circ Res. 2001;89:923–929. doi: 10.1161/hh2201.100315. [DOI] [PubMed] [Google Scholar]
- 54.Nicholls DG. Simultaneous monitoring of ionophore- and inhibitor-mediated plasma and mitochondrial membrane potential changes in cultured neurons. J Biol Chem. 2006;281:14864–14874. doi: 10.1074/jbc.M510916200. [DOI] [PubMed] [Google Scholar]
- 55.Nicholls DG. Fluorescence measurement of mitochondrial membrane potential changes in cultured cells. Methods Mol Biol. 2012;810:119–133. doi: 10.1007/978-1-61779-382-0_8. [DOI] [PubMed] [Google Scholar]
- 56.Nowikovsky K, Froschauer EM, Zsurka G, Samaj J, Reipert S, Kolisek M, Wiesenberger G, Schweyen RJ. The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf-Hirschhorn syndrome. J Biol Chem. 2004;279:30307–30315. doi: 10.1074/jbc.M403607200. [DOI] [PubMed] [Google Scholar]
- 57.Nowikovsky K, Pozzan T, Rizzuto R, Scorrano L, Bernardi P. Perspectives on: SGP symposium on mitochondrial physiology and medicine: the pathophysiology of LETM1. J Gen Physiol. 2012;139:445–454. doi: 10.1085/jgp.201110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oancea E, Meyer T. Reversible desensitization of inositol trisphosphate-induced calcium release provides a mechanism for repetitive calcium spikes. J Biol Chem. 1996;271:17253–17260. doi: 10.1074/jbc.271.29.17253. [DOI] [PubMed] [Google Scholar]
- 59.Olson ML, Chalmers S, McCarron JG. Mitochondrial Ca2+ uptake increases Ca2+ release from inositol 1,4,5-trisphosphate receptor clusters in smooth muscle cells. J Biol Chem. 2010;285:2040–2050. doi: 10.1074/jbc.M109.027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Olson ML, Sandison ME, Chalmers S, McCarron JG. Microdomains of muscarinic acetylcholine and InsP3 receptors create InsP3 junctions and sites of Ca2+ wave initiation in smooth muscle. J Cell Sci. 2012;125((Pt 22)):5315–5328. doi: 10.1242/jcs.105163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Reilly CM, Fogarty KE, Drummond RM, Tuft RA, Walsh JV., Jr Quantitative analysis of spontaneous mitochondrial depolarizations. Biophys J. 2003;85:3350–3357. doi: 10.1016/S0006-3495(03)74754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park MK, Ashby MC, Erdemli G, Petersen OH, Tepikin AV. Perinuclear, perigranular and sub-plasmalemmal mitochondria have distinct functions in the regulation of cellular calcium transport. EMBO J. 2001;20:1863–1874. doi: 10.1093/emboj/20.8.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol. 1999;113:229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pitter JG, Maechler P, Wollheim CB, Spat A. Mitochondria respond to Ca2+ already in the submicromolar range: correlation with redox state. Cell Calcium. 2002;31:97–104. doi: 10.1054/ceca.2001.0264. [DOI] [PubMed] [Google Scholar]
- 65.Rasband WS. Image J. Bethesda, Maryland, USA: U.S. National Institutes of Health; 1997. –2005, http://rsb.info.nih.gov/ij/ [Google Scholar]
- 66.Reers M, Smith TW, Chen LB. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991;30:4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- 67.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 68.Ross MF, Da Ros T, Blaikie FH, Prime TA, Porteous CM, Severina II, Skulachev VP, Kjaergaard HG, Smith RA, Murphy MP. Accumulation of lipophilic dications by mitochondria and cells. Biochem J. 2006;400:199–208. doi: 10.1042/BJ20060919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rottenberg H. Membrane potential and surface potential in mitochondria: uptake and binding of lipophilic cations. J Membr Biol. 1984;81:127–138. doi: 10.1007/BF01868977. [DOI] [PubMed] [Google Scholar]
- 70.Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997;411:77–82. doi: 10.1016/s0014-5793(97)00669-8. [DOI] [PubMed] [Google Scholar]
- 71.Scaduto RC, Jr, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76:469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomembr. 2000;32:97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 73.Simpson PB, Russell JT. Mitochondria support inositol 1,4,5-trisphosphate-mediated Ca2+ waves in cultured oligodendrocytes. J Biol Chem. 1996;271:33493–33501. doi: 10.1074/jbc.271.52.33493. [DOI] [PubMed] [Google Scholar]
- 74.Smith IF, Wiltgen SM, Parker I. Localization of puff sites adjacent to the plasma membrane: functional and spatial characterization of Ca2+ signaling in SH-SY5Y cells utilizing membrane-permeant caged IP3. Cell Calcium. 2009;45:65–76. doi: 10.1016/j.ceca.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Szalai G, Csordas G, Hantash BM, Thomas AP, Hajnoczky G. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem. 2000;275:15305–15313. doi: 10.1074/jbc.275.20.15305. [DOI] [PubMed] [Google Scholar]
- 76.Szanda G, Koncz P, Varnai P, Spat A. Mitochondrial Ca2+ uptake with and without the formation of high-Ca2+ microdomains. Cell Calcium. 2006;40:527–537. doi: 10.1016/j.ceca.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 77.Thomason PA, Wolanin PM, Stock JB. Signal transduction: receptor clusters as information processing arrays. Curr Biol. 2002;12:R399–R401. doi: 10.1016/s0960-9822(02)00885-0. [DOI] [PubMed] [Google Scholar]
- 78.Vallot O, Combettes L, Lompre AM. Functional coupling between the caffeine/ryanodine-sensitive Ca2+ store and mitochondria in rat aortic smooth muscle cells. Biochem J. 2001;357:363–371. doi: 10.1042/0264-6021:3570363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z. Na+/K+-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell. 2005;16:4034–4045. doi: 10.1091/mbc.E05-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhuge R, Fogarty KE, Baker SP, McCarron JG, Tuft RA, Lifshitz LM, Walsh JV., Jr Ca2+ spark sites in smooth muscle cells are numerous and differ in number of ryanodine receptors, large-conductance K+ channels, and coupling ratio between them. Am J Physiol Cell Physiol. 2004;287:C1577–C1588. doi: 10.1152/ajpcell.00153.2004. [DOI] [PubMed] [Google Scholar]
- 81.Zhuge R, Fogarty KE, Tuft RA, Walsh JV., Jr Spontaneous transient outward currents arise from microdomains where BK channels are exposed to a mean Ca2+ concentration on the order of 10 microM during a Ca2+ spark. J Gen Physiol. 2002;120:15–27. doi: 10.1085/jgp.20028571. [DOI] [PMC free article] [PubMed] [Google Scholar]