Abstract
People with Down syndrome, a frequent genetic disorder in humans, have increased risk of health problems associated with this condition. One clinical feature of Down syndrome is the increased prevalence and severity of periodontal disease in comparison with the general population. Because saliva plays an important role in maintaining oral health, in the present study the salivary proteome of Down syndrome subjects was investigated to explore modifications with respect to healthy subjects. Whole saliva of 36 Down syndrome subjects, divided in the age groups 10–17 yr and 18–50 yr, was analyzed by a top-down proteomic approach, based on the high performance liquid chromatography-electrospray ionization–MS analysis of the intact proteins and peptides, and the qualitative and quantitative profiles were compared with sex- and age-matched control groups. The results showed the following interesting features: 1) as opposed to controls, in Down syndrome subjects the concentration of the major salivary proteins of gland origin did not increase with age; as a consequence concentration of acidic proline rich proteins and S cystatins were found significantly reduced in older Down syndrome subjects with respect to matched controls; 2) levels of the antimicrobial α-defensins 1 and 2 and histatins 3 and 5 were significantly increased in whole saliva of older Down syndrome subjects with respect to controls; 3) S100A7, S100A8, and S100A12 levels were significantly increased in whole saliva of Down syndrome subjects in comparison with controls. The increased level of S100A7 and S100A12 may be of particular interest as a biomarker of early onset Alzheimer's disease, which is frequently associated with Down syndrome.
Down syndrome (DS)1 is a frequent genetic disorder in humans characterized by premature aging (1). A clinical feature of people with DS is the increased prevalence and severity of periodontal disease compared with age-matched subjects of similar levels of intellectual impairment and compared with the general population (2). Common conditions observed in DS are marginal gingivitis, acute and subacute necrotizing gingivitis, advanced periodontitis, gingival recession, and pocket formation (3, 4). It is known that saliva plays an important role in maintaining oral and dental health, because of the presence of a variety of antimicrobial peptides mainly derived from gland secretion, oral epithelial cells, and neutrophils (5). Several papers reported that neutrophils and T-lymphocyte function is impaired in people with DS (6–9). However, the salivary secretion of the antimicrobial LL-37 in young individuals with DS was found normal (10). A review of the literature (11, 12) reveals only sporadic and contradictory reports that attempt to explain the role of saliva in the oral health of subjects with DS, and on the whole, information on the biochemical composition of their saliva is scarce. On the basis of the above information, in the present study, we proposed to investigate the salivary proteome of DS subjects by an intact protein-based “top-down” approach. The spectrum of salivary peptides of DS subjects was compared with that of sex and age-matched healthy control groups to determine qualitative and quantitative differences. Interestingly, the results showed that several members of the S100A family, which possess different biological functions, and also described as potential markers of the Alzheimer Disease, were significantly increased in saliva of Down syndrome subjects with respect to controls.
EXPERIMENTAL PROCEDURES
All chemicals and reagents were of analytical grade and were purchased from Farmitalia-Carlo Erba, (Milan, Italy), Sigma Aldrich (St. Louis, MI) and Merck (Damstadt, Germany). Low-resolution high performance liquid chromatography-electrospray ionization-ion trap-MS (HPLC-ESI-ion trap-MS) measurements were carried out by a Surveyor HPLC system (ThermoFisher, San Jose, CA) connected by a T splitter to a diode-array detector and to an LCQ Advantage mass spectrometer (ThermoFisher). The mass spectrometer was equipped with an ESI source. The chromatographic column was a Vydac (Hesperia, CA) C8 column with 5 μm particle diameter (column dimensions 150 × 2.1 mm). High-resolution HPLC-ESI-MS/MS experiments were carried out by an Ultimate 3000 Micro HPLC apparatus (Dionex, Sunnyvale, CA) equipped with a FLM-3000-Flow manager module coupled to LTQ Orbitrap XL apparatus (ThermoFisher). A Zorbax 300SB-C8 column (3.5 μm particle diameter; column dimension 1.0 × 150 mm) was used for these experiments.
Ethics Statements and Subjects under Study
The study protocol and written consent forms were approved by the Medical Ethics Committee of the Faculty of Medicine of the Catholic University of Rome (according to the instructions of the Declaration of Helsinki). The study protocol was explained to both parents and children, and informed written consent to participate in the study was obtained from a parent. Down syndrome subjects were enrolled at the Department of Surgery, Medical, Molecular, and Critical Area Pathology, University of Pisa and at the Institute of Pediatric Clinic of the Faculty of Medicine of the Catholic University of Rome. The 36 patients enrolled for the study were divided in two age-groups: 10–17 years (mean age ± S.D.: 12.6 ± 2.3; 3 females (F), 15 males (M)) and 18–50 years (mean age ± S.D.: 27.5 ± 8.6; 5F, 13 M). The two control groups comprised the same number of subjects: 10–17 years (mean age ± S.D.: 12.7 ± 2.3; 5F, 13 M) and 18–50 years (mean age ± S.D.: 27.6 ± 8.0; 11F, 7 M).
Sample Collection
Unstimulated whole saliva was collected according to a standard protocol. Donors did not eat or drink at least 2 h before the collection, which was performed in the morning between 10:00 a.m. and 12:00 p.m. with a soft plastic aspirator. Saliva was transferred to a plastic tube in an ice bath and an acidic solution (0.2% 2,2,2-trifluoroacetic acid (TFA)) was immediately added in 1:1 v/v ratio. The solution was then centrifuged at 8000 × g for 10 min at 4 °C. The acidic supernatant was separated from the precipitate and either immediately analyzed by HPLC-ESI-MS (100 μl, corresponding to 50 μl of saliva) or stored at - 80 °C until the analysis.
HPLC-ESI-IT-MS Analysis
The following solutions were used for reversed phase (RP)-HPLC-ESI-MS analysis: (eluent A) 0.056% (v/v) aqueous TFA and (eluent B) 0.05% (v/v) TFA in acetonitrile-water 80/20. The gradient applied for the analysis of saliva was linear from 0 to 55% of B in 40 min, and from 55% to 100% of B in 10 min, at a flow rate of 0.30 ml/min. For tryptic digests the gradient was from 0 to 65% of B in 40 min, and from 65% to 100% of B in 5 min. The T splitter resulted in a flow-rate of 0.20 ml/min toward the diode array detector and 0.10 ml/min toward the ESI source. During the first 5 min of separation, the eluate was diverted to waste to avoid instrument damage because of the high salt concentration. The photodiode array detector was set at 214 and 276 nm. Mass spectra were collected every 3 ms in the positive ion mode. The MS spray voltage was 5.0 kV, and the capillary temperature was 260 °C.
High-resolution HPLC-ESI-MS/MS experiments were performed on a LTQ Orbitrap XL apparatus, using the same eluents as for the HPLC-ESI-MS analysis. The applied gradient was: 0–4 min 5% B, 4–38 min from 5% to 50% B (linear), 38–41 min from 50% to 90% B (linear), at a flow rate of 80 μl/min. MS/MS spectra were collected in data dependent scan mode with a capillary temperature of 250 °C, a source voltage of 3.6 kV and a capillary voltage of 40 V. Measurements were performed in the positive ion mode and mass accuracy (FT) was calibrated before measurements. Ions were isolated with a width of 6–10 m/z and activated for 30 ms using 35% normalized collision energy and an activation q of 0.25.
Characterization of Salivary Peptides and Proteins
Proteins (salivary acidic proline-rich proteins, histatins, salivary cystatins, statherin, proline-rich peptide P-B, α-defensins 1–4, cystatins A, B, C, beta-thymosins (4 and 10), S100A7 (D27), S100A8, S100A9, and S100A12 proteins) and several derivatives (acetylated, cysteinylated, glutathionylated, phosphorylated, and oxidized forms) have been already identified in our previous studies (13–21). Experimental mass values were obtained by deconvolution of averaged ESI-MS spectra automatically performed using MagTran 1.0 software (22). Experimental and theoretical average mass values, available at the Swiss-Prot Data Bank (http://us.expasy.org/tools), are reported in Table I.
Table I. Elution times, experimental and theoretical average mass values (Da) of the proteins and peptides detected and quantified in saliva of DS subjects and controls. The term PRP-1 type includes the three entire isoforms PRP-1, PRP-2, and Pif-s, with a mass difference of 1 Da. The term PRP-3 type includes the truncated isoforms PRP-3, PRP-4, and Pif-f.
| Protein (Swiss-Prot code) | El. time (min) | Exp. av mass (Th. av mass) |
|---|---|---|
| PRP-1 type 2P (P02810) | 22.9–23.6 | 15,515 ± 2 (15,514–15,515) |
| PRP-3 type 2P (P02810) | 23.3–24.2 | 11,161 ± 1 (11,161–11,162) |
| P-C peptide (P02810) | 13.6–14.5 | 4,370.9 ± 0.4 (4,370.8) |
| Histatin 1 (P15515) | 23.3–23.8 | 4,928.2 ± 0.5 (4,928.2) |
| Histatin 3 (P15516) | 17.6–17.9 | 4,062.2 ± 0.4 (4,062.4) |
| Histatin 5 (P15516) | 14.2–14.7 | 3,036.5 ± 0.3 (3,036.3) |
| Histatin 6 (P15516) | 14.0–14.4 | 3,192.4 ± 0.3 (3,192.5) |
| Cystatin S nonP (P01036) | 37.4–38.2 | 14,186 ± 2 (14,185.8) |
| Cystatin S 1P (S1) | 37.7–38.4 | 14266 ± 2 (14,265.8) |
| Cystatin S 2P (S2) | 37.8–38.4 | 14,346 ± 2 (14,345.8) |
| Cystatin SN (P01037) | 35.8–36.4 | 14,312 ± 2 (14,313.1) |
| Cystatin SA (P09228) | 38.7–39.4 | 14,347 ± 2 (14,346.1) |
| Cystatin B dimeric form (P04080) | 33.6–34.4 | 22,362 ± 3 (22,361.2) |
| Cystatin B glutathionylated | 32.5–33.1 | 11,487 ± 2 (11,486.7) |
| Cystatin C (P01034) | 38.2–38.9 | 13,343 ± 2 (13,343.1) |
| Cystatin C methionine sulfoxide | 38.1–38.7 | 13,360 ± 2 (13,359.1) |
| Statherin 2P (P02808) | 28.9–29.5 | 5,380.0 ± 0.5 (5,379.7) |
| P-B (P02814) | 29.7–30.6 | 5,792.9 ± 0.5 (5,792.7) |
| Thymosin β4 (P62328) | 20.7–21.0 | 4,963.7 ± 0.4 (4,963.5) |
| α-defensin 1 (P59665) | 24.9–25.4 | 3,442.1 ± 0.4 (3,442.1) |
| α-defensin 2 (P59665 and P59666) | 24.9–25.4 | 3,371.0 ± 0.4 (3,371.0) |
| α-defensin 3 (P59666) | 24.9–25.4 | 3,486.1 ± 0.4 (3,486.1) |
| S100A7 (D27) (P31151a) | 37.4–38.0 | 11,367 ± 2 (11,367.8) |
| S100A8 (P05109) | 39.1–39.7 | 10,833 ± 2 (10,834.5) |
| S100A9 short 1P (P06702) | 41.3–42.0 | 12,770 ± 2 (12,769.2) |
| S100A9 short 1P methionine sulfoxide | 41.3–42.0 | 12,786 ± 2 (12,785.2) |
| S100A9 short | 41.3–42.0 | 12,690 ± 2 (12,689.2) |
| S100A9 short methionine sulfoxide | 41.3–42.0 | 12,707 ± 2 (12,705.2) |
| S100A9 long glutathionylated | 41.1–41.8 | 13,458 ± 2 (13,456.8) |
| S100A12 (P80511) | 39.5–40.2 | 10,443 ± 2 (10,443.9) |
a The Swiss-Prot code refers to the variant E27.
Cystatin C and its oxidized derivative were confirmed in the present study by high resolution HPLC-ESI-MS/MS analysis of trypsin digestion products. Semi-purified fractions of cystatin C and its derivative were collected at the T-splitter during HPLC-ESI-MS experiments and submitted to digestion using the kit “Trypsin Singles Proteomic Grade” (Sigma-Aldrich) according to the manufacturer's instructions. The reaction was stopped after 12 h of incubation by acidification with 0.1% TFA (final concentration) and the solution stored at −80 °C until the analysis. HPLC-ESI-MS/MS data were analyzed by the Proteome Discoverer 1.2 program, based on SEQUEST cluster as a search engine (University of Washington, licensed to Thermo Electron Corp., San Jose, CA) against Swiss-Prot human proteome (March 3rd, 2011 released; Swiss Prot human complete.fasta; 34,765 nonredundant protein sequences). The decoy database included the reversed version of the human proteome, and the false discovery rate (FDR) was set to 0.01 for identifications. For peptide matching the limits were Xcorr scores greater than 1.5 for singly charged ions and 2.0 and 2.5 for doubly and triply charged ions, respectively, one missed cleavage site.
Precursor mass search tolerance was set to 10 ppm and fragment mass tolerance was set to 0.8 Da. Different searches were carried out to determine post-translational modifications (PTMs). Peptide sequences and sites of covalent modifications were also validated by manual spectra annotation. The results of HPLC-ESI-MS/MS experiments (supplemental Table S1) and annotated spectra are reported in the Supplemental Material. Our data were in agreement with the results of Ryan et al. (23) that demonstrated by a top-down approach that the mature form of cystatin C contains 120 amino acids and includes two disulfide bonds. In our samples the presence of a naturally occurring methionine sulfoxide derivative of cystatin C was also demonstrated for the first time.
Quantification
Quantification was based on the area of the low resolution RP-HPLC-ESI-MS eXtracted Ion Current (XIC) peaks, and considered when the S/N ratio was at least 5. The XIC analysis selectively reveals a protein in the chromatographic profile by extracting the ion current associated with the multiply charged ions characteristic of the protein. The area of the XIC peaks is proportional to the peptide/protein concentration under constant analytical conditions (24). The ions used to quantify the proteins and peptides were carefully selected to exclude values in common with other co-eluting proteins, and they are reported in the supplemental Table S2. A window of ± 0.5 Da was used to extract ion chromatograms.
Data Analysis
The software GraphPad Prism (version 4.0) was used for statistical analysis. Ranges, medians, means, standard deviations, and standard errors were calculated for all the peptide and protein XIC peak area. Means and standard deviations are reported in Table II. To compare the groups we used the opportune statistical test taking into account the distribution of the data (normal or skewed) and the variances (homogeneous or unequal). According to these characteristics the tests used were the following: parametric t test (variance homogeneous); t test with Welch correction (normal distribution, variance unequal), and the nonparametric Mann-Whitney test (skewed distribution, variance unequal). Statistical analysis was considered significant when the p value was less than 0.05 (two-tailed).
Table II. Extracted ion current peak areas of salivary proteins/peptides of glandular origin determined in DS subjects and healthy controls (mean values ± S.D. ×108).
| Protein | Ctrlsa (n = 18) | Patientsa (n = 18) | p valueb | Ctrlsc (n = 18) | Patientsc (n = 18) | p valueb | Ctrlsd p value | Patientsd p value |
|---|---|---|---|---|---|---|---|---|
| PRP-1 2P | 19.1 ± 13.7 | 15.2 ± 10.3 | ns | 40.7 ± 22.8 | 19.8 ± 8.1 | 0.001 | 0.002 | ns |
| PRP-3 2P | 5.5 ± 4.0 | 7.1 ± 6.1 | ns | 15.2 ± 9.5 | 8.4 ± 5.2 | 0.01 | 0.0002 | ns |
| P-C | 5.3 ± 4.2 | 8.4 ± 7.4 | ns | 13.3 ± 5.7 | 11.0 ± 10.0 | ns | 0.0001 | ns |
| Hst-1 | 2.9 ± 1.9 | 2.2 ± 1.8 | ns | 3.4 ± 2.4 | 4.8 ± 5.0 | ns | ns | 0.04 |
| Hst-3 | 0.7 ± 0.6 | 0.7 ± 0.8 | ns | 0.8 ± 1.5 | 1.6 ± 1.6 | 0.02 | ns | ns |
| Hst-5 | 1.7 ± 1.1 | 3.0 ± 3.3 | ns | 2.3 ± 2.1 | 5.3 ± 4.0 | 0.019 | ns | ns |
| Hst-6 | 0.8 ± 0.5 | 0.8 ± 0.9 | ns | 1.2 ± 2.0 | 2.2 ± 1.8 | ns | ns | 0.03 |
| Cyst S | 0.2 ± 0.3 | 0.1 ± 0.1 | ns | 0.5 ± 0.4 | 0.1 ± 0.2 | 0.0004 | 0.003 | ns |
| Cyst S1 | 2.7 ± 3.9 | 1.3 ± 1.4 | ns | 6.6 ± 5.1 | 1.3 ± 1.7 | 0.0001 | 0.002 | ns |
| Cyst S2 | 0.8 ± 1.3 | 0.3 ± 0.5 | ns | 1.6 ± 1.1 | 0.4 ± 0.8 | 0.0002 | 0.004 | ns |
| Cyst SN | 4.2 ± 6.2 | 3.5 ± 4.9 | ns | 12.0 ± 9.2 | 3.7 ± 6.2 | 0.001 | 0.001 | ns |
| Cyst SA | 1.0 ± 0.9 | 0.2 ± 0.3 | 0.004 | 1.4 ± 1.7 | 0.2 ± 0.3 | 0.002 | ns | ns |
| Statherin | 6.1 ± 4.1 | 7.8 ± 6.2 | ns | 9.5 ± 6.1 | 12.7 ± 12.0 | ns | ns | ns |
| P-B | 4.1 ± 2.7 | 9.3 ± 7.1 | 0.03 | 14.9 ± 8.3 | 15.4 ± 13.9 | ns | <0.0001 | ns |
a Age 10–17 yr group.
b Controls vs DS subjects.
c Age 18–50 yr group.
d Age 10–17 vs age 18–50 yr group; ns: not significant.
RESULTS
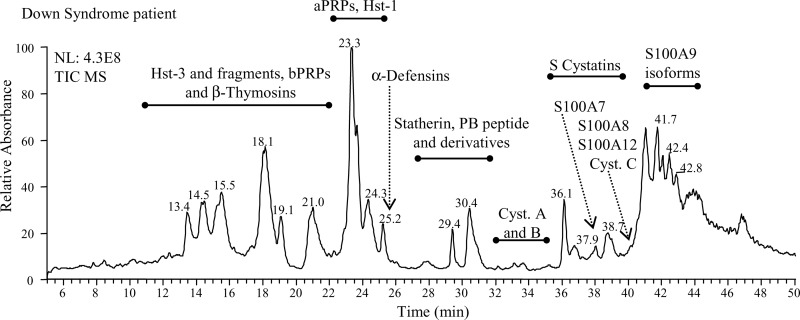
Unstimulated whole saliva was rapidly collected and treated with aqueous TFA to reduce protein degradation by oral proteinases after collection, and only the acidic soluble fraction was considered for the RP-HPLC-ESI-MS investigation. The salivary proteins and peptides detected and quantified are reported in Table I, and their chromatographic position is shown in Fig. 1. Characterization of these proteins, mainly based on the RP-HPLC-ESI-MS and Tandem/MS analysis, has been previously described by some of us (13–21). Cystatin C and its sulfoxide derivative on methionine 14 were confirmed in the present study by high resolution HPLC-ESI-MS/MS analysis of trypsin digestion products and data are reported in supplemental Table S1. The level of peptides and proteins under study, established on the basis of the extracted ion current peak area, is reported in Tables II and III. Because it is known that the level of some salivary proteins and peptides is age-dependent (25), and in particular that bPRPs are not fully expressed until after the pubertal age, we excluded bPRPs from our analyses and divided the subjects in the following two age groups: 10–17 years and 18–50 years.
Fig. 1.
Typical total ion current profile (TIC) of the acidic soluble fraction of whole saliva from Down syndrome subjects. The elution ranges of the salivary proteins investigated in the study are indicated.
Table III. Extracted ion current peak areas of other salivary proteins and peptides (mean values ± S.D. ×108) and results of the comparison between the groups.
| Protein | Ctrlsa (n = 18) | Patientsa (n = 18) | p valueb | Ctrlsc (n = 18) | Patientsc (n = 18) | p valueb | Ctrlsd p value | Patientsd p value |
|---|---|---|---|---|---|---|---|---|
| Cyst B dimer | 0.33 ± 0.43 | 0.23 ± 0.13 | ns | 0.33 ± 0.19 | 0.19 ± 0.12 | 0.01 | ns | ns |
| Cyst B glut | 0.24 ± 0.28 | 0.51 ± 0.45 | 0.04 | 0.55 ± 0.53 | 0.39 ± 0.21 | ns | 0.03 | ns |
| Cyst C | 0.15 ± 0.23 | 0.28 ± 0.20 | 0.02 | 0.49 ± 0.23 | 0.33 ± 0.41 | 0.02 | 0.0002 | ns |
| Cyst C sulfox | 0.03 ± 0.07 | 0.11 ± 0.15 | ns | 0.03 ± 0.09 | 0.17 ± 0.16 | 0.004 | ns | ns |
| Cyst C tot* | 0.18 ± 0.23 | 0.39 ± 0.30 | 0.02 | 0.52 ± 0.25 | 0.51 ± 0.45 | ns | 0.0002 | ns |
| Thymosin β4 | 0.15 ± 0.14 | 0.30 ± 0.31 | ns | 0.43 ± 0.25 | 0.61 ± 0.58 | ns | 0.0003 | 0.05 |
| α-defensin 1 | 0.5 ± 0.6 | 0.9 ± 1.0 | ns | 0.5 ± 0.4 | 2.4 ± 2.7 | 0.006 | ns | 0.03 |
| α-defensin 2 | 0.4 ± 0.4 | 0.7 ± 0.7 | ns | 0.4 ± 0.3 | 1.6 ± 1.5 | 0.003 | ns | 0.03 |
| α-defensin 3 | 0.3 ± 0.4 | 0.4 ± 0.5 | ns | 0.2 ± 0.2 | 0.8 ± 0.8 | 0.004 | ns | ns |
| S100A7 (D27) | 0.07 ± 0.12 (5/18) | 0.43 ± 0.55 (18/18) | 0.0004 | 0.03 ± 0.10 (2/18) | 0.28 ± 0.47 (14/18) | 0.001 | ns | ns |
| S100A8 | 0.04 ± 0.1 (4/18) | 0.14 ± 0.37 (3/18) | ns | 0.04 ± 0.09 (4/18) | 0.17 ± 0.17 (10/18) | 0.02 | ns | ns |
| S100A9 short | 0.41 ± 0.49 (12/18) | 0.27 ± 0.36 (11/18) | ns | 0.39 ± 0.44 (10/18) | 0.16 ± 0.18 (12/18) | 0.05 | ns | ns |
| S100A9 short sulfox | 0.22 ± 0.24 (11/18) | 0.17 ± 0.22 (11/18) | ns | 0.11 ± 0.18 (6/18) | 0.21 ± 0.16 (14/18) | 0.04 | ns | ns |
| S100A9 short tot* | 0.63 ± 0.69 (12/18) | 0.44 ± 0.50 (15/18) | ns | 0.50 ± 0.52 (11/18) | 0.37 ± 0.30 (14/18) | ns | ns | ns |
| S100A9 1P short | 0.26 ± 0.34 (11/18) | 0.14 ± 0.22 (10/18) | ns | 0.20 ± 0.27 (8/18) | 0.06 ± 0.07 (8/18) | 0.04 | ns | ns |
| S100A9 1P short sulfox | 0.11 ± 0.18 (8/18) | 0.07 ± 0.16 (6/18) | ns | 0.05 ± 0.11 (4/18) | 0.11 ± 0.19 (10/18) | ns | ns | ns |
| S100A9 1P short tot* | 0.38 ± 0.47 (11/18) | 0.22 ± 0.27 (12/18) | ns | 0.24 ± 0.34 (8/18) | 0.17 ± 0.22 (11/18) | ns | ns | ns |
| S100A9 long glut | 0.17 ± 0.35 (6/18) | 0.19 ± 0.32 (7/18) | ns | 0.27 ± 0.39 (8/18) | 0.14 ± 0.18 (9/18) | ns | ns | ns |
| S100A12 | 0.009 ± 0.03 (1/18) | 0.04 ± 0.13 (2/18) | - | 0.12 ± 0.34 (2/18) | 0.34 ± 0.32 (14/18) | 0.002 | - | 0.001 |
a Age 10–17 yr group.
b Controls vs DS subjects.
c Age 18–50 yr group.
d Age 10–17 vs 18–50 yr group.
* Oxidized plus not oxidized forms; ns: not significant.
Differences in the Salivary Levels of aPRPs, Cystatins, and P-B Peptide
In agreement with the already reported increase of salivary concentration of aPRPs with age (25) the comparison between the age 10–17 yr and the age 18–50 yr control groups (Table II) resulted in a statistically significant difference of the level of the major isoforms of aPRPs, namely diphosphorylated PRP-1 type (p value 0.002) and PRP-3 type aPRPs (p value 0.0002), as well as of the peptide PC which is generated from PRP-1 type proteins by proteolytic cleavage before secretion (p value 0.0001). Unlike controls, aPRPs concentration was only slightly affected by age in the DS subjects. This different behavior resulted in a statistically significant lower concentration of aPRPs in the age 18–50 yr patients with respect to control group (p value 0.001 and 0.01, respectively). Even more significant was the difference observed in the levels of salivary cystatins S, S1, S2, and SN, which were lower in saliva of the age 18–50 yr patients with respect to control group with the same age (p value <0.001, Table II). Also this result originated from the significant increase of cystatin concentration with age in the controls (p values <0.004). Interestingly, cystatin SA did not show a quantitative variation with age, and its level was significantly lower in patients than in controls of both age-groups (p value 0.004 and 0.002, respectively). Cystatin B was detected in two different isoforms: dimeric and glutathionylated. Only the salivary concentration of dimeric cystatin B was found significantly lower in the oldest DS subjects with respect to the corresponding control group (Table III). Cystatin C showed a statistically significant higher concentration in the age 18–50 yr group with respect to the age 10–17 yr control group, but not in DS subjects (Table III). The difference may be attributed to an increase with age of the oxidized form in the DS subjects; the same was also observed for S100A9 short isoform (see below).
As observed for the other salivary proteins with glandular secretion origin, P-B peptide concentration increased significantly with age in control subjects (p value <0.0001). Moreover, the peptide was found significantly more concentrated in saliva of the young patients with respect to controls. However, this result may reflect the high variability of peptide concentration in the control group.
Antimicrobial Peptides: Histatins, and α-Defensins
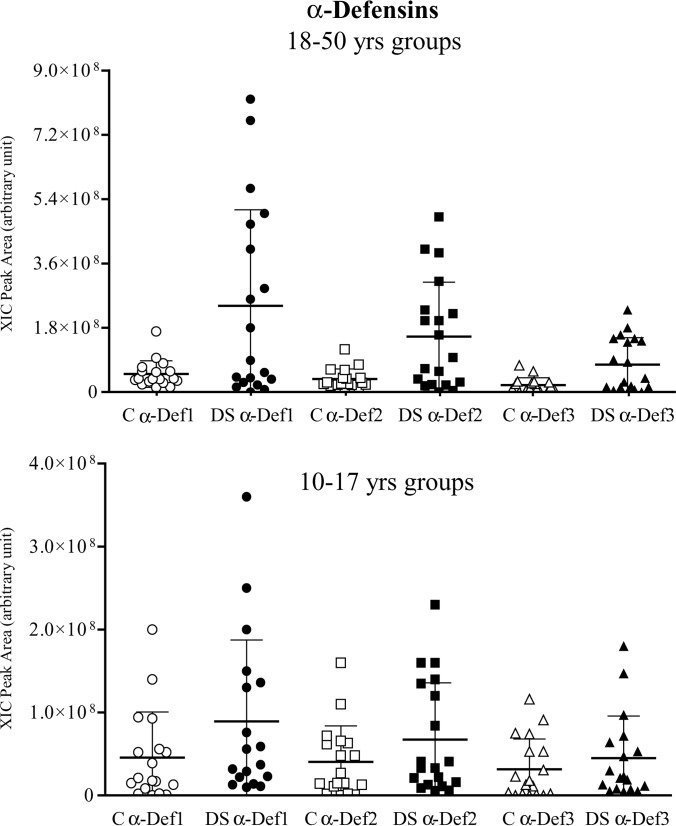
The antimicrobial peptides histatins 3 and 5 (the peptide of the histatin family with higher antimicrobial activity) showed similar salivary levels in the 10–17 year old patient and control groups (Table II), but the increase in concentration with age in the patients, even not statistically significant, resulted in a significant difference between DS subjects and controls (p value 0.02 for both histatins). A similar behavior was observed for α-defensins 1, 2, and 3, that showed a trend to increase with age in DS subjects. This was most significant for α-defensins 1 and 2 (p value 0.03 for both), but not in controls (Fig. 2, Table III). Thus, the levels of the α-defensins 1–3 increase in the 18–50 year old patient group with respect to controls (p value < 0.006).
Fig. 2.
Distribution of the XIC peak areas of α-defensins 1, 2, and 3 in the two age groups of controls and Down syndrome subjects.
S100A Family Proteins and Thymosin β4
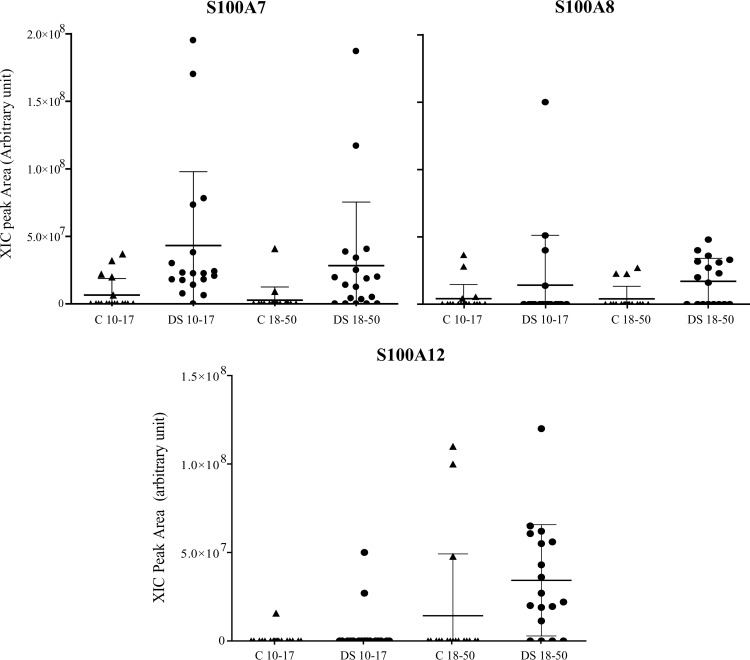
Several proteins belonging to the family of S100A were detected and quantified in saliva of the subjects affected by Down syndrome; they were S100A7 (D27), S100A8, S100A9, and S100A12. In the age 10–17 yr patient group, S100A7 (D27) was detected in all the samples (Fig. 3), whereas it was present only in 14 samples of the age 18–50 yr group. In the youngest patient group S100A7 (D27) was the most represented member of the S100A family proteins, together with S100A9 that was detected in the following different isoforms: short, short phosphorylated (at Thr-108 residue), and long S-glutathionylated at the Cys-2 residue (26). Part of the S100A9 short forms were oxidized at the Met-89 residue (19). A comparison between age 18–50 yr DS subjects and controls showed a statistically significant different level of both S100A9 short phosphorylated and nonphosphorylated isoforms (p values 0.04 and 0.05, respectively). The difference was mainly because of the increase with age of the corresponding oxidized derivatives in the DS subjects. Differently, the levels of S100A9 long glutathionylated were not affected by the age. DS subjects showed a lower level of phosphorylated S100A9 short isoforms with respect to controls, even if the difference has not been demonstrated to be statistically significant.
Fig. 3.
Distribution of the XIC peak areas of S100A7, S100A8, and S100A12 in the two age groups of controls and Down syndrome subjects.
S100A8 was rarely detected in the controls and in the young patients. The frequency was highest in the age 18–50 yr patient group (10 out of 18 samples, Fig. 3), that showed a significantly increased level of S100A8 with respect to matched controls (p value 0.02). A similar behavior was observed for S100A12 protein (Fig. 3); namely it was rarely detected in the controls, and found to increase in the age 18–50 yr DS subject group (p value 0.002). Because S100A12 was detected only in one sample of the age 10–17 yr control group it was not possible to perform a statistical analysis for comparison with the corresponding DS group (Fig. 3).
Thymosin β4 salivary concentration increased with age both in DS subjects and controls, but the increase was less significant in the former. Nonetheless, no significant differences were found between DS subjects and controls.
DISCUSSION
A proteomic investigation of saliva of DS subjects revealed several interesting differences with respect to control subjects. The increase in concentration with age of the major salivary proteins of glandular origin, seen in healthy subjects and confirmed by the present data, was not observed in the DS subjects. Interestingly, in a previous study Chaushu et al. (27) showed the statistically significant reduction of parotid gland salivary secretion in subjects with DS compared with a healthy control group. Moreover, the mean flow rate of the older patients was found to be decreased by 50% as compared with the younger group, demonstrating a decrease in stimulated parotid saliva flow rate across the adult age spectrum in these subjects, differently from control group. The authors suggested that a possible explanation for the decline of the salivary secretion in the older subjects with DS could be the occurrence of age-related neuropathological changes, consistent with Alzheimer's disease (AD). Indeed, a longitudinal study on oral health in subjects with AD demonstrated that salivary flow rates decreases in these subjects (28). It has been shown that DS is associated with early development of dementia (29), and it has also been suggested that oxidative stress could play a role in the pathogenesis of the clinical features of DS (30). As a result of over expression of SOD1 encoded by genes on chromosome 21 in DS, there is an imbalance of the ratio of anti-oxidant enzymes Cu-Zn superoxide dismutase, glutathione peroxidase, and catalase, which results in oxidative damage of different molecules and might be related to the degree of intellectual disability, premature aging, and dementia observed in DS (31–33). Interestingly, it has been observed recently that overexpression of amyloid β (Aβ) protein, whose gene is located on chromosome 21, may be at the basis of the susceptibility to oxidative stress observed in DS subjects (34). Indeed, it has been demonstrated that Aβ protein induces mitochondrial oxidative stress, suggesting a novel pro-oxidant role for Aβ protein, which may be relevant in AD and DS disease pathologies (34).
Most likely oxidative stress conditions are also responsible for the increase of concentration in the DS subjects of methionine sulfoxide derivatives of cystatin C and S100A9. Methionine oxidation of cystatin C has been already characterized in the recombinant protein expressed in E. coli by Berti et al. (35). The same authors demonstrated that oxidation does not affect the structure of the protease-binding region, and thus the interaction with substrates.
Oxidation of S100A9 has been already detected in saliva from children affected by type 1 diabetes (19). Sroussi et al. (36) reported that in the healthy mucosal tissue, expression of S100A9 by the epithelium may serve to inhibit leukocyte recruitment, and observed that oxidation of S100A9 abolished the chemo-repulsive effect on peripheral neutrophils, concluding that S100A9 serves as a molecular switch for oxidative control of inflammation regulated by its oxidative alteration. Oxidized Met63, Met81, and Met94 were also found to be variously present in S100A9 from asthmatic sputum by Gomes et al. (37). According to these authors, finding of oxidized S100A9 in a human inflammatory condition supports the notion of its oxidant scavenging capacity in vivo.
The most interesting differences observed between DS subjects and controls concerned the levels of S100A7, S100A8 and S100A12 proteins. Interestingly in a recent study, Qin et al. (38) identified and characterized by proteomic approaches the protein S100A7 in the CSF and brain of Alzheimer's disease. The authors demonstrated that S100A7 content was elevated in the cerebrospinal fluid (CSF) of AD dementia cases compared with neurological control cases, and these elevated S100A7 levels selectively identified AD clinical severity, concluding that S100A7 could be an early biomarker of AD. They also hypothesized that changes in S100A7 levels in the CSF of AD patients may reflect the oxidative stress changes, and thus they could be explored to monitor oxidative stress changes associated to altered Aβ metabolism in AD (39). In this study we found that salivary concentration of S100A7 is significantly higher in Down syndrome subjects with respect to controls, but we did not observe a significant increase of the protein level with the age.
Conversely, the salivary concentration of S100A12, and in particular the frequency of observation, significantly increased in DS subjects with age. S100A12 is a potent chemoattractant for monocytic cells (40), and it activates various cell types by binding the receptor for advanced glycosylation end products (RAGE), a multiligand member of the immunoglobulin superfamily of cell surface molecules, inducing expression of adhesion molecules and pro-inflammatory cytokines (41). It has been reported that the RAGE pathway is also involved in amyloidosis (42), and increased RAGE expression has been associated with the lesions of AD (43).
Similarly to S100A12, the frequency of S100A8 also increased with age. The level of all the forms of short S100A9 did not change, and age only affected the relative quantity of the oxidized and nonoxidized forms, resulting in the increased relative amount of the latter.
This study was not able to characterize S100B, which is related to neuropathology in brain regions, probably because of levels in the salivary fluid being below the sensitivity of the method applied (44).
Interestingly, in the present study we detected only the variant D27 of S100A7. Previously, on whole saliva of newborns the isoform E27 was also detected, but in minor quantities with respect to the D27 isoform (20). Protein sequence polymorphisms arising from single nucleotide polymorphisms has been also shown for other human salivary proteins, i.e. P-C peptide (45), cystatins (23), and several bPRPs (46–48). The top-down proteomic approach applied in this study offers the advantage to contemporaneously control a great number of peptide and proteins and characterize specific polymorphisms and unusual PTMs, which could be connected in different ways to pathological conditions.
Even though it has been reported an impaired function of neutrophils in DS subjects, our study showed the higher levels of the antimicrobial α-defensins 1–3 with respect to controls. A contribution to this result may derive from the reported relationship between S100A7 and α-defensins. In fact, Zheng et al. demonstrated that S100A7 enhances messenger RNA expression of α-defensins 1–3 and induces their extracellular release (49).
On the whole the results of the present investigation confirm that the use of saliva for diagnostic and prognostic purposes is promising. The different concentration of several salivary peptides recognized as important biomarkers of different pathologies and in particular of some complications of DS confirms that saliva, a simply and not invasively collectable biofluid, may reflect systemic disorders. As the lifespan for those with DS continues to increase, the ability to prevent or delay the progression of neurodegenerative diseases will promote healthy aging and improve quality of life of DS subjects.
Supplementary Material
Footnotes
* This work was supported by Cagliari University, Catholic University of Rome, MIUR, Italian National Research Council (CNR), Regione Sardegna, and Nando Peretti Foundation.
 This article contains supplemental Tables S1 and S2.
This article contains supplemental Tables S1 and S2.
1 The abbreviations used are:
- DS
- Down syndrome
- Aβ
- amyloid β
- AD
- Alzheimer's disease
- CSF
- cerebrospinal fluid
- PRP
- proline rich proteins
- RAGE
- receptor for advanced glycosylation end products
- TIC
- total ion current
- XIC
- eXtracted ion current.
REFERENCES
- 1. Hill D.A., Gridley G., Cnattingius S., Mellemkjaer L., Linet M., Adami H.O., Olsen J.H., Nyren O., Fraumeni J.F., Jr. (2003) Mortality and cancer incidence among individuals with Down syndrome. Arch. Intern. Med 163, 705–711 [DOI] [PubMed] [Google Scholar]
- 2. Barnett M.L., Press K.P., Friedman D., Sonnenberg E.M. (1986) The prevalence of periodontitis and dental caries in a Down's syndrome population. J. Periodontol 57, 288–293 [DOI] [PubMed] [Google Scholar]
- 3. Shapira J., Stabholz A., Schurr D., Sela M. N., Mann J. (1991) Caries levels, Streptococcus mutant counts, salivary pH, and periodontal treatment needs of adult Down syndrome patients. Spec. Care Dentist. 11, 248–251 [DOI] [PubMed] [Google Scholar]
- 4. Cichon P., Crawford L., Grimm W.D. (1998) Early-onset periodontitis associated with Down's syndrome - clinical interventional study. Ann. Periodontol 3, 370–380 [DOI] [PubMed] [Google Scholar]
- 5. Gorr S.U. (2012) Antimicrobial peptides in periodontal innate defense. Front. Oral Biol. 15, 84–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Deas D.E., Mackey S.A., McDonnell H.T. (2003) Systemic disease and periodontitis: manifestations of neutrophil dysfunction. Periodontol. 2000 32, 82–104 [DOI] [PubMed] [Google Scholar]
- 7. Izumi Y., Sugiyama S., Shinozuka O., Yamazaki T., Ohyama T., Ishikawa I. (1989) Defective neutrophil chemotaxis in Down's syndrome patients and its relationship to periodontal destruction. J. Periodontol. 60, 238–242 [DOI] [PubMed] [Google Scholar]
- 8. Yavuzyilmaz E., Ersoy F., Sanal O., Tezcan I., Erçal D. (1993) Neutrophil chemotaxis and periodontal status in Down's syndrome patients. J. Nihon Univ. Sch. Dent. 35, 91–95 [DOI] [PubMed] [Google Scholar]
- 9. Søhoel D.C., Jonsson R., Johannessen A.C., Nilsen R. (1995) Gamma/delta T lymphocytes in marginal periodontitis in patients with Down's syndrome. Adv. Exp. Med. Biol. 371B, 1135–1136 [PubMed] [Google Scholar]
- 10. Bachrach G., Chaushu G., Zigmond M., Yefenof E., Stabholz A., Shapira J., Merrick J., Chaushu S. (2006) Salivary LL-37 secretion in individuals with Down syndrome is normal. J. Dent. Res. 85, 933–936 [DOI] [PubMed] [Google Scholar]
- 11. Coburn S.P., Seidenberg M., Smith C.E., Mertz E.T. (1967) Nonprotein nitrogenous metabolites in saliva in Down's syndrome. J. Dent. Res. 46, 1476. [DOI] [PubMed] [Google Scholar]
- 12. Cutress T.W. (1972) Composition, flow rate and pH of mixed and parotid salivas from trisomic 21 and other mentally retarded subjects. Arch. Oral. Biol. 17, 1081–1094 [DOI] [PubMed] [Google Scholar]
- 13. Inzitari R., Cabras T., Onnis G., Olmi C., Mastinu A., Sanna M. T., Pellegrini M. G., Castagnola M., Messana I. (2005) Different isoforms and post-translational modifications of human salivary acidic proline-rich proteins. Proteomics 5, 805–815 [DOI] [PubMed] [Google Scholar]
- 14. Castagnola M., Inzitari R, Rossetti D. V., Olmi C., Cabras T., Piras V., Nicolussi P., Sanna M. T., Pellegrini M., Giardina B., Messana I. (2004) A cascade of 24 Histatins (Histatin 3 fragments) in human saliva: suggestions for a pre-secretory sequential cleavage pathway. J. Biol. Chem. 279, 41436–41443 [DOI] [PubMed] [Google Scholar]
- 15. Pisano E., Cabras T., Montaldo C., Piras V., Inzitari R., Olmi C., Castagnola M., Messana I. (2005) Peptides of human gingival crevicular fluid determined by HPLC-ESI-MS. Eur. J. Oral. Sci. 113, 462–468 [DOI] [PubMed] [Google Scholar]
- 16. Lupi A., Messana I., Denotti G., Schininà M. E., Gambarini G., Fadda M. B., Vitali A., Cabras T., Piras V., Patamia M., Cordaro M., Giardina B., Castagnola M. (2003) Identification of the human salivary Cystatin complex by the coupling of high-performance liquid chromatography and ion-trap mass spectrometry. Proteomics 3, 461–467 [DOI] [PubMed] [Google Scholar]
- 17. Inzitari R., Cabras T., Rossetti D. V., Fanali C., Vitali A., Pellegrini M., Paludetti G., Manni A., Giardina B., Messana I., Castagnola M. (2006) Detection in human saliva of different statherin and P-B fragments and derivatives. Proteomics 6, 6370–6379 [DOI] [PubMed] [Google Scholar]
- 18. Inzitari R., Cabras T., Pisano E., Fanali C., Manconi B., Scarano E., Fiorita A., Paludetti G., Manni A., Nemolato S., Faa G., Castagnola M., Messana I. (2009) HPLC-ESI-MS analysis of oral human fluids reveals that gingival crevicular fluid is the main source of oral thymosins beta(4) and beta(10). J. Sep. Sci. 32, 57–63 [DOI] [PubMed] [Google Scholar]
- 19. Cabras T., Pisano E., Mastinu A., Denotti G., Pusceddu P.P., Inzitari R., Fanali C., Nemolato S., Castagnola M., Messana I. (2010) Alterations of the salivary secretory peptidome profile in children affected by type 1 diabetes. Mol. Cell. Proteomics 9, 2099–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Castagnola M., Inzitari R., Fanali C., Iavarone F., Vitali A., Desiderio C., Vento G., Tirone C., Romagnoli C., Cabras T., Manconi B., Sanna M.T., Boi R., Pisano E., Olianas A., Pellegrini M., Nemolato S., Heizmann C.W., Faa G., Messana I. (2011) The surprising composition of the salivary proteome of preterm human newborn. Mol. Cell. Proteomics 10, M110.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cabras T., Manconi B., Iavarone F., Fanali C., Nemolato S., Fiorita A., Scarano E., Passali G.C., Manni A., Cordaro M., Paludetti G., Faa G., Messana I., Castagnola M. (2012) RP-HPLC-ESI-MS evidenced that salivary cystatin B is detectable in adult human whole saliva mostly as S-modified derivatives: S-glutathionyl, S-cysteinyl and S-S 2-mer. J. Proteomics 75, 908–913 [DOI] [PubMed] [Google Scholar]
- 22. Zhang Z., Marshall A.G. (1998) A universal algorithm for fast and automated charge state deconvolution of electrospray mass-to-charge ratio spectra. J. Am. Soc. Mass Spectrom. 9, 225–233 [DOI] [PubMed] [Google Scholar]
- 23. Ryan C.M., Souda P., Halgand F., Wong D.T., Loo J.A., Faull K.F., Whitelegge J.P. (2010) Confident assignment of intact mass tags to human salivary cystatins using top-down Fourier-transform ion cyclotron resonance mass spectrometry. J. Am. Soc. Mass Spectrom. 21, 908–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ong S. E., Mann M. (2005) Mass spectrometry-based proteomics turns quantitative. Nat. Chem. Biol. 1, 252–262 [DOI] [PubMed] [Google Scholar]
- 25. Cabras T., Pisano E., Boi R., Olianas A., Manconi B., Inzitari R., Fanali C., Giardina B., Castagnola M., Messana I. (2009) Age-dependent modifications of the human salivary secretory protein complex. J Proteome Res. 8, 4126–4134 [DOI] [PubMed] [Google Scholar]
- 26. Castagnola M., Cabras T., Iavarone F., Fanali C., Messana I. (2013) Calcium-Binding Proteins and RAGE: from Structural Basics to Clinical Applications. Detection of Ca2+-binding S100 Proteins in Human Saliva by HPLC-ESI-MS, Heizmann C.W. Editor, pp. 357–372, Humana Press [Google Scholar]
- 27. Chaushu S., Becker A., Chaushu G., Shapira J. (2002) Stimulated parotid salivary flow rate in patients with Down syndrome. Spec. Care Dentist 22, 41–44 [DOI] [PubMed] [Google Scholar]
- 28. Ship J.A., Puckett S.A. (1994) Longitudinal study on oral health in subjects with Alzheimer's disease. J. Am. Geriatr.Soc. 42, 57–63 [DOI] [PubMed] [Google Scholar]
- 29. Roizen N.J., Patterson D. (2003) Down's syndrome. Lancet 361, 1281–1289 [DOI] [PubMed] [Google Scholar]
- 30. Dickinson M.J., Singh I. (1993) Down's syndrome, dementia, and superoxide dismutase. Br. J. Psychiatry 162, 811–817 [DOI] [PubMed] [Google Scholar]
- 31. Jovanovic S.V., Clements D., MacLeod K. (1998) Biomarkers of oxidative stress are significantly elevated in Down syndrome. Free Radic. Biol. Med. 25, 1044–1048 [DOI] [PubMed] [Google Scholar]
- 32. de Haan J.B., Susil B., Pritchard M., Kola I. (2003) An altered antioxidant balance occurs in Down syndrome fetal organs: implications for the “gene dosage effect” hypothesis. J. Neural Transm. Suppl. (67), 67–83 [DOI] [PubMed] [Google Scholar]
- 33. Perluigi M., Butterfield D.A. (2012) Oxidative Stress and Down Syndrome: A Route toward Alzheimer-Like Dementia. Curr. Gerontol. Geriatr. Res. 2012:724904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bartley M.G., Marquardt K., Kirchhof D., Wilkins H.M., Patterson D., Linseman D.A. (2012) Overexpression of amyloid-β protein precursor induces mitochondrial oxidative stress and activates the intrinsic apoptotic cascade. J. Alzheimers Dis. 28, 855–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berti P.J., Ekiel I., Lindahl P., Abrahamson M., Storer AC. (1997) Affinity purification and elimination of methionine oxidation in recombinant human cystatin C. Protein Expr. Purif. 11, 111–118 [DOI] [PubMed] [Google Scholar]
- 36. Sroussi H.Y., Berline J., Palefsky J.M. (2007) Oxidation of methionine 63 and 83 regulates the effect of S100A9 on the migration of neutrophils in vitro. J. Leukoc. Biol. 81, 818–824 [DOI] [PubMed] [Google Scholar]
- 37. Gomes L.H., Raftery M.J., Xing Yan W., Goyette J.D., Thomas P.S., Geczy C.L. (2012) S100A8 and S100A9-oxidant scavengers in inflammation. Free Radic. Biol. Med. doi:pii: S0891–5849(12)01862-X [DOI] [PubMed] [Google Scholar]
- 38. Qin W., Ho L., Wang J., Peskind E., Pasinetti G.M. (2009) S100A7, a novel Alzheimer's disease biomarker with non-amyloidogenic alpha-secretase activity acts via selective promotion of ADAM-10. PLoS One 4, e4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gustaw K.A., Garrett M.R., Lee H.G., Castellani R.J., Zagorski M.G., Prakasam A., Siedlak S.L., Zhu X., Perry G., Petersen R.B., Friedland R.P., Smith M.A. (2008) Antigen-antibody dissociation in Alzheimer disease: a novel approach to diagnosis. J. Neurochem. 106, 1350–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Miranda L.P., Tao T., Jones A., Chernushevich I., Standing K.G., Geczy C.L., Alewood P.F. (2001) Total chemical synthesis and chemotactic activity of human S100A12 (EN-RAGE). FEBS Lett. 488, 85–90 [DOI] [PubMed] [Google Scholar]
- 41. Hofmann M.A., Drury S., Fu C., Qu W., Taguchi A., Lu Y., Avila C., Kambham N., Bierhaus A., Nawroth P., Neurath M.F., Slattery T., Beach D., McClary J., Nagashima M., Morser J., Stern D., Schmidt A.M. (1999) RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell 97, 889–901 [DOI] [PubMed] [Google Scholar]
- 42. Yan S.D., Zhu H., Zhu A., Golabek A., Du H., Roher A., Yu J., Soto C., Schmidt A.M., Stern D., Kindy M. (2000) Receptor dependent cell stress and amyloid accumulation in systemic amyloidosis. Nat. Med. 6, 643–651 [DOI] [PubMed] [Google Scholar]
- 43. Sasaki N., Toki S., Chowei H., Saito T., Nakano N., Hayashi Y., Takeuchi M., Makita Z. (2001) Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer's disease. Brain Res. 888, 256–262 [DOI] [PubMed] [Google Scholar]
- 44. Van Eldik L.J., Griffin W.S.T. (1994) S100 beta expression in Alzheimer's disease: relation to neuropathology in brain regions. Biochim. Biophys. Acta 1223, 398–403 [DOI] [PubMed] [Google Scholar]
- 45. Halgand F., Zabrouskov V., Bassilian S., Souda P., Wong D.T., Loo J.A., Faull K.F., Whitelegge J.P. (2010) Micro-heterogeneity of human saliva Peptide P-C characterized by high-resolution top-down Fourier-transform mass spectrometry. J. Am. Soc. Mass Spectrom. 21, 868–877 [DOI] [PubMed] [Google Scholar]
- 46. Halgand F., Zabrouskov V., Bassilian S., Souda P., Loo J.A., Faull K.F., Wong D.T., Whitelegge J.P. (2012) Defining intact protein primary structures from saliva: a step toward the human proteome project. Anal. Chem. 84, 4383–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Messana I., Inzitari R., Fanali C., Cabras T., Castagnola M. (2008) Facts and artifacts in proteomics of body fluids. What proteomics of saliva is telling us? J. Sep. Sci. 31, 1948–1963 [DOI] [PubMed] [Google Scholar]
- 48. Oppenheim F.G., Salih E., Siqueira W.L., Zhang W., Helmerhorst E.J. (2007) Salivary proteome and its genetic polymorphisms. Ann. N.Y. Acad. Sci. 1098, 22–50 [DOI] [PubMed] [Google Scholar]
- 49. Zheng Y., Niyonsaba F., Ushio H., Ikeda S., Nagaoka I., Okumura K., Ogawa H. (2008) Microbicidal protein psoriasin is a multifunctional modulator of neutrophil activation. Immunology 124, 357–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.