Abstract
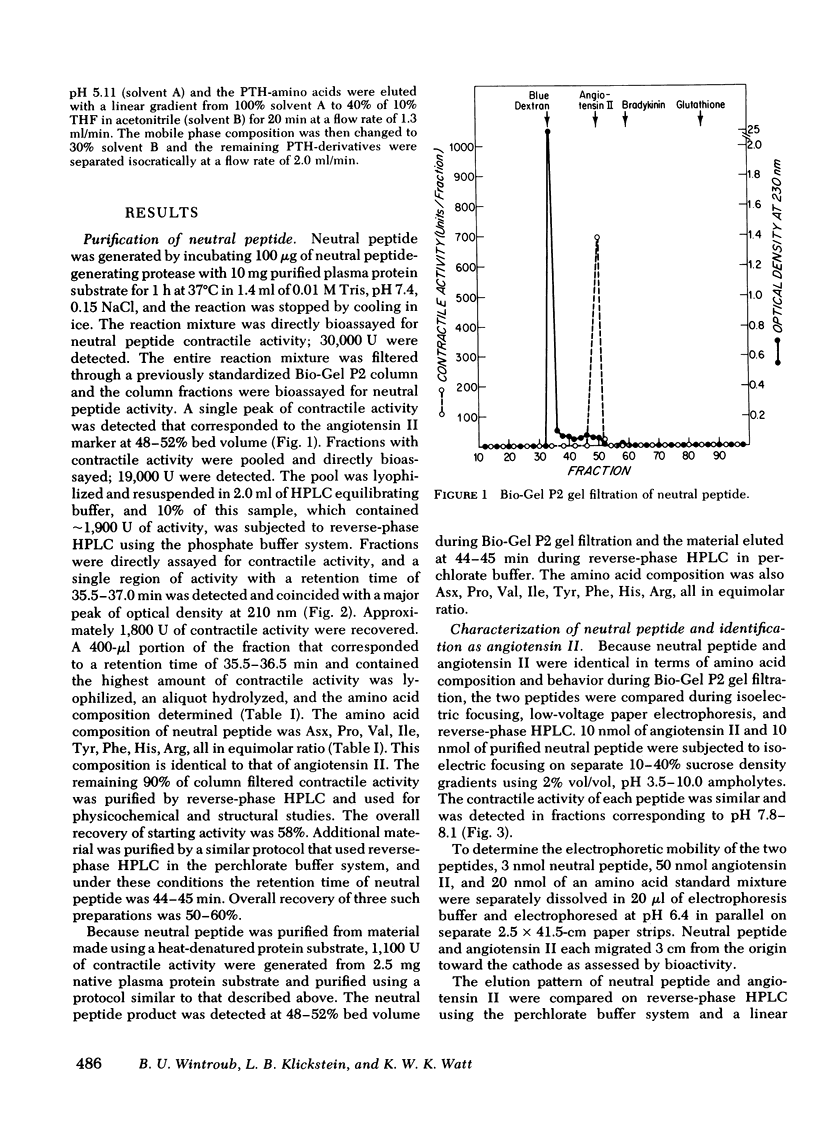
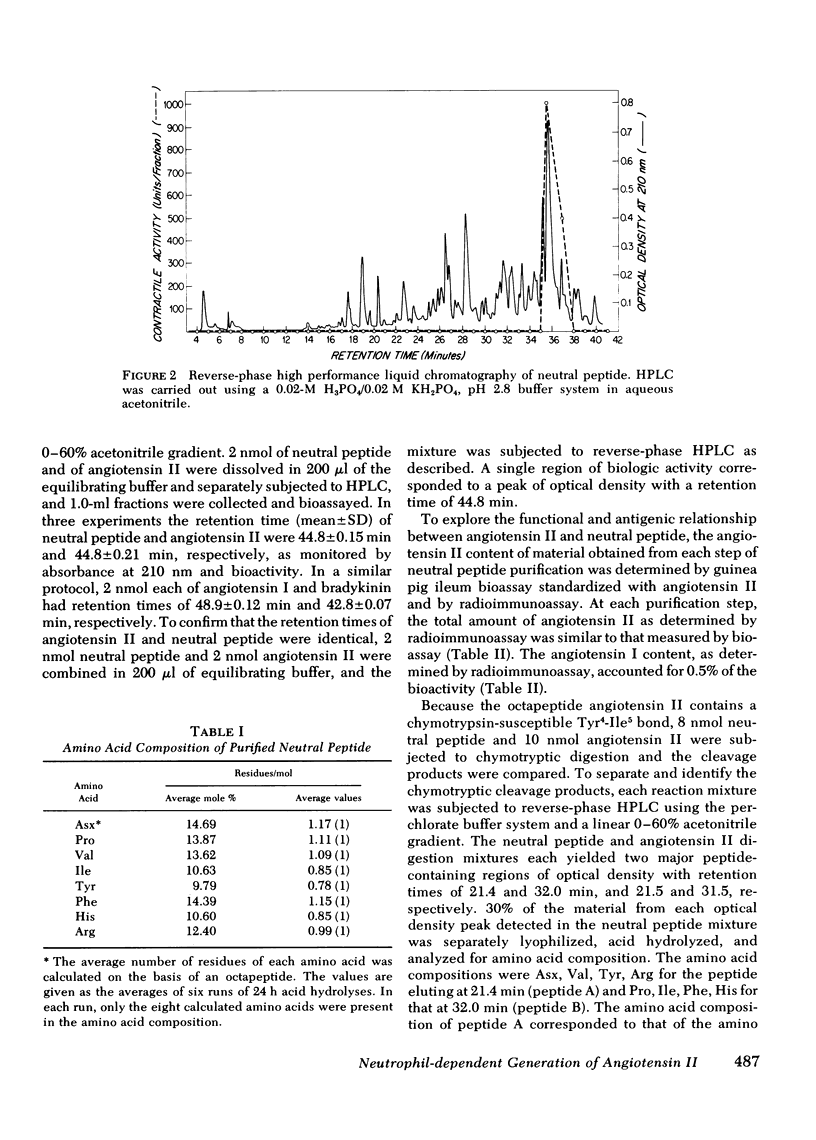
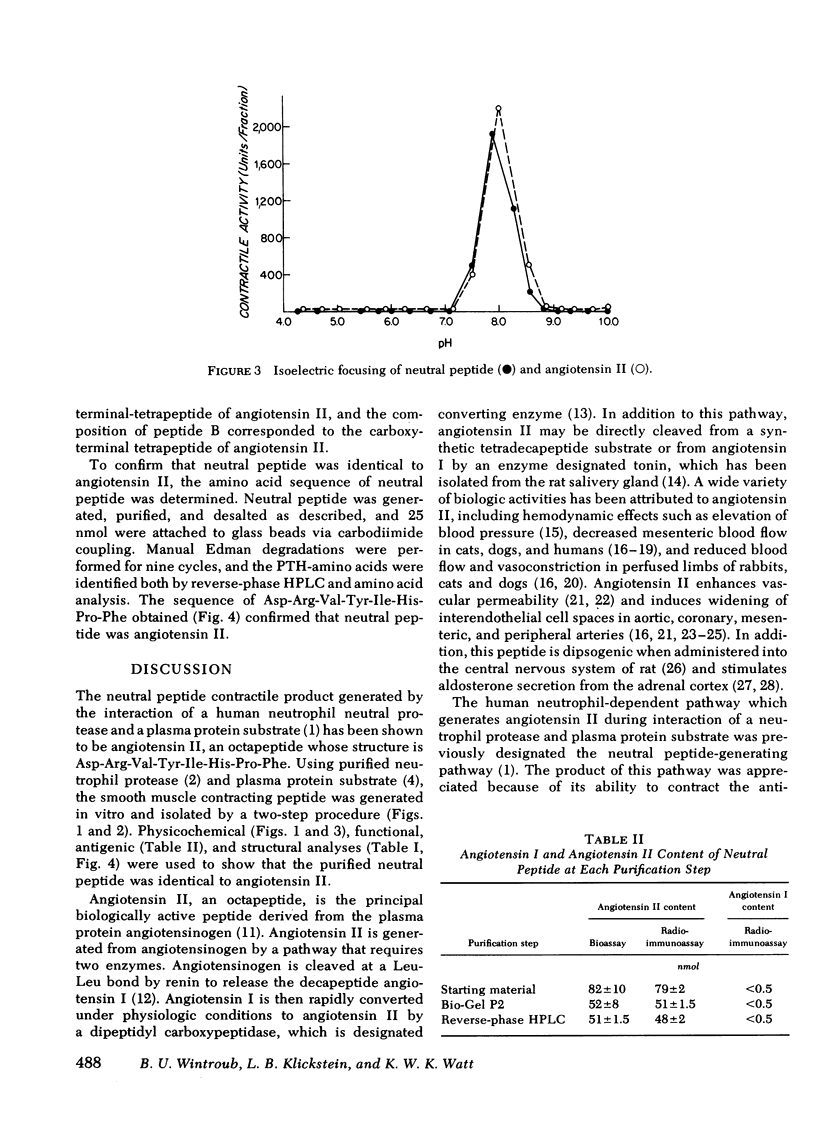
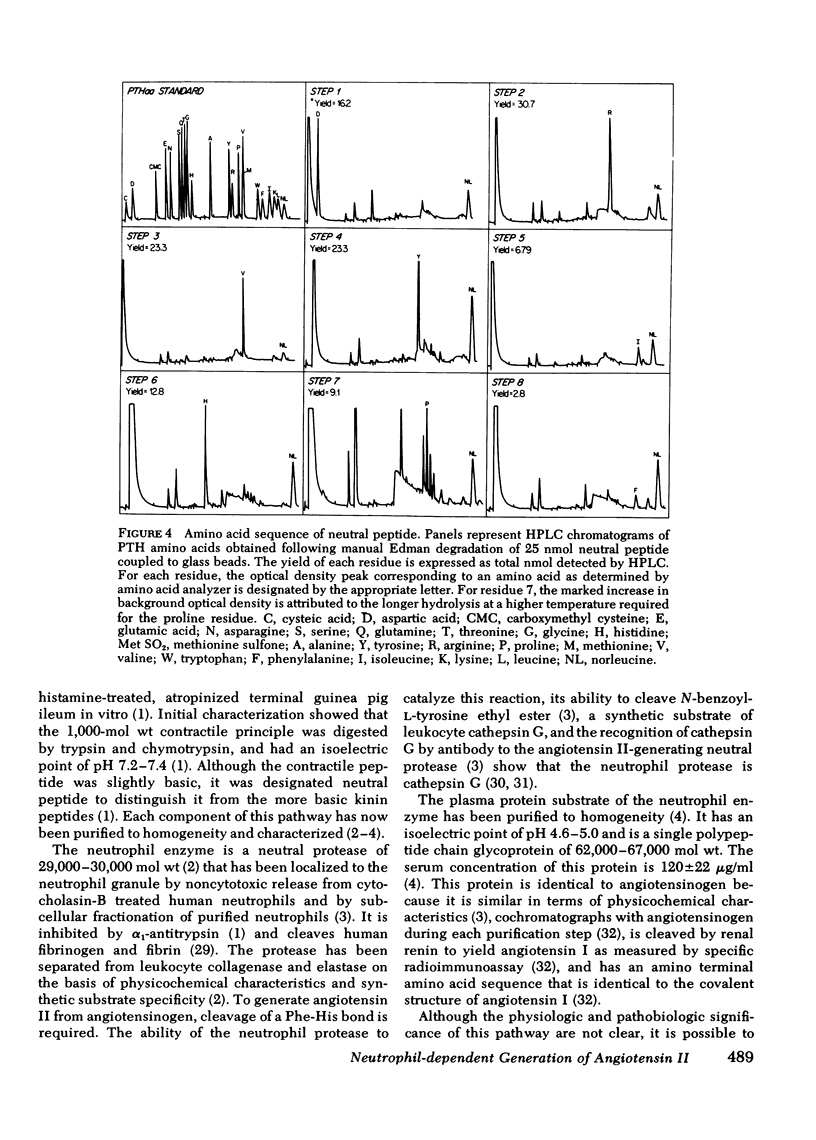
A human neutrophil lysosomal protease interacts at physiologic pH with a 62,000--67,000-mol wt plasma protein substrate to generate a vasoactive, smooth muscle-contracting "neutral" peptide. The peptide product of this system, previously designated the "neutral" peptide-generating pathway, was generated from purified components and purified by Bio-Gel P2 gel filtration and reverse-phase high performance liquid chromatography with a 50--60% yield of starting activity. The purified peptide had an amino acid composition of Asx, Pro, Val, Ile, Tyr, Phe, His, Arg, a composition identical to that of angiotensin II. The peptide and synthetic angiotensin II each filtered at 48--52% bed volume on Bio-Gel P2, had an isoelectric point of Ph 7.8--8.1 at 4 degrees C, migrated 3 cm toward the cathode during pH 6.4 low-voltage paper electrophoresis, and had a retention time of 44.8 min during reverse-phase high-performance liquid chromatography. In addition, the functional activity of the peptide at each purification step correlated with angiotensin II content determined by specific radioimmunoassay. The amino acid sequence of 25 nmol of the peptide was Asp-Arg-Val-Try-Ile-His-Pro-Phe, the same covalent structure as that of angiotensin II. Therefore, under physiologic conditions, in the absence of renin or angiotensin converting enzyme, a human neutrophil neutral protease cleaves a plasma protein to yield angiotensin II. This pathway provides a mechanism through which the neutrophil may alter local blood flow during inflammation by generation of a potent vasoactive peptide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES R. P., BORKOWSKI A. J., SICINSKI A. M., LARAGH J. H. PROLONGED INFUSIONS OF ANGIOTENSIN II AND NOREPINEPHRINE AND BLOOD PRESSURE, ELECTROLYTE BALANCE, AND ALDOSTERONE AND CORTISOL SECRETION IN NORMAL MAN AND IN CIRRHOSIS WITH ASCITES. J Clin Invest. 1965 Jul;44:1171–1186. doi: 10.1172/JCI105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron P., Koiw E., Nowaczynski W., Brouillet J., Genest J. THE EFFECTS OF INTRAVENOUS INFUSIONS OF VALINE-5 ANGIOTENSIN II AND OTHER PRESSOR AGENTS ON URINARY ELECTROLYTES AND CORTICOSTEROIDS, INCLUDING ALDOSTERONE. J Clin Invest. 1961 Feb;40(2):338–347. doi: 10.1172/JCI104261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brod J., Hejl Z., Hornych A., Jirka J., Slechta V., Buriánová B. Comparison of haemodynamic effects of equipressor doses of intravenous angiotensin and noradrenaline in man. Clin Sci. 1969 Apr;36(2):161–172. [PubMed] [Google Scholar]
- Coblyn J. S., Austen K. F., Wintroub B. U. Purification and characterization of a human neutrophil neutral protease. The neutral peptide-generating protease. J Clin Invest. 1979 May;63(5):998–1005. doi: 10.1172/JCI109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Cassman K. G., Cottrell B. A., Friezner S. J., Hucko J. T., Takagi T. Amino acid sequence studies on the alpha chain of human fibrinogen. Characterization of 11 cyanogen bromide fragments. Biochemistry. 1977 Apr 19;16(8):1703–1709. doi: 10.1021/bi00627a028. [DOI] [PubMed] [Google Scholar]
- Fitzsimons J. T. Thirst. Physiol Rev. 1972 Apr;52(2):468–561. doi: 10.1152/physrev.1972.52.2.468. [DOI] [PubMed] [Google Scholar]
- Forsyth R. P., Hoffbrand B. I., Melmon K. L. Hemodynamic effects of angiotensin in normal and environmentally stressed monkeys. Circulation. 1971 Jul;44(1):119–129. doi: 10.1161/01.cir.44.1.119. [DOI] [PubMed] [Google Scholar]
- Giacomelli F., Anversa P., Wiener J. Effect of angiotensin-induced hypertension on rat coronary arteries and myocardium. Am J Pathol. 1976 Jul;84(1):111–138. [PMC free article] [PubMed] [Google Scholar]
- Greenway C. V., Stark R. D. The vascular responses of the spleen to intravenous infusions of catecholamines, angiotensin and vasopressi in the anaesthetized cat. Br J Pharmacol. 1970 May;38(3):583–592. doi: 10.1111/j.1476-5381.1970.tb10599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järhult J. Comparative effects of angiotensin and noradrenaline on resistance, capacitance, and precapillary sphincter vessels in cat skeletal muscle. Acta Physiol Scand. 1971 Mar;81(3):315–324. doi: 10.1111/j.1748-1716.1971.tb04906.x. [DOI] [PubMed] [Google Scholar]
- Krasney J. A. Effects of angiotensin on stroke volume and regional blood flow and resistance. Am J Physiol. 1968 Dec;215(6):1454–1461. doi: 10.1152/ajplegacy.1968.215.6.1454. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Peach M. J. Renin-angiotensin system: biochemistry and mechanisms of action. Physiol Rev. 1977 Apr;57(2):313–370. doi: 10.1152/physrev.1977.57.2.313. [DOI] [PubMed] [Google Scholar]
- Robertson A. L., Jr, Khairallah P. A. Arterial endothelial permeability and vascular disease. The "trap door" effect. Exp Mol Pathol. 1973 Apr;18(2):241–260. doi: 10.1016/0014-4800(73)90022-1. [DOI] [PubMed] [Google Scholar]
- Robertson A. L., Khairallah P. A. Effects of angiotensin II and some analogues on vascular permeability in the rabbit. Circ Res. 1972 Dec;31(6):923–931. doi: 10.1161/01.res.31.6.923. [DOI] [PubMed] [Google Scholar]
- SKEGGS L. T., Jr, MARSH W. H., KAHN J. R., SHUMWAY N. P. The existence of two forms of hypertensin. J Exp Med. 1954 Mar;99(3):275–282. doi: 10.1084/jem.99.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W., Havemann K. Chymotrypsin-like neutral protease from lysosomes of human polymorphonuclear leukocytes. Hoppe Seylers Z Physiol Chem. 1977 May;358(5):555–564. doi: 10.1515/bchm2.1977.358.1.555. [DOI] [PubMed] [Google Scholar]
- Skeggs L. T., Lentz K. E., Gould A. B., Hochstrasser H., Kahn J. R. Biochemistry and kinetics of the renin-angiotensin system. Fed Proc. 1967 Jan-Feb;26(1):42–47. [PubMed] [Google Scholar]
- Starkey P. M., Barrett A. J. Human cathepsin G. Catalytic and immunological properties. Biochem J. 1976 May 1;155(2):273–278. doi: 10.1042/bj1550273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wintroub B. U., Coblyn J. S., Kaempfer C. E., Austen K. F. Cleavage of fibrinogen by the human neutrophil neutral peptide-generating protease. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5448–5452. doi: 10.1073/pnas.77.9.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintroub B. U., Goetzl E. J., Austen K. F. A neutrophil-dependent pathway for the generation of a neutral peptide mediator: partial characterization of components and control by alpha-1-antitrypsin. J Exp Med. 1974 Sep 1;140(3):812–824. doi: 10.1084/jem.140.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintroub B. U., Klickstein L. B., Kaempfer C. E., Austen K. F. A human neutrophil-dependent pathway for generation of angiotensin II: purification and physicochemical characterization of the plasma protein substrate. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1204–1208. doi: 10.1073/pnas.78.2.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S., Itano H. Phenanthrenequinone as an analytical reagent for arginine and other monosubstituted guanidines. Biochim Biophys Acta. 1966 Dec 28;130(2):538–540. doi: 10.1016/0304-4165(66)90256-x. [DOI] [PubMed] [Google Scholar]


