Abstract
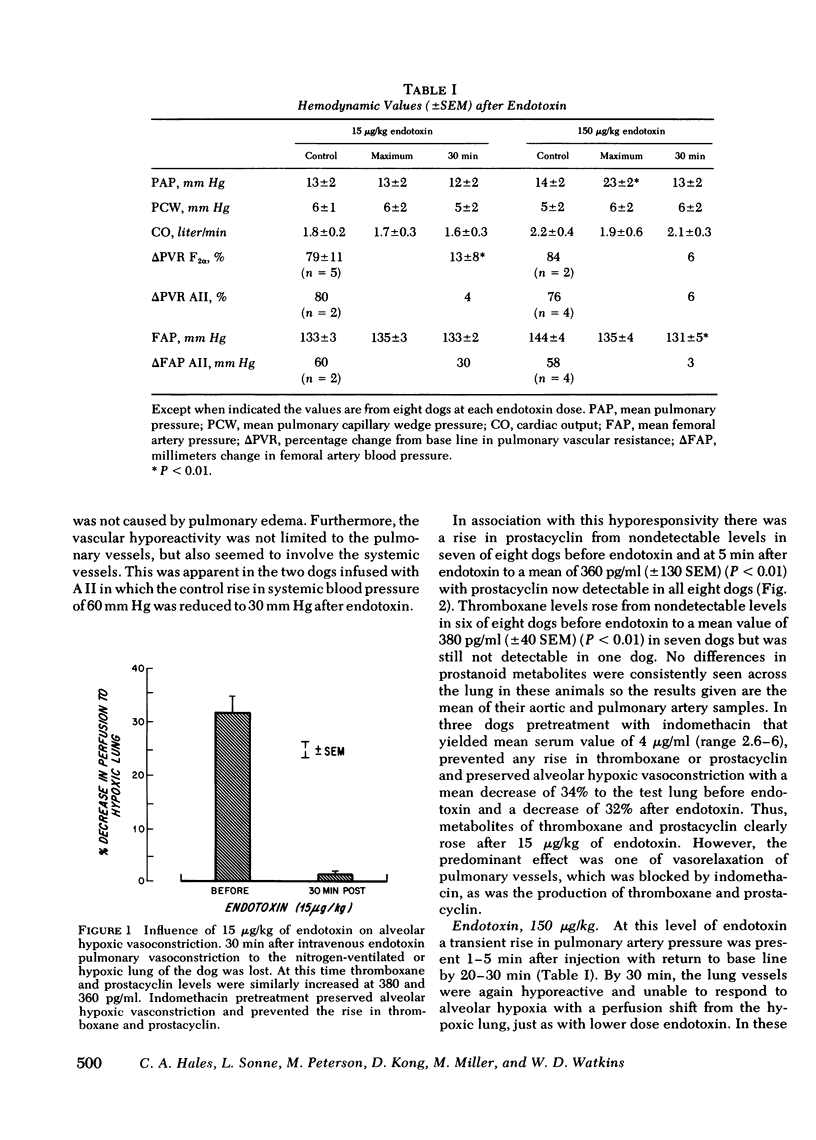
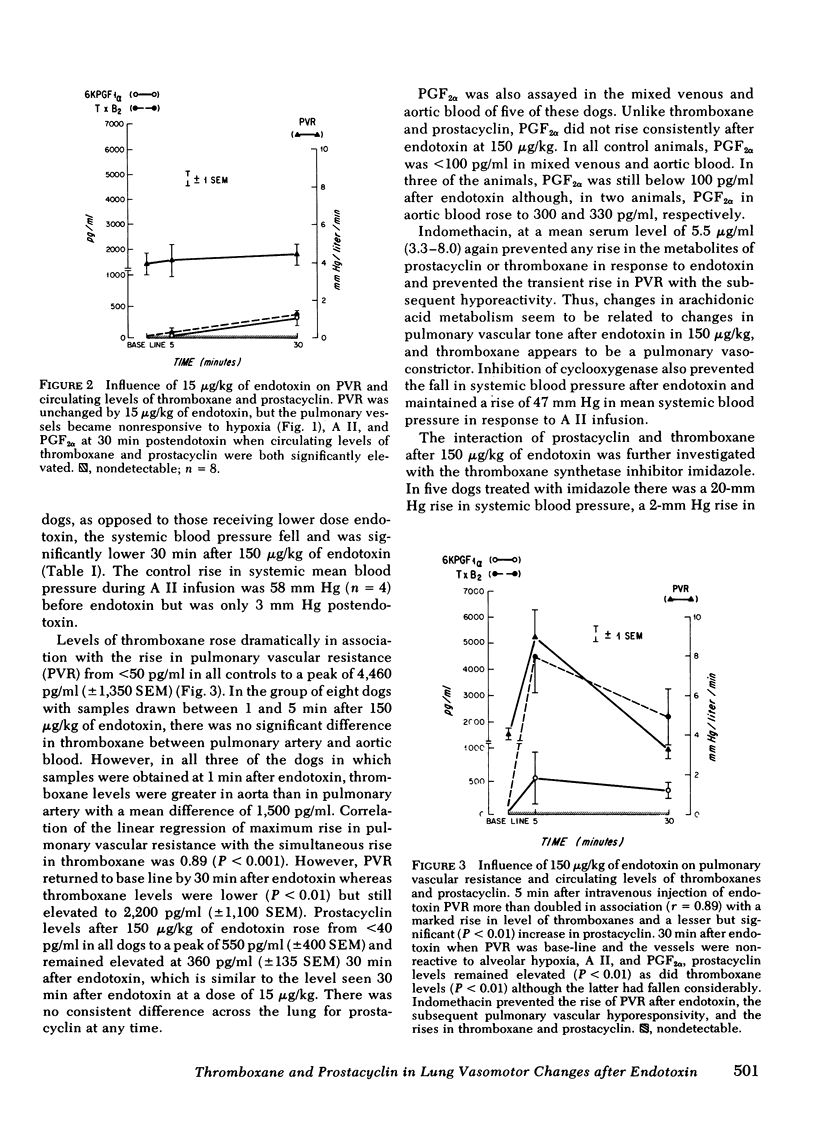
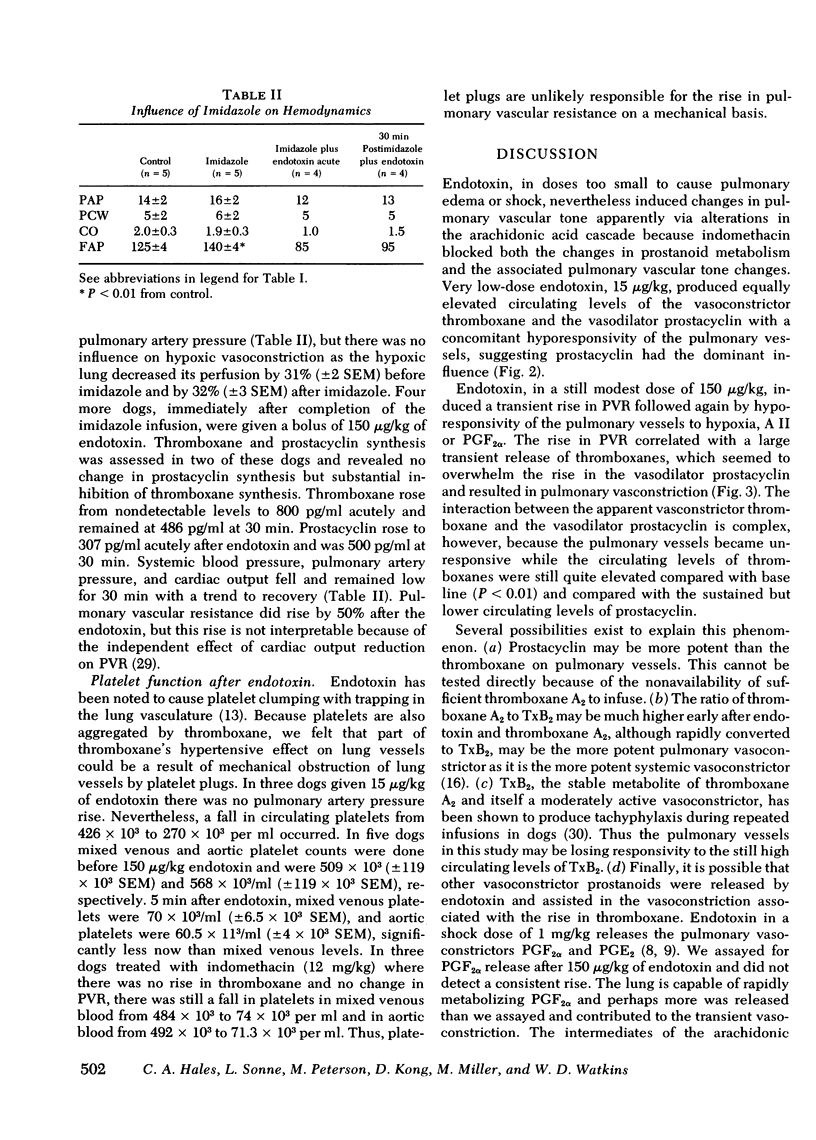
Cyclooxygenase inhibitors prevent the pulmonary vasomotor changes in response to low-dose endotoxin. We, therefore, explored the role of two highly vasoactive prostanoids, thromboxane A2, a vasoconstrictor, and prostacyclin, a vasodilator, in the transient pulmonary vasoconstriction and subsequent loss of alveolar hypoxis vasoconstriction (AHPV) that follows endotoxin. AHPV was tested in the dog with a double-lumened endotracheal tube allowing ventilation of one lung with nitrogen as a hypoxic challenge while the other lung was ventilated with oxygen to maintain systemic oxygenation. Relative distribution of perfusion to the two lungs was assessed with intravenous 133Xe and external scintillation detectors. The stable metabolites of thromboxane and prostacyclin, i.e., thromboxane B2 and 6-keto-prostaglandin F1α were measured in plasma with radioimmunoassay. 15 μg/kg i.v. of endotoxin induced no rise in pulmonary vascular resistance (PVR), but prevented AHPV so that the initial 33% (±2 SEM) decrease in perfusion to the hypoxic lung became only a 2% (±1) decrease. Circulating levels of thromboxane and prostacyclin concurrently rose (P < 0.01) from nondetectable levels to 380 pg/ml (±40) and 360 pg/ml (±130). 150 μg/kg of endotoxin induced a transient rise in PVR from 4.09 to 9.00 mm Hg/liter per min in association (r = 0.89, P < 0.01) with a sharp rise in thromboxane levels to 4,460 pg/ml (±1,350) whereas prostacyclin levels were elevated less markedly to 550 pg/ml (±400). Prostaglandin F2α, another vasoconstrictor, was not elevated. 30 min after endotoxin when PVR was again base line and AHPV lost, thromboxane fell significantly (P < 0.01) to 2,200 pg/ml (±1,100) whereas prostacyclin remained elevated at 360 pg/ml (±135), a level similar to that seen when 15 μg/kg of endotoxin induced loss of AHPV. Indomethacin prevented the rise in thromboxane and prostacyclin after endotoxin as well as the changes in pulmonary vasomotor tone. Thus, a complex interaction between thromboxane and prostacyclin is involved in the pulmonary vasomotor response to low-dose endotoxin.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATHENS J. W., HAAB O. P., RAAB S. O., MAUER A. M., ASHENBRUCKER H., CARTWRIGHT G. E., WINTROBE M. M. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest. 1961 Jun;40:989–995. doi: 10.1172/JCI104338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson F. L., Jubiz W., Tsagaris T. J., Kuida H. Endotoxin-induced prostaglandin E and F release in dogs. Am J Physiol. 1975 Feb;228(2):410–414. doi: 10.1152/ajplegacy.1975.228.2.410. [DOI] [PubMed] [Google Scholar]
- Anderson F. L., Tsagaris T. J., Jubiz W., Kuida H. Prostaglandin F and E levels during endotoxin-induced pulmonary hypertension in calves. Am J Physiol. 1975 May;228(5):1479–1482. doi: 10.1152/ajplegacy.1975.228.5.1479. [DOI] [PubMed] [Google Scholar]
- Berti F., Folco G. C., Giachetti A., Malandrino S., Omini C., Viganò T. Atropine inhibits thromboxane A2 generation in isolated lungs of the guinea-pig. Br J Pharmacol. 1980 Mar;68(3):467–472. doi: 10.1111/j.1476-5381.1980.tb14560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers R. E., Ellis E. F., Brigham K. L., Oates J. A. Effects of prostaglandin cyclic endoperoxides on the lung circulation of unanesthetized sheep. J Clin Invest. 1979 Jan;63(1):131–137. doi: 10.1172/JCI109266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult H., Beetens J., Herman A. G. Blood levels of 6-oxo-prostaglandin F 1 alpha during endotoxin-induced hypotension in rabbits. Eur J Pharmacol. 1980 Apr 11;63(1):47–56. doi: 10.1016/0014-2999(80)90115-6. [DOI] [PubMed] [Google Scholar]
- Cho Y. W. Direct cardiac action of E. coli endotoxin. Proc Soc Exp Biol Med. 1972 Nov;141(2):705–707. doi: 10.3181/00379727-141-36856. [DOI] [PubMed] [Google Scholar]
- Cook J. A., Wise W. C., Halushka P. V. Elevated thromboxane levels in the rat during endotoxic shock: protective effects of imidazole, 13-azaprostanoic acid, or essential fatty acid deficiency. J Clin Invest. 1980 Jan;65(1):227–230. doi: 10.1172/JCI109655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. D., McDonald J. W., Ali M., Menkes E., Masterson J., Klement P. Prostaglandin production associated with the pulmonary vascular response to complement activation. Surgery. 1980 Aug;88(2):215–221. [PubMed] [Google Scholar]
- DAVIS R. B., MEEKER W. R., McQUARRIE D. G. Immediate effects of intravenous endotoxin on serotonin concentrations and blood platelets. Circ Res. 1960 Jan;8:234–239. doi: 10.1161/01.res.8.1.234. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Ellis E. F., Oelz O., Roberts L. J., 2nd, Payne N. A., Sweetman B. J., Nies A. S., Oates J. A. Coronary arterial smooth muscle contraction by a substance released from platelets: evidence that it is thromboxane A2. Science. 1976 Sep 17;193(4258):1135–1137. doi: 10.1126/science.959827. [DOI] [PubMed] [Google Scholar]
- Faden A. I., Holaday J. W. Naloxone treatment of endotoxin shock: stereospecificity of physiologic and pharmacologic effects in the rat. J Pharmacol Exp Ther. 1980 Mar;212(3):441–447. [PubMed] [Google Scholar]
- Fine D. P. Activation of the classic and alternate complement pathways by endotoxin. J Immunol. 1974 Feb;112(2):763–769. [PubMed] [Google Scholar]
- Friedman L. S., Fitzpatrick T. M., Bloom M. F., Ramwell P. W., Rose J. C., Kot P. A. Cardiovascular and pulmonary effects of thromboxane B2 in the dog. Circ Res. 1979 Jun;44(6):748–751. doi: 10.1161/01.res.44.6.748. [DOI] [PubMed] [Google Scholar]
- Glauser F. L., Palmer J., Cecconi S., Schoolcraft W., Wells I., Novey H., Egan P., Smeltzer D. The effect of endotoxin on the mast cell c'AMP system. Ann Allergy. 1977 Feb;38(2):104–106. [PubMed] [Google Scholar]
- Gryglewski R. J., Korbut R., Ocetkiewicz A. Generation of prostacyclin by lungs in vivo and its release into the arterial circulation. Nature. 1978 Jun 29;273(5665):765–767. doi: 10.1038/273765a0. [DOI] [PubMed] [Google Scholar]
- HOROWITZ H. I., DES PREZ R. M., HOOK E. W. Effects of bacterial endotoxin on rabbit platelets. II. Enhancement of platelet factor 3 activity in vitro and in vivo. J Exp Med. 1962 Nov 1;116:619–633. doi: 10.1084/jem.116.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales C. A., Kanarek D. J., Ahluwalia B., Latty A., Erdmann J., Javaheri S., Kazemi H. Regional edema formation in isolated perfused dog lungs. Circ Res. 1981 Jan;48(1):121–127. doi: 10.1161/01.res.48.1.121. [DOI] [PubMed] [Google Scholar]
- Hales C. A., Kazemi H. Hypoxic vascular response of the lung: effect of aminophylline and epinephrine. Am Rev Respir Dis. 1974 Aug;110(2):126–132. doi: 10.1164/arrd.1974.110.2.126. [DOI] [PubMed] [Google Scholar]
- Kadowitz P. J., Chapnick B. M., Feigen L. P., Hyman A. L., Nelson P. K., Spannhake E. W. Pulmonary and systemic vasodilator effects of the newly discovered prostaglandin, PGI2. J Appl Physiol Respir Environ Exerc Physiol. 1978 Sep;45(3):408–413. doi: 10.1152/jappl.1978.45.3.408. [DOI] [PubMed] [Google Scholar]
- Kazemi H., Bruecke P. E., Parsons E. F. Role of the autonomic nervous system in the hypoxic response of the pulmonary vascular bed. Respir Physiol. 1972 Jun;15(2):245–254. doi: 10.1016/0034-5687(72)90101-6. [DOI] [PubMed] [Google Scholar]
- Levine L., Gjtierrez Cernosek R. M., Van Vunakis H. Specificities of prostaglandins B 1 , F 1 , and F 2 antigen-antibody reactions. J Biol Chem. 1971 Nov 25;246(22):6782–6785. [PubMed] [Google Scholar]
- McCosh E. J., Meyer D. L., Dupont J. Radioimmunoassay of prostaglandins E1, E2, and F2alpha in unextracted plasma, serum and myocardium. Prostaglandins. 1976 Oct;12(4):471–486. doi: 10.1016/0090-6980(76)90028-9. [DOI] [PubMed] [Google Scholar]
- Miller M. A., Hales C. A. Stability of alveolar hypoxic vasoconstriction with intermittent hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1980 Nov;49(5):846–850. doi: 10.1152/jappl.1980.49.5.846. [DOI] [PubMed] [Google Scholar]
- Mlczoch J., Weir E. K., Grover R. F., Reeves J. T. Pulmonary vascular effects of endotoxin in leukopenic dogs. Am Rev Respir Dis. 1978 Dec;118(6):1097–1099. doi: 10.1164/arrd.1978.118.6.1097. [DOI] [PubMed] [Google Scholar]
- Murray J. F., Karp R. B., Nadel J. A. Viscosity effects on pressure-flow relations and vascular resistance in dogs' lungs. J Appl Physiol. 1969 Sep;27(3):336–341. doi: 10.1152/jappl.1969.27.3.336. [DOI] [PubMed] [Google Scholar]
- Proctor R. A. Endotoxin in vitro interactions with human neutrophils: depression of chemiluminescence, oxygen consumption, superoxide production, and killing. Infect Immun. 1979 Sep;25(3):912–921. doi: 10.1128/iai.25.3.912-921.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves J. T., Grover R. F. Blockade of acute hypoxic pulmonary hypertension by endotoxin. J Appl Physiol. 1974 Mar;36(3):328–332. doi: 10.1152/jappl.1974.36.3.328. [DOI] [PubMed] [Google Scholar]
- Strieder D. J., Barnes B. A., Aronow S., Russell P. S., Kazemi H. Xenon 133 study of ventilation and perfusion in normal and transplanted dog lungs. J Appl Physiol. 1967 Sep;23(3):359–366. doi: 10.1152/jappl.1967.23.3.359. [DOI] [PubMed] [Google Scholar]
- Sun F. F., Chapman J. P., McGuire J. C. Metabolism of prostaglandin endoperoxide in animal tissues. Prostaglandins. 1977;14(6):1055–1074. doi: 10.1016/0090-6980(77)90285-4. [DOI] [PubMed] [Google Scholar]
- Svensson J., Hamberg M. Thromboxane A2 and prostaglandin H2: potent stimulators of the swine coronary artery. Prostaglandins. 1976 Dec;12(6):943–950. doi: 10.1016/0090-6980(76)90128-3. [DOI] [PubMed] [Google Scholar]
- Weir E. K., Mlczoch J., Reeves J. T., Grover R. F. Endotoxin and prevention of hypoxic pulmonary vasoconstriction. J Lab Clin Med. 1976 Dec;88(6):975–983. [PubMed] [Google Scholar]
- Weir E. K., Mlczoch J., Seavy J., Cohen J. J., Grover R. F. Platelet antiserum inhibits hypoxic pulmonary vasoconstriction in the dog. J Appl Physiol. 1976 Aug;41(2):211–215. doi: 10.1152/jappl.1976.41.2.211. [DOI] [PubMed] [Google Scholar]


