Abstract
Antimicrobial treatment strategies must improve to reduce the high mortality rates in septic patients. In noninfectious models of acute inflammation, activation of A2B adenosine receptors (A2BR) in extracellular adenosine-rich microenvironments causes immunosuppression. We examined A2BR in antibacterial responses in the cecal ligation and puncture (CLP) model of sepsis. Antagonism of A2BR significantly increased survival, enhanced bacterial phagocytosis, and decreased IL-6 and MIP-2 (a CXC chemokine) levels after CLP in outbred (ICR/CD-1) mice. During the CLP-induced septic response in A2BR knockout mice, hemodynamic parameters were improved compared with wild-type mice in addition to better survival and decreased plasma IL-6 levels. A2BR deficiency resulted in a dramatic 4-log reduction in peritoneal bacteria. The mechanism of these improvements was due to enhanced macrophage phagocytic activity without augmenting neutrophil phagocytosis of bacteria. Following ex vivo LPS stimulation, septic macrophages from A2BR knockout mice had increased IL-6 and TNF-α secretion compared with wild-type mice. A therapeutic intervention with A2BR blockade was studied by using a plasma biomarker to direct therapy to those mice predicted to die. Pharmacological blockade of A2BR even 32 h after the onset of sepsis increased survival by 65% in those mice predicted to die. Thus, even the late treatment with an A2BR antagonist significantly improved survival of mice (ICR/CD-1) that were otherwise determined to die according to plasma IL-6 levels. Our findings of enhanced bacterial clearance and host survival suggest that antagonism of A2BRs offers a therapeutic target to improve macrophage function in a late treatment protocol that improves sepsis survival.
Despite >30 y of significant advances in understanding the pathological mechanisms of sepsis, only activated protein C has proven to be effective (1, 2). Anti-inflammatory treatment strategies to reduce the hypothesized overzealous inflammation responsible for high sepsis mortality rates have uniformly failed clinical trials (3). These past failures provide a strong impetus for the development of alternative treatment approaches (reviewed in Ref. 4). In addition to improving survival of septic patients, the emergence of drug-resistant bacteria provides a rationale for additional alternative approaches to treat bacterial infections recalcitrant to standard antimicrobial therapy.
The clinical syndrome of sepsis involves dysfunction of the immune and cardiovascular systems (5–8). Sepsis is characterized by an inappropriate inflammatory response to infection that may result in impaired microbial clearance that contributes to mortality. The underlying pathogenesis of sepsis is poorly understood because of the complex nature it presents as a systemic disease. Past studies attempting to identify individual cytokines responsible for sepsis mortality have proven difficult. Their role as causal agents of disease remains to be proven, although it is unlikely because blocking and supplementation with inflammatory cytokines have largely proven ineffective in clinical trials (reviewed in Ref. 9).
Recent studies direct attention to immunosuppressive signals during infection that increase susceptibility to sepsis mortality. Immunoparalyzed cells present at the septic foci have been termed “Zombie” cells, immune cells that are physically, but not functionally present (10). Moreover, phagocytic impairment in neutrophils and macrophages is an important contributor to septic dysfunction (11–13), demonstrating pathogenic defects within the innate immune response to sepsis. Recently, a surprising new role for programmed death-1 receptor, a suppressor of T cell activation, was identified on macrophages to suppress innate responses to bacteria (11). In this study, genetic deficiency of the programmed death-1 receptor augmented antipathogen responses to increase bacterial clearance and survival in septic mice (11). This study highlights the importance of defining the role of immunosuppressive signals as a mechanistic basis of disease. Redefining immunosuppressive signaling to restore innate function represents a rational treatment approach for sepsis.
Immunosuppressive signals are present in inflamed tissue to protect normal tissue from cellular damage. Tight regulation of the immune response to infection minimizes tissue damage while eliminating the inciting agent. The role of physiological immunomodulators, such as extracellular adenosine signaling through A2A and A2B adenosine receptors (A2AR/A2BR, respectively) to reduce collateral tissue damage during inflammation, is now well established (14–16). There are four subtypes of adenosine receptors. The A1 and A3 receptor subtypes are inhibitory Gi protein coupled, and the A2 receptors are subdivided into subtypes that are stimulatory Gs protein coupled (17). Adenosine signaling through A2AR/A2BR is generally anti-inflammatory (16, 18). Pharmacological agonism of the A2AR (16, 19) results in inhibition of overactivated immune cells. In contrast, the genetic elimination or pharmacological antagonism of A2AR/A2BRs results in higher levels of proinflammatory mediators and extensive collateral tissue damage in models of inflammation-induced tissue injury (20–22).
cAMP-triggered intracellular pathways mediate the immunosuppressive properties of the Gs protein-coupled A2A and A2BRs. In addition, the A2BR has proinflammatory properties mediated by Gq pathways (23), but the overall effect of A2B receptors appears to be immunosuppressive in sepsis (24).
Interestingly, genetic deficiency of the high-affinity Gs protein-coupled A2AR resulted in better survival and bacterial clearance during sepsis (25), confirming a pathogenic role of the A2AR. This study also suggests that mice were dying from reduced antibacterial immunity as A2AR knockout (KO) mice had less bacterial load than their littermate controls. This indicates that acute inflammation is quelled by A2AR/A2BR signaling to reduce tissue damage when the innate system is activated in response to stress such as infection. However, the A2BR is a low-affinity Gs protein-coupled receptor, making it unclear whether extracellular levels of adenosine are sufficiently high to have any role in rescuing or promoting mice from sepsis mortality.
The ability of A2BRs to mediate immune suppression as a mechanism of impaired antimicrobial defenses was suggested by data showing that A2BR KO mice have increased circulating TNF-α levels that are required for proper bacterial clearance in sepsis (20, 26). However, the role of A2BRs during true infections has not been extensively studied (reviewed in Refs. 27, 28). We propose that the consequence of this cytoprotective mechanism during infection is ineffective removal of the septic foci, and that this persistence could drive septic morbidity and mortality.
Adenosine signaling through A2BRs may affect sepsis-induced morbidity and mortality by the regulation of nonimmunological mechanisms as well. The A2BR is up-regulated on nonimmune cell types such as vascular smooth muscle (VSM) cells in response to cytokine stimulation (29). This suggests that A2BRs could affect hemodynamic responses during sepsis. Our studies were designed to test this by measuring multiple cardiac parameters in septic mice.
We hypothesized that the A2BR directly contributes to inadequate bacterial clearance and immune dysfunction seen in bacterial sepsis, suggesting that the host bacterial clearance could be improved by blockade of the immunosuppressive A2BR. We tested this hypothesis in the clinically relevant model of cecal ligation and puncture (CLP)–induced sepsis using a combination of pharmacological experiments in wild-type (WT) mice, and genetic studies in mice deficient of A2BRs.
Materials and Methods
Mice
Male mice 8–12 wk old were used for all experiments, except female mice were included in the experiments using myeloid-specific A2BR KO mice (Fig. 5). C57BL/6 and ICR/CD-1 were used as aged matched controls and housed in a pathogen-free facility at Northeastern University (Boston, MA). Total and myeloid-specific A2BR-deficient mice were developed directly on C57BL/6 background by Ozgene (Australia). Importantly, we studied sepsis not only in the inbred C57BL/6 mouse, but also the outbred ICR/CD-1 mouse strain. The genetically heterogeneous strain allowed us to test the effects of A2BR antagonism on survival in a more clinically relevant mouse model of sepsis, because the patients’ genetic background is mixed. All animal experiments were conducted in accordance with Institutional Animal Care and Use Committee guidelines of Northeastern University and Boston University School of Medicine.
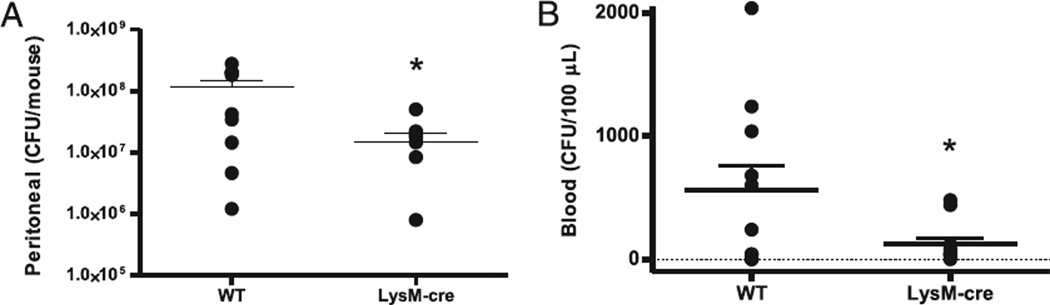
FIGURE 5.
A2BR deficiency in myeloid cells improves local and systemic bacteria. The contribution of A2BRs on myeloid cells to bacterial clearance during sepsis was analyzed in A2BRflox/flox LysM-cre+/− mice that are selectively lacking the A2BR in myeloid cells. A, Bacteria in the peritoneum were decreased in A2BRflox/flox LysM-cre+/− mice. In the same mice, blood was analyzed for the presence of bacteria. B, Bacteremia was decreased in A2BRflox/flox LysM-cre+/− mice. Bacterial counts were determined 24 h after CLP. Data are expressed as mean ± SEM of 11 or greater mice in two independent experiments, and each symbol is an individual animal. *p < 0.05, WT versus A2BRflox/flox LysM-cre+/− mice.
A2BR-deficient mice were developed on C57BL/6 background by Ozgene. In these mice, exon II of the A2BR gene was deleted, resulting in an A2BR mRNA that encodes only the first 78 aa of the A2BR protein. Transcripts lacking the poly(A) signal sequence encoded in exon II and are unstable. The locus of the A2BR exon II was targeted by vector containing the sequence of exon II flanked by loxP sites to allow Cre-mediated deletion, and phosphoglycerate kinase-neo selection cassette flanked by FLP recombinase target sites for Saccharomyces cerevisiae recombinase (Flippase)-recombinase (Supplemental Fig. 1A). Properly targeted embryonic stem (ES) cell clones (Supplemental Fig. 1B) were transfected with Crerecombinase. ES cell clones with deleted exon II (Supplemental Fig. 1C) were microinjected into C57BL/6 blastocysts to generate chimeric mice. Pups were screened for presence or deletion of exon II by PCR with tail DNA. KO versus WT was detected by PCR using the following primers (Supplemental Fig. 1D): 5′-TCCTGGGGTGGAACAGTAAAGAC-3′ and 5′-GTAGGCATAGACAATGGGGTTGAC-3′. These primers allow the amplification of a 455-bp fragment from the region in the WT allele that was deleted in the KO allele. In KO mice, no product was produced. The 5′-TAACAGACCTGTGTTCCAGCCG-3′ and 5′-AAGTGCAAGAAGC-CAGTGCG-3′ primers allowed the amplification of a 861-bp fragment from the KO allele and a 2259-bp fragment from the WT allele (which is usually undetectable due to the short time of PCR cycle).
To create myeloid tissue-specific A2BR KO mice, A2BR-floxed mice were generated first. A2BR-floxed allele-containing ES cells were used to generate A2BR-floxed mice. The floxed allele was detected by PCR using 5′-CGACCACCAAGCGAAACATC-3′ and 5′-CCACCCCCCAGAAT-AGAATGAC-3′ primers to allow the amplification of a 589-bp fragment from the floxed allele, whereas in WT mice no product was produced. A2BR-floxed homozygous mice were crossed with LysM-Cre transgenic mice (from The Jackson Laboratory) to generate A2BR-floxed/floxed_LysMCre+/−. A2BR-floxed/floxed_LysMCre−/− siblings were used as a WT control. The tissue-specific deletion of A2BR mRNA expression in myeloid cell lines was confirmed using real-time PCR analysis of the A2BR genomic DNA in macrophages and thymocytes (Supplemental Fig. 2).
Surgical procedures: CLP
Polymicrobial sepsis was induced by subjecting mice to CLP. To achieve the desired final outcomes in the studies outlined below, it was necessary to use multiple needle sizes to adjust the severity of our CLP model. Briefly, mice were anesthetized with a 3% isoflurane-oxygen mixture prior to surgery. While under anesthesia, 80% of the cecum was ligated distal to the ileocecal valve to prevent bowel obstruction. Fecal material was manually extruded from the punctured cecum into the abdominal cavity. Antibiotics and lactated Ringers plus 5% dextrose for fluid resuscitation were administered in all pharmacological studies to create a more clinically relevant sepsis model as this is standard care for humans. A larger diameter needle was used for cecal puncture in mice that received antibiotics (25 mg/kg imipenem), because antibiotic administration is known to reduce sepsis mortality rates (30). To achieve a survival rate of ~50% with antibiotic therapy, it was necessary to use a 16-gauge needle for cecal puncture in CD-1 and a 21-gauge needle in C57BL/6 mice. In studies in which antibiotics were withheld, a smaller 25-gauge needle was sufficient to achieve the desired mortality. Antibiotic therapy was administered by s.c. injection every 12 h for 4 d. Mice were treated with the A2BR antagonist (MRS 1754 [8-[4-[((4-cyanophenyl)carbamoylmethyl)oxy]phenyl]-1,3-di(n-propyl)xanthine hydrate]: 0.5–10 mg/kg) by s.c. injection once daily for 3 d, and control mice were given vehicle (lactated Ringers plus 5% dextrose + imipenem) only. Administration of antibiotics and MRS 1754 was delayed 1.5 h after CLP for studies described in Fig. 1. MRS 1754 was delayed until 32 h after CLP (antibiotics started at 1.5 h) for the delayed treatment studies described in Fig. 6.
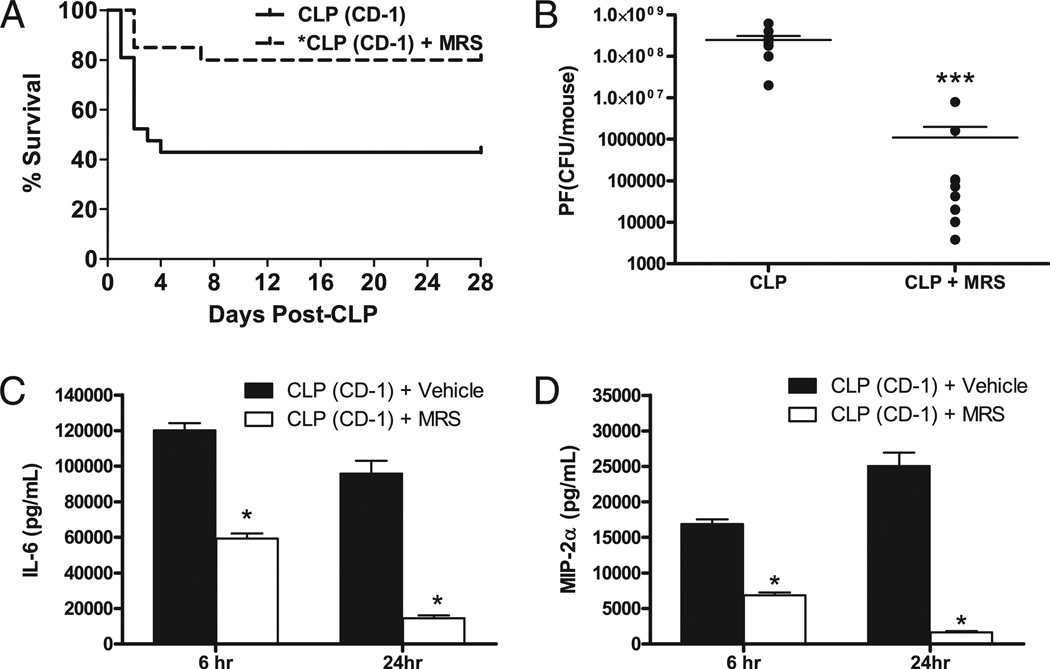
FIGURE 1.
A2BR antagonism improves CLP survival and bacterial clearance, and decreases plasma cytokine levels. Outbred CD-1 mice were given antibiotics (25 mg/kg imipenem) twice daily with fluid resuscitation (Lactated Ringer + 5% dextrose) for 4 d to evaluate the ability of the A2BR antagonist (10 mg/kg MRS 1754) to improve outcome after CLP-induced sepsis. A, 28-d survival in mice treated with the A2BR antagonist (MRS 1754) (broken line; n = 20) had significantly (p < 0.05) better survival than vehicle-treated mice (solid line; n = 21). MRS 1754 therapy was initiated 1.5 h after CLP and given once daily for 3 d. B, The effect of MRS 1754 on PF bacterial load 24 h after CLP. Mice were subjected to CLP and given vehicle with or without MRS 1754 via s.c. injection. Each symbol is an individual animal. Only one dose of MRS 1754 therapy was given to mice that received the antagonist. A2BR antagonism significantly reduced peritoneal bacterial counts. Plasma cytokine levels for IL-6 (C) or TNF (D). Plasma was collected 6 and 24 h after CLP. A2BR antagonism significantly reduced plasma levels of these inflammatory mediators. Data are expressed as mean ± SEM of at least two independent experiments. The survival study data were pooled from three independent studies. *p < 0.01, ***p < 0.001, comparing untreated to treated mice.
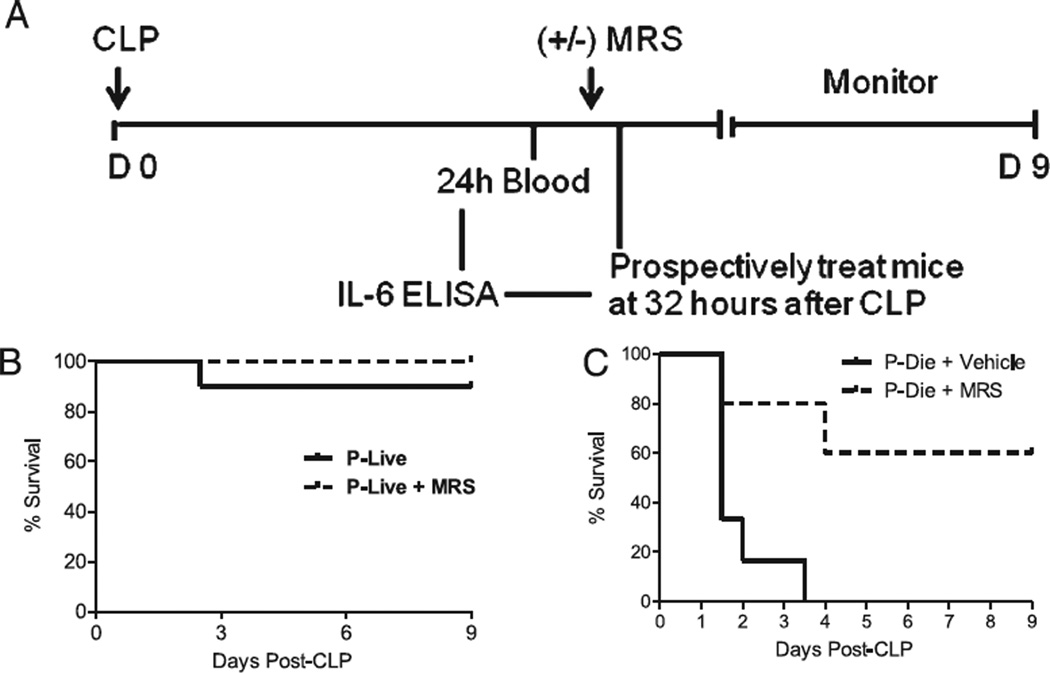
FIGURE 6.
A2BR antagonism saves mice predicted to die of CLP-induced lethality. A, Experimental protocol for the identification and treatment strategy for mice P-live and mice P-die based on plasma IL-6 levels 24 h after CLP. Mice are predicted to live or die during the first 5 d after CLP. B, Survival curve of CD-1 mice P-live treated 32 h post-CLP with the A2BR antagonist MRS 1754, 10 mg/kg (broken line; n = 10) or vehicle (solid line; n = 10). C, Survival curve of mice P-die treated 32 h post-CLP with MRS 1754 (broken line; n = 9) or vehicle (solid line; n = 7). p < 0.01 untreated versus treated mice (P-die) by log rank test for C.
Telemetry
A PAC-10 radio transmitter (DataSci International, St. Paul, MN) was surgically placed into the aortic arch of mice to monitor hemodynamic changes after CLP in C57BL/6 and A2BR KO mice. Briefly, a 1.5-cm cervical incision was made to expose the left carotid artery. The artery was cannulated and the transmitter catheter was threaded through the artery to reside in the aortic arch. Once in place, the catheter was secured with two sutures. Lastly, the transmitter was housed s.c. in the flank of mice and their telemetry was tracked by a receiver placed beneath each cage.
Predictive value of IL-6 stratification of mice predicted to live versus mice predicted to die
This study was designed to determine whether delayed administration of the A2BR antagonist could “rescue” mice from CLP lethality in mice predicted to die. IL-6 is a well-established marker of sepsis mortality (5, 8), which we used to stratify mice into two groups, as follows: mice predicted to live (P-live) and mice predicted to die (P-die). To determine our IL-6 cutoff values, a small aliquot of blood was collected in a nonterminal fashion from a subgroup of mice 24 h after CLP and subsequently analyzed for plasma IL-6 levels. Mice were then monitored for mortality over a 5-d period. In a retrospective analysis, we generated a receiver operator curve (GraphPad Prism 4.0) and determined an IL-6 cutoff value of 33 ng/ml or greater to predict 5-d mortality (Table II). Based on this IL-6 value, mice were separated into four experimental groups 32 h after CLP: P-live± antagonist and P-die± antagonist. Plasma was collected 24 and 48 h after MRS 1754 administration for cytokine analysis by ELISA. These studies were terminated after 9 d.
Table II.
Accuracy for IL-6 to predict 5-d survival of septic mice by receiver operator curve statistics
| Marker | Sensitivity (%) | Specificity (%)a | Area Under Curveb | SEM | 95% Confidence Interval |
|---|---|---|---|---|---|
| IL-6 | 77 | 97 | 0.974 | 0.019 | 0.936–1.01 |
A plasma IL-6 concentration of 33 ng/ml provided excellent discrimination between the mice that would live and the mice that would die in the first 5 d of sepsis.
Cutoff values were determined by favoring specificity to exclude the inclusion of animals with a low risk of lethality in the A2BR antagonist treatment subgroup.
The area under the curve gives the diagnostic value of IL-6 levels to determine outcome.
Blood collection and peritoneal content/cytology
The peritoneum was lavaged with a total of 5 ml ice-cold HBSS collected in 1-ml intervals. Each milliliter collected was strained using a 70-µm nylon filter (BD Biosciences, San Jose, CA) to remove peritoneal debris. The peritoneal fluid (PF) was centrifuged at 450 × g for 5 min to isolate the cell pellet. The cell pellet was immediately resuspended in 2 ml ice-cold RPMI 1640 media and kept on ice until further analysis. Cytospin slides were prepared (Shandon, Pittsburgh, PA) and stained with Diff-quick (Dade Behring, Newark, DE). An aliquot of cells was kept at room temperature in serum-free media for the phagocytosis assay. Trypan blue dye (Life Technologies, Grand Island, NY) exclusion was used to determine the total number of viable cells present in the PF. A maximum volume of 45 ml whole blood was collected via the submandibular vein. Whole blood was used to determine bacteremia and circulating cytokine levels after CLP, as described below. Serum or plasma for cytokine analysis was separated and stored at −80°C until future analysis.
Aerobic bacteria quantification
Bacteremia in CLP mice was determined in whole blood taken from the facial vein. First, the mandible was shaven and cleansed with 70% ethanol to prevent contamination of cultures by bacteria present on the skin. Then, the submandibular vein was punctured using a sterile 23-gauge needle to collect blood. A total of 25 µl undiluted whole blood was immediately plated for culture unless collected from a severely moribund mouse, which was diluted 2-fold in sterile saline for counting. Local bacterial load in CLP mice was determined by diluting 100 µl PF with sterile HBSS in 10-fold increments to a maximum dilution of 107. All samples were plated on sheep blood agar 5% tryptic soy agar monoplates (Remel, Lenexa, KS) and incubated overnight at 37°C under aerobic conditions. CFUs were determined by manual counting and multiplication by their dilution factor.
Cytokine production in peritoneal macrophages
To determine the effect of A2BR function on immune cell function, septic macrophages were isolated from PF obtained 24 h after CLP and stimulated with LPS ex vivo. Macrophages were isolated by culturing 1 × 106 peritoneal cells for 90 min in a 24-well culture plate. Cell cultures were washed three times with sterile PBS to remove nonadherent cells. Isolated, adherent macrophages were then stimulated with 100 ng/ml LPS in RPMI 1640 supplemented 10% FCS for 24 h. Supernatants were collected and stored at −80°C for future cytokine analysis.
IL-6, TNF-α, MIP-2, and IL-1β ELISA
Circulating cytokine levels and cytokines released by LPS-stimulated septic macrophages were determined by IL-6–, TNF-α–, and IL-1β–specific ELISA. Plasma MIP-2 levels were determined by MIP-2–specific ELISA (R&D Systems, Minneapolis, MN).
Cell culture media
FCS was heat inactivated in a 56°C water bath for 30 min. RPMI 1640 was added to make a 10% FCS culture media solution. Our media was supplemented with Pen-strep (100×) and gentamicin (50 mg/ml) to prevent microbial growth.
Opsonization/Phagocytosis assay
Heat-killed fluorescein-conjugated pHrodo Escherichia coli (Invitrogen, Molecular Probes, Carlsbad, CA) is designed specifically to assess phagocytosis with the use of flow cytometry. We used this technology to test for A2BR modulation of phagocytosis in sepsis. First, pHrodo E. coli were opsonized with purified rabbit polyclonal IgG Abs specific for the E. coli particles (Invitrogen, Molecular Probes) at 37°C for 1 h, according to manufacturer’s instructions. Approximately 4.0 × 105 PF cells isolated from septic mice were incubated with opsonized pHrodo E. coli at 37°C for 45 min at a ratio of 1:20 (PF cell:bacteria). The phagocytosis assay was stopped according to the manufacturer’s instructions. Flow cytometry was used to discriminate the phagocytic capacity of PF neutrophils and macrophages from septic mice. All steps of the phagocytosis assay were performed in serum-free media to avoid artificial alteration of phagocytic activity by exogenous factors present in serum.
Flow cytometric (FACS) analysis
FACS was used to analyze cell surface marker expression to identify and determine the phagocytic activity of macrophages and neutrophils obtained from the peritoneal cavity of septic mice. PF cells were washed, blocked with CD16/32 for 15 min, and stained for granulocyte marker FITC anti–Gr-1 (BD Biosciences, Franklin Lakes, NJ) and macrophage marker allophycocyanin anti-F4/80 (AbD Sertotec, Raleigh, NC) for 20 min at 4°C following the phagocytosis step in the phagocytosis assay. Stained cells were analyzed using BD FACSCalibur (BD Biosciences). Data were analyzed using BD CellQuest Pro software (BD Biosciences).
Statistical analysis
Survival significance was determined by Kaplan-Meier curve and log rank test. All values are expressed in mean ± SE. Student t test was used to compare data between two groups (GraphPad Prism 4.0).
Results
Pharmacological inhibition of A2BRs increases survival, enhances bacterial clearance, and decreases inflammation during polymicrobial sepsis in outbred mice
To test the hypothesis that A2BR signaling increases sepsis mortality and protects bacteria by decreasing antipathogen activity, we measured bacterial clearance and inflammatory mediators, and monitored survival in outbred mice treated with the A2BR antagonist, MRS 1754, and CLP mice given vehicle during bacterial sepsis. MRS 1754 (31) was shown to be selective for the A2BR in parallel assays in in vitro studies using A2AR KO and A2BR KO mice (data not shown). MRS 1754 administration was delayed until 1.5 h post-CLP, and was given simultaneously with antibiotic therapy once per day for 3 d. Combining the A2BR antagonist with antibiotic therapy mimics care that would be given in human clinical trials, in which all septic patients would also receive antibiotics. Pharmacological A2BR blockade significantly improved 28-d survival (Fig. 1A; MRS therapy 80% survival, vehicle therapy 43% survival, p < 0.05 by log rank survival). Insight into the mechanism of improved survival was sought by culturing the PF collected 24 h after CLP to determine the local bacterial load. All vehicle-treated mice had >107 bacterial CFUs/mouse, whereas none of the MRS 1754-treated mice exceeded this level (Fig. 1B). Blockade of the A2BR resulted in a >2-log reduction of peritoneal bacterial growth. Bacteremia is not observed in this model due to antibiotic administration (32), and bacteria were not detectable in the bloodstream (lower limit of detection 10 CFUs; data not shown).
Previous studies using the CLP model showed that plasma cytokine levels in the first 24 h correlate with mortality (33). A2BR antagonism reduced IL-6 and MIP-2 in the septic mice at both 6 and 24 h post-CLP when treated with a single dose of MRS 1754 therapy (Fig. 1C, 1D). This is consistent with our observation that A2BR blockade decreases sepsis mortality. Pharmacologic studies may lack specificity, and the dose of MRS 1754 may have also blocked A2AR. A2BR KO mice were studied to further verify the role of A2BR in sepsis mortality.
Genetic deficiency of the A2BR improves survival and cardiovascular function during sepsis
To confirm the findings using the A2BR antagonist in Fig. 1, we examined whether mice genetically deficient in the A2BR would have enhanced survival. Survival studies with the A2BR antagonist in WT C57BL/6 mice were performed to confirm the reproducibility of the findings in the same background strain as our A2BR KO mice. The ability of A2BR antagonism with MRS 1754 to decrease CLP lethality was indeed reproduced in C57BL/6 mice (Fig. 2A). We found administration of the A2BR antagonist MRS 1754 to be very effective in protecting C57BL/6 mice from CLP lethality over 28 d. Mice that received MRS (0.5 mg/kg) once per day for 3 d had a 71% survival (10 of 14 mice) compared with a 21% survival (3 of 14 mice) of those that did not receive the antagonist.
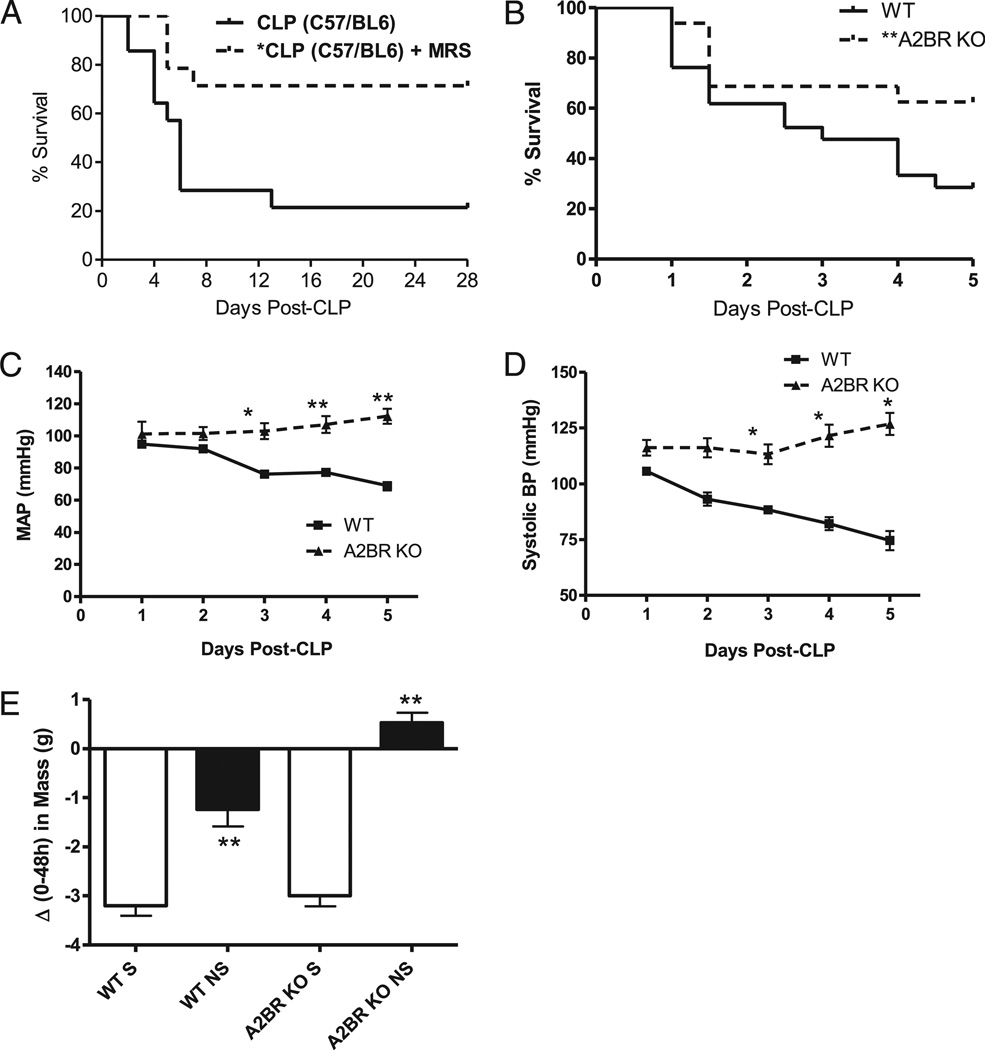
FIGURE 2.
A2BR-deficient mice are resistant to CLP mortality and cardiovascular dysfunction. A, The ability of MRS 1754 (0.5 mg/kg) to improve CLP survival in C57BL/6 mice given antibiotics plus fluid resuscitation was confirmed in this mouse strain (n = 14 per group; p < 0.01). B, Survival curve for WT (solid line; n = 21) and A2BR KO (broken line; n = 27) mice subjected to CLP. Antibiotics were withheld from this group to evaluate A2BR’s role in bacterial clearance. The CLP survival graph depicts data pooled from at least three independent studies. Log rank analysis shows a significant improvement in survival, p < 0.05. A2BR deficiency improves cardiovascular parameters in CLP mice. CLP was performed in WT (n = 6) and A2BR KO (n = 3) mice previously implanted with a radiotelemetry transmitter. Cardiovascular parameters including MAP (C), and systolic BP (D) were collected over a 24-h time period and quantified. E, Body mass was recorded at the time of CLP, and every 24 h thereafter, for 5 d. The graph depicts a post hoc analysis of the change in body mass over 48 h after CLP of mice stratified into either 5-d survivors (S) or nonsurvivors (NS). Data are expressed as mean ± SEM of at least three independent experiments. *p < 0.05, **p < 0.01 compared with control.
To understand the mechanisms of A2BR pathology in sepsis, antibiotic treatment was withheld in all studies with the genetically modified mice. A2BR KO mice were monitored for CLP mortality over 5 d, the time frame in which the majority of CLP-induced deaths occur (Figs. 1A, 2A). Because sepsis is a complex disease affecting multiple organ systems (reviewed in Ref. 4), we monitored not only immunological and antibacterial, but also the hemodynamic stability of infected animals. Indeed, in addition to immune dysfunction, the sepsis syndrome also includes septic shock, a profound decrease in cardiovascular function responsible for the high sepsis mortality rates. Parallel measurements of different immunological and cardiovascular parameters were made to allow better insights into multiorgan failure and its prevention by targeting the A2BR.
A2BR KO mice were resistant to CLP lethality compared with WT mice (Fig. 2B), and the A2BR-deficient mice had significantly better cardiovascular function after CLP-induced sepsis. Using an implantable radiotelemetry device, we determined the effect of A2BR deficiency on multiple cardiovascular parameters over a 24-h time period after CLP. Significant hemodynamic differences were observed within this time period. A2BR-deficient mice maintained their mean arterial blood pressure (MAP; Fig. 2C), and also maintained their systolic blood pressure (BP) (Fig. 2D). Additionally, A2BR mice had an increased heart rate compared with WT mice (data not shown). All of these changes were apparent within the first 6–12 h of sepsis, which corresponds to the time interval when inflammatory changes differentiate between mice that live and die (34). The changes also persisted throughout the entire 24 h of the study. Importantly, baseline differences in MAP and systolic BP were not found between WT and A2BR-deficient mice, and these differences only became apparent under the stress of the septic response. Moribund mice characteristically retain the resuscitation fluid as a result of hemodynamic failure, which may be easily followed by daily measurements of body weight. For this analysis, mice were divided into those who survived the septic insult and those who did not survive. No differences in the change in body weight between WT and A2BR KO survivors were detected. Interestingly, the A2BR KO nonsurvivors retained more body fluid compared with WT nonsurvivors, supporting the role of A2BR regulation of fluid transport across the vascular endothelium (35). Thus, studies of mice with A2BR deficiency show that targeting A2BR for elimination decreased the CLP-induced mortality and adverse hemodynamic alterations associated with the progression to septic shock.
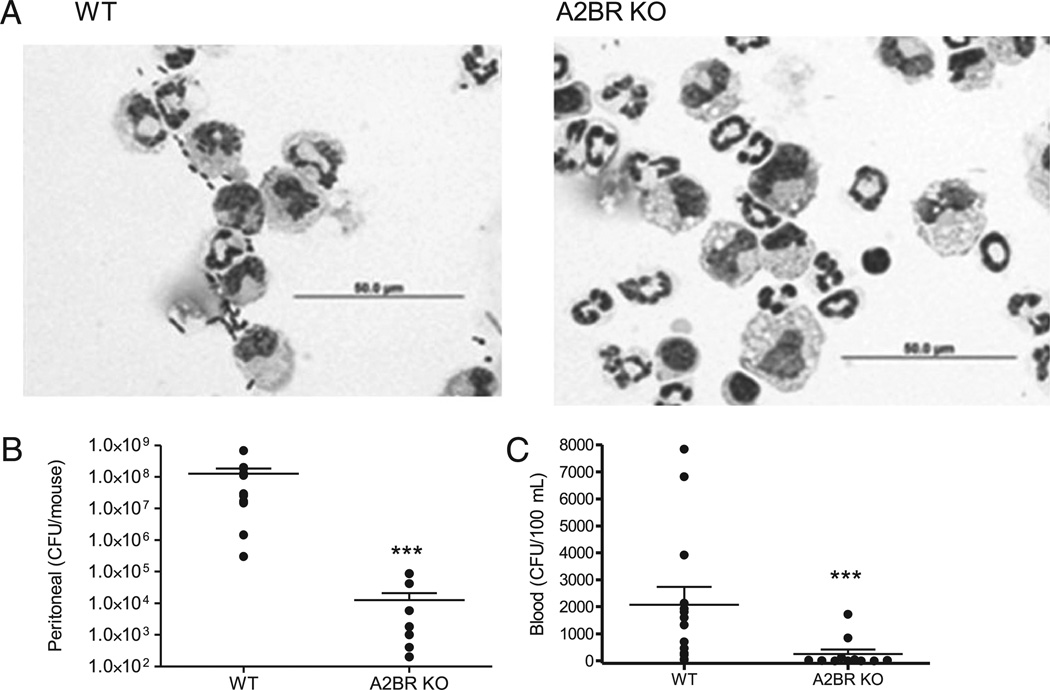
A2BR deficiency enhances bacterial clearance in sepsis
To determine whether A2BR deficiency increased pathogen elimination, local and systemic bacteria levels were quantified 24 h after CLP. In the CLP model, antibiotics prevent detectable bacteremia, but do not completely eradicate the local growth of bacteria. Thus, antibiotics were withheld to quantify bacteremia. A2BR deficiency profoundly enhanced bacterial clearance in septic mice. Photomicrographs of lavage fluid from the peritoneal cavity show the presence of bacteria in WT mice that were virtually absent in A2BR KO mice (Fig. 3A). Further analysis showed an astounding 4-log reduction in peritoneal bacterial load in A2BR KO mice compared with WT mice (Fig. 3B). Because antibiotics were not used in this model, bacteremia could be quantified. Bacteremia was significantly reduced in A2BR KO mice compared with WT mice (expressed as CFUs/100 µl blood; Fig. 3C). To determine whether the reduced bacterial levels were due to increased local cell recruitment, inflammatory cells within the peritoneum were measured. A2BR deficiency did not increase recruitment of macrophages or neutrophils to the peritoneum of septic mice. Interestingly, there was an increase in the total number of lymphocytes recovered from A2BR-deficient mice compared with WT mice, which may be the result of enhanced polyclonal T cell expansion or reduced lymphocyte apoptosis (Table I).
FIGURE 3.
A2BR deficiency improves local and systemic bacterial clearance. A, Photomicrographs of peritoneal cells from WT or A2BR KO mice showing the presence of neutrophils, macrophages, and bacteria. Fewer bacteria are visible in the A2BR KO mice. Scale bar, 50 µm. B, Peritoneal bacterial load was decreased in A2BR-deficient mice. The local bacterial load was determined in the peritoneal lavage collected from CLP mice 24 h after CLP. C, Bacteremia was decreased 24 h after CLP in A2BR-deficient mice. Data in B and C are expressed as mean ± SEM of 11 or more mice in at least three independent experiments, and each symbol is an individual animal. ***p < 0.001 WT versus A2BR KO mice.
Table I.
Genetic deletion of A2BR does not significantly decrease recruitment of innate inflammatory cells to the site of infection
| Total Cell Count | Neutrophil | Macrophage | Lymphocyte | |
|---|---|---|---|---|
| WT | 10.9 ± 1.5 × 106 | 6.3 ± 0.1 × 106 | 3.7 ± 0.6 × 106 | 0.4 ± 0.1 × 105 |
| A2BR KO | 8.5 ± 0.8 × 106 | 6.2 ± 0.7 × 106 | 2.0 ± 0.3 × 106 | 1.7 ± 0.4 × 105* |
PF was analyzed from A2BR KO and WT mice 24 h post-CLP, and the number of inflammatory cells was quantified. Data were expressed as mean ± SEM of two independent experiments (n = 6).
p < 0.05 between groups.
A2BR deficiency enhances macrophage-mediated, but not neutrophil-mediated microbicidal activity
The results in Table I demonstrate that phagocyte recruitment to the site of infection was not increased in the A2BR KO mice, suggesting that the KO mice have improved innate cell function leading to augmented bacterial clearance. Studies were performed on peritoneal macrophages and neutrophils collected from septic mice 24 h after CLP.
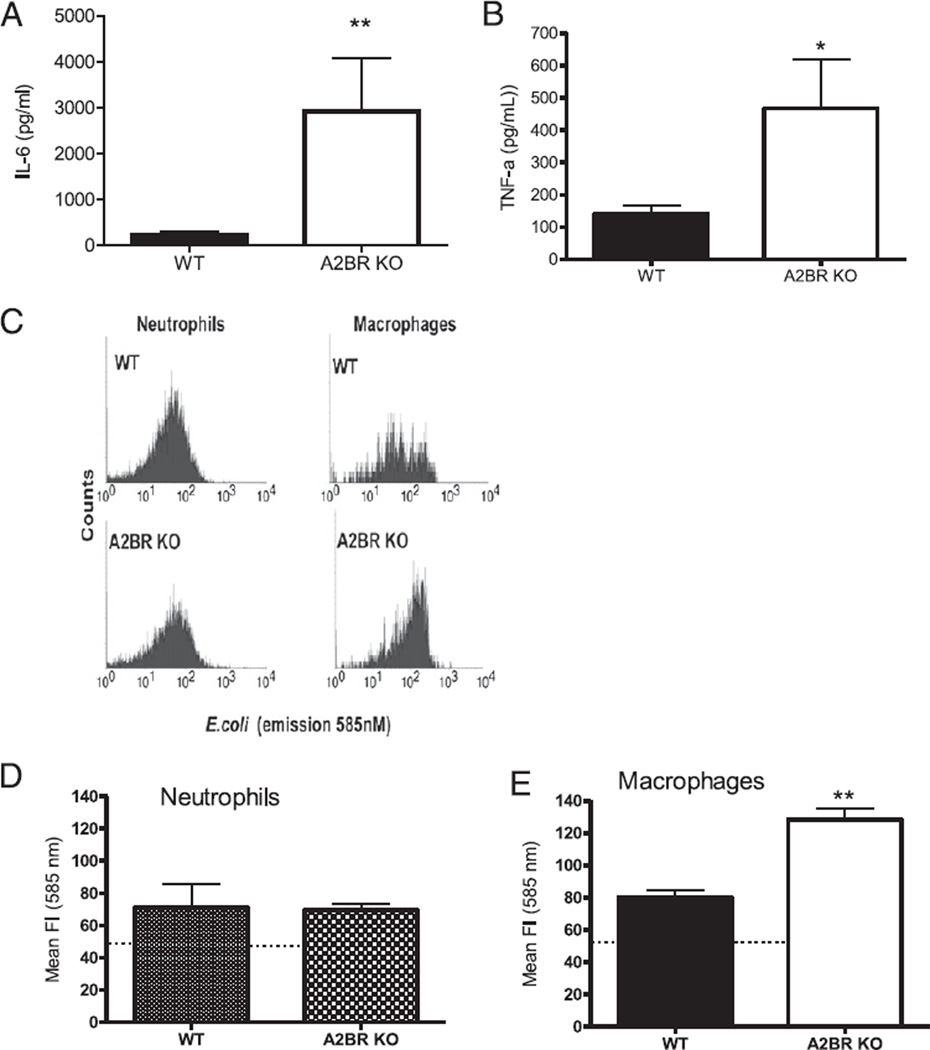
To test the effect of A2BRs on macrophage cytokine production, macrophages isolated from septic WT and A2BR KO mice were stimulated with LPS, and cytokine levels in the supernatant were determined after 24 h. The presence of A2BRs inhibited LPSstimulated cytokine release as supernatants from A2BR-deficient macrophages had increased IL-6 and TNF-α levels (Fig. 4A, 4B). Levels of IL-1β were not detectable (data not shown). Next, the effect of A2BR deficiency on the capacity of macrophages and neutrophils from septic mice to engulf bacteria was determined. Total PF cells were collected from septic mice and incubated with IgG-opsonized, pH-sensitive fluorescein-conjugated E. coli. In this assay, the fluorescent signal increases in low pH environments. The relatively low pH of the phagolysosome allows flow cytometric detection of E. coli that has been phagocytosed and incorporated into the phagolysosome as opposed to adherent/nonendocytosed bacteria. Bacteria present within the phagolysosome are destined for killing in this harsh environment. Flow cytometry with appropriate cell surface markers distinguishes neutrophil from macro-phage phagocytosis of bacteria. Representative histograms of neutrophil and macrophage bacterial phagocytosis are shown in Fig. 4C. Surprisingly, the capacity of neutrophils (Gr-1highF4/80negative) to phagocytize bacteria from WT mice was virtually identical to the A2BR-deficient mice (Fig. 4D). However, A2BR deficiency nearly doubled the phagocytosis activity of macrophages (Gr-1lowF4/80positive) compared with WT-derived macrophages (Fig. 4E). We believe that this is a novel finding, that macrophage A2BR expression inhibits bacterial phagocytosis in sepsis.
FIGURE 4.
A2BR deficiency enhances macrophage function. Peritoneal cells used in these experiments were analyzed from either A2BR KO or WT mice subjected to CLP and harvested 24 h later. LPS stimulation enhanced cytokine release from macrophages isolated from septic A2BR-deficient mice. Isolated peritoneal macrophages from septic mice were stimulated ex vivo with 100 ng/ml LPS for 24 h. Supernatants were measured for IL-6 (A) and TNF-α (B) by ELISA. Data are expressed as mean ± SEM of three independent experiments. Gated neutrophil and macrophage populations were identified as Gr-1highF4/80negative and Gr-1lowF4/80positive to discriminate between neutrophil and macrophage bacteria phagocytosis activity, respectively, from other cell types. C, Histogram of gated neutrophil and macrophage populations showing the phagocytic/endocytic capacity of phagocytes from septic WT and A2BR KO mice preincubated with the pH-sensitive fluorogenic E. coli. E. coli present inside the phagolysosome are highly fluorescent relative to nonendocytosed E. coli. Quantitative analysis of the phagocytic activity for neutrophils (D) (Gr-1highF4/80negative) and macrophages (E) (Gr-1lowF4/80positive) from septic WT and A2BR KO mice. *p < 0.05, **p < 0.01 compared with WT CLP mice.
Myeloid-specific deletion of the A2BR enhances bacterial clearance
To confirm the contribution of A2BR expression on myeloid cells (i.e., macrophages and neutrophils) in impairing bacterial clearance in vivo, we generated mice that selectively lack the A2BR in myeloid cell lineages (A2BRflox/flox × LysM cre+/−) and quantified local and systemic bacteria levels 24 h after CLP. The specificity of A2BR deletion in myeloid cells was confirmed using RT-PCR (Supplemental Fig. 2). The absence of A2BRs on myeloid cells significantly enhanced bacterial clearance in septic mice. A2BRflox/flox LysM-cre+/− mice had a significantly reduced peritoneal bacterial load compared with WT (A2BRflox/flox) mice (Fig. 5A). These findings are consistent with the reduction of peritoneal bacteria (Fig. 3B) and enhanced macrophage phagocytosis (Fig. 4E) in total A2BR KO septic mice. The use of antibiotics was withheld in this model, so bacteremia could be quantified. Bacteremia was significantly reduced in A2BRflox/flox LysM-cre+/− mice compared with WT (A2BRflox/flox) mice (expressed as CFUs/100 µl; Fig. 5B).
Delayed A2BR antagonist treatment reduces mortality in mice predicted to die of sepsis
To determine whether administration of an A2BR antagonist can rescue mice with a high probability of dying from sepsis, mice were separated by the proven sepsis mortality biomarker, IL-6 (5, 8). Plasma levels >33 ng/ml obtained 24 h after CLP predicted death, and this cutoff value was chosen to reduce false positives (Table II; 97% specificity for mortality). After stratification based on predicted mortality, mice were randomly divided into vehicle or antagonist therapy. This resulted in four groups, as follows: P-live given vehicle, P-live treated with MRS 1754 (P-live + MRS), P-die given vehicle, and P-die treated with MRS 1754 (P-die + MRS 1754). All mice received fluid resuscitation and antibiotic therapy before the therapeutic intervention (MRS 1754) was given to determine the effects of A2BR blockade beyond the standard of care for sepsis trials. Therapeutic intervention was given 32 h after CLP, that is, at a time point when the majority of mice would die within the next 16 h; Fig. 6A displays the experimental protocol. In P-live mice, A2BR antagonism did not alter mortality (Fig. 6B), indicating that the treatment did not demonstrate significant toxicity. In P-die mice, A2BR antagonism improved survival from 0% in vehicle-treated mice to 56% (Fig. 6C; p < 0.01). Furthermore, A2BR antagonist therapy protection lasted past the P-die 5-d period, conferring lasting protection against CLP lethality.
Discussion
Sepsis is a clinical syndrome that carries a high financial burden and an unacceptably high mortality rate (36, 37). The current study took advantage of recent insights in the regulation of inflammation by hypoxia and adenosine that established a detrimental role of A2BRs in sepsis. The validity of the role of adenosine was tested in the clinically relevant model of sepsis that allows detailed examination of therapeutic interventions and determination of mechanisms to improve survival.
These studies uncovered a critical pathogenic role for the A2BR in promoting cellular dysfunction that impairs the innate immune system in sepsis. Modulation of A2BRs, either by genetic ablation or a receptor antagonist, documented that A2BRs inhibited bacterial clearance, promoted circulatory collapse, and decreased resistance to CLP lethality. Our in vivo and ex vivo findings that A2BR-mediated immune suppression prevented adequate bacterial clearance highlight an important pathophysiological dilemma posed by A2BR-mediated mechanisms. Our data are in accord with previous work demonstrating the immunosuppressive characteristic of adenosine as a physiological mechanism to prevent inflammatory-induced tissue damage (16, 20, 38). The apparent paradox of adenosine to protect the host while safeguarding pathogenic bacteria can be explained by the conserved role for adenosine to prevent self-inflicted tissue damage during inflammation. In most cases, the immune system effectively eliminates the infection. However, as inflammation ensues, microvascular blood flow may be impaired, leading to increases in extracellular adenosine levels subsequent to local tissue hypoxia (reviewed in Ref. 39). Additional cellular contributions of extracellular adenosine are derived from adenosine-generating enzymes expressed on the surface of host cells that are up-regulated upon the stabilization of hypoxia inducible factor 1 in response to hypoxia and reactive oxygen species (40). Further amplification of adenosine signaling is mediated by the transcriptional activation of the A2BR promoter by hypoxia inducible factor 1 to increase A2BR surface expression (41). The energy expended to activate the hypoxia–adenosinergic pathway in inflamed tissue is an evolutionarily conserved negative feedback mechanism aimed to quell the host’s immune system.
Numerous studies demonstrate the important tissue-protective role of A2AR/A2BRs during acute inflammation. However, adenosine signaling can be a double-edged sword. Indeed, we identify that A2BR signaling impairs host antimicrobial defenses in septic mice, which is explained by increases in extracellular adenosine subsequent to normal physiological responses to inflammation and infection. Some bacteria have adapted to exploit this immunosuppressive pathway to increase their chances of survival (42).
In contrast to our findings, a similar study by Csóka et al. (24) showed that A2BRs dampened the systemic inflammatory response syndrome and improved survival. The authors did not report a significant effect of the A2BR on bacterial clearance as in our study. There are similarities in the studies, because a portion of both studies was performed in mice with a C57BL/6 background. However, methodological differences to induce peritonitis may provide an explanation for the disparate results because we document substantially greater numbers of bacteria in the peritoneal cavity and greater 5-d mortality. A2BR activation may lead to different outcomes that are dependent on the number of bacteria and expected outcome. In experiments with a high bacterial load and high mortality (current studies), A2BR activation increases mortality, whereas opposite data are obtained with a smaller inflammatory challenge. This phenomenon of differential outcome based on the intensity of the inflammation has been previously reported in septic patients (43).
The concept that microbes use a normal host response to subvert the immune system has been previously reported. In the late 1980s, the vaccinia virus was found to encode viral proteins that are secreted from infected host cells to inhibit components of the complement system to escape host immunity (44). Since then, viral genes encoding human anti-inflammatory cytokine analogs, termed virokines, have been identified in an array of viruses, as follows: CMV viral (v)IL-10, parapoxvirus (vIL-10), HSV-8 (vIL-6), orthopoxvirus (vIL-1R), etc. (reviewed in Ref. 45).
Bacteria are not foreign to utilizing self-preservation mechanisms to exploit immunosuppressive pathways while occupying its host. A recent study by Thammavongsa et al. (42) identified bacterial genes encoding surface adenosine synthase A (AdsA) that converts AMP to adenosine in Staphylococcus aureus and an AdsA analog in Bacillus anthracis DNA. Genetic deletion of bacterial AdsA decreased abscess formation and numbers of bacteria recovered from S. aureus-infected mice. This study identified pathogenic sources to generate extracellular adenosine as a means to subvert the immune system to increase bacterial survival. Based on our findings, A2BR-mediated immune suppression participates in this mechanism of immune evasion. Cytokine changes over the first 5 d after CLP are relatively stable. Low levels of IL-6 remain stable in mice that live for 5 d (8, 33, 46). The ability of A2BR blockade to reduce circulating IL-6 levels by 24 h may be explained by the reduction of bacterial load, and reflects that mice are going to live. Hence, immunosuppressive signaling by the A2BR is partially responsible for the protection of not only host tissue, but also bacteria, from the immune system that promotes sepsis mortality. Although past studies support the recruitment of A2BRs to prevent excessive collateral tissue damage during inflammation, we suggest pharmacological intervention using an A2BR antagonist is appropriate to enhance functions of the innate immune system in clinical situations in which immune evasion is a threat.
To better understand the mechanisms of A2BR-mediated immune suppression in the presence of bacterial infection, we studied the phagocytic capacity of neutrophils and macrophages in the absence of A2BRs. In addition to past studies showing the immunosuppressive effects of A2BR signaling on the immune system, insights that A2BRs impair host defenses can be extrapolated from previous studies showing that A2BRs promote allergic-type immune responses (23). One of our unexpected findings was that neutrophil-mediated phagocytosis of bacteria was not affected by A2BR deficiency, indicating that neutrophils are not responsible for the enhanced microbial clearance seen in A2BR-deficient mice. A2BR-mediated suppression of myeloid cell antimicrobial defenses was confirmed by selectively knocking out A2BRs on myeloid cells. The overall reduction of bacteria in A2BRflox/flox LysM-cre+/− mice was not as dramatic as the bacterial clearance seen in total A2BR KO mice. These data suggest synergy between myeloid and nonmyeloid cell defenses against bacteria is suppressed by A2BR signaling. Our studies identify A2BRs as contributors to macrophage dysfunction that can be prevented by the removal of A2BR signaling pathways, and results in enhanced phagocytosis of bacteria. The finding that A2BRs suppress macrophage function during bacterial infections is novel.
These findings are supported by previous studies demonstrating abundant A2BR mRNA (47) and protein expression by murine macrophages (20), but not neutrophils. Furthermore, the oxidative burst of neutrophils stimulated with fMLP is reduced specifically by A2AR agonism, and not by A2BR agonism (47), thereby adding specificity of A2BR modulation of innate responses. Conversely, A2BRs have been shown to regulate reactive oxygen species generation in nonimmune cell types in response to inflammation (18, 29), suggesting that the mechanism of enhanced bacterial killing in A2BR-deficient mice may include increases in reactive oxygen species generation. Recently, netrin-1 regulation of the innate immune system was shown to be dependent on A2BR expression. Netrin-1 requires the A2BR to dampen neutrophil endothelial transmigration to hypoxic tissue (48). This study attests to the critical role that A2BRs play to downregulate inflammation. In accord, our studies show impaired phagocytosis via the A2BR protects bacteria from elimination. Perhaps abolishing netrin-1 signaling by A2BR blockade has an additive effect to enhance antibacterial defenses in our studies. Future studies of netrin-1 signaling in septic mice would be informative. Identification of A2BRs that reduce the phagocytic capacity of septic macrophages has potential implications for the treatment of sepsis and other immunological disorders that share an infectious etiology, such as Crohn’s disease (49).
In addition to the immunological alterations, the cardiovascular system adapts in response to infection. Dysregulation of cardiovascular parameters may be detrimental to the host. The regulation of cardiovascular responses during infection points to the downstream effects of A2BR activation on VSM cells, because proinflammatory cytokines are known to induce its A2BR expression (29). Activation of other Gs protein-coupled receptors such as β2-adrenergic on VSM cells leads to vasodilation that is caused by the downstream effects of the intracellular signaling molecule cAMP. Previous studies show convergence of β2-adrenergic and A2BR signaling cascades to work synergistically as these two receptor pathways function independently to increase cAMP in a model of ventilator-induced acute lung injury (35). In fact, we show that A2BR KO mice are more resistant to hypotension caused by CLP that is associated with poor prognosis. Therefore, it is plausible that A2BRs on VSM cells promote the progression toward mortality by increasing intracellular cAMP levels, resulting in decreased vascular tone, widespread vasodilation, septic shock, and death. Our results that suggest a role for A2BRs to promote septic shock are novel.
We believe that therapy aimed to restore innate and cardiovascular function targeted through A2BR antagonism may represent a novel treatment strategy for the treatment of sepsis, and potentially other infectious diseases. The studies show that the biological role of adenosine as a tissue protector also mediates bacterial evasion of innate defenses via A2BR signaling. Additionally, decreased hemodynamic stability in the presence of A2BRs suggests that A2BR antagonist treatment to vasopress may stabilize falling BP, and reduce rates of septic shock. Furthermore, our pharmacological studies show that adenosine signaling through the A2BR is both an early and late mediator of sepsis mortality, providing a unique opportunity to successfully treat sepsis by late administration of an A2BR antagonist.
In this study, we identified an important A2BR-adenosinergic mechanism that limits antibacterial clearance and thereby promotes the pathogenesis of sepsis. Our preclinical studies in mice provide the proof of principle for a novel approach to enhance survival during sepsis through blockade of the A2BR.
Supplementary Material
Acknowledgments
This work was supported by Grants R21 AT002788 (to M.S.), R01 GM 82962 (to D.G.R.), and T32 HL007501 by the National Institutes of Health.
We thank Louis Vaickus for helpful suggestions in the preparation of this manuscript.
Abbreviations used in this article
- A2AR
A2A adenosine receptor
- A2BR
A2B adenosine receptor
- AdsA
adenosine synthase A
- BP
blood pressure
- CLP
cecal ligation and puncture
- ES
embryonic stem
- KO
knockout
- MAP
mean arterial blood pressure
- MRS 1754
8-[4-[((4-cyanophenyl)carbamoylmethyl)oxy]phenyl]-1,3-di(n-propyl)xanthine hydrate
- P-die
predicted to die
- PF
peritoneal fluid
- P-live
predicted to live
- v
viral
- VSM
vascular smooth muscle
- WT
wild-type
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Bernard GR, Margolis BD, Shanies HM, Ely EW, Wheeler AP, Levy H, Wong K, Wright TJ Extended Evaluation of Recombinant Human Activated Protein C United States Investigators. Extended evaluation of recombinant human activated protein C United States Trial (ENHANCE US): a single-arm, phase 3B, multicenter study of drotrecogin alfa (activated) in severe sepsis . Chest. 2004;125:2206–2216. doi: 10.1378/chest.125.6.2206. [DOI] [PubMed] [Google Scholar]
- 2.Laterre PF, Levy H, Clermont G, Ball DE, Garg R, Nelson DR, Dhainaut JF, Angus DC. Hospital mortality and resource use in subgroups of the Recombinant Human Activated Protein C Worldwide Evaluation in Severe Sepsis (PROWESS) trial. Crit. Care Med. 2004;32:2207–2218. doi: 10.1097/01.ccm.0000145231.71605.d8. [DOI] [PubMed] [Google Scholar]
- 3.Remick DG. Pathophysiology of sepsis. Am. J. Pathol. 2007;170:1435–1444. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buras JA, Holzmann B, Sitkovsky M. Animal models of sepsis: setting the stage. Nat. Rev. Drug Discov. 2005;4:854–865. doi: 10.1038/nrd1854. [DOI] [PubMed] [Google Scholar]
- 5.Osuchowski MF, Connett J, Welch K, Granger J, Remick DG. Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Crit. Care Med. 2009;37:1567–1573. doi: 10.1097/CCM.0b013e31819df06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward PA, Gao H. Sepsis, complement, and the dysregulated inflammatory response. J. Cell Mol. Med. 2009;13:4154–4160. doi: 10.1111/j.1582-4934.2009.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yano K, Liaw PC, Mullington JM, Shih SC, Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, et al. Vascular endothelial growth factor is an important determinant of sepsis morbidity and mortality. J. Exp. Med. 2006;203:1447–1458. doi: 10.1084/jem.20060375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbull IR, Javadi P, Buchman TG, Hotchkiss RS, Karl IE, Coopersmith CM. Antibiotics improve survival in sepsis independent of injury severity but do not change mortality in mice with markedly elevated interleukin 6 levels . Shock. 2004;21:121–125. doi: 10.1097/01.shk.0000108399.56565.e7. [DOI] [PubMed] [Google Scholar]
- 9.Marshall JC. Such stuff as dreams are made on: mediator-directed therapy in sepsis. Nat. Rev. Drug Discov. 2003;2:391–405. doi: 10.1038/nrd1084. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N. Engl. J. Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc. Natl. Acad. Sci. USA. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayala A, Chaudry IH. Immune dysfunction in murine polymicrobial sepsis: mediators, macrophages, lymphocytes and apoptosis. Shock. 1996;6(Suppl. 1):S27–S38. [PubMed] [Google Scholar]
- 13.Munoz C, Carlet J, Fitting C, Misset B, Blériot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. J. Clin. Invest. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cronstein BN, Kubersky SM, Weissmann G, Hirschhorn R. Engagement of adenosine receptors inhibits hydrogen peroxide (H2O2−) release by activated human neutrophils. Clin. Immunol. Immunopathol. 1987;42:76–85. doi: 10.1016/0090-1229(87)90174-7. [DOI] [PubMed] [Google Scholar]
- 15.Reutershan J, Cagnina RE, Chang D, Linden J, Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J. Immunol. 2007;179:1254–1263. doi: 10.4049/jimmunol.179.2.1254. [DOI] [PubMed] [Google Scholar]
- 16.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 17.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol. Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 18.Yang D, Koupenova M, McCrann DJ, Kopeikina KJ, Kagan HM, Schreiber BM, Ravid K. The A2b adenosine receptor protects against vascular injury. Proc. Natl. Acad. Sci. USA. 2008;105:792–796. doi: 10.1073/pnas.0705563105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linden J. New insights into the regulation of inflammation by adenosine. J. Clin. Invest. 2006;116:1835–1837. doi: 10.1172/JCI29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St. Hilaire C, Seldin DC, Toselli P, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J. Clin. Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thiel M, Chouker A, Ohta A, Jackson E, Caldwell C, Smith P, Lukashev D, Bittmann I, Sitkovsky MV. Oxygenation inhibits the physiological tissue-protecting mechanism and thereby exacerbates acute inflammatory lung injury. PLoS Biol. 2005;3:e174. doi: 10.1371/journal.pbio.0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryzhov S, Zaynagetdinov R, Goldstein AE, Novitskiy SV, Dikov MM, Blackburn MR, Biaggioni I, Feoktistov I. Effect of A2B adenosine receptor gene ablation on proinflammatory adenosine signaling in mast cells. J. Immunol. 2008;180:7212–7220. doi: 10.4049/jimmunol.180.11.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CsÓka B, Németh ZH, Rosenberger P, Eltzschig HK, Spolarics Z, Pacher P, Selmeczy Z, KoscsÓ B, Himer L, Vizi ES, et al. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J. Immunol. 2010;185:542–550. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Németh ZH, CsÓka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch EA, Pacher P, Spolarics Z, HaskÓ G. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J. Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Echtenacher B, Männel DN, Hültner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 27.HaskÓ G, CsÓka B, Németh ZH, Vizi ES, Pacher P. A(2B) adenosine receptors in immunity and inflammation. Trends Immunol. 2009;30 doi: 10.1016/j.it.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.HaskÓ G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.St. Hilaire C, Koupenova M, Carroll SH, Smith BD, Ravid K. TNF-alpha upregulates the A2B adenosine receptor gene: the role of NAD(P)H oxidase 4. Biochem. Biophys. Res. Commun. 2008;375:292–296. doi: 10.1016/j.bbrc.2008.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall JC, Innes M. Intensive care unit management of intraabdominal infection. Crit. Care Med. 2003;31:2228–2237. doi: 10.1097/01.CCM.0000087326.59341.51. [DOI] [PubMed] [Google Scholar]
- 31.Kim SA, Marshall MA, Melman N, Kim HS, Mu¨ller CE, Linden J, Jacobson KA. Structure-activity relationships at human and rat A2B adenosine receptors of xanthine derivatives substituted at the 1-, 3-, 7-, and 8-positions. J. Med. Chem. 2002;45:2131–2138. doi: 10.1021/jm0104318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newcomb D, Bolgos G, Green L, Remick DG. Antibiotic treatment influences outcome in murine sepsis: mediators of increased morbidity. Shock. 1998;10:110–117. doi: 10.1097/00024382-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Osuchowski MF, Welch K, Yang H, Siddiqui J, Remick DG. Chronic sepsis mortality characterized by an individualized inflammatory response. J. Immunol. 2007;179:623–630. doi: 10.4049/jimmunol.179.1.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemzek JA, Xiao HY, Minard AE, Bolgos GL, Remick DG. Humane endpoints in shock research. Shock. 2004;21:17–25. doi: 10.1097/01.shk.0000101667.49265.fd. [DOI] [PubMed] [Google Scholar]
- 35.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J. Clin. Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit. Care Med. 2007;35:1244–1250. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 37.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Day YJ, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J. Immunol. 2005;174:5040–5046. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- 39.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu. Rev. Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 40.Eltzschig HK, Thompson LF, Karhausen J, Cotta RJ, Ibla JC, Robson SC, Colgan SP. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 41.Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 42.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J. Exp. Med. 2009;206:2417–2427. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reinhart K, Menges T, Gardlund B, Harm Zwaveling J, Smithes M, Vincent JL, Tellado JM, Salgado-Remigio A, Zimlichman R, Withington S, et al. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: The RAMSES Study. Crit. Care Med. 2001;29:765–769. doi: 10.1097/00003246-200104000-00015. [DOI] [PubMed] [Google Scholar]
- 44.Kotwal GJ, Moss B. Vaccinia virus encodes a secretory polypeptide structurally related to complement control proteins. Nature. 1988;335:176–178. doi: 10.1038/335176a0. [DOI] [PubMed] [Google Scholar]
- 45.Smith SA, Kotwal GJ. Virokines: novel immunomodulatory agents. Expert Opin. Biol. Ther. 2001;1:343–357. doi: 10.1517/14712598.1.3.343. [DOI] [PubMed] [Google Scholar]
- 46.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect. Immun. 2006;74:5227–5235. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 48.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat. Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 49.Smith AM, Rahman FZ, Hayee B, Graham SJ, Marks DJ, Sewell GW, Palmer CD, Wilde J, Foxwell BM, Gloger IS, et al. Disordered macrophage cytokine secretion underlies impaired acute inflammation and bacterial clearance in Crohn’s disease. J. Exp. Med. 2009;206:1883–1897. doi: 10.1084/jem.20091233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.