Abstract
Advances in our understanding of stem cells in the gastrointestinal tract include the identification of molecular markers of stem and early progenitor cells in the small intestine. Although gastric epithelial stem cells have been localized, little is known about their molecular biology. Recent reports describe the use of inducible Cre recombinase activity to indelibly label candidate stem cells and their progeny in the distal stomach, (ie, the antrum and pylorus). No such lineage labeling of epithelial stem cells has been reported in the gastric body (corpus). Among stem cells in the alimentary canal, those of the adult corpus are unique in that they lie close to the lumen and increase proliferation following loss of a single mature progeny lineage, the acid-secreting parietal cell. They are also unique in that they neither depend on Wnt signaling nor express the surface marker Lgr5. Because pathogenesis of gastric adenocarcinoma has been associated with abnormal patterns of gastric differentiation and with chronic tissue injury, there has been much research on the response of stomach epithelial stem cells to inflammation. Chronic inflammation, as induced by infection with Helicobacter pylori, affects differentiation and promotes metaplasias. Several studies have identified cellular and molecular mechanisms in spasmolytic polypeptide–expressing (pseudopyloric) metaplasia. Researchers have also begun to identify signaling pathways and events that take place during embryonic development that eventually establish the adult stem cells to maintain the specific features and functions of the stomach mucosa. We review the cytologic, molecular, functional, and developmental properties of gastric epithelial stem cells.
Keywords: Stomach Stem Cells, Epithelial Self-Renewal, Tissue Metaplasia
The self-renewing epithelium of the stomach body contains 4 types of terminally differentiated cells that are replaced at different rates: oxyntic (parietal) cells, zymogenic (chief) cells, surface mucous foveolar (pit) cells, and hormone-secreting enteroendocrine cells. Mucous neck cells can function in a secretory capacity and as an intermediate progenitor for chief cells (Figures 1–3).1–10 The gastric antrum has few parietal or chief cells but has a separate population of alkaline, mucus-producing cells near the base of gland units that, in terms of molecular marker expression, resemble corpus mucous neck cells (Figure 1).11,12 A transitional zone separates the stereotypic corpus and antral/pyloric epithelia and has features of each.13 There is consensus that all gastric mucosal cells originate from stem cells,2,14 although stem cell properties differ in the corpus and antrum. Gastric epithelial stem cells share many properties with intestinal epithelial stem cells, which have been studied more extensively, but it is also becoming clear that they differ in fundamental respects.
Figure 1.
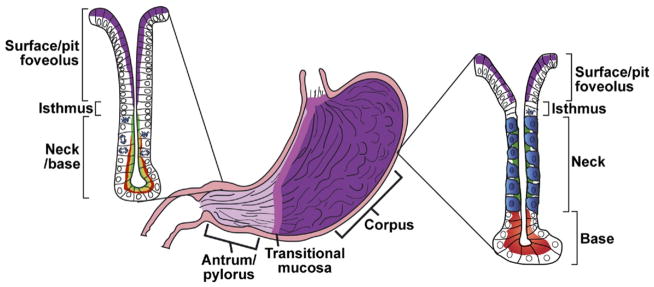
Typical anatomy and histology of a mammalian stomach. There are a number of variations in mammalian gastric anatomy. For example, mice have a forestomach with keratinized squamous epithelium, whereas humans have a pronounced cardiac region with simpler mucous glands that mark the transition region between the esophagus and corpus. However, the most prominent regions in most mammals are a proximal corpus, encompassing most of the stomach volume, and a distal antrum or pylorus. The corpus epithelium is organized into repeating gastric units that are invaginations from the surface and contain multiple cell lineages in 4 distinct zones. In the diagram, acid-secreting parietal cells are blue, digestive enzyme secreting zymogenic (chief) cells are red, mucous neck cells are green, and the mucus-secreting pit cells nearest the surface are purple. In the antrum, the gastric units are simpler, with few parietal or zymogenic cells. Antral units contain 2 distinct types of mucous cells: those lining the surface (purple) are similar to the surface cells of the corpus, and those nearer to the base have properties intermediate between zymogenic cells and mucous neck cells of the corpus (red-yellow). The interfaces between esophagus and corpus and between corpus and antrum are not abrupt but marked by transitional mucosae. Endocrine cells (not depicted) are also present throughout the corpus and antrum epithelium.
Figure 3.
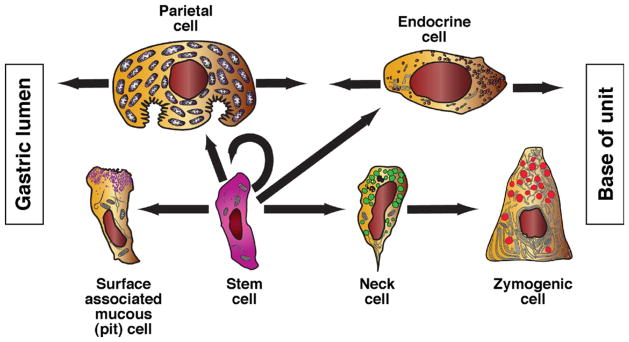
Origins of principal corpus epithelial lineages. The self-renewing stem cell gives rise to each of the principal epithelial lineages of the corpus. There is ultrastructural evidence for the transient intermediates for each lineage (eg, presurface cells depicted in Figure 2); however, available evidence indicates greater complexity in the zymogenic lineage, which arises from a long-lived (≥1 week in mice) intermediate, the mucous neck cell, with its own distinct ultrastructure and probable function.
Stem cells in adult tissues can regenerate all the resident cell types within a lineage, and stem cell research is often motivated by the desire to harness their potential for regeneration of lost or damaged tissue. In developed countries, there is little need for therapeutic replacement of stomach mucosa, but aberrant differentiation of the gastric epithelium occurs during tumorigenesis. Thus, understanding normal and abnormal gastric epithelial stem cell biology may help reveal the origins of gastric cancer, the second leading cause of cancer death worldwide.15 We review how stem cells might contribute to metaplasia, and eventually cancer, following inflammation or injury.
Tissue-based adult stem cells can be defined by 3 properties we describe as location, allocation, and relocation (Figure 4); in other words, characterizing their anatomic and molecular niches (location within the tissue), identifying molecular markers (allocation from other cells), and establishing assays for their regenerative properties (experimental relocation to a new niche to show the capacity to regenerate all lineages of that tissue).16 We know most about the location of stomach epithelial stem cells but less about their molecular properties or ways to measure their regenerative potential.
Figure 4.
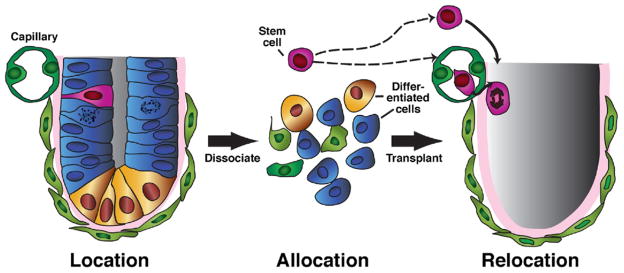
Approaches to investigate stem cell biology. A stem cell (pink) can be characterized by understanding its niche (location), meaning the cells that surround it and affect its activity. Stem cells can also be characterized by identification of molecular markers, which allow their distinction and isolation (allocation) from differentiated cells. Finally, stem cells can be assayed for functional activity based on their ability to regenerate all the normal lineages of a specific tissue (relocation). Ideally, relocation experiments are performed in vivo (eg, serial transfer of hematopoietic stem cells to irradiated recipients). However, tissue culture approaches have also been useful for isolating and demonstrating the properties of stem cells. In several tissues, including the gastric antrum, single cells have been shown to function as stem cells. However, isolation procedures in all tissues only enrich the fraction of single cells that have stem cell capacity; pure populations of single cells, each with complete stem cell activity, have not yet been isolated.
Stem Cell Properties
Location
In the late 1940s, Leblond et al identified the location of 32P-labeled nucleotides that were incorporated into nuclei of live cells.17 In the stomach, radiolabeled cells appeared just below the pits or foveolae, the microscopic openings of gastric gland units into the stomach lumen. The investigators concluded that this region of anatomic narrowing, the isthmus, was the site of cellular renewal in undamaged tissue (Figures 1 and 2). The isthmus is found toward the upper third of the typical glandular unit in the gastric corpus and in the lower third of typical units in the antrum. These studies indicated that one or a few cells in the isthmus constantly regenerate cells that migrate bidirectionally, up to the mucosal surface and down to the gland base, as they differentiate into mature cells of the gastric unit (Figure 3). However, because earlier studies had focused on regeneration after tissue injury, researchers did not investigate the continuous renewal of healthy mucosa until years later.18
Figure 2.
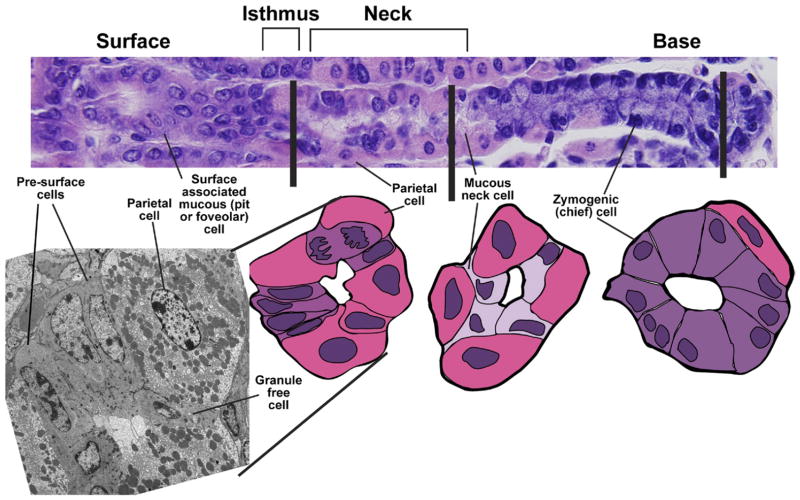
Microanatomy of a corpus gastric unit. (Top) A typical mouse gastric unit, stained by H&E, with the gastric lumen to the left and musculature to the right. Below that are transverse sections through the isthmus (note the dividing cell), neck, and base. Cartoons traced from actual H&E tissue sections are shown. Note the close apposition of progenitor cells in the isthmus and mucous neck cells in the neck with mature parietal cells. (Left) A transverse transmission electron micrograph of a section through the isthmus. A granule-free (presumptive gastric epithelial stem) cell is notable for its high nuclear-cytoplasmic ratio and absence of distinguishing granules. Presurface cells, the precursors to surface mucous (pit or foveolar) cells, are distinguished by early granules that are characteristic of this lineage. Again, mature parietal cells are prominent in the isthmus.
In 1966, Richard Corpron analyzed his own findings with those from the few available ultrastructural studies of the rat gastric corpus and concluded that “nondifferentiated cells” in the isthmus were the source of all other mucosal cells.19 These cells had a high nucleus-to-cytoplasm ratio, open chromatin, lack of granules, underdeveloped rough endoplasmic reticulum, many free ribosomes, and few mitochondria (Figure 2). Although Corpron did not use the term “stem cell,” he did localize and identify cells with undifferentiated morphology as the probable origin of all other epithelial cells. Leblond et al continued in this area of research, using light and electron microscopy to identify different cell types and patterns of differentiation and migration in gastric antral units of mice.12,20 They proposed that undifferentiated, “granule-free” cells in the isthmus represented gastric stem cells and, based on their abundance, that each unit contained, on average, a single stem cell. Karam and Leblond and others used morphologic and labeled nucleotide incorporation assays in tissues from mouse6 –9 and rat21 gastric bodies to study the bidirectional migration and differentiation from morphologically undifferentiated cells in the isthmus. Karam et al subsequently used morphologic and ultrastructural analyses to delineate those patterns in the human gastric body.22 Again, cells with stem-like activity were found in the isthmus and appeared immature, with high nucleus/cytoplasm ratios, open chromatin, small and scant organelles, and many ribosomes. Unlike their granule-free counterparts in rodents, the most undifferentiated cells in humans had mini-granules, without distinct morphologic features to allow assignment to any mature lineage.
We know little about the gastric epithelial stem cell niche. Myofibroblasts in the scant mesenchyme between gland units are proposed to regulate stem cell activity,23 but this has not been tested. Endothelial cells or pericytes within the capillary network might also interact with the stem cell compartment because the extensive capillary network throughout the normal corpus is reduced as stem cells expand during metaplasia.4 In the isthmus, undifferentiated cells and precursors, especially of the mucous neck lineage, usually appear close or immediately adjacent to mature parietal cells5,24 (Figure 2). The significance of this proximity is unclear, but parietal cells might influence stem cell proliferation, at least following certain types of injury.
Allocation
Surface markers of hematopoietic stem and progenitor cells allow their isolation by flow cytometry, setting a standard for stem cells in other tissues. Molecular markers of gastric epithelial stem cells have not been sufficiently characterized for isolation from or identification within their niche. In early studies,25,26 Gordon et al used microarrays to detect increased expression of factors in the insulin-like growth factor signaling pathways and RNA-binding proteins in stomachs of mice with increased stem/progenitor cell proliferation relative to normal controls.5 Subsequently, microdissected isthmus regions from parietal cell–deficient mice (which have increased progenitor/stem cell proliferation) showed increased transcript levels of the doublecortin-like serine-threonine kinase (Dcamkl1) gene, a proposed marker of intestinal stem cells.27 Dcamkl1 is expressed in cells scattered throughout the isthmus of normal corpus units and in a larger population following parietal cell ablation. Although these cells lack molecular markers of advanced differentiation, they have dendritic processes, tufted microvilli (in the small intestine), and a relatively low nucleus/cytoplasm ratio, which are features distinct from normal isthmal progenitors.
The state-of-the-art way to identify stem cell activity in an adult tissue without purifying stem cells in vitro and subsequently testing their regenerative capacity is by lineage labeling (lineage tracing). Candidate stem cells are marked genetically by indelibly inducing expression of a reporter gene using genetic recombination of genomic sequence that otherwise would prevent expression (eg, inducing lacZ in the ROSA26 locus). After recombination, any cells derived from the labeled cell can be traced by their shared expression of the reporter.28 If recombination occurs in a stem cell with constant turnover and traceable migration of cell lineages, such as the gastric epithelium, all the cells in a unit will eventually reveal their origin from a stem cell expressing the reporter gene. Provided the initial recombination event occurs only in a certain cell and not in any of its progeny, this approach indicates stem cell activity in that cell. Lineage labeling studies should thus help determine whether DCAMKL1 or other putative markers specifically mark a gastric stem cell population.29
Using such lineage labeling, Qiao et al found rare cells that expressed a transgene regulated by an intestine-specific promoter (villin, not usually expressed at detectable levels in stomach) at varying positions between the isthmus and base of some antral units. Following crosses to the R26 reporter line, the investigators showed that stimulation with interferon gamma caused these cells to regenerate all the cells within a given antral unit,30 indicating stem cell activity. Because few gland units carry these cells and they seem to replicate only after cytokine stimulation, villin is not likely to be a marker of most antral stem cells. However, those cells that expressed Cre under control of the villin promoter in this study might represent a rare stem-like population that regulates the gastric epithelium in response to specific signals such as injury or inflammation.
More recently, Barker et al used lineage labeling to show that cells that express the intestinal stem cell marker LGR5 and are located at the base, rather than the isthmus, of glands can give rise to all antral unit cells.31 As with Lgr5+ intestinal stem cells, which replicate rapidly, Lgr5+ cells at the base of antral glands incorporate labeled nucleotides and express markers of cell proliferation. It is often assumed that stem cells in all tissues resemble hematopoietic stem cells, which are believed to divide infrequently.32 Although some researchers consider replicative quiescence to be a cardinal property of stem cells, stem cells in a rapidly renewing tissue might indeed divide rapidly, as Lgr5+ cells do. Mouse Lgr5+ cells have more differentiated morphology than granule-free isthmus cells, with more abundant basal endoplasmic reticulum and apical microvilli. Compared with the cells marked by expression of the villin-regulated trans-gene, they show stem cell properties more frequently but also lack the morphology or long-term nucleotide retention associated with native isthmal stem cells. As the antral epithelium expands in part by branching or fission from the base of gland units,11,14,33 basal Lgr5+ cells might contribute to formation of new units by gland fission from the base; studies are needed to determine if this is the case.
Although Lgr5-expressing cells are also detected in the neonatal mouse corpus, they disappear soon after birth and become confined to the antral-pyloric mucosa.31 It is important to identify the cells that replenish the corpus stomach epithelium.
Wang et al recently described a mouse line that expresses tamoxifen-inducible Cre recombinase under control of the Tff2 promoter. Crosses with the R26 reporter strain revealed that parietal and zymogenic, but not surface or foveolar, cells arose from cells that express the transgene,34 indicating that a population of corpus progenitor cells can give rise to parietal and zymogenic lineages. Transgene expression was confined to these progenitors, which are located in the isthmus, and normal mucous neck cells expressed trefoil factor 2 (TFF2) protein but not the transgene.
Relocation
Stem cells from the bone marrow or mammary gland regenerate tissues on transplantation in animal models.35,36 These types of experiments are not easy to perform with gastric epithelial stem cells because of the lack of markers and because it is difficult to create animal models where gastric or intestinal mucosa is denuded to host exogenous cells as a test of their regenerative capacity. Ex vivo assays have therefore been developed. Lgr5+ cells isolated from the antrum of adult mice generate long-lived colonies that resemble gastric units in morphology and carry all resident epithelial lineages.31 The best evidence for stem cell activity within these colonies comes from the isolation of single cells from primary outgrowths that generate secondary and tertiary colonies, indicating self-renewal. This finding presents opportunities to optimize culture conditions and characterize the minimal requirements for ex vivo growth and differentiation. Markers of each gastric lineage make it possible to assess the full differentiation potential of individual progenitors; additional markers might be identified to isolate and characterize corpus and antrum subpopulations with stem or progenitor properties. It is, however, possible that the greater cellular diversity and 3-dimensional complexity of corpus glands, compared with their antral counterparts,24 will limit their potential to be replicated in tissue culture.
Proliferation (Label Retention)
Because gastric stem cells might proliferate infrequently, investigators have investigated whether labeled nucleotides, incorporated during S phase, remain concentrated in the nuclei of stem cells for long periods. Although label retention has received much attention in characterizing intestinal stem cells in the +4 position,37,38 there is little evidence for long-term, label-retaining cells in the stomach. Labeled nucleotide analogues incorporated in the stomach epithelium usually mark cells near the isthmus in the short-term, followed by bidirectional migration into neighboring nuclei; only differentiated cells are labeled next, and the marker eventually disappears,20,39 indicating that gastric stem cells might proliferate rapidly. Because the isthmus in antral gland units lies close to the base, bidirectional migration is harder to monitor. Basal Lgr5+ cells seem to divide frequently and repopulate gland units in undamaged mucosa.31 After tissue injury, additional cells might be recruited that have progenitor or stem cell functions. For example, cytokines can induce quiescent cells that express the Cre transgene under control of the villin promoter to enter the cell cycle.30 Without stimulation, however, these cells do not differentiate in normal mice.
Unitary Origin and Clonality of Gastric Units
The concept that all mature gastrointestinal epithelial cell lineages arise from a common stem cell, once known as the Unitarian Theory,40 has been validated in the stomach.2 However, it is not known if an individual gastric unit is replenished from a single monoclonal stem cell. Evidence supporting the Unitarian Theory came from Thompson et al, who found that all lineages within a gland shared the same XX or XY genotype in XX-XY chimeric mice. Those studies indicated that gastric enteroendocrine cells also derive from the same stem cell and not from a lineage that originated in the embryonic neural crest.2 Nomura et al followed expression of an X-linked LacZ transgene that female mice inactivate randomly during development; cells are blue or white, depending on X chromosome inactivation in stem cells and their progeny.33 In adult mice, most units were totally blue or white, indicating monoclonality. However, because some units could derive from 2 (or more) stem cells of the same genotype, the investigators estimated that at least 3 of 4 mouse gastric glandular units are monoclonal. Because X-chromosome inactivation occurs in early embryos, Bjerknes and Cheng investigated whether cell lineage generating activity in the adult stomach might result from progenitors that remained after development. They used chemical mutagenesis experiments in adult mice to determine whether single adult stem cells yielded entire units that included the same mutation or if units arose from 2 or more stem cells, based on their mixture of mutant and wild-type cells.14 Although they found evidence that most glands arose from a single stem cell, even 48 weeks after mutagen exposure, some units carried mutant cells of only a single lineage, indicating that new cells had arisen continually along only that lineage. In light of known cell renewal rates, those cells could not have been derived from a multipotential stem cell. Hence, some units might maintain long-lived progenitors that are committed to replenishing cells of only a single lineage. Nomura et al33 did not report mixed glands with a single labeled lineage, perhaps because long-lived progenitors arise only in adults, long after X chromosome inactivation.
Human gastric gland units are architecturally more complex than units of mice, with multiple glands feeding like tributaries into a single pit. McDonald et al followed spontaneous mutations in a mitochondrial gene, Cytochrome c oxidase, to examine their propagation and stem cell and progenitor cell dynamics in human gastric units.41 Although the mutation was occasionally confined to a single cell type, adult gastric glands were found to derive from multipotential and clonal stem cells, at least in basal states. Local injury and cytokine stimulation might, of course, alter stem cell dynamics, affecting differentiation potential and clonality of stem and progenitor cells until homeostasis is restored.
Unique Aspects of the Gastric Stem Cell
Studies of other tissues that have continuously self-renewing cells, such as the skin, blood, intestine, and mammary gland, have led to better characterization of stem cells than in the stomach. In particular, identification of LGR5 and BMI-1 as markers of intestinal cells with stem cell–like properties,38,42 and Musashi and Prominin1/CD133 as markers of a broader crypt population,43,44 helped advance characterization of intestinal stem cells that respond to Wnt and Notch signals.45 Candidate molecular markers of small intestine stem cells label crypt cells either deep in the base, interspersed among Paneth cells, or just above the Paneth cell zone in the canonical +4 tier.45 In each case, labeled crypt cells also had the least differentiated morphology and were among the first to incorporate labeled nucleotides, providing a satisfying agreement between morphologic and molecular features. In the antral stomach, by contrast, LGR5, which marks cells with apparent stem function, does not coincide with the anticipated location, morphology, or nucleotide uptake, whereas the few cells marked by the villin transgene do have some of the predicted properties. These distinctions may force reconsideration of core assumptions in the field.
The gastric corpus epithelium differs from the rest of the digestive tract in important ways. The stem cell niche is nearer the lumen than in the base of the glands (Figure 1); hence, it is likely to be more exposed to surface irritants and requires bidirectional migration of its daughter cells. Cell lineages in the stomach epithelium vary greatly in life span: from 3 to 5 days for mouse surface-associated mucous cells to several months for zymogenic cells, whereas the life span of mature intestinal cells ranges from 3 to 5 days for enterocytes to about 2 weeks for Paneth cells.20 The marked variation in gastric corpus epithelial turnover rates exerts asymmetry in the demand for various stem cell progeny; stem cells must generate many more precursors of pit cells than of chief cells in each differentiation cycle. It is not clear how stem cells allocate progeny toward individual lineages or respond to differential demands for daughter cells in any tissue. Stem cell activities, or at least proliferative activities, in the gastric corpus mucosa are sensitive to loss of mature progeny; parietal cell injury causes pseudo-pyloric (spasmolytic polypeptide–expressing) metaplasia, an altered differentiation pattern wherein proliferative activity shifts deeper into the gland base (see the following text).
Although intestinal progenitors depend on Wnt stimulation and stop dividing immediately on withdrawal of Wnt signals,46 the steady-state gastric corpus does not depend on this signaling pathway. There have been few reports indicating that Wnt signaling occurs in most of the adult stomach and, unlike intestinal cancers, which have dysregulated Wnt signaling, gastric cancers rarely carry mutations in Wnt pathway genes or show signs of constitutive Wnt activation.47 In light of the fundamental similarities between stomach and intestinal epithelial organization and turnover, and the origin of each in a common primordium, it is surprising that each has distinct means of homeostatic regulation. Studies of antral stem cells indicate that the gastric antrum/pylorus might be a hybrid of corpus and intestine.48 These cells express the Wnt-dependent intestinal stem cell marker LGR5.26 Moreover, ApcMin and Apc1322T mice, which develop intestinal polyps as a result of inactivation of the Wnt-regulatory gene Apc, develop a few adenomas in the gastric antrum but not in the corpus.49,50 Furthermore, loss of Apc in Lgr5+ cells rapidly results in formation of antral but not corpus adenomas.31 These observations indicate that the antrum has Wnt-responsive stem cells that are distinct from those that mediate corpus mucosal self-renewal. Furthermore, antral stem cells rarely generate parietal or chief cells. On the other hand, the basal mucous neck cells they do generate resemble the duodenal Brunner’s gland cell in morphology and mucus production. Intestinal epithelial progenitors seem also to depend on Notch signaling, responding to chemical or genetic inhibition of the Notch pathway with profound cell cycle arrest and goblet call metaplasia51,52; a parallel dependence among gastric progenitors has not been investigated.
Response of Gastric Epithelial Progenitor Cells to Injury
Categories of Injury
For the purpose of this review, we consider gastric mucosal injury in 2 broad categories: focal (repairable damage that does not change the cellular differentiation pattern) and diffuse (chronic damage that alters cell differentiation). Toxin ingestion, bile reflux, and certain infectious agents usually induce the first type of injury, resulting in focal erosions or full-thickness ulcerations that are rapidly repaired by increased proliferation in neighboring units and migration of surface cells; these eventually reestablish normal differentiation in damaged units.53 It is unclear, though, how new stem cells emerge after extensive injury. The second pattern of injury results in abnormal differentiation (metaplasia) in humans, most commonly from chronic infection with Helicobacter pylori or from parietal cell destruction in autoimmune gastritis.54–57 In mice and other animals, metaplasia can be induced by Helicobacter species or by direct destruction of parietal cells.24,26,58–61 Metaplasias are associated with cancer and seem to reflect a permanent alteration in the behavior of stem and progenitor cells.
Intestinal Metaplasia
The gastric mucosa can adopt various aberrant differentiation patterns, resulting, in rare instances, in cells with pancreatic acinar or ciliated bronchial features; however, the most well-characterized pattern of metaplasia involves conversion of gastric into intestinal-type epithelium. This change is easily detected by analysis of specimens by histopathology, based on the markedly different cellular organization and histochemical staining patterns of gastric and intestinal epithelia. Patterns of intestinal metaplasia can vary from instances where the gastric mucosa mimics the morphology of small or large bowel epithelium perfectly to varying degrees of intestinal differentiation of indeterminate type. The extent of metaplasia can vary from partial intestinalization, in which patches of cells express intestinal markers but contain normal gastric epithelium,55 to diffuse, in which large portions of the stomach resemble the intestine. Gastric units can also be partially intestinal, with whole or parts of gland branches affected, with sharp interfaces between cells with gastric and intestinal morphologies.41,55 Most animal models for experimental or spontaneous metaplasia have limited tissue conversion, with only focal changes in mucin content or gene expression; they do not undergo the dramatic changes in differentiation patterns observed by histologic analysis of human intestinal metaplasia lesions. Although intestinal metaplasia causes changes in stem and progenitor cells, it is not clear whether native gastric stem cells are the initial source of the changes and metaplasia results from their reprogramming into an intestinal type or if differentiated gastric cells first acquire intestinal properties and then stem cell properties. The stomach epithelium of mice converts readily into the intestinal type on transgenic expression of CDX2, a transcription factor that regulates intestinal development and differentiation.62,63 This observation indicates that intestinalization of gastric stem cells might be the initiating event in intestinal metaplasia. Additional animal models are needed to improve research into the molecular and cellular pathogenesis of intestinal metaplasia.
The most common gastric adenocarcinomas have intestinal features; just as Barrett’s metaplasia is characterized by intestinalization of the esophagus or gastric cardia,64 intestinal metaplasia has been proposed as an intermediate step in the development of gastric cancer.57,65 However, this interpretation may be facile, because different types of intestinal metaplasia have different degrees of association with malignancy, and early-stage gastric cancers can arise in nonintestinalized epithelium.66–68
Spasomolytic Polypeptide (TFF2)-Expressing Metaplasia
Pathologists observed more than a century ago that certain types of gastric corpus injury alter the balance between enzyme- and mucus-secreting cells.69,70 Pseudopyloric metaplasia, so named because increases in mucus and loss of mature parietal and chief cells make the corpus resemble the pylorus or antrum, is associated with increased cell proliferation (Figure 5). Understanding of the condition advanced when Goldenring et al observed that human metaplastic stomach expressed high levels of spasmolytic polypeptide (TFF2) at the base of corpus gastric units, although TFF2 is normally expressed at lower levels in mucous neck cells in upper and mid portions of the units (Figure 5).54,55,71 Animal models have been developed with this pattern of pseudopyloric features (or spasmolytic polypeptide–expressing metaplasia [SPEM]) in Mongolian gerbils and mice; in all cases, including humans, parietal cells are lost, zymogenic cells are lost or dedifferentiate, and TFF2 expression extends from the isthmus to the gland base.24,55,60,72,73
Figure 5.
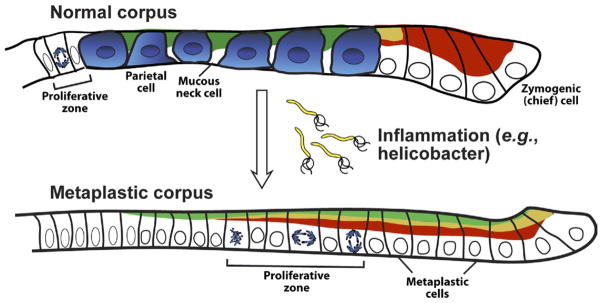
Cellular mechanisms of SPEM. Chronic inflammation of the corpus in mammals leads to characteristic changes in differentiation in the gastric unit. Parietal cells are lost (atrophy), and the zymogenic chief cell lineage is reprogrammed so that genes that are normally expressed only in mucous neck cells, such as spasmolytic polypeptide/TFF2 (shown in green), are expressed at high levels in cells at the base. The zymogenic cell–specific transcription factor MIST1 (not depicted) is lost, but other zymogenic cell markers (such as pepsinogen C; red) are coexpressed with neck cell markers. Proliferation is increased and occurs more basally in the unit. The pattern of basal proliferation and coexpression of neck and zymogenic cell genes is similar to the histologic pattern in the normal antrum and pylorus, which is why it is called pseudopyloric metaplasia. The most common metaplasia-inducing inflammation is caused by H pylori infection, although autoimmune gastritis (in which autoantibodies target parietal cells) can cause the same metaplasia pattern.
Some studies suggest that gastric tumorigenesis might have a stronger correlation with SPEM than with intestinal metaplasia,54,74 although these metaplasias often occur together.55,75 Also, SPEM is by definition a corpus lesion, whereas cancer is believed to arise more commonly in the antrum or in the transitional area between antrum and corpus. However, because SPEM resembles the antrum histologically, cancers arising in corpus tissue that converted to SPEM might appear to pathologists to arise in the antrum; this would result in overestimation of the prevalence of antral tumors.
Parietal cell loss in humans correlates with SPEM.74 Virtually any intervention that disrupts parietal cells in animals—parietal cell–specific expression of a toxic transgene,26,59 knockout of genes required for development or activity,61 or injection of agents that are toxic to parietal cells73—leads to SPEM. Parietal cell loss rapidly expands the proliferative compartment in the isthmus, including cells that are morphologically indistinguishable from normal granule-free presumptive stem cells (Figures 2 and 5).25,26 It is unclear if this change in stem cell behavior is associated with permanent or transient changes in gene expression. In addition, chief cells lose expression of at least one marker of maturity (the transcription factor MIST1, also known as BHLA15), reenter the cell cycle, re-express progenitor neck cell markers like TFF2, and begin to react with the lectin GSII.24,60 Expression of secretory chief cell markers (eg, pepsinogen C) is maintained in these TFF2-expressing metaplastic cells (Figure 5). A recent study of hundreds of specimens indicated that the same chief cell changes in expression of MIST1 and other genes occur in human tissues.55,75 It is unclear what proportion of the SPEM cells arise de novo, from altered stem cells, or from transdifferentiation of chief or even mucous neck cells (Figure 5). A recent study used genetic lineage tracing in tamoxifen-inducible, Mist1-Cre knock-in mice to show that mature, Mist1-expressing chief cells can give rise to metaplastic cells in SPEM.76
Although little is known about molecular mechanisms that control the fate of stem cells during SPEM, as mentioned previously, expansion of presumptive isthmal stem cells in SPEM was exploited to identify genes expressed by gastric stem and progenitor cells.5 Loss of the epidermal growth factor family member Amphiregulin leads to SPEM,77 and chief cells that differentiate in the absence of the transcription factor XBP1 or parietal cell–secreted Sonic Hedgehog have significantly increased numbers of basal cells that have a SPEM pattern of coexpression of mucous neck cell markers (such as TFF2) and chief cell markers. Sonic Hedgehog might normally signal chief cells to increase expression of XBP1, whose transcriptional targets inhibit SPEM.78,79 XBP1 induces MIST1 expression,79 and MIST1 is lost in SPEM.24,55 Studies in experimental models of SPEM will increase our understanding of its mechanism and mediators.
The Relation of Gastric Metaplasias to Helicobacter infection
Human gastric cancer is commonly associated with H pylori infection and intestinal metaplasia, and there has been much interest in identifying the mechanisms of their relationships. However, the role of gastric epithelial stem cells is uncertain.80 As for all epithelial tumors, it is unclear whether gastric adenocarcinoma develops through alterations in the native stem cell population or of less primitive cells in the transit-amplifying or mature cell compartments (eg, via progression from metaplasia; Figure 5). Distinguishing between these possibilities will help elucidate the role of stem cells in tumorigenesis and improve our ability to isolate and monitor these cells. Mice have different immune responses to Helicobacter infection from humans and rarely develop metastatic gastric tumors but continue to be used as models of gastric cancer because they can be genetically manipulated and infected with various Helicobacter species, and cell lineages can be traced from stem cell populations when stem cell–specific markers are available. H pylori infection initially and briefly increases apoptosis of surface and proliferative cells and then expands the proliferative cell zone to deeper in the gland, presumably as a compensatory response (similar to the pattern in chemically induced SPEM; Figure 5); mucosal atrophy and SPEM ensue in the next 12 to 18 months.58 Helicobacter felis infection, of C57/B6 mice in particular, leads more consistently to high-grade dysplasia and the development of large antral tumors months later.58 The parietal cell loss that is characteristic of SPEM precedes development of invasive cancer in mouse models and most humans, but it is not clear if tumors eventually arise from the resulting metaplastic stem and chief cells or if the pattern of metaplasia and achlorhydria associated with parietal cell loss merely increases the propensity of other cells to form tumors.80
Other Injury-Induced Changes in Progenitor Activity
Other progenitor cells have activity during chronic stomach injury that leads to metaplasia. In myeloablated C57BL/6 mice infected with H felis, bone marrow–derived cells contributed significantly to the resulting metaplastic epithelium.81 After these mice received transplants of bone marrow from ROSA26R (expressing LacZ as a reporter) or from β-actin-EGFP (expressing green fluorescent protein) mice, LacZ+ or green fluorescent protein–positive bone marrow–derived cells were detected in the gastric mucosa. However, more recent experiments suggest that those mesenchymal stem cells may not originate from the blood but from keratin-19–expressing mesenchymal cells.82 During chronic inflammation, failure of gastric stem cells might lead to recruitment and engraftment of mesenchymal cells, whether from bone marrow–derived populations or already resident in the stomach epithelial stem cell niche; these cells might contribute to metaplasia, dysplasia, and cancer. The study did not exclude the possibility of fusion between mucosal and bone marrow cells, a common source of the erroneous concept that stem cells in other tissues have a circulating source; although bone marrow–derived cells in the stomach mucosa were diploid, fused cells shed excess chromosomes.83 Importantly, even if cells that appear in response to gastric injury are fusions of mucosal and blood-derived cells, the observations indicate an unusual amalgamation of cell lineages that may ultimately affect stem cell properties.
Altered Gastric Epithelial Differentiation in Other Conditions
Certain uncommon chronic disorders also alter gastric epithelial differentiation in ways that alter stem cells. Ménétrier’s disease, which involves chronic over-stimulation by epidermal growth factor ligands, leads to expansion of foveolar pit cells.84 The Zollinger–Ellison syndrome of gastrin hypersecretion leads to excess proliferation of parietal and/or zymogenic precursors. Although these disorders can be modeled in mice, it is not clear if selected cell types are affected or if changes in stem cells promote their differentiation along a specific lineage.
Developmental Origins of the Gastric Stem Cell Compartment
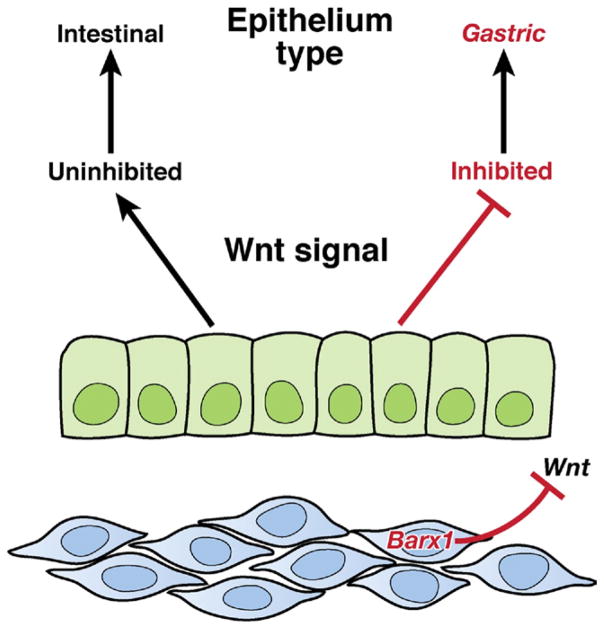
It is important to learn how prospective stem cells are distinguished from their neighbors during development, come to occupy a unique niche, and establish the property of self-renewal. Not much is known about this process in any tissue type; in the small intestine of fetal mice, cell proliferation is initially disseminated throughout nascent villi but becomes confined to the intervillus space over the span of 1 to 2 days in midgestation.85 These intervillus cells subsequently invade the underlying mesenchyme to form crypts during the first 2 to 3 weeks of life in mice and in the third trimester of pregnancy in humans. Although we can visualize this transition, we have limited insight into its molecular and physiologic basis and even less understanding of development of the gastric stem cell niche. Early stomach endoderm retains a certain plasticity; if juxtaposed experimentally with intestinal mesenchyme, it can develop into a villiform intestine.86 This developmental potential becomes restricted in midgestation, when the homeodomain transcription factor BARX1, whose expression in the digestive tract is restricted to the stomach mesenchyme, promotes stomach-specific differentiation of the overlying endoderm.87,88 This specification somehow determines the properties of future stem cells, creating an environment that promotes stomach-specific differentiation. Generation of gastric identity in the fetus requires inhibition of canonical Wnt signaling in the nascent epithelium (Figure 6). Stomach expression of Barx1 is restricted to embryos; the mesenchyme in neonatal and adult stomachs lacks Barx1 but still supports and regulates the overlying gastric epithelium in perpetuity. In adult transgenic mice, expression of the intestinal homeodomain protein CDX2 converts the stomach epithelium into the intestinal type, defined by the presence of intestine-specific goblet cells.62,63 Mechanisms that repress transcriptional regulation by CDX2 must therefore be important in maintaining gastric epithelial and, by extension, gastric stem cell identity.
Figure 6.
Molecular basis of stomach epithelial specification in embryos. Expression of the homeodomain transcription factor Barx1 is restricted to the developing mesenchyme, which underlies the nascent gastric epithelium. Barx1 regulates transcription of many factors, including the secreted inhibitors of Wnt signaling that repress the canonical Wnt pathway in the overlying endoderm. This repression promotes stomach epithelial differentiation at the expense of intestinal differentiation, which would occur in the absence of Barx1-induced Wnt blockade.
In the intestinal epithelium, stem or progenitor cell activity represents a balance between Wnt and Notch signaling, which promote cell replication, and bone morphogenetic protein BMP4 signaling, which reduces proliferation and promotes cell differentiation.45 The corresponding regulators of the adult gastric stem cell are not known. However, disruption of BMP2, BMP4, and BMP7 signaling, through deletion of their receptor BMPR1A, leads to hyperplastic polyp formation in the antrum, but not the corpus,89,90 indicating again the parallel between regulation of stem cell activity in the antrum and intestine.
Maturation of the stem cell compartment continues after birth. Nomura et al studied mosaic LacZ+ gastric glands33; although monoclonal gland units were observed in adult mice, most units in stomachs of fetal and newborn mice contained mixed populations of blue and white cells. These observations reflect a gradual and seemingly stochastic process of gland evolution toward monoclonality, which is sometimes called purification and is similar to the clonal restriction of intestinal crypts.91 One mechanism for progression toward monoclonality might be that, as gastric units propagate by branching or fission, those with 2 competing stem cells are more likely to branch, with one progenitor forming the new unit and the other supplying the original unit. Surprisingly, experimental and mathematical modeling studies have indicated that intestinal crypt stem cells divide symmetrically and stochastically, not with the asymmetry observed in stem cell divisions in some other tissues.92,93 Similarly, symmetric divisions among gastric stem cells probably account for the emergence of monoclonality within individual gastric glands; the pace is likely to be slower than in the intestine because stomach stem cells self-renew more slowly.
Future Directions
Each gastric unit in the stomach is served by a tiny population of monoclonal stem cells that enable lifelong epithelial self-renewal. Despite their fundamental similarities with intestinal stem cells, which are increasingly well characterized, gastric stem cells are poorly understood, although they are likely to be involved in the pathogenesis of gastric cancer, which is a global health problem. In the oxyntic mucosa of the gastric corpus, they probably lie in the isthmus and cycle slowly, generating progeny that migrate bidirectionally, differentiate into mature resident lineages, and have variable life spans. A lack of molecular markers presents the most significant barrier to research on corpus stem cells. Identification of markers of stem cells in normal and diseased states, and reliable methods for ex vivo culture and expansion of gastric corpus stem cells, are priorities for this field of research. In the simpler mucosa of the gastric antrum, stem cells lie closer to the gland base, produce fewer types of progeny, and seem to have hybrid characteristics between corpus and intestinal stem cells. At least one subset, if not the whole population, of antral stem cells bears the surface marker LGR5 and replicates briskly, perhaps daily, in adult mice, where it can contribute to all mature epithelial lineages over long periods. The recent expansion and differentiation of these cells in culture should lead to experiments to define their growth requirements and signaling pathways and determinants of whether these cells undergo continued replication or lineage commitment. Because stem cells throughout the stomach respond continually to external cues and local tissue injury, they must occupy a sophisticated niche that conveys homeostatic signals as well as information about infection and inflammation. Some combination of intrinsic and niche-derived cues likely converts gastric cells with proliferative potential into cells with aberrant, metaplastic differentiation patterns that lead to dysplasia and carcinoma. Although our knowledge about stem cell niches is limited, few areas in basic gastroenterology research present greater interest or challenges. It is important to develop methods to isolate and culture stem cells that express well-validated molecular markers. Such progress will advance understanding of stem cell properties and the responses to infection and tissue damage that induce metaplasia and cancer in the gastric epithelium.
Abbreviations used in this paper
- SPEM
spasmolytic polypeptide–expressing metaplasia
- TFF2
trefoil factor 2
Footnotes
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Suzuki S, Tsuyama S, Murata F. Cells intermediate between mucous neck cells and chief cells in rat stomach. Cell Tissue Res. 1983;233:475–484. doi: 10.1007/BF00212218. [DOI] [PubMed] [Google Scholar]
- 2.Thompson M, Fleming KA, Evans DJ, et al. Gastric endocrine cells share a clonal origin with other gut cell lineages. Development. 1990;110:477–481. doi: 10.1242/dev.110.2.477. [DOI] [PubMed] [Google Scholar]
- 3.Mills JC, Syder AJ, Hong CV, et al. A molecular profile of the mouse gastric parietal cell with and without exposure to Helicobacter pylori. Proc Natl Acad Sci U S A. 2001;98:13687–13692. doi: 10.1073/pnas.231332398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills JC, Andersson N, Stappenbeck TS, et al. Molecular characterization of mouse gastric zymogenic cells. J Biol Chem. 2003;278:46138–46145. doi: 10.1074/jbc.M308385200. [DOI] [PubMed] [Google Scholar]
- 5.Mills JC, Andersson N, Hong CV, et al. Molecular characterization of mouse gastric epithelial progenitor cells. Proc Natl Acad Sci U S A. 2002;99:14819–14824. doi: 10.1073/pnas.192574799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. III. Inward migration of neck cells followed by progressive transformation into zymogenic cells. Anat Rec. 1993;236:297–313. doi: 10.1002/ar.1092360204. [DOI] [PubMed] [Google Scholar]
- 7.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. V. Behavior of entero-endocrine and caveolated cells: general conclusions on cell kinetics in the oxyntic epithelium. Anat Rec. 1993;236:333–340. doi: 10.1002/ar.1092360206. [DOI] [PubMed] [Google Scholar]
- 8.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. II. Outward migration of pit cells. Anat Rec. 1993;236:280–296. doi: 10.1002/ar.1092360203. [DOI] [PubMed] [Google Scholar]
- 9.Karam SM. Dynamics of epithelial cells in the corpus of the mouse stomach. IV. Bidirectional migration of parietal cells ending in their gradual degeneration and loss. Anat Rec. 1993;236:314–332. doi: 10.1002/ar.1092360205. [DOI] [PubMed] [Google Scholar]
- 10.Hanby AM, Poulsom R, Playford RJ, et al. The mucous neck cell in the human gastric corpus: a distinctive, functional cell lineage. J Pathol. 1999;187:331–337. doi: 10.1002/(SICI)1096-9896(199902)187:3<331::AID-PATH241>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 11.Lee ER. Dynamic histology of the antral epithelium in the mouse stomach: I. Architecture of antral units. Am J Anat. 1985;172:187–204. doi: 10.1002/aja.1001720303. [DOI] [PubMed] [Google Scholar]
- 12.Lee ER, Leblond CP. Dynamic histology of the antral epithelium in the mouse stomach: II. Ultrastructure and renewal of isthmal cells. Am J Anat. 1985;172:205–224. doi: 10.1002/aja.1001720304. [DOI] [PubMed] [Google Scholar]
- 13.Lee ER, Trasler J, Dwivedi S, et al. Division of the mouse gastric mucosa into zymogenic and mucous regions on the basis of gland features. Am J Anat. 1982;164:187–207. doi: 10.1002/aja.1001640302. [DOI] [PubMed] [Google Scholar]
- 14.Bjerknes M, Cheng H. Multipotential stem cells in adult mouse gastric epithelium. Am J Physiol Gastrointest Liver Physiol. 2002;283:G767–G777. doi: 10.1152/ajpgi.00415.2001. [DOI] [PubMed] [Google Scholar]
- 15.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 16.Huh WJ, Pan XO, Mysorekar IU, et al. Location, allocation, relocation: isolating adult tissue stem cells in three dimensions. Curr Opin Biotechnol. 2006;17:511–517. doi: 10.1016/j.copbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Leblond CP, Stevens CE, Bogoroch R. Histological localization of newly-formed desoxyribonucleic acid. Science. 1948;108:531–533. doi: 10.1126/science.108.2811.531. [DOI] [PubMed] [Google Scholar]
- 18.Stevens CE, Leblond CP. Renewal of the mucous cells in the gastric mucosa of the rat. Anat Rec. 1953;115:231–245. doi: 10.1002/ar.1091150206. [DOI] [PubMed] [Google Scholar]
- 19.Corpron RE. The ultrastructure of the gastric mucosa in normal and hypophysectomized rats. Am J Anat. 1966;118:53–90. doi: 10.1002/aja.1001180105. [DOI] [PubMed] [Google Scholar]
- 20.Karam SM, Leblond CP. Dynamics of epithelial cells in the corpus of the mouse stomach. I. Identification of proliferative cell types and pinpointing of the stem cell. Anat Rec. 1993;236:259–279. doi: 10.1002/ar.1092360202. [DOI] [PubMed] [Google Scholar]
- 21.Yang DH, Tsuyama S, Ge YB, et al. Proliferation and migration kinetics of stem cells in the rat fundic gland. Histol Histopathol. 1997;12:719–727. [PubMed] [Google Scholar]
- 22.Karam SM, Straiton T, Hassan WM, et al. Defining epithelial cell progenitors in the human oxyntic mucosa. Stem Cells. 2003;21:322–336. doi: 10.1634/stemcells.21-3-322. [DOI] [PubMed] [Google Scholar]
- 23.Modlin IM, Kidd M, Lye KD, et al. Gastric stem cells: an update. Keio J Med. 2003;52:134–137. doi: 10.2302/kjm.52.134. [DOI] [PubMed] [Google Scholar]
- 24.Bredemeyer AJ, Geahlen JH, Weis VG, et al. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev Biol. 2009;325:211–224. doi: 10.1016/j.ydbio.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Syder AJ, Guruge JL, Li Q, et al. Helicobacter pylori attaches to NeuAc alpha 2,3Gal beta 1,4 glycoconjugates produced in the stomach of transgenic mice lacking parietal cells. Mol Cell. 1999;3:263–274. doi: 10.1016/s1097-2765(00)80454-2. [DOI] [PubMed] [Google Scholar]
- 26.Li Q, Karam SM, Gordon JI. Diphtheria toxin-mediated ablation of parietal cells in the stomach of transgenic mice. J Biol Chem. 1996;271:3671–3676. [PubMed] [Google Scholar]
- 27.Giannakis M, Stappenbeck TS, Mills JC, et al. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem. 2006;281:11292–11300. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- 28.Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- 29.May R, Sureban SM, Hoang N, et al. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27:2571–2579. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiao XT, Ziel JW, McKimpson W, et al. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–1998. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 33.Nomura S, Esumi H, Job C, et al. Lineage and clonal development of gastric glands. Dev Biol. 1998;204:124–135. doi: 10.1006/dbio.1998.9055. [DOI] [PubMed] [Google Scholar]
- 34.Quante M, Marrache F, Goldenring JR, et al. TFF2 mRNA transcript expression marks a gland progenitor cell of the gastric oxyntic mucosa. Gastroenterology. 2010;139:2018–2027.e2. doi: 10.1053/j.gastro.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 36.Shackleton M, Vaillant F, Simpson KJ, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 37.Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest. 2000;105:1493–1499. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramsey VG, Doherty JM, Chen CC, et al. The maturation of mucus-secreting gastric epithelial progenitors into digestive-enzyme secreting zymogenic cells requires Mist1. Development. 2007;134:211–222. doi: 10.1242/dev.02700. [DOI] [PubMed] [Google Scholar]
- 40.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–561. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 41.McDonald SA, Greaves LC, Gutierrez-Gonzalez L, et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–510. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 42.Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 43.Snippert HJ, van Es JH, van den Born M, et al. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology. 2009;136:2187–2194.e2181. doi: 10.1053/j.gastro.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Potten CS, Booth C, Tudor GL, et al. Identification of a putative intestinal stem cell and early lineage marker; musashi-1. Differentiation. 2003;71:28–41. doi: 10.1046/j.1432-0436.2003.700603.x. [DOI] [PubMed] [Google Scholar]
- 45.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sansom OJ, Meniel VS, Muncan V, et al. Myc deletion rescues Apc deficiency in the small intestine. Nature. 2007;446:676–679. doi: 10.1038/nature05674. [DOI] [PubMed] [Google Scholar]
- 47.Cervantes A, Rodriguez Braun E, Perez Fidalgo A, et al. Molecular biology of gastric cancer. Clin Transl Oncol. 2007;9:208–215. doi: 10.1007/s12094-007-0041-4. [DOI] [PubMed] [Google Scholar]
- 48.Li X, Udager AM, Hu C, et al. Dynamic patterning at the pylorus: formation of an epithelial intestine-stomach boundary in late fetal life. Dev Dyn. 2009;238:3205–3217. doi: 10.1002/dvdy.22134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomita H, Yamada Y, Oyama T, et al. Development of gastric tumors in Apc(Min/+) mice by the activation of the beta-catenin/Tcf signaling pathway. Cancer Res. 2007;67:4079–4087. doi: 10.1158/0008-5472.CAN-06-4025. [DOI] [PubMed] [Google Scholar]
- 50.Pollard P, Deheragoda M, Segditsas S, et al. The Apc 1322T mouse develops severe polyposis associated with submaximal nuclear beta-catenin expression. Gastroenterology. 2009;136:2204–2213.e2201–2213. doi: 10.1053/j.gastro.2009.02.058. [DOI] [PubMed] [Google Scholar]
- 51.van Es JH, Jay P, Gregorieff A, et al. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 52.Kazanjian A, Noah T, Brown D, et al. Atonal homolog 1 is required for growth and differentiation effects of notch/gamma-secretase inhibitors on normal and cancerous intestinal epithelial cells. Gastroenterology. 139:918–928.e916. doi: 10.1053/j.gastro.2010.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Starodub OT, Demitrack ES, Baumgartner HK, et al. Disruption of the Cox-1 gene slows repair of microscopic lesions in the mouse gastric epithelium. Am J Physiol Cell Physiol. 2008;294:C223–C232. doi: 10.1152/ajpcell.00395.2006. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. [PMC free article] [PubMed] [Google Scholar]
- 55.Lennerz JKM, Kim SH, Oates EL, et al. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia and carcinoma. Amer J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faller G, Kirchner T. Immunological and morphogenic basis of gastric mucosa atrophy and metaplasia. Virchows Arch. 2005;446:1–9. doi: 10.1007/s00428-004-1157-3. [DOI] [PubMed] [Google Scholar]
- 57.Correa P. Human gastric carcinogenesis: a multistep and multi-factorial process—First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 58.Cai X, Carlson J, Stoicov C, et al. Helicobacter felis eradication restores normal architecture and inhibits gastric cancer progression in C57BL/6 mice. Gastroenterology. 2005;128:1937–1952. doi: 10.1053/j.gastro.2005.02.066. [DOI] [PubMed] [Google Scholar]
- 59.Canfield V, West AB, Goldenring JR, et al. Genetic ablation of parietal cells in transgenic mice: a new model for analyzing cell lineage relationships in the gastric mucosa. Proc Natl Acad Sci U S A. 1996;93:2431–2435. doi: 10.1073/pnas.93.6.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nozaki K, Ogawa M, Williams JA, et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008;134:511–522. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spicer Z, Miller ML, Andringa A, et al. Stomachs of mice lacking the gastric H,K-ATPase alpha -subunit have achlorhydria, abnormal parietal cells, and ciliated metaplasia. J Biol Chem. 2000;275:21555–21565. doi: 10.1074/jbc.M001558200. [DOI] [PubMed] [Google Scholar]
- 62.Mutoh H, Hakamata Y, Sato K, et al. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem Biophys Res Commun. 2002;294:470–479. doi: 10.1016/S0006-291X(02)00480-1. [DOI] [PubMed] [Google Scholar]
- 63.Silberg DG, Sullivan J, Kang E, et al. Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology. 2002;122:689–696. doi: 10.1053/gast.2002.31902. [DOI] [PubMed] [Google Scholar]
- 64.Barbera M, Fitzgerald RC. Cellular mechanisms of Barrett’s esophagus development. Surg Oncol Clin North Am. 2009;18:393–410. doi: 10.1016/j.soc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 65.Jarvi O, Lauren P. On the role of heterotopias of the intestinal epithelium in the pathogenesis of gastric cancer. Acta Pathol Microbiol Scand. 1951;29:26–44. [PubMed] [Google Scholar]
- 66.Hattori T. Development of adenocarcinomas in the stomach. Cancer. 1986;57:1528–1534. doi: 10.1002/1097-0142(19860415)57:8<1528::aid-cncr2820570815>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 67.Kawachi H, Takizawa T, Eishi Y, et al. Absence of either gastric or intestinal phenotype in microscopic differentiated gastric carcinomas. J Pathol. 2003;199:436–446. doi: 10.1002/path.1323. [DOI] [PubMed] [Google Scholar]
- 68.Park do Y, Srivastava A, Kim GH, et al. Adenomatous and foveolar gastric dysplasia: distinct patterns of mucin expression and background intestinal metaplasia. Am J Surg Pathol. 2008;32:524–533. doi: 10.1097/PAS.0b013e31815b890e. [DOI] [PubMed] [Google Scholar]
- 69.Harvey BCH. A study of the structure of the gastric glands of the dog and of the changes which they undergo after gastroenterostomy and occlusion of the pylorus. Am J Anat. 1907;6:207–243. [Google Scholar]
- 70.Townsend SF. Regeneration of gastric mucosa in rats. Am J Anat. 1961;109:133–147. doi: 10.1002/aja.1001090204. [DOI] [PubMed] [Google Scholar]
- 71.Leys CM, Nomura S, Rudzinski E, et al. Expression of Pdx-1 in human gastric metaplasia and gastric adenocarcinoma. Hum Pathol. 2006;37:1162–1168. doi: 10.1016/j.humpath.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 72.Yoshizawa N, Takenaka Y, Yamaguchi H, et al. Emergence of spasmolytic polypeptide-expressing metaplasia in Mongolian gerbils infected with Helicobacter pylori. Lab Invest. 2007;87:1265–1276. doi: 10.1038/labinvest.3700682. [DOI] [PubMed] [Google Scholar]
- 73.Nomura S, Yamaguchi H, Ogawa M, et al. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G362–G375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 74.Goldenring JR, Nam KT, Wang TC, et al. Spasmolytic polypeptide-expressing metaplasia and intestinal metaplasia: time for reevaluation of metaplasias and the origins of gastric cancer. Gastroenterology. 2010;138:2207–2210. 2210.e1. doi: 10.1053/j.gastro.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee HJ, Nam KT, Park HS, et al. Gene expression profiling of metaplastic lineages identifies CDH17 as a prognostic marker in early stage gastric cancer. Gastroenterology. 2010;139:213–225.e3. doi: 10.1053/j.gastro.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nam KT, Lee HJ, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037.e9. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nam KT, Lee HJ, Mok H, et al. Amphiregulin-deficient mice develop spasmolytic polypeptide expressing metaplasia and intestinal metaplasia. Gastroenterology. 2009;136:1288–1296. doi: 10.1053/j.gastro.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiao C, Ogle SA, Schumacher MA, et al. Loss of parietal cell expression of Sonic hedgehog induces hypergastrinemia and hyperproliferation of surface mucous cells. Gastroenterology. 2010;138:550–561. 561e1–8. doi: 10.1053/j.gastro.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huh WJ, Esen E, Geahlen JH, et al. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology. 2010;139:2038–2049. doi: 10.1053/j.gastro.2010.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–1578. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 81.Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 82.Okumura T, Wang SS, Takaishi S, et al. Identification of a bone marrow-derived mesenchymal progenitor cell subset that can contribute to the gastric epithelium. Lab Invest. 2009;89:1410–1422. doi: 10.1038/labinvest.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quante M, Wang TC. Stem cells in gastroenterology and hepatology. Nat Rev Gastroenterol Hepatol. 2009;6:724–737. doi: 10.1038/nrgastro.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coffey RJ, Washington MK, Corless CL, et al. Menetrier disease and gastrointestinal stromal tumors: hyperproliferative disorders of the stomach. J Clin Invest. 2007;117:70–80. doi: 10.1172/JCI30491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim BM, Mao J, Taketo MM, et al. Phases of canonical Wnt signaling during the development of mouse intestinal epithelium. Gastroenterology. 2007;133:529–538. doi: 10.1053/j.gastro.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 86.Duluc I, Freund JN, Leberquier C, et al. Fetal endoderm primarily holds the temporal and positional information required for mammalian intestinal development. J Cell Biol. 1994;126:211–221. doi: 10.1083/jcb.126.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim BM, Buchner G, Miletich I, et al. The stomach mesenchymal transcription factor Barx1 specifies gastric epithelial identity through inhibition of transient Wnt signaling. Dev Cell. 2005;8:611–622. doi: 10.1016/j.devcel.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 88.Kim BM, Miletich I, Mao J, et al. Independent functions and mechanisms for homeobox gene Barx1 in patterning mouse stomach and spleen. Development. 2007;134:3603–3613. doi: 10.1242/dev.009308. [DOI] [PubMed] [Google Scholar]
- 89.Bleuming SA, He XC, Kodach LL, et al. Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Res. 2007;67:8149–8155. doi: 10.1158/0008-5472.CAN-06-4659. [DOI] [PubMed] [Google Scholar]
- 90.Huh WJ, Mysorekar IU, Mills JC. Inducible activation of Cre recombinase in adult mice causes gastric epithelial atrophy, metaplasia and regenerative changes in the absence of “floxed” alleles. Am J Physiol Gastrointest Liver Physiol. 2010;299:G368–G380. doi: 10.1152/ajpgi.00021.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schier S, Wright NA. Stem cell relationships and the origin of gastrointestinal cancer. Oncology. 2005;69(Suppl 1):9–13. doi: 10.1159/000086625. [DOI] [PubMed] [Google Scholar]
- 92.Snippert HJ, van der Flier LG, Sato T, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 93.Lopez-Garcia C, Klein AM, Simons BD, et al. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]