Abstract
Cataract affects 1 in 6 Americans over the age of 40, and is considered a global health problem. Cataract is caused by the aggregation of unfolded or damaged proteins in the lens, which accumulate as an individual ages. Currently, surgery is the only available treatment for cataract, however, small molecules have been suggested as potential preventative therapies. In this work, we study the effect of sodium citrate on the stability of Human γD Crystallin (HγD-Crys), a structural protein of the eye lens, and two cataract-related mutants, L5S HγD-Crys and I90F HγD-Crys. In equilibrium unfolding-refolding studies, the presence of 250 mM sodium citrate increased the transition midpoint of the N-td of WT HγD-Crys and L5S HγD-Crys by 0.3 M GuHCl, the C-td by 0.6M GuHCl, and the single transition of I90F HγD-Crys by 0.4M GuHCl. In kinetic unfolding reactions, sodium citrate demonstrates a measurable stabilization effect only for the mutant I90F HγD-Crys. In the presence of citrate, a kinetic unfolding intermediate of I90F HγD-Crys can be observed, which was not observed in the absence of citrate. Rate of aggregation was measured using solution turbidity, and sodium citrate demonstrates negligible effect on rate of aggregation of WT HγD-Crys, but considerably slows the rate of aggregation of both L5S HγD-Crys and I90F HγD-Crys. The presence of sodium citrate dramatically slows refolding of WT HγD-Crys and I90F HγD-Crys, but has a significantly smaller effect on the refolding of L5S HγD-Crys. The differential stabilizing effect of sodium citrate suggests that the ion is binding to a partially unfolded conformation of the C-td, but a solution-based Hofmeister effect cannot be eliminated as a possible explanation for the effects observed. These results suggest that sodium citrate may be a potential preventative agent for cataract.
Keywords: crystallin, protein folding, aggregation, citrate, stability
1. Introduction
Cataract, the accumulation of light-scattering aggregates in the eye lens, affects an estimated 20 million people, and is the leading cause of blindness worldwide (Brian and Taylor, 2001). In the United States, cataract affects one in six persons over the age of 40, and with prevalence increasing with age, affects 50% of persons over the age of 80 (Friedman et al., 2008). No successful preventive therapies have been developed, and surgery remains the only effective treatment for cataract (Nanavaty, 2006). Though cataract surgery has a high success rate, surgery is costly and remains unavailable to much of the developing world, leading to a disproportionate occurrence of blindness in poverty-stricken regions (Brian and Taylor, 2001).
The gamma crystallins are members of the βγ superfamily of structural proteins, and are a major component in the mature fiber cells of the vertebrate lens (McFall-Ngai et al., 1985; Siezen et al., 1987). These proteins are expressed early in lens development, and enriched in the nucleus, the oldest part of the lens (Graw, 2009; Siezen et al., 1988). The nucleus is densely packed with protein, where concentrations reach as high as 450 mg/ml, accounting for nearly 70% of the wet cell mass (Fagerholm et al., 1981). Packing of crystallin proteins into the fiber cells contributes structure to the lens tissue, and helps maintain the shape of the lens, which is critical for focusing light on the retina (Cooper et al., 1994; Delaye and Tardieu, 1983; Oyster, 1999).
HγD-Crys is one of the most abundant members of the βγ-crystallin family in the lens. This 173 residue protein has two homologous domains, each containing two greek-key motifs with intercalated anti-parallel beta sheet structure (Basak et al., 2003; Slingsby and Clout, 1999). The folding and unfolding pathways of HγD-Crys have been extensively characterized in vitro. Equilibrium experiments with HγD-Crys demonstrated that folding proceeds via an intermediate consisting of an unfolded N-terminal domain (N-td) and a folded C-terminal domain (C-td) (Flaugh et al., 2005a, b; Mills et al., 2007). Kinetic unfolding and refolding studies indicate that the folding of HγD-Crys is nucleated by the more stable C-td (Flaugh et al., 2005a, b;Mills, et al., 2007). Both hydrophobic and polar interactions at the domain interface helps to stabilize the N-td (Das et al., 2010; Flaugh et al., 2005a, b; Mills et al., 2007).
Analysis of human cataract indicated that proteins found in lens aggregates are heavily damaged and modified in comparison to soluble lens proteins (Hanson et al., 2000). As the lens ages, proteins in the lens can experience damage due to post-translational modifications such as truncation, deamidation, and glycation (Hains and Truscott, 2010; Hanson et al., 2000; Lampi et al., 1998; Takemoto et al., 1985; Ueda et al., 2002), as well as photo-oxidation of tryptophans and other residues (Kurzel et al., 1973; McCarty and Taylor, 2002). These damages lower the kinetic barrier to unfolding, and induce partial unfolding of lens proteins (David et al., 1996; Flaugh et al., 2006; Takata et al., 2008). The partial unfolding of the protein structure exposes hydrophobic residues normally buried in the native state, making them more susceptible to aggregation in vitro (Roder and Colón, 1997). Glutamine to glutamic acid replacement in HγD-Crys, which mimic oxidative damage in the lens, destabilize HγD-Crys in vitro, generating aggregation-prone partially unfolded intermediates (Flaugh et al., 2006). In age-related cataract, damaged proteins can accumulate over time without clearance from the lens, and these aggregation-prone conformations may seed the formation of cataract (Hains and Truscott, 2007; Harrington et al., 2007; Zigler and Goosey, 1984).
Lens protein modifications resulting from mutations can also provide insight into cataract formation. A progressive cataract phenotype in mice was found to be caused by a single point mutation F9→S, in Murine γS Crystallin (Everett et al., 1994). Histological studies of mutant homozygous mice revealed that F9S Murine γS Crystallin impeded the differentiation of lens epithelial cells to lens fiber cells, while biochemical experiments revealed the protein is temperature-sensitive to unfolding in vitro, which can lead to aggregation (Sinha et al., 2001). When the analogous mutation was made in HγD-Crys, in vitro folding studies demonstrated that the mutant destabilized the N-td, but had no effect on the stability of the C-td (Moreau and King, 2009). Furthermore, these studies showed that destabilization in the N-td accelerates unfolding in vitro, which suggests that reduced stability of the mutant is related to the pathology of the cataract (Lee et al., 2010; Moreau and King, 2009).
A congenital mutation shown to cause cataract, I90→F, was identified in Murine γD Crystallin (Graw et al., 2004). Studies on the stability of the analogous mutation in HγD-Crys demonstrated that the amino acid replacement disrupts the buried core of the C-td (Moreau and King, 2009). This replacement destabilized the partially-folded intermediate in the folding pathway, and induced a concerted unfolding-refolding mechanism (Moreau and King, 2009). In this study, WT HγD and the two HγD-Crys mutants, I90→F and L5→S were used to investigate the stabilizing effect of citrate on partially unfolded conformations of HγD-Crys.
Developing pharmacological agents that can stabilize damaged proteins or slow the aggregation of lens proteins may have a dramatic impact on public health. It has been estimated that retarding the progress of cataract by ten years may reduce the number of individuals affected by up to 50 percent (Livingston et al., 1995). Preliminary experiments in vivo suggested that the antioxidant carnosine suppressed radical oxygen species in the lens, and inhibited glycation of lens proteins in diabetic rats (Shi et al., 2009; Yan et al., 2008). Topical ocular application of a carnosine derivative, N-acetylcarnosine was used in a study of 75 patients with age-related cataract, which demonstrated that 9 months of treatment increased visual acuity and in some cases cleared cataract from treated eyes (Babizhayev et al., 2009).
Stabilization of proteins by ions in solution has been extensively studied (Street et al., 2006; Tadeo et al., 2009). For a number of proteins, the binding of ions has a stabilizing effect (Muzammil et al., 2000; Pace, 1992; Pace and McGrath, 1980; Ramos and Baldwin, 2002). Structural studies of the molten globule protein α-lactalbumin demonstrated that the binding of a single calcium ion induced a conformational change that stabilized the protein and increased the ΔG of unfolding by 40 kJ (Kronman et al., 1981; Van Ceunebroeck et al., 1985). Subsequent studies demonstrated that sodium, potassium, lithium and magnesium bound to the same site, and also induced a more stable conformation (Permyakov and Berliner, 2000). Similar studies using the enzyme superoxide dismutase showed that while zinc binding plays no catalytic role, it is critical to stabilizing flexible loops between the β-strands of the Greek key fold (Getzoff et al., 1989; Parge et al., 1992). The bound zinc ion forms a hydrogen-bond network that bridges the active site channel and stabilizes critical charged amino acids in Loop VII (Banci et al., 2003; Parge et al., 1992).
Recent studies of ancestral members of the βγ-crystallin superfamily revealed a common calcium binding motif (Aravind et al., 2009; Barnwal et al., 2009). Thermodynamic studies of several members of the crystallin family demonstrated that calcium binding increases stability by bridging the loops which connect the β-strands of the Greek key fold (Wenk et al., 1999; Kretschmar et al., 1999). These in vitro studies have demonstrated that ion binding can have a dramatic influence on tertiary structure and protein stability. In the physiological context, these finding suggest that the kinetic intermediates populated on these unfolding and aggregation pathways need to be considered, particularly for β-sheet proteins (Roder and Colón, 1997). Stabilization of kinetic precursors to off-pathway aggregation may slow unfolding and prevent aggregation. Conversely, stabilization of proteins on the aggregation pathway may accelerate aggregation. Elucidation of the mechanism by which ions stabilize crystallin to prevent unfolding may facilitate the development of new therapies that can prevent the progression of cataract.
Orally delivered citric acid was shown to slow the progression of cataract in diabetic rats, and also reduce glycation end products in the lenses of treated animals (Nagai et al., 2010). Recent studies demonstrated that sodium citrate slowed unfolding and aggregation of the protease inhibitor α1-Antitrypsin in vitro, and increased inhibitory activity of the enzyme (Bottomley and Tew, 2000; Pearce et al., 2008). The enzyme was crystallized in the presence of sodium citrate, and it was found that citrate binds to A and B β-sheets of the protein, a region known for maintaining stability. This result suggests that citrate binding induces stabilization of the protein, which can slow unfolding and prevent aggregation (Pearce et al., 2008). Sodium citrate was also found to stabilize soluble beta sheet structure of Human γC Crystallin and slow aggregation at low pH (Wang et al., 2010). The purpose of this work is to further investigate the stabilizing effect of sodium citrate and its potential as a small molecule inhibitor of aggregation of Human γD Crystallin (HγD-Crys).
2. Materials and Methods
2.1. Protein Expression and Purification
Plasmids for WT HγD-Crys, L5S HγD-Crys, and I90F HγD-Crys were the generous gift of K.M. N-terminal His-tagged constructs of WT HγD-Crys and I90F HγD-Crys were expressed as described previously (Kosinski-Collins et al., 2004). Briefly, M15 E.Coli was grown at 37°C to an OD600 of 1.0, and expression was induced with 1 mM IPTG for 4 hours. Cells were pelleted and resuspended in lysis buffer (50 mM NaPi, 300 mM NaCl, 18 mM Imidazole, 1 mM EDTA, pH 8.0) and stored at −80°C. L5S HγD-Crys was expressed as described previously (Moreau and King, 2009). M15 E.coli was grown at 37°C to OD600 of 1.0, then expression was induced overnight at 17°C with 1 mM IPTG. For all HγD-Crys variants, cells were lysed by sonication, and cell debris was pelleted by centrifugation at 20,000 × g for 45 minutes. DNA was precipitated using 0.12% PEI, followed by centrifugation at 20,000 × g for 10 minutes. Supernatant was filtered and loaded onto a Ni-NTA (Qiagen) affinity column, and purified using an AKTA fast protein liquid chromatography system (GE Healthcare) by eluting the HγD-Crys using an linear gradient of imidazole from 0–100%. Fractions containing pure HγD-Crys were pooled and dialyzed in 10 mM ammonium acetate, pH 7.0.
2.2. Equilibrium Unfolding-Refolding
Equilibrium unfolding-refolding experiments were performed as previously described (Flaugh et al., 2006). Buffers were prepared as appropriate, adjusted to pH 7.0, and then filtered. The unfolding-refolding curves were fit to a two-state (Greene and Pace, 1974) or three-state model (Clark et al., 1993) using Kaleidagraph (Synergy Software). Increase in fluorescence intensity due to light scattering at low concentrations of GuHCl in refolding samples were not included in data used to fit refolding curve. Average and standard deviation of transition midpoint, ΔG0, and m-values were calculated using three separate trials.
2.3. Unfolding Kinetics
Unfolding kinetics were performed as previously described (Moreau and King, 2009). Briefly, WT HγD-Crys was diluted into unfolding buffer (5.5 M GuHCl, 100 mM NaPi, 5 mM DTT, 1 mM EDTA, pH 7.0) at 18°C for a final protein concentration of 10 μg/ml. Concentration of GuHCl was reduced to 3.5 M GuHCl in unfolding buffer for mutant HγD-Crys. In citrate samples, 250 mM sodium citrate was added to unfolding buffer. Excitation wavelength was set to 295 nm, and fluorescence emission at 350 nm was measured over time using a Hitachi F-4500 fluorimeter. Kinetic unfolding data was fit to single or double exponentials using Kaleidagraph (Synergy Software). Best fits were chosen based on random distribution of fit residuals, and agreement of parameters with previous results (Moreau and King, 2009). Experiments were performed in triplicate for each condition, and fit parameters for the three trials were averaged.
2.4. Refolding Kinetics
WT, I90F and L5S HγD-Crys were unfolded in buffer containing 5.5 M GuHCl, 100 mM NaPi, 5 mM DTT, 1 mM EDTA, pH 7.0, at 37°C at a concentration of 100 μg/ml for 5 hours. Citrate samples were unfolded under identical conditions with the addition of 250 mM sodium citrate in the unfolding buffer. Samples were diluted to 10 μg/ml into refolding buffer containing 1 M GuHCl, 100 mM NaPi, 5 mM DTT, 1 mM EDTA, pH 7.0, at 37°C, and citrate samples were diluted into refolding buffer containing 250 mM sodium citrate. Excitation wavelength was set to 295 nm, and fluorescence emission at 350 nm was measured over time using a Hitachi F-4500 fluorimeter. Kinetic refolding data was fit to single or double exponentials using Kaleidagraph (Synergy Software). At high concentrations of sodium citrate, long-lived intermediates prevented the fitting of the data with a double exponential. In these cases, the discrete transitions were estimated, then fit separately with single exponential equations. Best fits were chosen based on random distribution of fit residuals, and agreement of parameters with previous results (Moreau and King, 2009). Experiments were performed in triplicate for each condition, and fit parameters for the three trials were averaged.
2.5. Aggregation Turbidity
Aggregation experiments were performed as described previously (Knee et al., 2011) (Acosta-Sampson and King, 2010) WT, L5S and I90F HγD-Crys were incubated in unfolding buffer (5.5 M GuHCl, 100 mM NaPi 1 mM EDTA, 5 mM DTT) for 16 hours at 37°C at a concentration of 0.5 mg/ml in the presence and absence of stabilizing anions. Unfolded protein was then rapidly diluted ten-fold into buffer (100 mM NaPi, 1 mM EDTA, 5 mM DTT) at 37°C in the presence and absence of stabilizing anions for a final protein concentration of 50 μg/ml. Solution turbidity was measured by apparent absorbance at 350 nm using a Cary 50 UV/Vis Spectrophotometer (Varian).
3. Results
3.1. Sodium Citrate Stabilizes the Equilibrium Unfolding of WT HγD-Crys
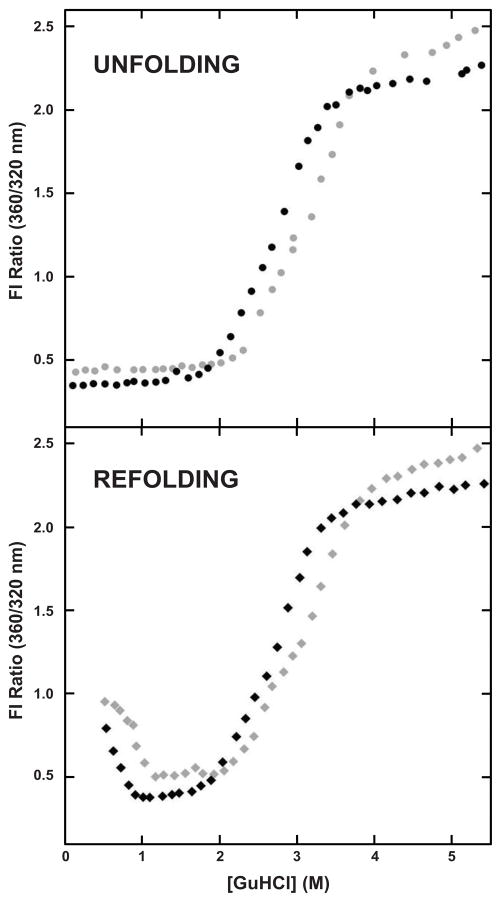
The unfolding-refolding pathway of WT HγD-Crys follows a three-state folding pathway, and populates a meta-stable intermediate consisting of an unfolded N-td and a folded C-td (Flaugh et al., 2005a; Kosinski-Collins et al., 2004; Kosinski-Collins and King, 2003; Mills et al., 2007). When either 100 mM sodium phosphate or 100 mM sodium phosphate and 250 mM sodium citrate was included in the buffer, the characteristic three state transition of HγD-Crys was observed. In the presence of 100 mM sodium phosphate, the N-td had an unfolding midpoint of 2.2 ± 0.1 M GuHCl, and the C-td had an unfolding midpoint of 2.8 ± 0.1 M GuHCl (Table 1), values consistent with previous reports (Kosinski-Collins et al., 2004). In the presence of 250 mM sodium citrate both of the unfolding midpoints increased, from 2.2 ± 0.1 to 2.5 ± 0.1 M GuHCl for the N-td and from 2.8±0.1 to 3.4±0.1 M GuHCl for the C-td (Figure 1, Table 1). Sodium citrate increased the ΔG of unfolding of WT HγD-Crys from 16.6 kcal/mol to 22.2 kcal/mol (Table 1). In these unfolding-refolding experiments, the ionic strength of the sample is dominated by the high concentration of GuHCl. The observed effect of the citrate ion must be through specific interactions with the protein or the solvent. Though not as effective as sodium citrate, increased stability was also observed for other kosmotropic anions in the Hofmeister series (Zhang and Cremer, 2010), including sodium phosphate and sodium sulfate (data not shown).
Table 1.
Thermodynamic Parameters for Equilibrium Unfolding/Refolding and Kinetic Unfolding of WT HγD-Crys
| Equilibrium Unfolding | |||||||
|---|---|---|---|---|---|---|---|
| t1 | t2 | m1 | m2 | ΔG1 | ΔG2 | ΔΔGt | |
|
|
|||||||
| 100 mM NaPia | 2.2 ± 0.1 | 2.8 ± 0.1 | 3.6 ± 0.1 | 3.1 ± 0.4 | 7.7 ± 0.2 | 8.9 ± 1.3 | N/A |
| 250 mM NaCit | 2.6 ± 0.1 | 3.4 ± 0.1 | 4.1 ± 1.2 | 3.5 ± 0.7 | 10.4 ± 3.2 | 11.8 ± 2.6 | 5.6 |
| Kinetic Unfolding | |||||||
|---|---|---|---|---|---|---|---|
| k1 | k2 | t1 | t2 | ||||
|
|
|||||||
| 100 mM NaPi | 0.0011 ± 7×10−5 | 0.00017 ± 9×10−6 | 900 ± 60 | 6000 ± 300 | |||
| 250 mM NaCit | 0.0011 ± 1×10−4 | 0.00016 ± 2×10−5 | 910 ± 90 | 6500 ± 900 | |||
From Flaugh et al., 2005b.
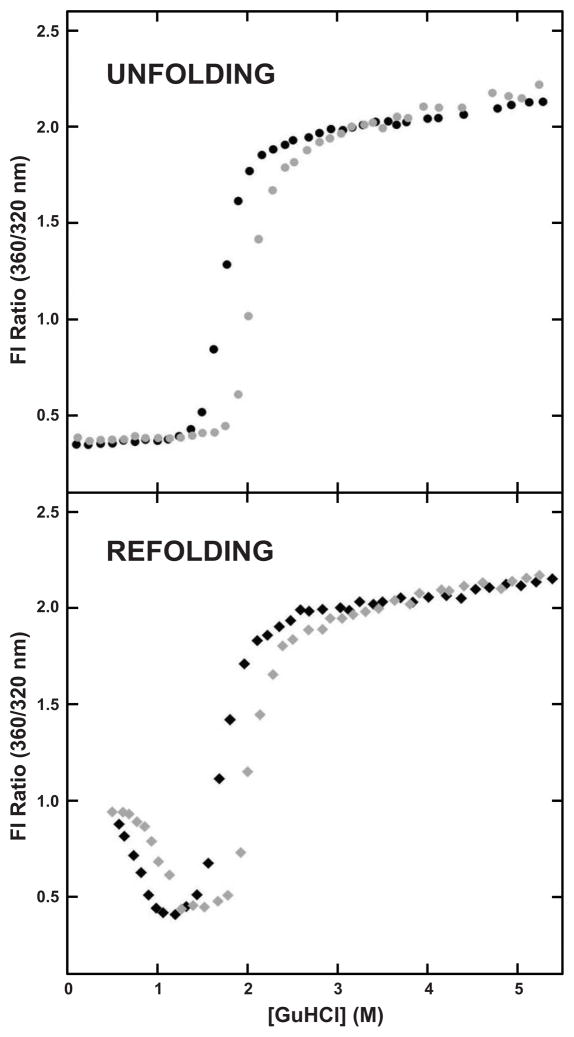
Figure 1.
Equilibrium Unfolding-Refolding of WT HγD-Crys. (Top) Equilibrium unfolding of WT HγD-Crys in the absence (●) and presence (●) of 250 mM sodium citrate. (Bottom) Equilibrium refolding of WT HγD-Crys in the absence (◆) and presence (◆) of 250 mM sodium citrate.
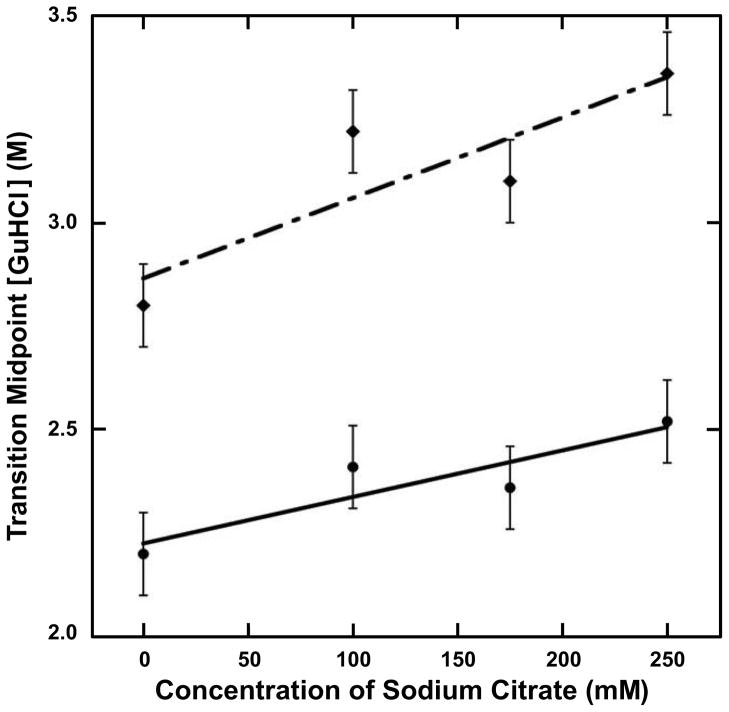
The stabilizing effect of the sodium citrate on the unfolding of HγD-Crys was further examined by varying the concentration of sodium citrate; the stability of each domain increased linearly with increasing concentration of sodium citrate, but the effect was more pronounced in the C-td than in the N-td (Figure 2, Table 1). The presence of sodium citrate had a greater effect on the C-td of WT HγD-Crys as shown by the 0.6 M GuHCl increase in the unfolding midpoint in the presence of sodium citrate, as compared to 0.3M GuHCl increase observed for the N-td.
Figure 2.
Linear Dependence of Transition Midpoint on Concentration of Sodium Citrate. WT HγD-Crys C-td unfolding midpoints (◆) and N-td unfolding midpoints (●) plotted as a function of concentration of sodium citrate. Midpoints were fit with linear fit for transition 1 (solid line) and transition 2 (dashed line).
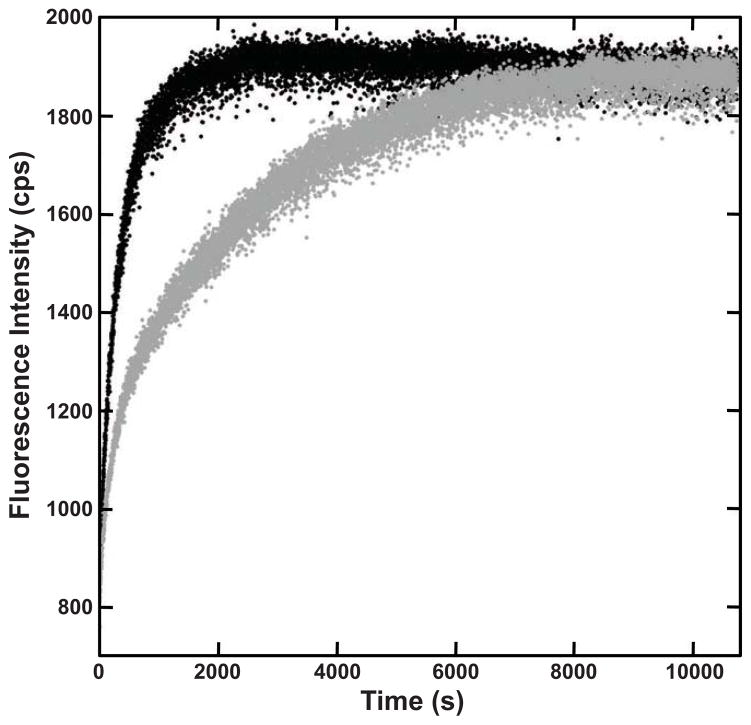
The kinetic unfolding of WT HγD-Crys was also measured in the presence and absence of sodium citrate. Previous studies have established that HγD-Crys has a 3 state unfolding pathway, in which the N-td unfolds, followed by the C-td (Kosinski-Collins and King, 2003; Mills et al., 2007). In 100 mM sodium phosphate buffer, the N-td unfolded with a half-time of 900 ± 60 seconds, followed by the more-stable C-td, which unfolded with a half-time of 6000 ± 300 seconds, consistent with previous reports (Moreau and King, 2009). When 250 mM sodium citrate was added to the buffer, no change was observed in the unfolding kinetics. In the presence of 250 mM sodium citrate, the N-td unfolded with a half time of 910 ± 90 seconds, and C-td unfolded with a half time of 6500±900 seconds. This result suggests that citrate does not act by stabilizing the native state, but more likely by an effect on a partially folded intermediate.
3.2. Stabilization of N-terminal Domain Mutant L5S HγD-Crys
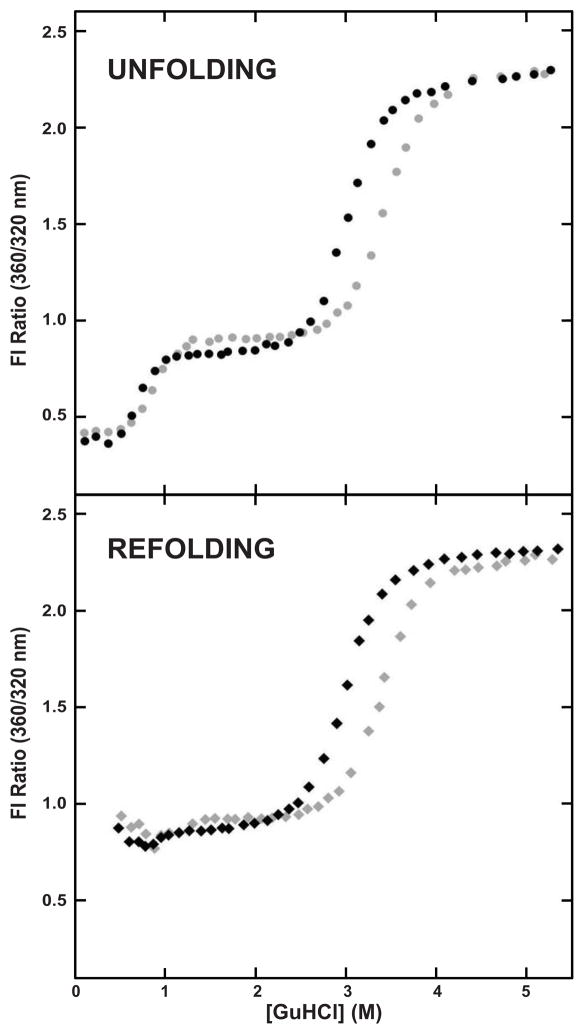
Previous work extensively characterized the thermodynamic properties of the congenital cataract associated mutant L5S, which has a destabilized N-td, but an unaffected C-td (Moreau and King, 2009).The unfolding of L5S HγD-Crys creates a long-lived folding intermediate consisting of an unfolded N-td and a folded C-td, as evidenced by a plateau in the equilibrium unfolding-refolding curves from 1 M to 2.3 M GuHCl (Figure 4). When L5S HγD-Crys was equilibrated with 250 mM sodium citrate, the stabilizing effect was of similar magnitude to the effect observed for WT HγD-Crys. In the presence of 250 mM sodium citrate, the unfolding midpoint of the N-td was increased by 0.3 M GuHCl, from 0.7 ± 0.1 M GuHCl, to 1.0 ± 0.1 M GuHCl, and the unfolding midpoint of the C-td was increased by 0.6 M GuHCl from 2.8 ± 0.1 M GuHCl to 3.4 ± 0.1 M GuHCl (Figure 4, Table 2).
Figure 4.
Equilibrium Unfolding-Refolding of L5S HγD-Crys. (Top) Equilibrium unfolding of L5S HγD-Crys in the absence (●) and presence (●) of 250 mM sodium citrate. (Bottom) Equilibrium refolding of L5S HγD-Crys in the absence (◆) and presence (◆) of 250 mM sodium citrate.
Table 2.
Thermodynamic Parameters for Equilibrium Unfolding/Refolding and Kinetic Unfolding of L5S HγD-Crys
| Equilibrium Unfolding | |||||||
|---|---|---|---|---|---|---|---|
| t1 | t2 | m1 | m2 | ΔG1 | ΔG2 | ΔΔG | |
|
|
|||||||
| 100 mM NaPia | 0.7 ± 0.1 | 2.9 ± 0.1 | 4.4 ± 0.2 | 3.1 ± 0.2 | 3.1 ± 0.2 | 8.9 ± 0.4 | N/A |
| 250 mM NaCit | 1.0 ± 0.1 | 3.4 ± 0.1 | 5.8 ± 1.6 | 2.8 ± 0.3 | 5.6 ± 1.7 | 9.6 ± 1.1 | 2.9 |
| Kinetic Unfolding | ||
|---|---|---|
| k1 | t1 | |
|
|
||
| 100 mM NaPi | 0.68 ± 0.08 | 1.5 ± 0.2 |
| 250 mM NaCit | 0.53 ± 0.01 | 1.9 ± 0.1 |
From Moreau and King, 2009.
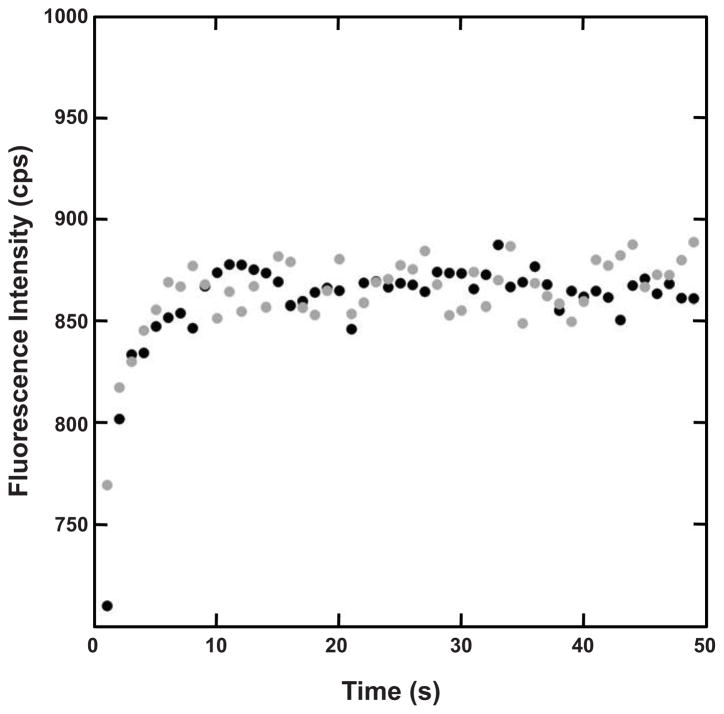
For kinetic unfolding experiments, the destabilization caused by the mutation necessitated a change in the concentration of denaturant from 5.5 M to 3.5 M GuHCl, in order to more accurately observe the rapid unfolding of the N-td. Dilution of L5S HγD-Crys into 100 mM sodium phosphate unfolding buffer resulted in rapid unfolding of the destabilized N-td with a half-time of 1.5 ± 0.2 seconds, which is in agreement with previous reports (Moreau and King, 2009). The addition of 250 mM sodium citrate to the unfolding buffer resulted in a half-time of unfolding of the N-td of 1.9 ± 0.1 seconds (Figure 5, Table 2). Thus, the presence of citrate did not appreciably slow the rate of unfolding of the N-td. As a result of the low concentration of denaturant used in the experiment, the C-td did not fully unfold, and therefore no observations could be made concerning the unfolding of the C-td of L5S HγD-Crys. These kinetic unfolding results are consist with those found for wild-type, suggesting that citrate does not act by stabilizing the native state.
Figure 5.
Kinetic Unfolding of the N-td of L5S HγD-Crys. Sample was rapidly diluted into unfolding buffer containing 3.5 M GuHCl in the absence (●) and presence (●) of 250 mM sodium citrate.
3.3. Stabilization of C-terminal Mutant I90F HγD-Crys
Previous studies demonstrated that the cataract associated I90F mutation in the C-td eliminates the intermediate in the folding pathway and induces a two-state concerted unfolding mechanism (Moreau and King, 2009). Equilibrium unfolding-refolding experiments indicated that in the presence of 100 mM sodium phosphate, the unfolding midpoint of I90F HγD-Crys was 1.7 ± 0.1 M GuHCl. In the presence of 250 mM sodium citrate, the unfolding midpoint was increased by 0.4 M GuHCl to 2.1 ± 0.1 M GuHCl (Figure 6, Table 3). In the presence of citrate, the increase in stability of the two-state unfolding midpoint of I90F HγD-Crys was larger than the increase in stability of the N-td of WT HγD-Crys, but smaller than the increase in stability of the C-td of WT HγD-Crys. This may be the result of conformational change in the C-td caused by perturbation of the hydrophobic core of I90F HγD-Crys.
Figure 6.
Equilibrium Unfolding-Refolding of I90F HγD-Crys. (Top) Equilibrium unfolding of I90F HγD-Crys in the absence (●) and presence (●) of 250 mM sodium citrate. (Bottom) Equilibrium refolding of I90F HγD-Crys in the absence (◆) and presence (◆) of 250 mM sodium citrate.
Table 3.
Thermodynamic Parameters for Equilibrium Unfolding/Refolding and Kinetic Unfolding of L5S HγD-Crys
| Equilibrium Unfolding | ||||
|---|---|---|---|---|
| t1 | m1 | ΔG1 | ΔΔG | |
|
|
||||
| 100 mM NaPia | 1.7± 0.1 | 5.5 ± 1.2 | 9.4 ± 2.1 | N/A |
| 250 mM NaCit | 2.1 ± 0.1 | 6.5 ± 0.6 | 13.4 ± 1.2 | 1.2 |
| Kinetic Unfolding | ||||
|---|---|---|---|---|
| k1 | k2 | t1 | t2 | |
|
|
||||
| 100 mM NaPi | 0.0026 ± 1×10−4 | N/A | 385 ± 17 | N/A |
| 250 mM NaCit | 0.0058 ± 1×10−4 | 0.00039 ± 3×10−5 | 173 ± 4 | 2595 ± 179 |
From Moreau and King, 2009.
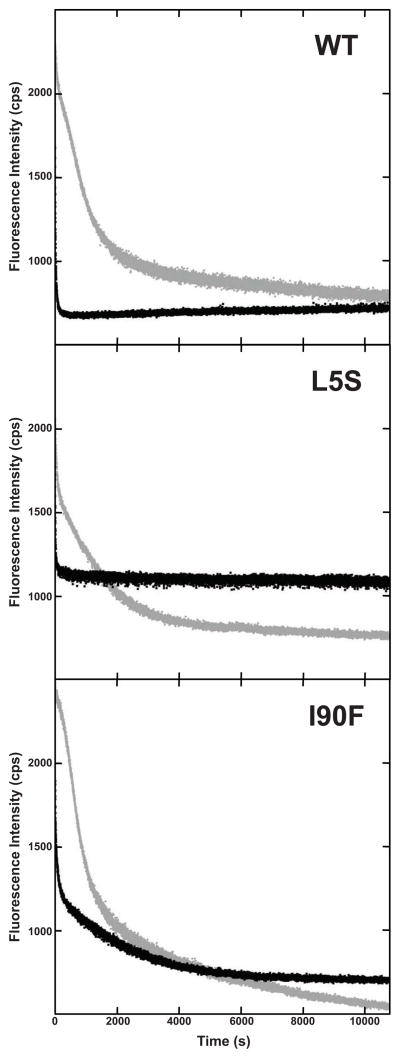
In the presence of 100 mM sodium phosphate, I90F HγD-Crys was observed to unfold with a half time of 380 ± 20 seconds (Figure 7), in agreement with previously published results (Moreau and King, 2009). When 250 mM sodium citrate was added, a folding intermediate appeared in the unfolding pathway, which was not apparent in the absence of citrate. In the presence of 250 mM sodium citrate, the first transition has a half time of 173 ± 4 seconds, while the second transition has a half time of 2600 ± 200 seconds (Figure 7, Table 3). Sequential unfolding of the N-td and C-td was previously observed in kinetic unfolding studies of WT HγD, therefore, it is likely that the two transitions observed for I90F HγD unfolding in the presence of sodium citrate can be attributed to the unfolding of N-td and C-td (Kosinski-Collins and King, 2003; Mills et al., 2007).
Figure 7.
Kinetic Unfolding of I90F HγD-Crys. Sample was rapidly diluted into unfolding buffer containing 3.5 M GuHCl in the absence (●) and presence (●) of 250 mM sodium citrate.
3.4. Sodium Citrate Slows Aggregation of Mutant HγD-Crys
When HγD is rapidly diluted from high to low denaturant concentration, the proteins do not spontaneously refold to a native state, but rather associate into amorphous aggregates. The rate of aggregation of HγD-Crys can be measured in vitro by solution turbidity, and can be used to quantify the effects of small molecules on the HγD-Crys aggregation pathway. WT, L5S, and I90F HγD-Crys were rapidly diluted from 5.5 M GuHCl to 0.55 M GuHCl, in the presence and absence of 250 mM sodium citrate, and the rate of aggregation was measured. For WT HγD-Crys, an aggregation rate of 45 ± 5 seconds was observed in 100 mM sodium phosphate, and a rate of 50 ± 10 seconds was observed in the presence of 250 mM sodium citrate (Table 4). These rates are consistent with previously published aggregation rates for HγD-Crys (Knee et al., 2011) and indicate that sodium citrate had little effect on the aggregation of WT HγD-Crys, a result similar to that seen in the kinetic unfolding experiments.
Table 4.
Half-time of Aggregation for HγD-Crys
| T ½ | T ½ | |
|---|---|---|
| 100 mM sodium phosphate | 250 mM sodium citrate | |
|
|
||
| WT | 45 ± 5 s | 46 ± 10 s |
| L5S | 21 ± 1 s | 46 ± 6 s |
| I90F | 34 ± 5 s | 76 ± 10 s |
For the N-td mutant L5S HγD-Crys, an aggregation rate of 21 ± 1 seconds was observed for 100 mM sodium phosphate. However, in the presence of 250 mM sodium citrate, the aggregation rate was slowed by 120%, to 46 ± 6 seconds (Table 4). The decreased rate suggests that the presence of the sodium citrate is influencing the aggregation of L5S HγD-Crys to a greater degree than WT HγD-Crys. The largest effect of sodium citrate on aggregation rate was seen with the C-td mutant I90F HγD-Crys. In 100 mM sodium phosphate, the rate of I90F HγD-Crys aggregation was 34 ± 5 seconds, while in the presence of 250 mM sodium citrate, the rate of aggregation was slowed by 135%, to 80 ± 10 seconds (Table 4), the slowest rate of aggregation for all three HγD variants used in this study. This result mirrors what was observed for the unfolding kinetics, in which the presence of the sodium citrate had the largest effect on the kinetic unfolding of I90F HγD-Crys.
3.5. Sodium Citrate Slows HγD Crystallin Refolding
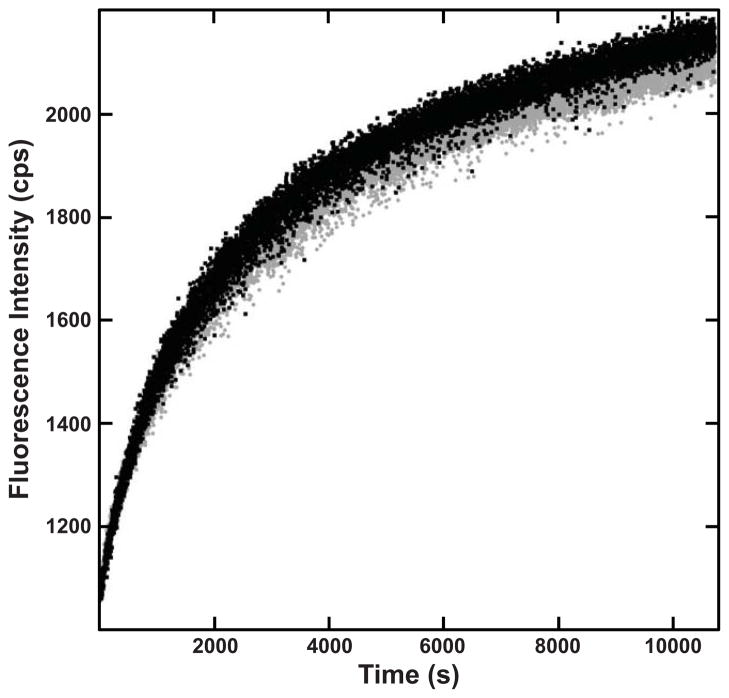
Previous reports have demonstrated that refolding of HγD-Crys begins with the folding of the C-td, followed by the folding of the N-td, passing through an intermediate likely consisting of a folded C-td and an unfolded N-td (Das et al., 2010; Kosinski-Collins and King, 2003; Mills et al., 2007). Rapid dilution of HγD-Crys into buffer containing varying amounts of sodium citrate initiated the refolding reaction. In phosphate buffer, the C-terminal domain of WT HγD-Crys refolded with a half-time of 10.9 ± 0.2 seconds, and the N-terminal domain refolds with a half-time of 60 ± 4 seconds (Figure 8, Table 5), consistent with previous reports (Kosinski-Collins and King, 2003; Mills et al., 2007). When refolded in the presence of 250 mM sodium citrate, an additional intermediate was observed in WT HγD-Crys. Initial collapse of the protein structure occurred with a half-time of 130 ± 3 seconds, which populated a stable intermediate. Following the formation of the stable intermediate, WT HγD-Crys followed a three-state folding pathway beginning with refolding of the C-td with a half-time of 600 ± 100 seconds, followed by refolding of the N-td with a half-time of 8000 ± 1000 seconds (Figure 8, Table 5). These results suggest that citrate interacts with and stabilizes a partially folded intermediate, thus retarding the rate of refolding.
Figure 8.
Kinetic Refolding of WT, L5S, and I90F HγD-Crys. (WT) Kinetic refolding of WT HγD-Crys in the absence (■) and presence (●) of 250 mM sodium citrate. (L5S) Kinetic refolding of L5S HγD-Crys in the absence (■) and presence (●) of 250 mM sodium citrate. (I90F) Kinetic refolding of I90F HγD-Crys in the absence (■) and presence (●) of 250 mM sodium citrate
Table 5.
Rate of Kinetic Refolding of HγD-Crys
| WT HγD-Crys | ||||||
|---|---|---|---|---|---|---|
| k1 | k2 | k3 | t1 | t2 | t3 | |
|
|
||||||
| 100mM NaPi | 0.092 ± 0.003 | 0.017 ± 0.001 | 10.9 ± 0.2 | 60 ± 4 | ||
| 250mM NaCit | 0.0077 ± 2×10−4 | 0.0016 ± 3×10−4 | 0.00013 ± 2×10−5 | 130 ± 3 | 630 ± 100 | 8200 ± 1400 |
| L5S HγD-Crys | ||||
|---|---|---|---|---|
| k1 | k2 | t1 | t2 | |
|
|
||||
| 100 mM NaPi | 0.070 ± 0.003 | 0.00051 ± 7×10−5 | 14.2 ± 0.7 | 1970 ± 280 |
| 250 mM NaCit | 0.026 ± 0.001 | 0.00061 ± 2×10−5 | 39 ± 2 | 1630 ± 60 |
| I90F HγD-Crys | ||||
|---|---|---|---|---|
| k1 | k2 | t1 | t2 | |
|
|
||||
| 100 mM NaPi | 0.0181 ± 2×10−4 | 0.00047 ± 5×10−5 | 55.2 ± 0.6 | 2270 ± 230 |
| 250 mM NaCit | 0.00184 ± 5×10−5 | 0.00018 ± 2×10−5 | 540 ± 10 | 5730 ± 590 |
In refolding studies of L5S HγD-Crys, the N-td of L5S HγD-Crys, which was significantly destabilized in equilibrium unfolding-refolding studies, refolded with a half-time of 1970 ± 280 seconds, thirty-three times slower than the rate of refolding of WT HγD-Crys N-td (Figure 8, Table 5). On the other hand, the C-td refolded on a similar time scale to WT HγD-Crys, with a half-time of 14.2 ± 0.7 seconds (Figure 8, Table 5), further demonstrating that the N-td mutation has no effect on the folding and stability of the C-td. In refolding studies of L5S HγD-Crys in the presence of 250 mM sodium citrate, the lag phase observed in the refolding of WT HγD-Crys was no longer present, and the C-td refolds rapidly with a half time 39 ± 2 seconds (Figure 8, Table 5). Folding of the N-td follows with a half time of 1630 ± 60 seconds. The decreased stability of the N-terminal domain of L5S HγD slowed the refolding, which influencing the populations of intermediate conformations in the folding pathway. As a result, citrate may interact differently with these intermediates than those which dominate the folding pathway of WT HγD-Crys.
In the case of I90F HγD-Crys, in which both the C-td and N-td are destabilized, kinetic refolding was slower when compared to WT HγD-Crys. In phosphate buffer, the C-td of I90F HγD-Crys refolded with a half-time 55.2 ± 0.6 seconds (Figure 8, Table 5), five times slower than the refolding of the C-td of WT HγD-Crys. The N-td of I90F HγD-Crys refolded with a half-time of 2200 ± 200 seconds in (Figure 8, Table 5), over thirty times slower than the refolding of the N-td in WT HγD-Crys. In the presence of 250 mM sodium citrate, the refolding of I90F HγD-Crys was further slowed when compared to refolding in phosphate. After an initial lag phase, the C-td refolded with a half time of 540 ± 10 seconds (Figure 8, Table 5), and the N-td followed with a half-time of 5700 ± 600 seconds (Figure 8, Table 5). Kinetic and equilibrium results indicate that the interaction of citrate is strongest when the buried hydrophobic core of the protein is exposed, and this interaction can interfere with the folding pathway of all three HγD-Crys variants.
4. Discussion
Numerous studies on the interaction of proteins with ions in solution have demonstrated that cosolutes are critical to maintaining protein solubility and stability (Busby et al., 1981; Ramos and Baldwin, 2002; Tadeo et al., 2009). In this study, equilibrium unfolding-refolding results with WT HγD-Crys demonstrated that the presence of sodium citrate increased protein stability. The free energy of unfolding increased 5.6 kcal/mol in the presence of sodium citrate, which is similar to the increase in ΔG of unfolding of Ribonuclease A in the presence of 200 mM sodium sulfate (Ramos and Baldwin, 2002). The presence of sodium sulfate has also been shown to increase the thermal stability of immunoglobulin light chain, increasing the Tm from 46.1°C to 59.3°C (Sikkink and Ramirez-Alvarado, 2008). A comp rehensive study of protein stability in the presence of salt demonstrated that a specific interaction with neutral salts increased the stability of Ribonuclease A in a linear manner as measured by thermal unfolding (Von Hippel and Wong, 1965).
Despite the increase in protein stability observed in equilibrium unfoldingrefolding studies, the presence of sodium citrate had no effect on the rate of kinetic unfolding of WT and L5S HγD-Crys. However, the presence of citrate did have an effect on the kinetic unfolding of I90F HγD-Crys. In the presence of citrate, unfolding of the mutant was slowed significantly, and a kinetic intermediate was observed that was not observed in the absence of citrate (Figure 7). Kinetic analysis of the folding and unfolding of I90F HγD-Crys reveal that the lifetimes and rates of the mutant intermediates differ from that of the wild-type (Figures 3, 5, 7, 8) (Moreau and King, 2009). It is also reasonable to infer that the conformation of the mutant intermediates differs from that of the wild-type. We propose that the kinetic unfolding data demonstrate that the I90F mutant has a longer-lived conformation accessible to citrate binding.
Figure 3.
Kinetic Unfolding of WT HγD-Crys. Sample was rapidly diluted into unfolding buffer containing 5M GuHCl in the absence (■) and presence (●) of 250 mM sodium citrate.
The I90F mutation destabilizes the hydrophobic core of HgD-Crys, leading to increased solvent exposure. I90F HγD-Crys is the most destabilized of the three HγD-Crys proteins studied, and thus it is reasonable that the sodium citrate would have the strongest stabilizing effect. Simulations by Das and colleagues show that folding of WT HγD-Crys is nucleated by the A and B strands in motif 4 of the C-td, which is stabilized by a non-native salt bridge between Q135 and R142 (Das et al., 2010). Citrate stabilization of these critical charge-charge interactions may play a role in slowing the unfolding of the perturbed hydrophobic core of the C-td of I90F HγD-Crys. A systematic study of surface mutants of Protein L from Streptococcal magnus demonstrated that an increase in solvent-exposed surface area increases the stabilization effect of solvated ions (Tadeo et al., 2009). Furthermore, studies have shown that the presence of sodium citrate slowed the unfolding of α1-Antitrypsin by direct binding (Pearce et al., 2008). These results indicate that citrate can bind directly to β-strands, thereby stabilizing the protein and slowing the unfolding.
To better understand the interaction of sodium citrate with folding intermediates, kinetic refolding of HγD-Crys was studied in the presence and absence of sodium citrate. In kinetic unfolding studies of WT and L5S HgD-Crys, presence of citrate showed no effect on the rate of unfolding. However, in the presence of citrate, the refolding kinetics of both WT and L5D HgD-Crys slowed dramatically. This suggests that citrate stabilizes partially folded intermediates in the folding pathway, which slows progress to the native state. A similar slowing of the refolding kinetics of I90F HgD-Crys is observed, further indicating that citrate interacts with partially folded conformations.
In aggregation experiments in this study, sodium citrate demonstrated negligible effect on rate of aggregation of WT HγD-Crys, but adding 250 mM sodium citrate considerably slows the rate of aggregation of both L5S HγD-Crys and I90F HγD-Crys. Similar results were reported in studies of α1-Antitrypsin, which showed that the presence of sodium citrate slowed aggregation in vitro (Bottomley and Tew, 2000; Pearce et al., 2008). These results suggest that citrate may slow the rate of aggregation of destabilized or damaged proteins by stabilizing partially unfolded conformations. If we assume that the precursor conformer in aggregation is derived from the partially unfolded intermediate on the productive pathway, then stabilization of partially unfolded intermediates would slow the rate of unfolding and aggregation. A second possibility is that citrate acts directly to inhibit the aggregation reaction without interacting with the productive intermediate. Recent studies have shown that molecules which do not interact strongly with the protein in solution can slow aggregation by increasing the energy required to exclude the solvent from hydrophobic interactions in the aggregated state (Trout, 2009; Vagenende et al., 2009).
There are two different mechanisms which may explain the observed stabilization of HgD-Crys by citrate; a global stabilization via the Hofmeister effect, or direct ion binding. Computational models suggest that solvated ions can stabilize native protein structure by increasing surface tension surrounding aliphatic side chains, and stabilizing the surface charge interaction by polarizing the first hydration shell of the protein (Baldwin, 1996; Dill et al., 2005; Ramos and Baldwin, 2002; Tadeo et al., 2009). These effects can combine to shift equilibrium to favor the folded state by stabilizing both surface charge interaction and hydrophobic interaction between aliphatic side chains without direct ion binding.
However, the domain-specific effect of citrate stabilization of HγD-Crys is not completely explained by the Hofmeister effect. In equilibrium unfolding-refolding studies of both WT and L5S HγD-Crys, the C-td was stabilized to a much higher degree than the N-td, suggesting that citrate preferentially interacts with the C-td. The presence of 250 mM sodium citrate had the same effect on the N-td of WT HγD-Crys as on the N-td of L5S HgD-Crys, which is significantly destabilized (Table 1, 2). The presence of sodium citrate increased the stability of the N-td of L5S HγD-Crys by 0.3 M GuHCl, and the C-td by 0.6 M GuHCl, the same effect as observed in WT HγD-Crys. This difference may be attributed to the increased dipole moment of the C-td, resulting in chargecharge interactions between the C-td and citrate anions (Purkiss et al., 2007), but direct binding of citrate to a partially unfolded conformation of the C-td cannot be excluded. Studies of the effect of ions on the equilibrium unfolding-refolding of human serum albumin (HSA) show that the first transition in unfolding of HSA is stabilized in the presence of 1 M potassium chloride, while the second transition remains unaffected (Muzammil et al., 2000). Muzammil and colleagues attribute this stabilization effect to a direct binding of the ion to the protein, rather than interaction at protein-water interface. Protein stabilization by sodium citrate was also observed in equilibrium unfolding studies of α1-Antitrypsin, and these studies concluded that the effect was the result of binding of citrate in the turn between strand A and B, a region of the protein known for importance to stability (Pearce et al., 2008).
Our studies on the effect of sodium citrate on HγD-Crys demonstrate that the presence of an anion can have a profound influence on both the stability of protein in solution and the intermediate conformations along the folding and unfolding pathways. Structural studies of α1-antitrypsin show that citrate can bind in the turn between two β-strands, which suggests that citrate may stabilize other β-sheet proteins in a similar manner (Bottomley and Tew, 2000; Pearce et al., 2008). Evolutionary studies of the βγ-crystallin superfamily have demonstrated that members of this family are known to bind ions, which stabilize tertiary structure (Aravind et al., 2009; Barnwal et al., 2009). Comparative analysis of protein ion binding sites revealed that ions bind tightly to charged amino acids buried within a hydrophobic shell (Yamashita et al., 1990). The hydrophobic interface of HγD-Crys contains a number of glutamine residues, and it is possible that citrate binds to these residues at the interface between the N-td and the C-td. Citrate may also bind to charged residues that are exposed in kinetic intermediates of HγD-Crys folding (Das et al., 2010; Flaugh et al., 2005b; Flaugh et al., 2006). Alternatively, many studies have demonstrated that solvated ions can strengthen hydrophobic interactions in the native state via the Hofmeister effect (Ramos and Baldwin, 2002; Zhang and Cremer, 2006).
Slowing the progression of cataract by preventing the formation of light-scattering aggregates is an important public health concern as life expectancy increases and the average age of the world population rises (Abraham et al., 2006). In a recently proposed model of cataract formation, damage to the Crystallins accumulates over time, inducing partially unfolded conformations which aggregate and scatter light (Acosta-Sampson and King, 2010). The stabilizing effect of sodium citrate on the unfolding of HγD-Crys may have implications for individuals who are at risk of developing cataract. These results demonstrate that citrate may slow the unfolding of damaged or partially unfolded conformations by stabilizing hydrophobic interactions of buried resides that are exposed to solvent over time, suggesting that this ubiquitous, non-toxic small molecule may be a possible treatment for cataract in the future.
Acknowledgments
The authors would like to acknowledge Dr. Kate Moreau for the L5S and I90F HgD-Crys, as well as technical assistance with data collection and analysis. We would also like to acknowledge Dr. Ligia Acosta-Sampson and Dr. Ishara Mills-Henry for technical assistance and general discussion. This work was supported by NEI grant EY015834 to J.K., and NEI Roadmap grant EY016525 to Wah Chiu and Judith Frydman.
Abbreviations
- HγD-Crys
Human γD Crystallin
- N-td
N-terminal Domain
- C-td
C-terminal domain
- GuHCl
guanidinium hydrochloride
- DTT
DL-Dithiothreitol
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham AG, Condon NG, West Gower E. The new epidemiology of cataract. Ophthalmology Clinics of North America. 2006;19:415–425. doi: 10.1016/j.ohc.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Acosta-Sampson L, King J. Partially folded aggregation intermediates of human gammaD-, gammaC-, and gammaS-crystallin are recognized and bound by human alphaB-crystallin chaperone. Journal of Molecular Biology. 2010;401:134–152. doi: 10.1016/j.jmb.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babizhayev MA, Burke L, Micans P, Richer SP. N-Acetylcarnosine sustained drug delivery eye drops to control the signs of ageless vision: Glare sensitivity, cataract amelioration and quality of vision currently available treatment for the challenging 50,000-patient population. Journal of Clinical Interventions in Aging. 2009;4:31–50. [PMC free article] [PubMed] [Google Scholar]
- Baldwin RL. How Hofmeister ion interactions affect protein stability. Biophysical Journal. 1996;71:2056–2063. doi: 10.1016/S0006-3495(96)79404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banci L, Bertini I, Cramaro F, Del Conte R, Viezzoli MS. Solution structure of Apo Cu,Zn superoxide dismutase: role of metal ions in protein folding. Biochemistry. 2003;42:9543–9553. doi: 10.1021/bi034324m. [DOI] [PubMed] [Google Scholar]
- Basak A, Bateman O, Slingsby C, Pande A, Asherie N, Ogun O, Benedek GB, Pande J. High-resolution X-ray crystal structures of human gammaD crystallin (1.25 A) and the R58H mutant (1.15 A) associated with aculeiform cataract. Journal of Molecular Biology. 2003;328:1137–1147. doi: 10.1016/s0022-2836(03)00375-9. [DOI] [PubMed] [Google Scholar]
- Bottomley SP, Tew DJ. The citrate ion increases the conformational stability of alpha(1)-antitrypsin. Biochimica et biophysica acta. 2000;1481:11–17. doi: 10.1016/s0167-4838(00)00118-7. [DOI] [PubMed] [Google Scholar]
- Brian G, Taylor H. Cataract blindness-Challenges for the 21st Century. Bulletin of the World Health Organization. 2001;79:249–256. [PMC free article] [PubMed] [Google Scholar]
- Busby TF, Atha DH, Ingham KC. Thermal denaturation of antithrombin III. Stabilization by heparin and lyotropic anions. Journal of Biological Chemistry. 1981;256:12140–12147. [PubMed] [Google Scholar]
- Clark AC, Sinclair JF, Baldwin TO. Folding of bacterial luciferase involves a non-native heterodimeric intermediate in equilibrium with the native enzyme and the unfolded subunits. Journal of Biological Chemistry. 1993;268:10773–10779. [PubMed] [Google Scholar]
- Cooper PG, Aquilina JA, Truscott RJ, Carver JA. Supramolecular order within the lens: 1H NMR spectroscopic evidence for specific crystallin-crystallin interactions. Experimental Eye Research. 1994;59:607–616. doi: 10.1006/exer.1994.1146. [DOI] [PubMed] [Google Scholar]
- Das P, King JA, Zhou R. beta-Strand interactions at the domain interface critical for the stability of human lens gammaD-crystallin. Protein Science. 2010;19:131–140. doi: 10.1002/pro.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David LL, Lampi KJ, Lund AL, Smith JB. The sequence of human betaB1-crystallin cDNA allows mass spectrometric detection of betaB1 protein missing portions of its N-terminal extension. Journal of Biological Chemistry. 1996;271:4273–4279. doi: 10.1074/jbc.271.8.4273. [DOI] [PubMed] [Google Scholar]
- Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- Dill KA, Truskett TM, Vlachy V, Hribar-Lee B. Modeling water, the hydrophobic effect, and ion solvation. Annual Review of Biophysical and Biomolecular Structure. 2005;34:173–199. doi: 10.1146/annurev.biophys.34.040204.144517. [DOI] [PubMed] [Google Scholar]
- Everett CA, Glenister PH, Taylor DM, Lyon MF, Kratochvilova-Loester J, Favor J. Mapping of six dominant cataract genes in the mouse. Genomics. 1994;20:429–434. doi: 10.1006/geno.1994.1197. [DOI] [PubMed] [Google Scholar]
- Fagerholm PP, Philipson BT, Lindstrom B. Normal human lens - the distribution of protein. Experimental Eye Research. 1981;33:615–620. doi: 10.1016/s0014-4835(81)80101-7. [DOI] [PubMed] [Google Scholar]
- Flaugh SL, Kosinski-Collins MS, King J. Contributions of hydrophobic domain interface interactions to the folding and stability of human gammaD-crystallin. Protein Science. 2005a;14:569–581. doi: 10.1110/ps.041111405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaugh SL, Kosinski-Collins MS, King J. Interdomain side-chain interactions in human gammaD crystallin influencing folding and stability. Protein Science. 2005b;14:2030–2043. doi: 10.1110/ps.051460505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaugh SL, Mills IA, King J. Glutamine deamidation destabilizes human gammaD-crystallin and lowers the kinetic barrier to unfolding. Journal of Biological Chemistry. 2006;281:30782–30793. doi: 10.1074/jbc.M603882200. [DOI] [PubMed] [Google Scholar]
- Getzoff ED, Tainer JA, Stempien MM, Bell GI, Hallewell RA. Evolution of CuZn superoxide dismutase and the Greek key beta-barrel structural motif. Proteins. 1989;5:322–336. doi: 10.1002/prot.340050408. [DOI] [PubMed] [Google Scholar]
- Graw J. Genetics of crystallins: cataract and beyond. Experimental Eye Research. 2009;88:173–189. doi: 10.1016/j.exer.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Graw J, Neuhauser-Klaus A, Klopp N, Selby PB, Loster J, Favor J. Genetic and allelic heterogeneity of Cryg mutations in eight distinct forms of dominant cataract in the mouse. Investigative Ophthalmology and Visual Science. 2004;45:1202–1213. doi: 10.1167/iovs.03-0811. [DOI] [PubMed] [Google Scholar]
- Greene RF, Pace CN. Urea and guanidine hydrochloride denaturation of ribonuclease, lysozyme, alpha-chymotrypsin, and beta-lactoglobulin. Journal of Biological Chemistry. 1974;249:5388–5393. [PubMed] [Google Scholar]
- Hains PG, Truscott RJ. Age-dependent deamidation of lifelong proteins in the human lens. Investigative Ophthalmology and Visual Science. 2010;51:3107–3114. doi: 10.1167/iovs.09-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hains PG, Truscott RJW. Post-translational modifications in the nuclear region of young, aged, and cataract human lenses. Journal of Proteome Research. 2007;6:3935–3943. doi: 10.1021/pr070138h. [DOI] [PubMed] [Google Scholar]
- Hanson SR, Hasan A, Smith DL, Smith JB. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Experimental Eye Research. 2000;71:195–207. doi: 10.1006/exer.2000.0868. [DOI] [PubMed] [Google Scholar]
- Harrington V, Srivastava OP, Kirk M. Proteomic analysis of water insoluble proteins from normal and cataractous human lenses. Molecular Vision. 2007;13:1680–1694. [PubMed] [Google Scholar]
- Knee KM, Goulet DR, Zhang J, Chen B, Chiu W, King JA. The group II chaperonin Mm-Cpn binds and refolds human γD crystallin. Protein Science. 2011;20:30–41. doi: 10.1002/pro.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski-Collins MS, Flaugh SL, King J. Probing folding and fluorescence quenching in human gammaD crystallin Greek key domains using triple tryptophan mutant proteins. Protein Science. 2004;13:2223–2235. doi: 10.1110/ps.04627004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosinski-Collins MS, King J. In vitro unfolding, refolding, and polymerization of human gammaD crystallin, a protein involved in cataract formation. Protein Science. 2003;12:480–490. doi: 10.1110/ps.0225503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronman MJ, Sinha SK, Brew K. Characteristics of the binding of Ca2+ and other divalent metal ions to bovine alpha-lactalbumin. Journal of Biological Chemistry. 1981;256:8582–8587. [PubMed] [Google Scholar]
- Kurzel RB, Wolbarsht M, Yamanashi BS, Staton GW, Borkman RF. Tryptophan excited states and cataracts in the human lens. Nature. 1973;241:132–133. doi: 10.1038/241132a0. [DOI] [PubMed] [Google Scholar]
- Lampi KJ, Ma Z, Hanson SR, Azuma M, Shih M, Shearer TR, Smith DL, Smith JB, David LL. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Experimental Eye Research. 1998;67:31–43. doi: 10.1006/exer.1998.0481. [DOI] [PubMed] [Google Scholar]
- Lee S, Mahler B, Toward J, Jones B, Wyatt K, Dong L, Wistow G, Wu Z. A Single Destabilizing Mutation (F9S) Promotes Concerted Unfolding of an Entire Globular Domain in gammaS-Crystallin. Journal of Molecular Biology. 2010;399:320–330. doi: 10.1016/j.jmb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston PM, Carson CA, Taylor HR. The epidemiology of cataract: a review of the literature. Ophthalmic Epidemiology. 1995;2:151–164. doi: 10.3109/09286589509057097. [DOI] [PubMed] [Google Scholar]
- McCarty CA, Taylor HR. A review of the epidemiologic evidence linking ultraviolet radiation and cataracts. Developmental Ophthalmology. 2002;35:21–31. doi: 10.1159/000060807. [DOI] [PubMed] [Google Scholar]
- McFall-Ngai MJ, Ding LL, Takemoto LJ, Horwitz J. Spatial and temporal mapping of the age-related changes in human lens crystallins. Experimental Eye Research. 1985;41:745–758. doi: 10.1016/0014-4835(85)90183-6. [DOI] [PubMed] [Google Scholar]
- Mills IA, Flaugh SL, Kosinski-Collins MS, King JA. Folding and stability of the isolated Greek key domains of the long-lived human lens proteins gammaD-crystallin and gammaS-crystallin. Protein Science. 2007;16:2427–2444. doi: 10.1110/ps.072970207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau KL, King J. Hydrophobic core mutations associated with cataract development in mice destabilize human gammaD-crystallin. Journal of Biological Chemistry. 2009;284:33285–33295. doi: 10.1074/jbc.M109.031344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzammil S, Kumar Y, Tayyab S. Anion-induced stabilization of human serum albumin prevents the formation of intermediate during urea denaturation. Proteins. 2000;40:29–38. doi: 10.1002/(sici)1097-0134(20000701)40:1<29::aid-prot50>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Nagai R, Nagai M, Shimasaki S, Baynes JW, Fujiwara Y. Citric acid inhibits development of cataracts, proteinuria and ketosis in streptozotocin (type 1) diabetic rats. Biochemical and Biophysical Research Communications. 2010;393:118–122. doi: 10.1016/j.bbrc.2010.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyster CW. The Human Eye: Structure and Function. Sinauer Associates, Inc; Sunderland: 1999. [Google Scholar]
- Pace CN. Contribution of the hydrophobic effect to globular protein stability. Journal of Molecular Biology. 1992;226:29–35. doi: 10.1016/0022-2836(92)90121-y. [DOI] [PubMed] [Google Scholar]
- Pace CN, McGrath T. Substrate stabilization of lysozyme to thermal and guanidine hydrochloride denaturation. Journal of Biological Chemistry. 1980;255:3862–3865. [PubMed] [Google Scholar]
- Parge HE, Hallewell RA, Tainer JA. Atomic structures of wild-type and thermostable mutant recombinant human Cu,Zn superoxide dismutase. Proceedings of the National Academy of Science. 1992;89:6109–6113. doi: 10.1073/pnas.89.13.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce MC, Morton CJ, Feil SC, Hansen G, Adams JJ, Parker MW, Bottomley SP. Preventing serpin aggregation: the molecular mechanism of citrate action upon antitrypsin unfolding. Protein Science. 2008;17:2127–2133. doi: 10.1110/ps.037234.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permyakov EA, Berliner LJ. alpha-Lactalbumin: structure and function. FEBS Letters. 2000;473:269–274. doi: 10.1016/s0014-5793(00)01546-5. [DOI] [PubMed] [Google Scholar]
- Purkiss A, Bateman O, Wyatt K, Wilmarth P, David L, Wistow G, Slingsby C. Biophysical Properties of γC-Crystallin in Human and Mouse Eye Lens: The Role of Molecular Dipoles. Journal of Molecular Biology. 2007;372:205–222. doi: 10.1016/j.jmb.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos C, Baldwin R. Sulfate anion stabilization of native ribonuclease A both by anion binding and by the Hofmeister effect. Protein Science. 2002;11:1771–1778. doi: 10.1110/ps.0205902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder H, Colon W. Kinetic role of early intermediates in protein folding. Current Opinion in Structural Biology. 1997;7:15–28. doi: 10.1016/s0959-440x(97)80004-8. [DOI] [PubMed] [Google Scholar]
- Shi Q, Yan H, Li MY, Harding JJ. Effect of a combination of carnosine and aspirin eye drops on streptozotocin -- induced diabetic cataract in rats. Molecular Vision. 2009;15:2129–2138. [PMC free article] [PubMed] [Google Scholar]
- Siezen RJ, Thomson JA, Kaplan ED, Benedek GB. Human lens gamma-crystallins: isolation, identification, and characterization of the expressed gene products. Proceedings of the National Academy of Science. 1987;84:6088–6092. doi: 10.1073/pnas.84.17.6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siezen RJ, Wu E, Kaplan ED, Thomson JA, Benedek GB. Rat lens gamma-crystallins. Characterization of the six gene products and their spatial and temporal distribution resulting from differential synthesis. Journal of Molecular Biology. 1988;199:475–490. doi: 10.1016/0022-2836(88)90619-5. [DOI] [PubMed] [Google Scholar]
- Sikkink LA, Ramirez-Alvarado M. Salts enhance both protein stability and amyloid formation of an immunoglobulin light chain. Biophysical Chemistry. 2008;135:25–31. doi: 10.1016/j.bpc.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha D, Wyatt MK, Sarra R, Jaworski C, Slingsby C, Thaung C, Pannell L, Robison WG, Favor J, Lyon M, Wistow G. A temperaturesensitive mutation of Crygs in the murine Opj cataract. Journal of Biological Chemistry. 2001;276:9308–9315. doi: 10.1074/jbc.M010583200. [DOI] [PubMed] [Google Scholar]
- Slingsby C, Clout NJ. Structure of the Crystallins. Eye (London) 1999;13:395–402. doi: 10.1038/eye.1999.113. [DOI] [PubMed] [Google Scholar]
- Street TO, Bolen DW, Rose GD. A molecular mechanism for osmolyte-induced protein stability. Proceedings of the National Academy of Science. 2006;103:13997–14002. doi: 10.1073/pnas.0606236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadeo X, Lopez-Mendez B, Castano D, Trigueros T, Millet O. Protein stabilization and the Hofmeister effect: the role of hydrophobic solvation. Biophysical Journal. 2009;97:2595–2603. doi: 10.1016/j.bpj.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata T, Oxford JT, Demeler B, Lampi KJ. Deamidation destabilizes and triggers aggregation of a lens protein, betaA3-crystallin. Protein Science. 2008;17:1565–1575. doi: 10.1110/ps.035410.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto LJ, Hansen JS, Zigler JS, Jr, Horwitz J. Characterization of polypetides from human nuclear cataracts by Western blot analysis. Experimental Eye Research. 1985;40:205–212. doi: 10.1016/0014-4835(85)90005-3. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Duncan MK, David LL. Lens proteomics: the accumulation of crystallin modifications in the mouse lens with age. Investigative Ophthalmology and Visual Science. 2002;43:205–215. [PubMed] [Google Scholar]
- Van Ceunebroeck JC, Hanssens I, Joniau M, Van Cauwelaert F. Thermodynamics of the Ca2+ binding to bovine alpha-lactalbumin. Journal of Biological Chemistry. 1985;260:10944–10947. [PubMed] [Google Scholar]
- Von Hippel PH, Wong KY. On the conformational stability of globular proteins. The effects of various electrolytes and nonelectrolytes on the thermal ribonuclease transition. Journal of Biological Chemistry. 1965;240:3909–3923. [PubMed] [Google Scholar]
- Wang Y, Petty S, Trojanowski A, Knee K, Goulet D, Mukerji I, King J. Formation of amyloid fibrils in vitro from partially unfolded intermediates of human gammaC-crystallin. Investigative Ophthalmology and Visual Science. 2010;51:672–678. doi: 10.1167/iovs.09-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita MM, Wesson L, Eisenman G, Eisenberg D. Where metal ions bind in proteins. Proceedings of the National Academy of Science. 1990;87:5648–5652. doi: 10.1073/pnas.87.15.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Guo Y, Zhang J, Ding Z, Ha W, Harding JJ. Effect of carnosine, aminoguanidine, and aspirin drops on the prevention of cataracts in diabetic rats. Molecular Vision. 2008;14:2282–2291. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cremer PS. Interactions Between Macromolecules and Ions: The Hofmeister Series. Current Opinion in Chemical Biology. 2006;10:658–663. doi: 10.1016/j.cbpa.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cremer PS. Chemistry of Hofmeister anions and osmolytes. Annual Review Physical Chemistry. 2010;61:63–83. doi: 10.1146/annurev.physchem.59.032607.093635. [DOI] [PubMed] [Google Scholar]
- Zigler JS, Jr, Goosey JD. Singlet oxygen as a possible factor in human senile nuclear cataract developement. Current Eye Research. 1984;3:59–65. doi: 10.3109/02713688408997187. [DOI] [PubMed] [Google Scholar]