Abstract
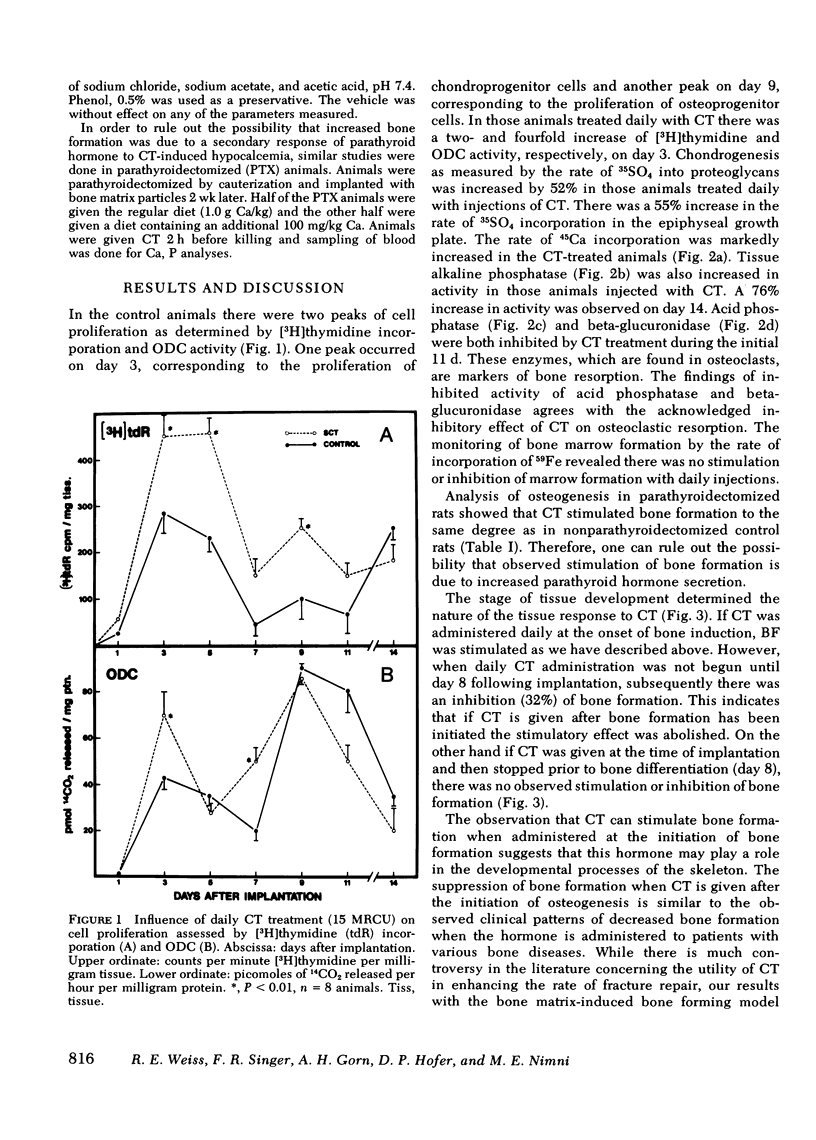
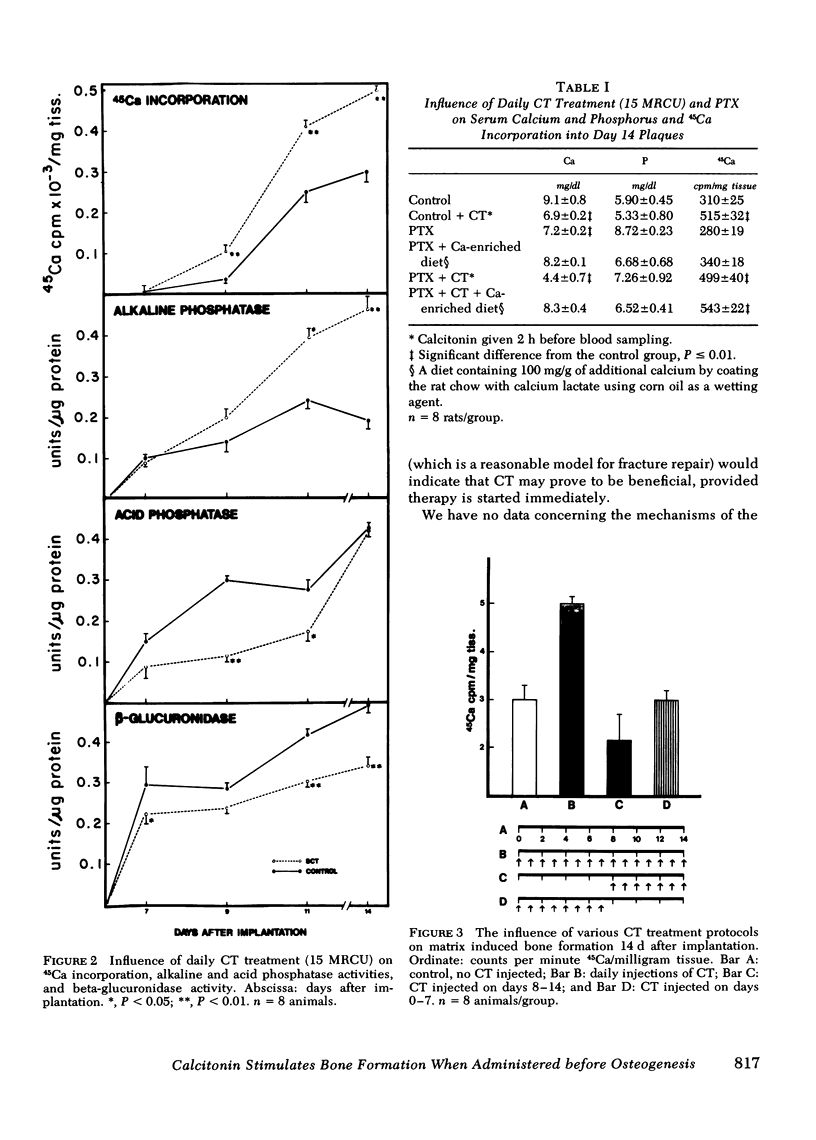
The influence of calcitonin (CT) on various stages of bone formation was investigated. A demineralized collagenous bone matrix-induced bone forming system in rats was used to temporally segregate chondrogenesis and osteogenesis. Administration of CT (15 Medical Research Council Units [MRCU]) daily) at the initiation of matrix-induced bone formation (BF) resulted in a 76% stimulation of BF as measured by 45Ca incorporation and alkaline phosphatase activity. This increase was due, in part, to a stimulation of cartilage and bone precursor cell proliferation monitored by the rate of [3H]thymidine incorporation and ornithine decarboxylase activity. Chondrogenesis on day 7 as measured by 35SO4 incorporation was increased by 52% with CT treatment. To rule out the possibility of a secondary response due to parathyroid hormone, similar studies were done in parathyroidectomized animals and CT stimulation of BF was still observed. However, when CT injections were started after cartilage formation (day 8) there was no stimulation of BF but a significant decrease in 45Ca incorporation was observed. These results indicate CT has two actions: (a) when CT is administered during the initial phases of bone formation, it increases BF due to a stimulation of proliferation of cartilage and bone precursor cells; and (b) when CT is administered after bone formation has been initiated, subsequent bone formation is suppressed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Foster G. V., Doyle F. H., Bordier P., Matrajt H. Effect of thyrocalcitonin on bone. Lancet. 1966 Dec 31;2(7479):1428–1431. doi: 10.1016/s0140-6736(66)90593-9. [DOI] [PubMed] [Google Scholar]
- Galante L., Colston K. W., MacAuley S. J., MacIntyre I. Effect of calcitonin on vitamin D metabolism. Nature. 1972 Aug 4;238(5362):271–273. doi: 10.1038/238271a0. [DOI] [PubMed] [Google Scholar]
- Holtrop M. E., Raisz L. G., Simmons H. A. The effects of parathyroid hormone, colchicine, and calcitonin on the ultrastructure and the activity of osteoclasts in organ culture. J Cell Biol. 1974 Feb;60(2):346–355. doi: 10.1083/jcb.60.2.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. On the purification of L-ornithine decarboxylase from rat prostate and effects of thiol compounds on the enzyme. J Biol Chem. 1971 Mar 25;246(6):1725–1732. [PubMed] [Google Scholar]
- Kallio D. M., Garant P. R., Minkin C. Ultrastructural effects of calcitonin on osteoclasts in tissue culture. J Ultrastruct Res. 1972 May;39(3):205–216. doi: 10.1016/s0022-5320(72)90017-2. [DOI] [PubMed] [Google Scholar]
- McWhinnie D. J. In vivo effects of mammalian thyrocalcitonin on bone growth and alkaline phosphatase activity in the chick embryo. Comp Biochem Physiol A Comp Physiol. 1975 Jan 1;50(1A):169–175. doi: 10.1016/s0010-406x(75)80221-0. [DOI] [PubMed] [Google Scholar]
- Nunez E. A., Horwith M., Krook L., Whalen J. P. An electron microscopic investigation of human familial bone dysplasia. Inhibition of osteocytic osteolysis and induction of osteocytic formation of elastic fibers following calcitonin treatment. Am J Pathol. 1979 Jan;94(1):1–18. [PMC free article] [PubMed] [Google Scholar]
- Puzas J. E., Drivdahl R. H., Howard G. A., Baylink D. J. Endogenous inhibitor of bone cell proliferation. Proc Soc Exp Biol Med. 1981 Jan;166(1):113–122. doi: 10.3181/00379727-166-41032. [DOI] [PubMed] [Google Scholar]
- Rath N. C., Reddi A. H. Changes in ornithine decarboxylase activity during matrix-induced cartilage, bone and bone marrow differentiation. Biochem Biophys Res Commun. 1978 Mar 15;81(1):106–113. doi: 10.1016/0006-291x(78)91636-4. [DOI] [PubMed] [Google Scholar]
- Reddi A. H., Anderson W. A. Collagenous bone matrix-induced endochondral ossification hemopoiesis. J Cell Biol. 1976 Jun;69(3):557–572. doi: 10.1083/jcb.69.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi A. H., Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1601–1605. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M., Jowsey J. Failure of calcitonin to prevent disuse osteopenia: An experimental study in rabbits. Endocrinology. 1970 Jul;87(1):183–186. doi: 10.1210/endo-87-1-183. [DOI] [PubMed] [Google Scholar]
- Whalen J. P., Krook L., MacIntyre I., Nunez E. A. Calcitonin, parathyroidectomy and modelling of bones in the growing rat. J Endocrinol. 1975 Aug;66(2):207–212. doi: 10.1677/joe.0.0660207. [DOI] [PubMed] [Google Scholar]
- Ziegler R., Delling G. Effect of calcitonin on the regeneration of a circumscribed bone defect (bored hole in the rat tibia). Acta Endocrinol (Copenh) 1972 Mar;69(3):497–506. doi: 10.1530/acta.0.0690497. [DOI] [PubMed] [Google Scholar]


