Abstract
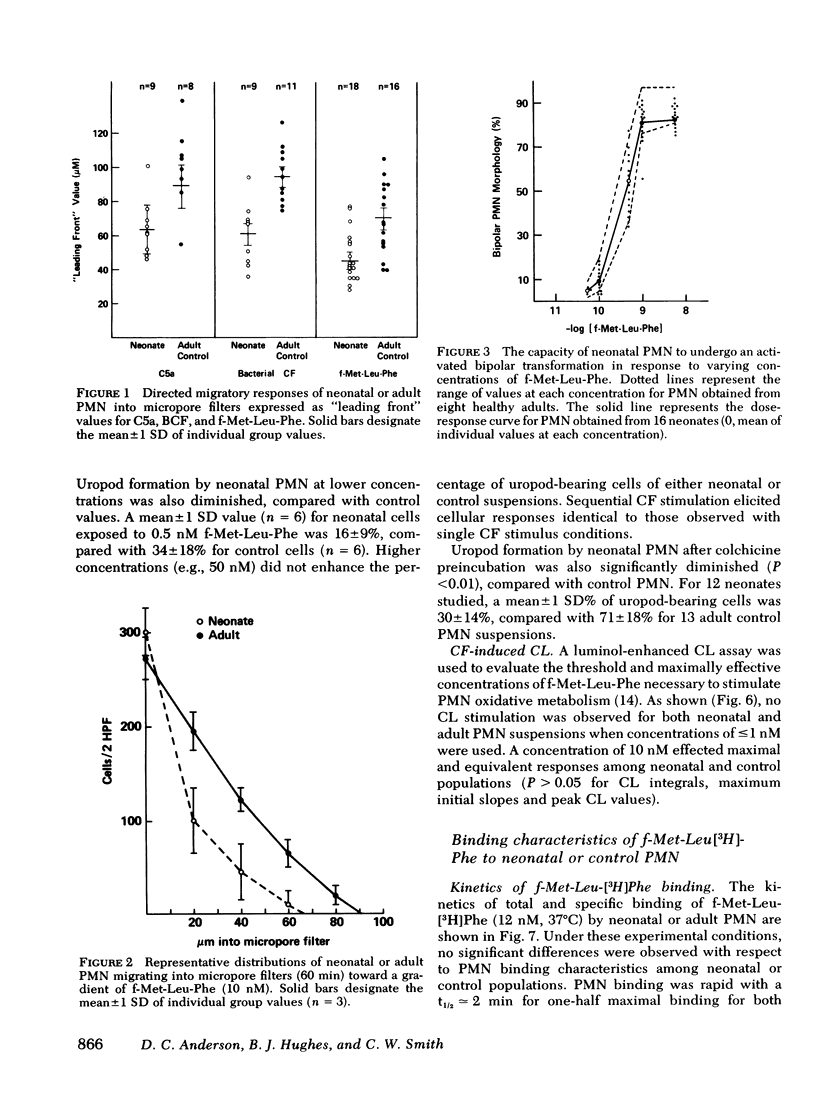
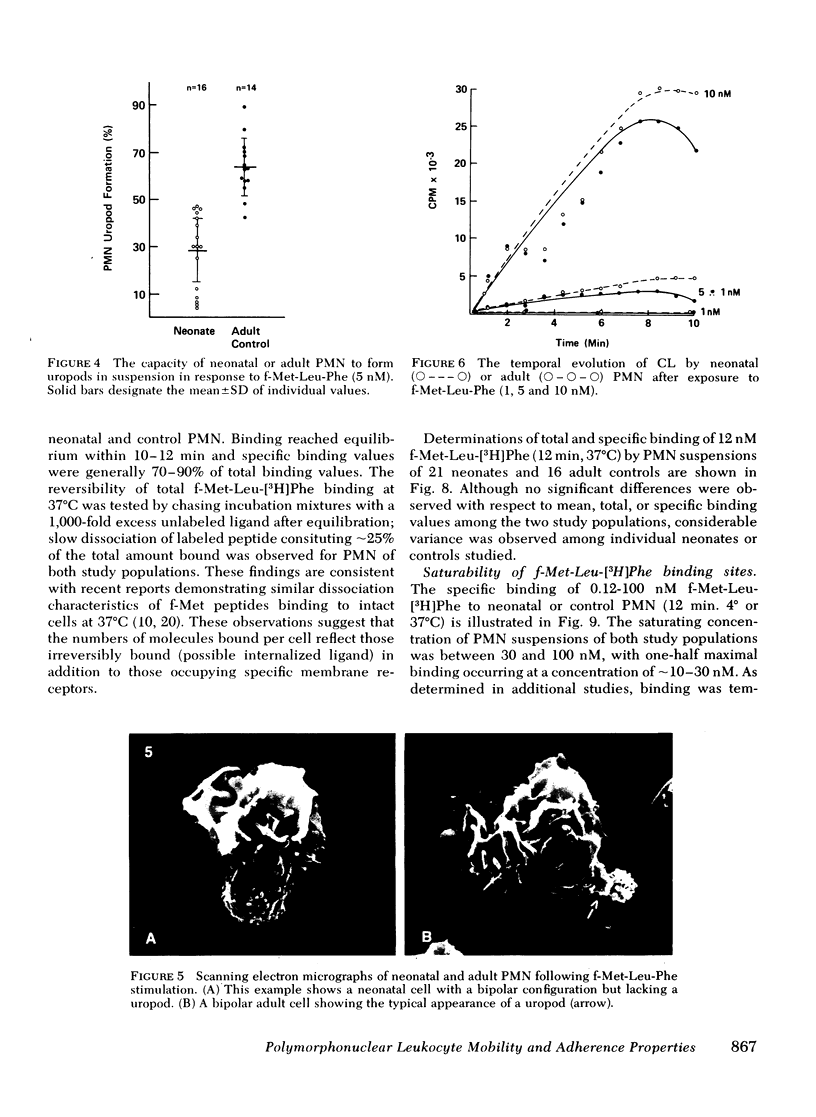
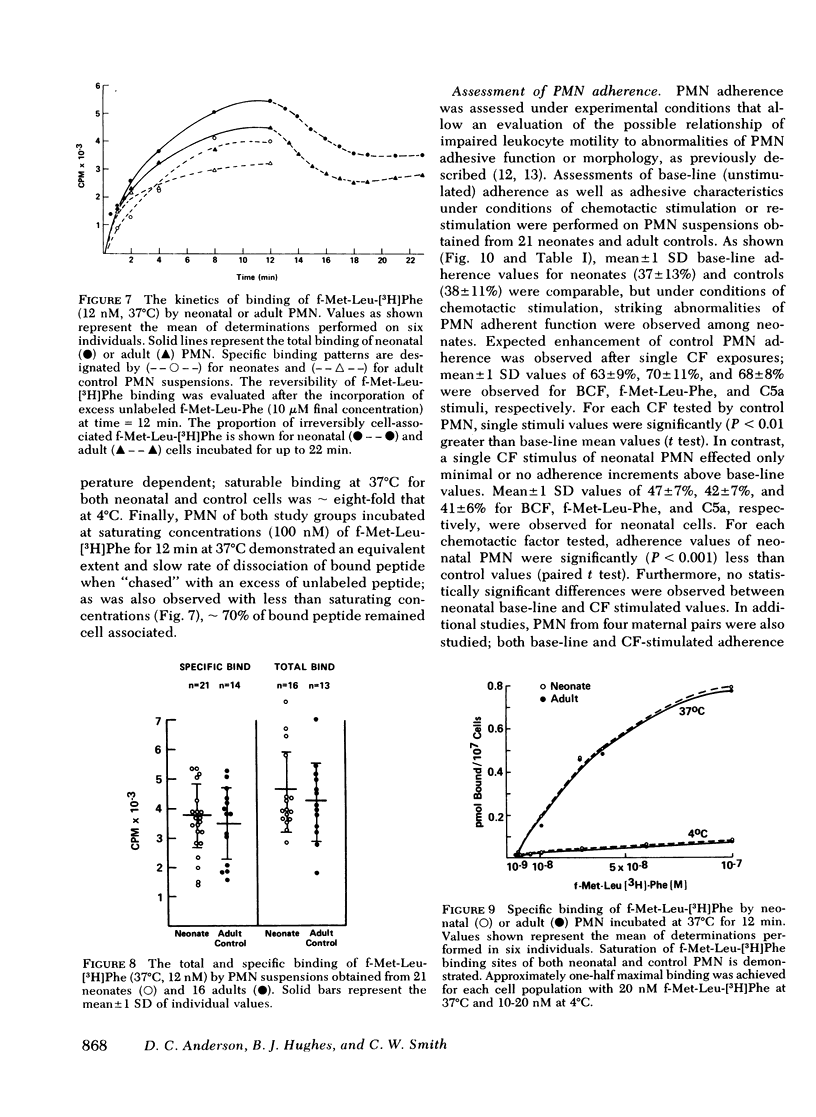
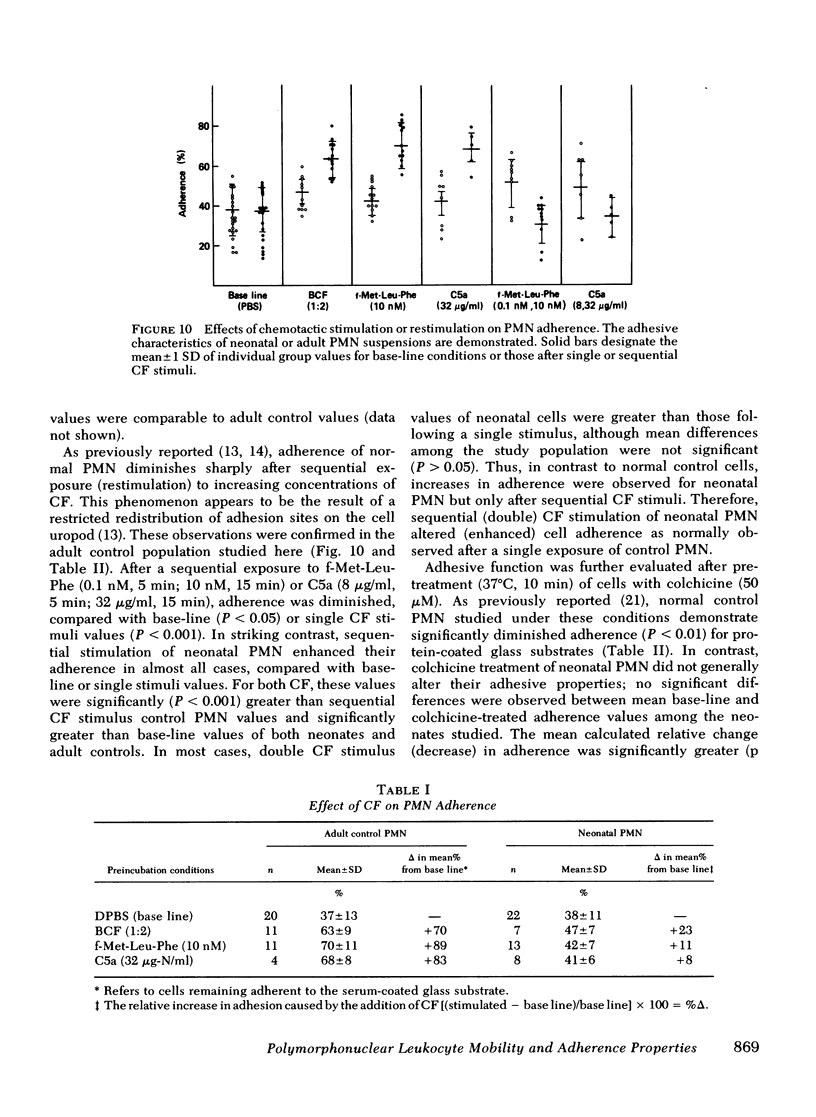
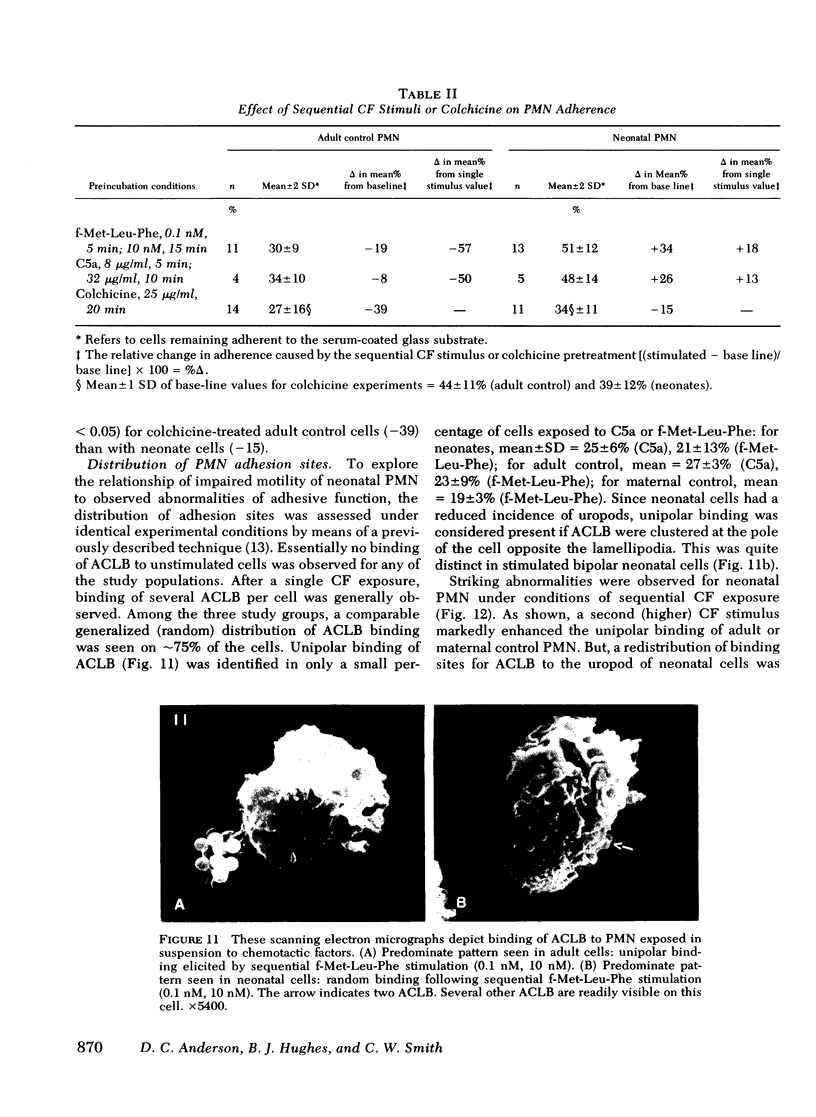
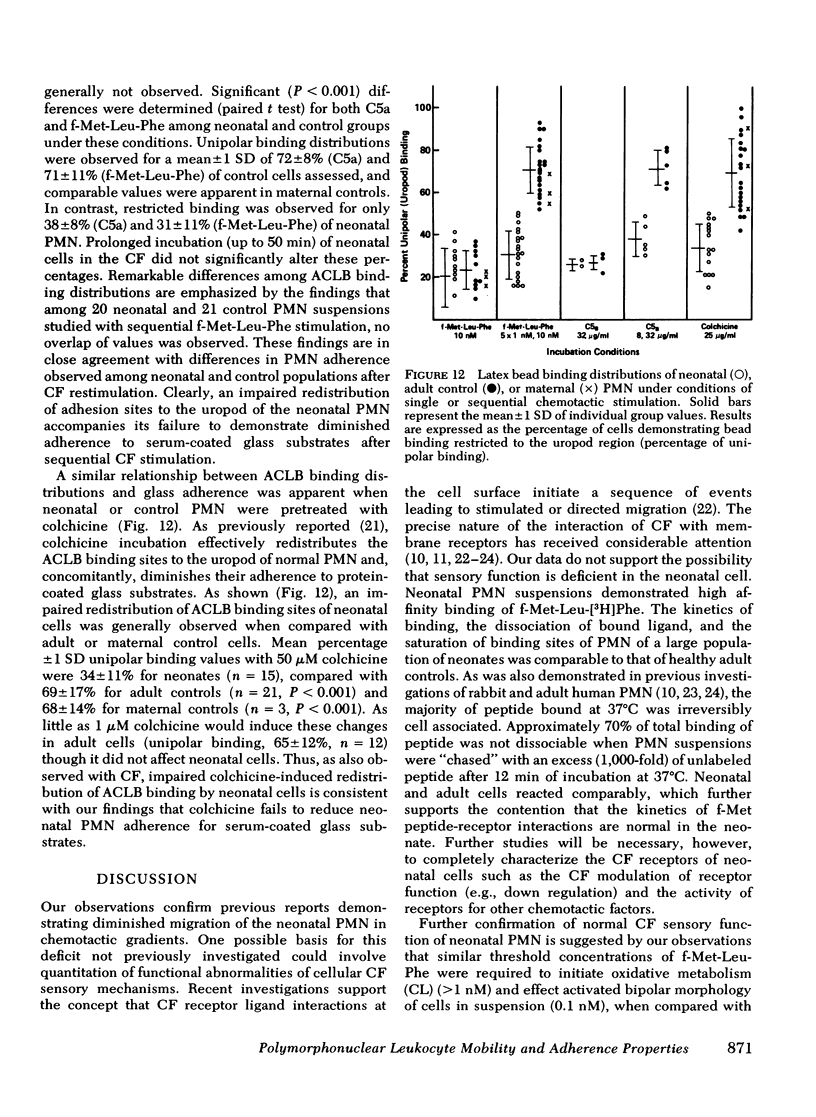
To determine the mechanism(s) of diminished, stimulated, and directed migration of neonatal (N) polymorphonuclear leukocytes (PMN), chemotactic factor (CF) sensory and PMN effector functions were studied in healthy N and adult or maternal controls (C). N PMN demonstrated high affinity binding for N-formyl-methionyl-leucyl-[3H]phenylalanine (fMLP), which was saturable between 40 and 100 nM as observed with C PMN. The kinetics of binding and the characteristics of dissociation of binding by N PMN were equivalent to control PMN. Both "threshold" and "peak" concentrations (1 and 10 nM, respectively) of fMLP effected comparable PMN chemiluminescence among neonates and controls. An equivalent threshold concentration (0.05 nM) of fMLP effected N and C PMN shape change in suspension, and a maximally effective concentration (5 nM) induced comparable bipolar configuration, although uropod formation was only 38 +/- 8% of N PMN, compared with 73 +/- 11% of C PMN (P less than 0.01). Striking abnormalities of N PMN adherence were identified: mean +/- SD base-line (unstimulated) N adherence values (39 +/- 8%) were equal to C (38 +/- 9%), but diminished increments in response to single CF stimuli were noted among N (fMLP: 42 +/- 7% (N), 70 +/- 11% (C); C5a: 41 +/- 6% (N), 68 +/- 6% (C); BCF: 41 +/- 6% (N), 63 +/- 9% (C), P less than 0.01 for each CF). On sequential exposure to increasing concentrations of CF N PMN failed to demonstrate expected decreased adherence values; sequential stimuli with fMLP (0.1 nM, 10 nM) or C5a (8 microgram protein/ml, 32 microgram protein/ml) effected mean +/- 1 SD values of 51 +/- 9% (N), 30 +/- 9% (C), and 34 +/- 10 (N), 48 +/- 14% (C), respectively. As demonstrated with a latex bead-binding technique, N PMN failed to redistribute adhesion sites to the cell's tail under the same experimental conditions; in 21 N samples studied, restricted unipolar binding occurred in 33 +/- 8% (fMLP) or 37 +/- 7% (C5a) of PMN in contrast to C values of 70% (fMLP), or 71% (C5a), P less than 0.001. Similar findings were observed when PMN were preincubated with colchicine (25 microgram/ml); expected diminished adherence scores (compared with base-line values) were demonstrated with C PMN but not with N cells, P less than 0.01. Additionally colchicine-induced redistribution of adhesion sites as was observed with C samples (72 +/- 14% unipolar binding) was significantly (P less than 0.001) less among N PMN (31 +/- 11% unipolar binding). These investigations indicate that CF sensory mechanisms of N PMN are normal, compared with healthy adult or maternal controls. Diminished stimulated locomotion of the N PMN may be functionally related to reduced modulation of cell adhesiveness by chemotactic stimulation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini D. F., Berlin R. D., Oliver J. M. The mechanism of concanavalin A cap formation in leukocytes. J Cell Sci. 1977 Aug;26:57–75. doi: 10.1242/jcs.26.1.57. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Edwards M. S., Baker C. J. Luminol-enhanced chemiluminescence for evaluation of type III group B streptococcal opsonins in human sera. J Infect Dis. 1980 Mar;141(3):370–381. doi: 10.1093/infdis/141.3.370. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Mace M. L., Brinkley B. R., Martin R. R., Smith C. W. Recurrent infection in glycogenosis type Ib: abnormal neutrophil motility related to impaired redistribution of adhesion sites. J Infect Dis. 1981 Mar;143(3):447–459. doi: 10.1093/infdis/143.3.447. [DOI] [PubMed] [Google Scholar]
- Anderson D. C., Pickering L. K., Feigin R. D. Leukocyte function in normal and infected neonates. J Pediatr. 1974 Sep;85(3):420–425. doi: 10.1016/s0022-3476(74)80134-4. [DOI] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M. Analogous ultrastructure and surface properties during capping and phagocytosis in leukocytes. J Cell Biol. 1978 Jun;77(3):789–804. doi: 10.1083/jcb.77.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Albertini D. F., Baehner R. L., Oliver J. M. Impaired microtubule assembly and polymorphonuclear leucocyte function in the Chediak-Higashi syndrome correctable by ascorbic acid. Br J Haematol. 1979 Oct;43(2):207–213. doi: 10.1111/j.1365-2141.1979.tb03743.x. [DOI] [PubMed] [Google Scholar]
- Boxer L. A., Allen J. M., Baehner R. L. Diminished polymorphonuclear leukocyte adherence. Function dependent on release of cyclic AMP by endothelial cells after stimulation of beta-receptors by epinephrine. J Clin Invest. 1980 Aug;66(2):268–274. doi: 10.1172/JCI109853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Allen J. M., Watanabe A. M., Besch H. R., Jr, Baehner R. L. Role of microtubules in granulocyte adherence. Blood. 1978 Jun;51(6):1045–1050. [PubMed] [Google Scholar]
- Boxer L. A., Hedley-Whyte E. T., Stossel T. P. Neutrophil action dysfunction and abnormal neutrophil behavior. N Engl J Med. 1974 Nov 21;291(21):1093–1099. doi: 10.1056/NEJM197411212912101. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Crowley C. A., Curnutte J. T., Rosin R. E., André-Schwartz J., Gallin J. I., Klempner M., Snyderman R., Southwick F. S., Stossel T. P., Babior B. M. An inherited abnormality of neutrophil adhesion. Its genetic transmission and its association with a missing protein. N Engl J Med. 1980 May 22;302(21):1163–1168. doi: 10.1056/NEJM198005223022102. [DOI] [PubMed] [Google Scholar]
- Dretschmer R. R., Stewardson R. B., Papierniak C. K., Gotoff S. P. Chemotactic and bactericidal capacities of human newborn monocytes. J Immunol. 1976 Oct;117(4):1303–1307. [PubMed] [Google Scholar]
- Fehr J., Dahinden C. Modulating influence of chemotactic factor-induced cell adhesiveness on granulocyte function. J Clin Invest. 1979 Jul;64(1):8–16. doi: 10.1172/JCI109466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontan G., Lorente F., Rodriguez M. G., Ojeda J. A. Granulocyte adherence in umbilical cord blood. J Pediatr. 1979 Jun;94(6):969–970. doi: 10.1016/s0022-3476(79)80238-3. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Malech H. L., Wright D. G., Whisnant J. K., Kirkpatrick C. H. Recurrent severe infections in a child with abnormal leukocyte function: possible relationship to increased microtubule assembly. Blood. 1978 May;51(5):919–933. [PubMed] [Google Scholar]
- Gallin J. I., Rosenthal A. S. The regulatory role of divalent cations in human granulocyte chemotaxis. Evidence for an association between calcium exchanges and microtubule assembly. J Cell Biol. 1974 Sep;62(3):594–609. doi: 10.1083/jcb.62.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. B., Fischer T. J., Gard S. E., Biberstein M., Rich K. C., Stiehm E. R. Decreased mononuclear and polymorphonuclear chemotaxis in human newborns, infants, and young children. Pediatrics. 1977 Oct;60(4):467–472. [PubMed] [Google Scholar]
- Laurenti F., Ferro R., Marzetti G., Rossini M., Bucci G. Neutrophil chemotaxis in preterm infants with infections. J Pediatr. 1980 Mar;96(3 Pt 1):468–470. doi: 10.1016/s0022-3476(80)80700-1. [DOI] [PubMed] [Google Scholar]
- Lichtman M. A., Weed R. I. Alteration of the cell periphery during granulocyte maturation: relationship to cell function. Blood. 1972 Mar;39(3):301–316. [PubMed] [Google Scholar]
- Maderazo E. G., Woronick C. L. Micropore filter assay of human granulocyte locomotion: problems and solutions. Clin Immunol Immunopathol. 1978 Oct;11(2):196–211. doi: 10.1016/0090-1229(78)90044-2. [DOI] [PubMed] [Google Scholar]
- McGillen J. J., Phair J. P. Adherence, augmented adherence, and aggregation of polymorphonuclear leukocytes. J Infect Dis. 1979 Jan;139(1):69–73. doi: 10.1093/infdis/139.1.69. [DOI] [PubMed] [Google Scholar]
- Mease A. D., Fischer G. W., Hunter K. W., Ruymann F. B. Decreased phytohemagglutinin-induced aggregation and C5a-induced chemotaxis of human newborn neutrophils. Pediatr Res. 1980 Feb;14(2):142–146. doi: 10.1203/00006450-198002000-00015. [DOI] [PubMed] [Google Scholar]
- Miler I., Vondrácek J., Hromádková L. The decreased in vitro chemotactic activity of polymorphonuclear leukocytes of newborns and infants. Folia Microbiol (Praha) 1979;24(3):247–252. doi: 10.1007/BF02926456. [DOI] [PubMed] [Google Scholar]
- Miller M. E. Phagocyte function in the neonate: selected aspects. Pediatrics. 1979 Nov;64(5 Pt 2 Suppl):709–712. [PubMed] [Google Scholar]
- Niedel J., Wilkinson S., Cuatrecasas P. Receptor-mediated uptake and degradation of 125I-chemotactic peptide by human neutrophils. J Biol Chem. 1979 Nov 10;254(21):10700–10706. [PubMed] [Google Scholar]
- O'Flaherty J. T., Kreutzer D. L., Ward P. A. Chemotactic factor influences on the aggregation, swelling, and foreign surface adhesiveness of human leukocytes. Am J Pathol. 1978 Mar;90(3):537–550. [PMC free article] [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa S. G., Pahwa R., Grimes E., Smithwick E. Cellular and humoral components of monocyte and neutrophil chemotaxis in cord blood. Pediatr Res. 1977 May;11(5):677–680. doi: 10.1203/00006450-197705000-00010. [DOI] [PubMed] [Google Scholar]
- Shurin S. B., Socransky S. S., Sweeney E., Stossel T. P. A neutrophil disorder induced by capnocytophaga, a dental micro-organism. N Engl J Med. 1979 Oct 18;301(16):849–854. doi: 10.1056/NEJM197910183011601. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C. Motility and adhesiveness in human neutrophils. Redistribution of chemotactic factor-induced adhesion sites. J Clin Invest. 1980 Apr;65(4):804–812. doi: 10.1172/JCI109731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C., Patrick R. A., Hassett C. Motility and adhesiveness in human neutrophils. Effects of chemotactic factors. J Clin Invest. 1979 Feb;63(2):221–229. doi: 10.1172/JCI109293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. P., Lackie J. M., Wilkinson P. C. The effects of chemotactic factors on the adhesiveness of rabbit neutrophil granulocytes. Exp Cell Res. 1979 Aug;122(1):169–177. doi: 10.1016/0014-4827(79)90571-8. [DOI] [PubMed] [Google Scholar]
- Spagnuolo P. J., Ellner J. J., Hassid A., Dunn M. J. Thromboxane A2 mediates augmented polymorphonuclear leukocyte adhesiveness. J Clin Invest. 1980 Sep;66(3):406–414. doi: 10.1172/JCI109870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S. J., Zigmond S. H. Chemotactic peptide receptor modulation in polymorphonuclear leukocytes. J Cell Biol. 1980 Jun;85(3):703–711. doi: 10.1083/jcb.85.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tono-Oka T., Nakayama M., Uehara H., Matsumoto S. Characteristics of impaired chemotactic function in cord blood leukocytes. Pediatr Res. 1979 Mar;13(3):148–151. doi: 10.1203/00006450-197903000-00002. [DOI] [PubMed] [Google Scholar]
- Vitkauskas G., Showell H. J., Becker E. L. Specific binding of synthetic chemotactic peptides to rabbit peritoneal neutrophils: effects on dissociability of bound peptide, receptor activity and subsequent biologic responsiveness (deactivation). Mol Immunol. 1980 Feb;17(2):171–180. doi: 10.1016/0161-5890(80)90069-3. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Sullivan S. J. Sensory adaptation of leukocytes to chemotactic peptides. J Cell Biol. 1979 Aug;82(2):517–527. doi: 10.1083/jcb.82.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]




