Abstract
Siderophores play a central role in iron metabolism and virulence of most fungi. Both Aspergillus fumigatus and Aspergillus nidulans excrete the siderophore triacetylfusarinine C (TAFC) for iron acquisition. In A. fumigatus, green fluorescence protein-tagging revealed peroxisomal localization of the TAFC biosynthetic enzymes SidI (mevalonyl-CoA ligase), SidH (mevalonyl-CoA hydratase) and SidF (anhydromevalonyl-CoA transferase), while elimination of the peroxisomal targeting signal (PTS) impaired both, peroxisomal SidH-targeting and TAFC biosynthesis. The analysis of A. nidulans mutants deficient in peroxisomal biogenesis, ATP import or protein import revealed that cytosolic mislocalization of one or two but, interestingly, not all three enzymes impairs TAFC production during iron starvation. The PTS motifs are conserved in fungal orthologues of SidF, SidH and SidI. In agreement with the evolutionary conservation of the partial peroxisomal compartmentalization of fungal siderophore biosynthesis, the SidI orthologue of coprogen-type siderophore-producing Neurospora crassa was confirmed to be peroxisomal. Taken together, this study identified and characterized a novel, evolutionary conserved metabolic function of peroxisomes.
Introduction
Iron is an essential nutrient for all eukaryotes and nearly all prokaryotes (Kaplan and Kaplan, 2009). Moreover, the control over iron access is one of the central battlefields during infection as pathogens have to ‘steal’ the iron from the host – in particular as the mammalian innate immune system restricts iron access to pathogens via a variety of mechanisms (Ganz, 2009; Weinberg, 2009).
Aspergillus fumigatus is a ubiquitous saprophytic fungus, which has become the most common air-borne fungal pathogen of humans (Tekaia and Latge, 2005). Clinical manifestations range from allergic reactions to life-threatening invasive disease, termed aspergillosis, particularly in immunocompromised patients. The identification and functional characterization of more than 20 genes that are involved in iron homeostasis maintenance in A. fumigatus and/or its less pathogenic relative Aspergillus nidulans have made them models for iron metabolism in filamentous fungi (Haas, 2012). Both Aspergillus species employ low-affinity ferrous iron acquisition as well as siderophore-assisted iron uptake, a high-affinity ferric iron uptake system (Eisendle et al., 2003; Schrettl et al., 2004). In contrast to A. nidulans, A. fumigatus possesses a second high-affinity iron uptake system, termed reductive iron assimilation. Interestingly, the fungal model system Saccharomyces cerevisiae differs from most other fungi due to the lack of siderophore biosynthesis and employment of iron regulators that are not conserved in most other fungal species (Haas et al., 2008). Both Aspergillus species excrete the fusarinine-type siderophore triacetylfusarinine C (TAFC) to mobilize extracellular iron. Iron-chelated TAFC is taken up by siderophore-iron transporters (Haas et al., 2003; 2008; Philpott and Protchenko, 2008). Intracellular release of iron from TAFC involves hydrolysis of the siderophore backbone catalysed in part by the esterase EstB (Kragl et al., 2007). Furthermore, both Aspergillus species produce the intracellular siderophore ferricrocin (FC) for hyphal iron storage and distribution (Schrettl et al., 2007; Wallner et al., 2009; Blatzer et al., 2011).
TAFC consists of three N2-acetyl-N5-anhydromevalonyl-N5-hydroxyornithine residues cyclically linked by ester bonds; FC is a cyclic hexapeptide with the structure Gly–Ser–Gly–(N5-acetyl-N5-hydroxyornithine)3 (Haas et al., 2008). The siderophore biosynthetic pathway is shown in Fig. 1. The first committed step in the biosynthesis of these siderophores is the hydroxylation of ornithine catalysed by the ornithine monooxygenase SidA (Eisendle et al., 2003; Schrettl et al., 2004; Olucha et al., 2011). Subsequently, the pathways for biosynthesis of extra- and intracellular siderophores split. For biosynthesis of extracellular siderophores, the transacylase SidF transfers anhydromevalonyl to N5-hydroxyornithine (Schrettl et al., 2007). The required anhydromevalonyl-CoA moiety is derived from mevalonate by CoA ligation and dehydration catalysed by SidI and SidH respectively (Yasmin et al., 2011). The acetylation of N5-hydroxyornithine for FC biosynthesis involves two transacetylases, the constitutively expressed SidL (Blatzer et al., 2011) and an unidentified enzyme, the activity of which is upregulated by iron starvation. Assembly of Fusarinine C (FsC) and FC is catalysed by two different non-ribosomal peptide synthetases (NRPS), SidD and SidC respectively. Subsequently, SidG catalyses N2-acetylation of FsC for forming TAFC. As typical for NRPS, SidD and SidC depend on activation by 4′-phosphopantetheinyl transferase (Oberegger et al., 2003). Both extra- and intracellular siderophores are crucial for growth during iron limitation in A. fumigatus and A. nidulans (Eisendle et al., 2003; Schrettl et al., 2004). Elimination of the entire siderophore biosynthesis (ΔsidA mutant) results in absolute avirulence of A. fumigatus in a murine model of invasive pulmonary aspergillosis (Schrettl et al., 2004; Hissen et al., 2005), while deficiency in either extracellular (ΔsidI, ΔsidH, ΔsidF or ΔsidD mutants) or intracellular siderophores (ΔsidC mutants) causes partial attenuation of virulence (Schrettl et al., 2007; Yasmin et al., 2011).
Fig. 1.
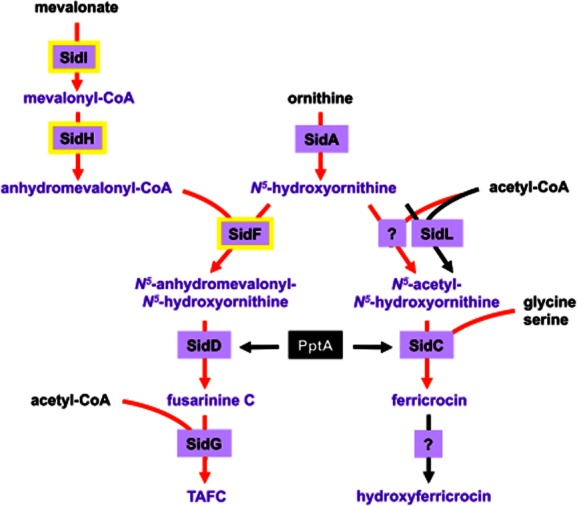
The siderophore biosynthetic pathway of A. fumigatus and A. nidulans. The enzymes boxed in purple are described in the text. Enzymes transcriptionally upregulated during iron starvation are marked by red arrows. The enzymes identified in this study to localize to peroxisomes are framed in yellow. Adapted from Haas (2012).
Peroxisomes are single membrane organelles, which compartmentalize a wide range of metabolic functions in eukaryotic cells. Important functions of peroxisomes include fatty acid β-oxidation, the glyoxylate cycle, metabolisms of cholesterol and reactive oxygen species, as well as methanol oxidation (Schrader and Fahimi, 2008). Due to the diversity of metabolic pathways in peroxisomes, their content varies depending on the species, cell or tissue type, as well as on environmental conditions (Brown and Baker, 2008). In contrast to mitochondria, peroxisomes are devoid of DNA and protein synthesis machinery. Therefore, all peroxisomal proteins are encoded by nuclear genes and are post-translationally imported into peroxisomes. In S. cerevisiae, more than 60 peroxisomal proteins have been identified including the peroxins (Pex), which are essential for peroxisomal biogenesis and maintenance as well as matrix protein import (Schrader and Fahimi, 2008).
This study revealed peroxisomal targeting signal of type one (PTS1) or type two (PTS2) in SidI, SidH and SidF of Aspergillus spp. and their orthologues in other Ascomycetes. Peroxisomal localization of all three enzymes was confirmed by green fluorescence protein (GFP) tagging and by PTS1 mutation of SidH in A. fumigatus. The analysis of A. nidulans mutants deficient in peroxisomal biogenesis, ATP import and protein import depending on either PTS1, PTS2, or both, indicated that cytosolic mislocalization of individual enzymes but not of the entire TAFC biosynthetic machinery impairs TAFC production during iron starvation.
Results
A. fumigatus SidI, SidH and SidF carry PTS and localize to peroxisomes
The majority of peroxisomal matrix proteins are post-translationally directed to the lumen of the organelle by peroxisomal targeting signals (PTS) and two different motifs have been characterized (Olivier and Krisans, 2000). PTS1 is a tri-peptide with the consensus sequence (S/A/C)(K/H/R)(L/M) located at the extreme C-terminus, whereas PTS2 is a nona-peptide of the consensus sequence (R/K)(L/V/I)X5(H/Q)(L/A) located near the N-terminus of a matrix protein. Sequence analysis of the A. fumigatus TAFC biosynthetic enzymes revealed the presence of the putative PTS1 motifs SKL and AKL in SidH and SidF, respectively, and a putative PTS2, RLQTLSQHL, localized at amino acid 6–14 in SidI.
To confirm the peroxisomal localization of SidH and SidF, ΔsidH and ΔsidF mutant strains were complemented with respective N-terminally GFP-tagged versions as described in Experimental procedures. SidI was C-terminally GFP-tagged in a wild-type (wt) strain via integration of the GFP-encoding gene at the sidI locus. Consequently, the respective mutant produces only GFP-tagged SidI. Consistent with the predicted peroxisomal localization, the GFP-tagged versions of all three enzymes localized to punctate dots in the cytoplasm (Fig. 2A). ΔsidH and ΔsidF mutant strains lack TAFC production (Schrettl et al., 2007), while the expression of the respective GFP-fusion proteins increased TAFC production to 86 ± 11% and 91 ± 8%, respectively, of the wild type (wt). Similarly, the SidI–GFP carrying strain displayed 95 ± 19% of the wt TAFC production. These data indicate correct enzymatic activity and subcellular localization of the GFP-tagged protein versions. Truncation of the putative PTS1 motif of GFP–SidH (GFP–SidHΔPTS1) caused cytosolic localization, which strongly suggests that the C-terminus of SidH is indeed a targeting sequence required for peroxisomal localization (Fig. 2A). In contrast to GFP–SidH, GFP–SidHΔPTS1 did not support TAFC synthesis, i.e. TAFC biosynthesis was not detectable in the respective mutant strain (data not show), indicating that peroxisomal localization of SidH is essential for TAFC biosynthesis.
Fig. 2.
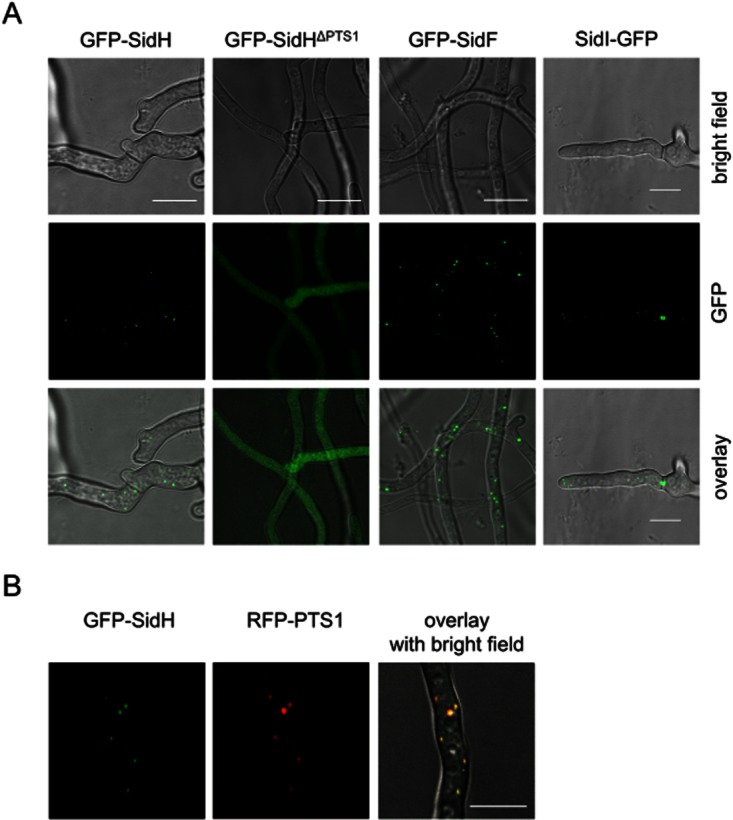
A. In A. fumigatus, GFP-tagged versions of SidH (GFP–SidH), SidF (GFP–SidF) and SidI (SidI–GFP) localize to peroxisomes while PTS1 truncation (GFP–SidHΔPTS1) blocks peroxisomal localization of SidH.
B. GFP-tagged SidH (GFP–SidH) colocalizes with peroxisomal RFP (RFP–PTS1). Fungal strains were grown in iron-depleted minimal medium for 18 h at 37°C.
Scale bar, 10 μm.
Recently, red fluorescence protein (RFP) C-terminally tagged with a PTS1 motif (RFP–PTS1) has been shown to localize to peroxisomes in A. fumigatus (Beck and Ebel, 2013). In a strain producing both GFP–SidH and RFP–PTS1, we found virtually perfect colocalization (Fig. 2B). These data underline the peroxisomal localization of SidH.
Peroxisomal targeting signals are conserved in fungal SidI, SidH and SidF orthologues
With few exceptions such as S. cerevisiae, Candida albicans or Cryptoccoccus neoformans, most fungi produce siderophores and encode respective genes (Yasmin et al., 2011). Genome mining by blast searches and sequence analysis revealed that putative SidI, SidH and SidF orthologues from various Ascomycetes carry putative PTS motifs. Table 1 lists the closest homologues to SidI, SidH and SidF, which were identified by blast searches. Genes involved in siderophore biosynthesis (including sidI, sidH and sidF) tend to be organized in gene clusters (Schrettl et al., 2008). The orthologues of sidI, sidH and sidF listed in Table 1 are colocalized in the genome with other putative siderophore biosynthetic gene (Table S1), which emphasizes that these genes are indeed the orthologues. Besides these orthologues, all analysed species possess additional homologues (see below). All identified SidF and SidH orthologues possess PTS1 motifs. Importantly, SidI orthologues from Sordariomycetes such as Neurospora crassa possess a PTS1 in contrast to the PTS2 found in other Ascomycetes.
Table 1.
Peroxisomal targeting signals in orthologues of SidI, SidH and SidF
| SidI | SidH | SidF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Organism | Accession | PTS | Score (PTS1) | Accession | PTS | Score (PTS1) | Accession | PTS | Score (PTS1) | |
| Eurotiomycetes | Aspergillus fumigatus | XP_753087.1 | RLQQTLSHL | – | XP_748661.1 | SKL | 10.8 | XP_748660.1 | AKL | 7.5 |
| Neosartorya fischeri | XP_001264038.1 | RLQQTLSHL | – | XP_001259092.1 | SKL | 10.5 | XP_001259091.1 | AKL | 7.5 | |
| Aspergillus niger | XP_001390954.1 | RLQQTLSHF | – | XP_001390237.1 | SKL | 9.6 | XP_001390236.1 | AKL | 6.9 | |
| Aspergillus clavatus | XP_001268556.1 | RLQQTLSHL | – | XP_001273567.1 | SNL | 9.3 | XP_001273568.1 | AKL | 7.3 | |
| Aspergillus oryzae | XP_001821069.1 | RLQQTLSHI | – | XP_001826764.1 | SNL | 5.4 | XP_001826765.1 | AKL | 5.2 | |
| Penicillium chrysogenum | XP_002569340.1 | RLQQTLSHV | – | XP_002565937.1 | SKL | 8.1 | XP_002565938.1 | AKL | 6.9 | |
| Aspergillus nidulans | XP_658213.1 | RLQQTLSHL | – | XP_663839.1 | SNLd | 5.9 | XP_663838.1 | AKL | 7.7 | |
| Ajellomyces capsulatus | EEH02760.1 | RLQQTLNHI | – | EEH07298.1 | SKL | 12.4 | XP_001536540.1 | SKL | 14.0 | |
| Talaromyces stipitatus | XP_002484634 | RLQQTQRHI | – | XP_002486020.1 | SKL | 8.4 | XP_002486021.1 | AKL | 6.9 | |
| Leotiomycetes | Botryotinia fuckeliana | XP_001546797.1 | PKLa | 13.5 | XP_001551006 | SKL | 9.1 | XP_001551005.1 | PKLa | 6.5 |
| Sclerotinia sclerotiorum | XP_001585101.1 | PKL | 12.7 | XP_001594442.1 | SKLb | 9.1 | XP_001594441.1 | AKL | 6.9 | |
| Sordariomycetes | Neurospora crassa | XP_959826.1 | SKL | 6.2 | XP_962600.1 | SKL | 6.3 | XP_959825.1 | PKL | 6.5 |
| Gibberella zeae | XP_384509.1 | SKL | 3.7 | XP_383922.1 | SKL | 10.4 | XP_383921.1 | PKL | 2.2 | |
| Podospora anserina | XP_001905332.1 | AKL | 12.6 | XP_003437360.1 | SKL | 8.6 | XP_001905331.1 | PKL | 8.0 | |
| Chaetomium globosum | XP_001226170.1 | AKL | 0.0 | XP_001227399.1 | SKL | 8.6 | XP_001227400.1 | AKL | 6.9 | |
| Magnaporthe oryzae | EHA55987 | AKL | 10.1 | EHA47011.1 | SKL | 9.3 | XP_362759.1 | PKL | 8.2 | |
| Dothideomycetes | Phaeosphaeria nodorum | XP_001804551.1 | RLNQTLLQIc | – | XP_001790987.1 | SKL | 11.7 | XP_001804552.1 | ARL | 8.5 |
| Basidiomycota | Ustilago maydis | XP_760950.1 | – | – | XP_757580.1 Fer4 | – | – | XP_757579.1 Fer5 | – | – |
Updated genome data from Botrytis cinerea strain B05.10 with the locus tag B0510_2726 (SidI) and B0510_7543 (SidF) reveal gene products containing a PTS1 (PKL). http://www.broadinstitute.org/annotation/genome/botrytis_cinerea/FeatureSearch
The annotated gene is organized in four exons, whereas all other orthologues have two. Manual reannotating of the intron/exon structure increased the sequence similarity to its orthologues and led to a PTS1-containing C-terminus (EWYPRLVKSPNFAEGIQAYVDKRPPKWVNSKL).
The correct start codon is most likely 414 bp upstream and in frame of the annotated start, which leads to a gene product with higher similarity to its orthologues and contains the quoted PTS2.
The deposited sequence most likely contains a sequencing error leading to a false C-terminus. Contig 1.106, which was used for gene assembly, misses in contrast to contig 1.107 a cytosine after nt 745 of the cds. Correction of the sequence generated a C-terminus showing 80% identity with A. fumigatus SidH (EEASSALVDEWYPKLIAGENFHEGVKAFVEKRQPRWRASNL).
Variants of the classical PTS1 SKL sequence such as –ARL, –AKL or –PKL were shown to be functional peroxisomal targeting signals in human, yeast and Penicillium chrysogenum (Amery et al., 1998; Kiel et al., 2009). The PTS1 scores of proteins were obtained using the PTS1-predictor program http://mendel.imp.ac.at/mendeljsp/sat/pts1/PTS1predictor.jsp (Neuberger et al., 2003). Positive scores indicate high probability of peroxisomal targeting, sequences with scores < −10 are unlikely to function as PTS1, and motifs with scores in between have unclear function. PTS2 motifs were identified using the PTS2 finder http://www.peroxisomedb.org/diy_PTS2.html, which does not provide reliability scores.
A. fumigatus SidH and SidI are targeted to peroxisomes via their corresponding PTS1 and PTS2 receptors PexE and PexG, respectively, in A. nidulans
Aspergillus fumigatus and A. nidulans produce the same siderophores and the proteins involved in their biosynthesis are highly conserved (Haas et al., 2008; Schrettl et al., 2008). The amino acid sequence identities of the corresponding TAFC biosynthetic enzymes of A. fumigatus and A. nidulans are given in Table S2. Moreover, the PTS are perfectly conserved (Table 1). To investigate the peroxisomal localization of TAFC biosynthesis in more detail, we switched from A. fumigatus to A. nidulans, because the peroxisomal system of the latter has been characterized in great detail and a number of respective peroxin mutants, described in Table S3, is available (Hynes et al., 2008). Peroxins are proteins required for the assembly and function of peroxisomes. These are highly conserved among fungal species (Kiel et al., 2006); a comparison of relevant peroxins of A. fumigatus and A. nidulans is given in Table S3 (Hynes et al., 2008).
In a first step, localization of A. fumigatus GFP–SidH was heterologously studied in A. nidulans wt and mutants lacking peroxisomes (pexC::bar), PTS1-dependent peroxisomal import (ΔpexE, missing the PTS1 receptor PexE) or PTS2-dependent peroxisomal targeting (pexG14, lacking a functional PTS2 receptor PexG). In perfect agreement with PTS1 receptor-mediated peroxisomal localization, GFP–SidH localized in cytosolic spots in A. nidulans wt and pexG14 strains but mislocalized to the cytosol in ΔpexE and pexC::bar mutants (Fig. 3). The peroxisomal localization of GFP–SidF in A. fumigatus together with its evolutionary conserved PTS1 (Fig. 2, Table 1) strongly suggests that SidF is directed to peroxisomes in the same manner.
Fig. 3.
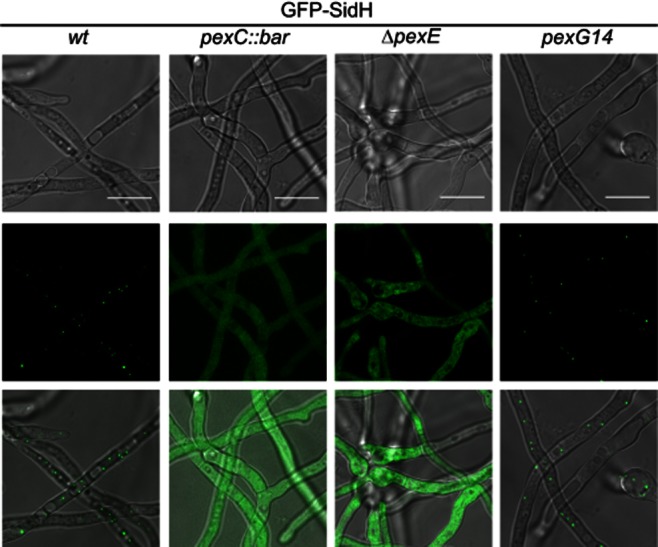
In A. nidulans, peroxisomal localization of A. fumigatus GFP–SidH is blocked by inactivation of PexC (pexC::bar) or PexE (ΔpexE) but not PexG (pexG14). Fungal strains were grown in iron-depleted minimal medium for 18 h at 37°C. Upper panel, bright-field image; mid-panel, confocal fluorescence microscopy; lower panel, overlay. Scale bar, 10 μm.
In contrast to GFP–SidH, SidI–GFP was mislocalized to the cytosol in a pexG14 strain missing the PTS2 receptor, which demonstrates a PTS2-dependent peroxisomal targeting of SidI (Fig. 4).
Fig. 4.
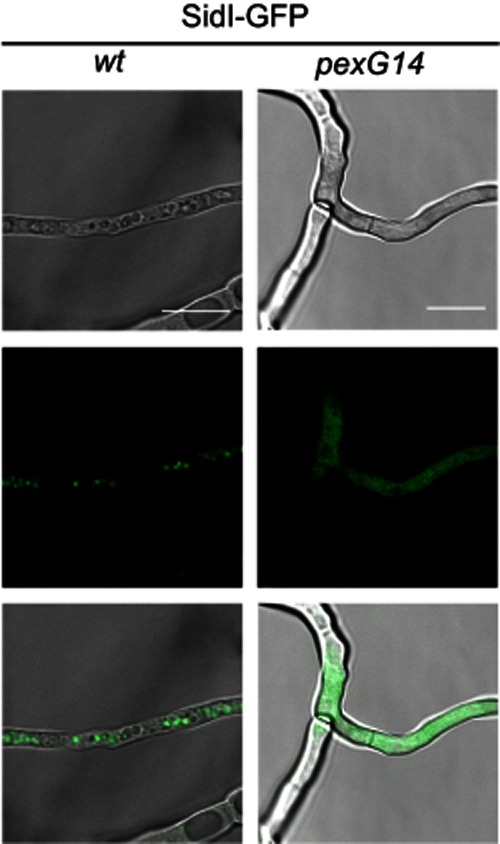
In A. nidulans, peroxisomal localization of A. fumigatus SidI–GFP is blocked by inactivation of PexG (pexG14). Fungal strains were grown in iron-depleted minimal medium containing 200 μM BPS to generate harsh iron starvation for 24 h at 37°C. Upper panel, bright-field image; mid-panel, confocal fluorescence microscopy; lower panel, overlay. Scale bar, 10 μm.
These data confirm that PTS1 and PTS2 receptors function independently in filamentous fungi, which contrasts with the situation in mammals and plants (Kiel et al., 2006; Hynes et al., 2008). Moreover, the data suggest that inactivation of the PTS1 receptor mislocalizes SidH and SidF, while inactivation of the PTS2 receptor mislocalizes SidI to the cytosol.
Deficiency in either PTS1- or PTS2-dependent peroxisomal protein import or AntA-mediated peroxisomal ATP import decreases TAFC production in A. nidulans
To characterize the role of peroxisomes in siderophore biosynthesis at the metabolite level, production of TAFC and FC was analysed in the A. nidulans wt and mutants affected in peroxisomal import (ΔpexE, pexG14, pexF23, ΔpexE/pexG14), peroxisomal proliferation (ΔpexK), peroxisomal biogenesis (pexC::bar) or peroxisomal ATP import (antA14) respectively (Fig. 5).
Fig. 5.
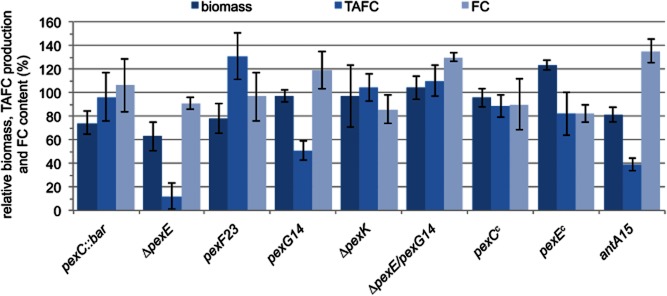
Inactivation of PexE, PexG or AntA but not of PexC, PexK or both PexE and PexG impairs TAFC biosynthesis. Production of TAFC and FC was measured after growth for 24 h in iron-depleted minimal medium. The values represent the means ± STD of three experiments normalized to biomass and wt (100%).
In accordance with the importance of PexE-mediated peroxisomal transport of SidH and SidF, the TAFC production of the pexE deletion mutant (ΔpexE) was decreased to 12% of the wt during iron starvation (Fig. 5). The decreased TAFC production of ΔpexE is most likely the reason for the 37% reduced biomass production compared with wt (Fig. 5) because the lack of siderophore production has been shown to decrease growth during iron limitation (Schrettl et al., 2004; 2007). The TAFC and biomass production defects during iron starvation are cured by pexE gene complementation in strain pexEc (Fig. 5). Inactivation of PexG-mediated peroxisomal import (strain pexG14) reduced TAFC production to 51% of the wt, which suggests a crucial role for PTS2-dependent peroxisomal import of SidI in TAFC biosynthesis. In agreement with the higher TAFC production compared with ΔpexE, pexG14 biomass production was also higher. Taken together, these data indicate that cytosolic mislocalization of SidF and SidH or of SidI via inactivation of PTS1 or PTS2 receptors, respectively, impairs TAFC biosynthesis in A. nidulans.
The peroxisomal membrane is impermeable to ATP (Palmieri et al., 2001; van Roermund et al., 2001; Hynes et al., 2008). In A. nidulans, inactivation of the peroxisomal ATP-importer AntA in the antA15 strain reduces growth on fatty acids (Hynes et al., 2008) because ATP is required to fuel peroxisomal β-oxidation by activation of fatty acyl-CoA synthetases, which belong to the acyl-CoA-ligase family. SidI belongs also to the acyl-CoA ligase protein family and converts mevalonate to mevalonyl-CoA in an ATP-dependent manner (Yasmin et al., 2011). Consistent with the requirement for ATP within peroxisomes for SidI activity, TAFC production was reduced to 39% of wt in antA15, a similar reduction to that observed for pexG14. Both β-oxidation and TAFC biosynthesis were not blocked completely in antA15 suggesting an AntA-independent peroxisomal ATP delivery system. In this respect it can be noted that glycolytic/gluconeogenic enzymes have recently been shown to have dual localization in fungi, i.e. in the cytoplasm and peroxisomes (Freitag et al., 2012). This metabolic network may function in redox/ATP shuttling or as a buffer system to cope with perturbations of redox/ATP equivalents (Freitag et al., 2012).
Remarkably, neither blocking of peroxisome biogenesis (pexC::bar) nor inactivation of entire peroxisomal protein import by simultaneous inactivation of both PexE and PexG (ΔpexE/pexG14), or inactivation of pexF (pexF26) impaired TAFC production (Fig. 5). Remarkably, the TAFC production of pexF26, which lacks PexF and consequently both PTS1- and PTS2-dependent peroxisomal protein targeting (Erdmann and Schliebs, 2005; Koek et al., 2007; Hynes et al., 2008), was even 31% increased compared with wt (Fig. 5). Peroxisomes are generated either de novo from the endoplasmatic reticulum, which requires PexC, or by PexK-dependent peroxisomal division of the novo-generated peroxisomes (Hoepfner et al., 2005; Hettema and Motley, 2009). PexK deficiency (ΔpexK) also did not affect TAFC production (Fig. 5).
The FC content of ΔpexE, pexF23, pexC::bar and ΔpexK was between 85% and 105% of the wt indicating that FC biosynthesis does not rely on peroxisomes (Fig. 5). In agreement, SidI, SidH and SidF are not involved in FC biosynthesis (Fig. 1). Interestingly, FC production was increased between 19% and 35% compared with wt in pexG14, ΔpexE/pexG14 and antA15.
Inactivation of PexE, PexG or AntA impairs growth under iron-depleted conditions
Consistent with the extremely reduced TAFC production and biomass production in liquid culture (Fig. 5), PexE deficiency dramatically decreased growth on plates under iron starvation but not iron sufficiency (Fig. 6). All mutants lacking peroxisomes or PTS1-dependent peroxisomal protein import display reduced conidiation (Hynes et al., 2008). However, deficiency in PexG or AntA completely blocked sporulation during iron starvation, most likely due to the decreased TAFC production because siderophore-mediated iron supply has previously been shown to be crucial for sporulation (Schrettl et al., 2007; Wallner et al., 2009). Consistently, siderophore cross-feeding from wt increased growth and conidiation of ΔpexE and pexG14 (Fig. 7).
Fig. 6.
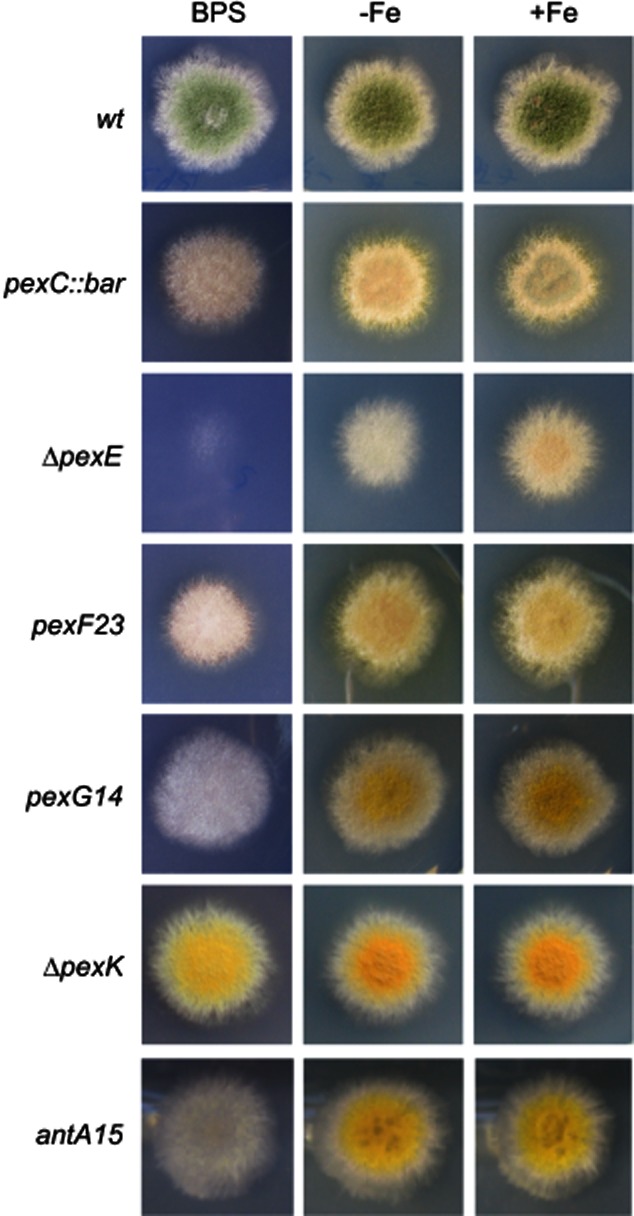
Growth phenotypes of A. nidulans wt and peroxisomal mutants. Fifty conidia of the fungal strains were point-inoculated onto minimal medium plates with different iron supply (BPS, 200 μM BPS; −Fe, without addition of iron; +Fe, sufficient iron supply with 30 μM FeSO4) and incubated for 48 h. BPS is a chelator generating harsh iron starvation. The wt produces green conidia, while the mutant strains produce yellow conidia, due to the genetic marker yA1.
Fig. 7.
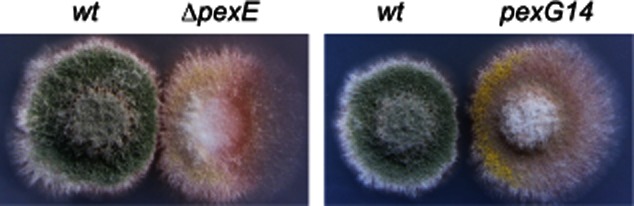
In A. nidulans, cross-feeding from wt cures the growth and sporulation defects of ΔpexE and pexG14 respectively. Fifty conidia of the wt and respective mutant strain were spotted in near distance onto minimal medium agar containing 200 μM BPS to generate harsh iron starvation and incubated for 48 h.
The N. crassa SidI orthologue localizes to peroxisomes in Aspergillus spp. and assists TAFC biosynthesis
In contrast to A. fumigatus and A. nidulans, N. crassa secretes the siderophore coprogen instead of TAFC, but shares the same hyphal siderophore FC (Matzanke et al., 1987). Similar to TAFC, coprogen contains anhydromevalonyl residues (Haas et al., 2008). Therefore, it is not surprising that N. crassa possesses orthologues to SidH, SidF and SidI. The SidI orthologues from A. fumigatus and N. crassa share 60% identity at the amino acid sequence level, but in contrast to the PTS2-carrying A. fumigatus SidI, N. crassa SidI harbours a PTS1 motif (Table 1). To confirm its role in siderophore biosynthesis and its peroxisomal localization, the N. crassa sidI was expressed as an N-terminal GFP-tagged protein in A. nidulans wt and the A. nidulans ΔpexE strain (Fig. 8). The N. crassa GFP–SidI localized to peroxisomes in A. nidulans wt, which emphasizes conservation of peroxisomal localization. In accordance with the PTS1 motif, it was mislocalized to the cytoplasm in A. nidulans ΔpexE. Remarkably, the expression of N. crassa GFP–SidI increased TAFC production from 11% of the parental ΔpexE strain to 86% of the wt. In this strain, GFP–SidI, SidH and SidF are now all cytosolic, which clearly shows that colocalization of all three enzymes, either in peroxisomes or in the cytosol, is essential for efficient TAFC production.
Fig. 8.
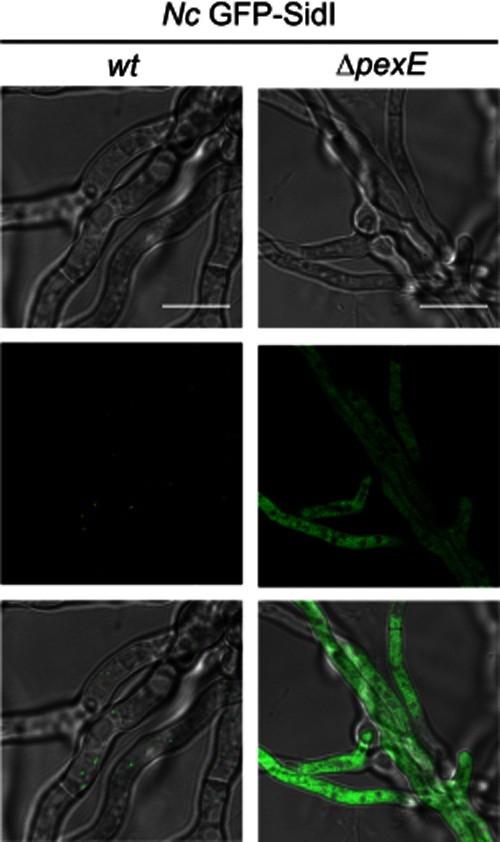
Peroxisomal localization of N. crassa GFP–SidI in A. nidulans is PexE-dependent. Fungal strains were grown in iron-depleted minimal medium for 18 h at 37°C. Upper panel, bright-field image; mid-panel, confocal fluorescence microscopy; lower panel, overlay. Scale bar, 10 μm.
Discussion
This study has revealed peroxisomal localization of the three TAFC biosynthetic enzymes from A. fumigatus: PTS1-containing SidH and SidF as well as PTS2-containing SidI. All other identified components of the siderophore biosynthetic machinery lack PTS motifs indicating cytosolic localization. However, peroxisomal localization of other siderophore biosynthetic enzymes via unidentified targeting sequences, unidentified import systems or cryptic PTS motifs that are unmasked after post-transcriptional processes cannot be completely excluded (Freitag et al., 2012). Nevertheless, the acetyl transferase SidL, required for FC biosynthesis, as well as the esterase EstB, which hydrolyses TAFC after uptake, have been shown to be cytosolic (Kragl et al., 2007; Blatzer et al., 2011). Cytosolic mislocalization of one to two of the three peroxisomal TAFC biosynthetic enzymes due to PTS1 truncation of SidH in A. fumigatus or blocking either PTS1- (strain ΔpexE) or PTS2-dependent (strain pexG14) peroxisomal protein import in A. nidulans, which employs the same siderophore biosynthetic machinery, blocked or at least decreased TAFC biosynthesis. Moreover, blocking peroxisomal ATP import (strain antA14), which is required for SidI function, decreased TAFC production in A. nidulans. These data suggest that the siderophore pathway intermediates synthesized by SidI and SidH, mevalonyl-CoA and anhydromevalonyl-CoA, cannot efficiently pass the peroxisomal membrane (Fig. 1). Furthermore, the peroxisomal localization of SidI, SidH and SidF indicates that TAFC biosynthesis requires peroxisomal import of N5-hydroxyornithine and peroxisomal export of N5-anhydromevalonyl-N5-hydroxyornithine. Peroxisomal membranes are likely to be permeable to molecules with a molecular mass (Mr) < 400 via channels/pores (Antonenkov et al., 2009). Consequently, it is likely that the siderophore precursors mevalonate (Mr = 148) and N5-hydroxyornithine (Mr = 148) can enter and the SidF product N5-anhydromevalonyl-N5-hydroxyornithine (Mr = 260) can freely pass the peroxisomal membrane. In contrast, the CoA-intermediates mevalonyl-CoA (Mr = 916) and anhydromevalonyl-CoA (Mr = 898) are unlikely to be able to freely exit peroxisomes. This feature of the peroxisomal membrane is expected to increase the local concentration of the CoA-intermediates of TAFC biosynthesis, which might enhance the efficiency of the involved enzymatic reactions and consequently provides a rational for the localization in peroxisomes. The decrease in TAFC biosynthesis varied between the different ways of cytosolic mislocalization and can be explained by the different, in part pleiotropic, effects: SidH PTS1 truncation mislocalizes only SidH, PTS1 inactivation mislocalizes SidH and SidF together with all other PTS1-dependent peroxisomal proteins, and PTS2 inactivation mislocalizes SidI together with all PTS2-dependent peroxisomal proteins. Moreover, differences in peroxisomal import and export efficiency of N5-hydroxyornithine and N5-anhydromevalonyl-N5-hydroxyornithine might play a role. In this respect it is interesting to note that the vast majority of peroxisomal matrix proteins are PTS1 receptor-dependent and SidI is one of the few exceptions (Kiel et al., 2006). Despite the clear peroxisomal localization of SidI, SidH and SidF, it cannot be excluded that low levels of these enzymes are present and operate in the cytosol.
In agreement with the dramatically reduced TAFC production, the PexE-deficient mutant displayed reduced growth during iron starvation on solid and in liquid growth media (Figs 5 and 6). However, growth of mutants lacking PexC or PexF was also somehow reduced despite their TAFC production not being reduced. These data indicate that siderophore-independent peroxisomal functions are additionally important for adaptation to iron starvation.
Orthologues of SidI, SidH and SidF are found in numerous Ascomycetes as these enzymes are not only required for fusarinine-type but also coprogen-type siderophores, which also contain anhydromevalonyl moieties (Haas et al., 2008). Genome mining indicated PTS motif conservation in SidI, SidH and SidF orthologues of numerous Ascomycetes (Table 1). In agreement, the SidF orthologue of the coprogen producer Penicillium chrysogenum was recently identified as a peroxisomal matrix protein in a proteomic approach (Kiel et al., 2009). Interestingly, SidI orthologues of Sordariomycetes possess a PTS1 in contrast to the PTS2 motif found in other Ascomycetes and the PTS1 motif of the SidI orthologue from coprogen-producing N. crassa was confirmed to be functional here. Therefore, all three siderophore enzymes in Sordariomycetes rely on PexE-dependent peroxisomal import only.
The enoyl-CoA hydratase Fer4 (UMO1433) and the SidF orthologue Fer5 (UM01432.1), which are essential for ferrichrome A biosynthesis in the Basidiomycete Ustilago maydis (Winterberg et al., 2010), lack PTS indicating non-peroxisomal localization (Table 1). Due to the differences in siderophore structure and biosynthesis, U. maydis lacks a SidI orthologue. A BlastP search (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi?organism=fungi) identified SidF homologues in various Basidiomycetes besides U. maydis (Table S7; Malassezia globosa, Serpula lacrymans, Schizophyllum commune, Puccinia graminis, Melampsora larici-populina) but none of these proteins contained PTS1 or PTS2 motifs, which suggests non-peroxisomal localization of siderophore biosynthesis in Basidiomycetes. Moreover, BlastP searches (http://fungidb.org/fungidb/) failed to identify homologues to SidF or SidA in Chytridiomycetes (Batrachochytrium dendrobatidis), Oomycetes (Hyaloperonospora arabidopsis, Phytophthora infestans, Pythium ultimum) or Zygomyctes (Rhizopus oryzae), which indicates the inability of these species to produce hydroxamate-type siderophores.
Nevertheless, the evolutionary conservation of peroxisomal localization of siderophore biosynthetic enzymes in Ascomycetes indicates its importance. Therefore, it is remarkable that cytosolic mislocalization in A. nidulans of all three TAFC biosynthetic enzymes by (i) inactivation of peroxisomal biogenesis (strain pexC::bar), (ii) simultaneous inactivation of both PTS1- and PTS2- dependent transport (strains pexG14/ΔpexE and ΔpexF), or (iii) expression of PTS1-containing SidI from N. crassa in a mutant lacking PTS1-dependent peroxisomal protein import (strain GFP–SidIΔpexE) did not decrease TAFC biosynthesis.
Taken together these data suggest that siderophore biosynthesis can efficiently work in both peroxisomes and the cytosol as long as SidI, SidH and SidF share the same compartment. In contrast, peroxisomes are essential for biotin biosynthesis in A. nidulans, because it has originally been shown that pex mutants defective in PTS1 protein import were found to be auxotrophic for biotin due to an inability to synthesize the intermediate pimelic acid (Hynes et al., 2008). Further studies confirmed peroxisomal localization of BioF, a biotin synthetic enzyme in A. nidulans and A. oryzae (Magliano et al., 2011; Tanabe et al., 2011). Peroxisomes are indispensible for Ak toxin biosynthesis in the fungal plant pathogen Alternaria alternate (Imazaki et al., 2010) and β-oxidation in A. nidulans (Hynes et al., 2008). Similarly, cytosolic mislocalization of the single peroxisomal penicillin biosynthetic enzyme completely blocks penicillin biosynthesis in P. chrysogenum (Muller et al., 1992). Nevertheless, there are known pathways that are localized in peroxisomes but also work outside. For example, the acyl-CoA transferase IAT (containing a PTS1 motif), which is involved in penicillin biosynthesis, works better in peroxisome but is still functional in the cytosol in A. nidulans (Sprote et al., 2009). Moreover, in the glyoxylate cycle both malate synthase (using acetyl-coA and glyoxylate) and isocitrate lyase (producing glyoxylate and succinate) normally operate in peroxisomes, but cytoplasmic localization of both allows growth on acetate (Hynes et al., 2008). These data raise the general question for the rationale of peroxisomal localization of pathways that are also functional outside. A possible explanation is that all the mentioned pathways include CoA-ligands and peroxisomes appear to provide the optimal conditions for this, which might be favoured by the distinct permeability feature of the peroxisomal membrane mentioned above and the high local CoA concentration. Another possible explanation for the siderophore biosynthetic enzymes could be their homology to and evolution from peroxisomal enzymes involved in β-oxidation, e.g. SidI and SidH display significant similarity to acyl-CoA synthase and enoyl-CoA hydratase (Figs S1 and S2).
Peroxisomes are generated either de novo from the endoplasmatic reticulum or by PexK-dependent peroxisomal division (Hettema and Motley, 2009). PexK deficiency (strain ΔpexK) did not affect TAFC production (Fig. 5) which indicates that de novo peroxisome biogenesis from the endoplasmatic reticulum is sufficient for TAFC biosynthesis. In contrast, PexK is required for the increase of peroxisomes during growth on fatty acids and optimal β-oxidation (Valenciano et al., 1996) (Hynes et al., 2008). Deficiency in peroxisomal biogenesis, protein import or ATP import did not decrease FC production (strains ΔpexE, pexF23, pexC::bar and ΔpexK). Intriguingly, all investigated deficiencies impairing SidI localization or activity (pexG14, ΔpexE/pexG14 and antA15) displayed even increased FC contents (Fig. 5). Inactivation of SidI blocks mevalonate consumption for siderophore biosynthesis (Yasmin et al., 2011). It is likely that N5-hydroxyornithine, the common precursor for TAFC and FC, and the mevalonate precursor acetyl-CoA is redirected to FC biosynthesis.
Taken together, this study has demonstrated for the first time that particular siderophore biosynthetic enzymes are localized in peroxisomes and that this compartmentalization is evolutionary conserved. Remarkably, the peroxisomal part of siderophore biosynthesis is an example of a metabolic pathway that functions as long as all three components are localized in the same compartment independent of peroxisomes, which is indicated by the fact that siderophore production was impaired by cytoplasmic mislocalization of individual enzymes but not the complete loss of peroxisomes. The importance of iron acquisition in the pathogenicity of both plant and animal fungal pathogens has been shown in many studies (Haas et al., 2008). Our results indicate that peroxisomes play a crucial role in the production of virulence-determining siderophores.
Experimental procedures
Strains, oligonucleotides, plasmids, growth conditions
The fungal strains, plasmids and oligonucleotides used in this study are listed in Table S4, Table S5 and Table S6. Generally, A. fumigatus and A. nidulans strains were grown at 37°C in Aspergillus minimal medium according to Pontecorvo et al. (1953) containing 1% glucose as the carbon source and 20 mM glutamine as the nitrogen source. Iron-replete media (+Fe) contained 30 μM FeSO4. For iron-depleted conditions (−Fe), addition of iron was omitted. The final concentration for required supplements was 1 mg l−1 biotin, 4 mg l−1 p-aminobenzoic acid, 2.5 mg l−1 pyridoxine, 2.5 mg l−1 riboflavin. The bathophenanthroline disulphonate (BPS) concentration used was 200 μM. N. crassa was grown in standard Vogel's medium (Vogel, 1956) containing 2% sucrose as carbon source and 20 mM glutamine as the nitrogen source and 1 mg l−1 biotin. For iron-depleted conditions, iron was omitted.
DNA manipulations
For extraction of genomic DNA, mycelia were homogenized and DNA was isolated according to Sambrook et al. (1989). For general DNA propagations Escherichia coli DH5α strain was used as a host.
Generation of fungal strains expressing GFP-tagged versions of the studied enzymes
The sidH (AFUA_3g03410) and sidF (AFUA_3g03400) coding regions including introns were amplified from cosmid psidD-COS as template using primers osidHgfp1 and osidHgfp3 with add-on BglII and NotI sites for sidH and ogfpsidF3 and osidHgfp2 with add-on BamHI and NotI sites for sidF respectively. The PCR products were cloned in frame with the 5′-preceding GFP-encoding region into the BglII/NotI restriction sites of the plasmid pCAME703-AoHapX-full, replacing the original hapX fusion (Goda et al., 2005). This expression vector for the GFP-fusion protein is driven by a constitutive XAH promoter from Chaetomium gracile. A PTS1 lacking version of SidH was constructed with the primers osidHgfp1 and osidHgfp2 in the same manner. In order to express the sidI orthologue from N. crassa, RNA from mycelia grown for 72 h under iron starvation was transcribed into cDNA (SuperScript II Kit, Invitrogen). This cDNA was then used as a template for PCR-amplification of the N. crassa sidI coding region excluding introns using the primers oNcSidI-1/oNcSidI-2 containing add-on BglII and /NotI sites. The resulting fragment was cloned into the BglII/NotI sites of the plasmid pCAME703-AoHapX-full resulting in plasmid pGFP–NcSidI. The resulting plasmids pGFP–SidH, pGFP–SidF, pGFP–SidHΔPTS1 and pGFP–NcSidI were then used to transform the respective A. fumigatus and A. nidulans strains. Transformation of Aspergillus spp. was carried out as described by Tilburn et al. (1995). The selection was ensured by co-transformation with the plasmid pSK275, which carries the pyrithiamine resistance gene ptrA using 0.1 μg ml−1 pyrithiamine (Sigma). The screening for transformants was performed by PCR (ogfp1/ oAf538RAC1-f for gfp-sidH and ogfp4/oAf538-AT1-r for gfp-sidF), TAFC production and GFP-fluorescence.
To visualize localization of SidI, a sidI–gfp gene fusion encoding a fusion of SidI C-terminus with the enhanced green fluorescent protein (EGFP) was constructed. Therefore, a 1.2 kb fragment encoding the C-terminal region of SidI was amplified using oligonucleotides oAfsidI-1 and oAfsidI-2, thereby replacing the sidI stop codon with a BamHI site. A 2.2 kb SmaI–SacI GFP-encoding fragment was subcloned from plasmid pUCGH (Langfelder et al., 2001) into the compatible EcoRV–SacI sites of plasmid pGEM-5zf(+) (Promega), yielding plasmid pGfp. The pGfp and the PCR product were digested with SphI and BamHI respectively. Both the insert and the vector were made blunt ended using Klenow fragment and ligated to give plasmid pSidI–Gfp. A 2.2 kb BssHII fragment of ptrA gene from psk275 was inserted into the BssHII site of pGem-sidI, yielding pSPGfp. pSPGfp was introduced into the wt strain by protoplast transformation. Pyrithiamine-resistant transformants containing the SidI–GFP in-frame fusion of the sidI and EGFP-encoding genes (sidIgfp strain) were selected and used for the subcellular localization of SidI. Single homologous recombination was confirmed by Southern blot analysis of XhoI digested DNA.
To gain a plasmid carrying the gene plus promoter encoding the entire SidI–GFP fusion protein, a 5 kb fragment was amplified from genomic DNA of the sidIgfp strain using oligonucleotides oAfsidIgfp1 and oAfsidIgfp2 with add-on EcoRV and ClaI sites respectively. The PCR product was cloned into the EcoRV/ClaI site of the plasmid pphleo. The resulting plasmid pSidI–gfp2 carries the endogenous promotor with sidI–gfp codons and was transformed in A. nidulans strains. Phleomycin-resistant transformants containing the SidI–GFP were selected and used for the subcellular localization of SidI.
For the colocalization studies of GFP–SidH and RFP–PTS1, the RFP–PTS1-encoding plasmid pSK379–RFP–PTS1 was integrated into the GFP–SidH producing A. fumigatus mutant by co-transformation with the plasmid pphleo, which confers phleomycine resistance. Transformants were screened via resistance to pyrithiamine and phleomycin.
Analysis of siderophores
Analysis of siderophore was carried out by reversed phase HPLC as described previously (Oberegger et al., 2001). To quantify extracellular or intracellular siderophores, culture supernatants or cellular extracts were saturated with FeSO4 and siderophores were extracted with 0.2 volumes of phenol. The phenol phase was separated and subsequent to addition of five volumes of diethylether and one volume of water, the siderophore concentration of the aqueous phase was measured photometrically using a molar extinction factor of 2996/440 nm (M−1 cm−1) for TAFC and 2460/434 nm (M−1 cm−1) for FC.
Fluorescence microscopy
For confocal microscopy strains were grown in glass bottom dishes (MatTec) with supplemented minimal media for 17 h at 37°C. Images were taken on a confocal laser scanning microscope (SP5, Leica) equipped with a 63×/1.40 oil immersion objective. Z series of optical sections were obtained and projected along the z axis to obtain a general view of the specimen. The acquisition software was LAS AF software (Leica Microsystems). Images were processed using ImageJ (http://rsbweb.nih.gov/ij/).
Computational analysis
The following databases were used for gene comparisons, NCBI: http://www.ncbi.nlm.nih.gov/; Broad Institute: http://www.broadinstitute.org/. PTS1 predictions were performed using the general function of the PTS1 Predictor (http://mendel.imp.ac.at/jspcgi/cgi-bin/pts1/pts1.cgi). PTS2 motifs were identified using a PTS2 finder (http://www.peroxisomedb.org/diy_PTS2.html).
Acknowledgments
This work was supported by the Austrian Science Foundation grant (FWF P-18606-B11 and P21643-B11 to H.H.). We are grateful to Dr Frank Ebel for providing the plasmid pSK379–RFP–PTS1.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web-site.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amery L, Brees C, Baes M, Setoyama C, Miura R, Mannaerts GP, Van Veldhoven PP. C-terminal tripeptide Ser–Asn–Leu (SNL) of human D-aspartate oxidase is a functional peroxisome-targeting signal. Biochem J. 1998;336(Part 2):367–371. doi: 10.1042/bj3360367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenkov VD, Mindthoff S, Grunau S, Erdmann R, Hiltunen JK. An involvement of yeast peroxisomal channels in transmembrane transfer of glyoxylate cycle intermediates. Int J Biochem Cell Biol. 2009;41:2546–2554. doi: 10.1016/j.biocel.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Beck J, Ebel F. Characterization of the major Woronin body protein HexA of the human pathogenic mold Aspergillus fumigatus. Int J Med Microbiol. 2013;303:90–97. doi: 10.1016/j.ijmm.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Blatzer M, Schrettl M, Sarg B, Lindner HH, Pfaller K, Haas H. SidL, an Aspergillus fumigatus transacetylase involved in biosynthesis of the siderophores ferricrocin and hydroxyferricrocin. Appl Environ Microbiol. 2011;77:4959–4966. doi: 10.1128/AEM.00182-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakhage AA, Turner G. L-lysine repression of penicillin biosynthesis and the expression of penicillin biosynthesis genes acvA and ipnA in Aspergillus nidulans. FEMS Microbiol Lett. 1992;77:123–127. doi: 10.1016/0378-1097(92)90142-b. [DOI] [PubMed] [Google Scholar]
- Brown LA, Baker A. Shuttles and cycles: transport of proteins into the peroxisome matrix (review) Mol Membr Biol. 2008;25:363–375. doi: 10.1080/09687680802130583. [DOI] [PubMed] [Google Scholar]
- Eisendle M, Oberegger H, Zadra I, Haas H. The siderophore system is essential for viability of Aspergillus nidulans: functional analysis of two genes encoding l-ornithine N 5-monooxygenase (sidA) and a non-ribosomal peptide synthetase (sidC. Mol Microbiol. 2003;49:359–375. doi: 10.1046/j.1365-2958.2003.03586.x. [DOI] [PubMed] [Google Scholar]
- Erdmann R, Schliebs W. Peroxisomal matrix protein import: the transient pore model. Nat Rev Mol Cell Biol. 2005;6:738–742. doi: 10.1038/nrm1710. [DOI] [PubMed] [Google Scholar]
- Freitag J, Ast J, Bolker M. Cryptic peroxisomal targeting via alternative splicing and stop codon read-through in fungi. Nature. 2012;485:522–525. doi: 10.1038/nature11051. [DOI] [PubMed] [Google Scholar]
- Ganz T. Iron in innate immunity: starve the invaders. Curr Opin Immunol. 2009;21:63–67. doi: 10.1016/j.coi.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda H, Nagase T, Tanoue S, Sugiyama J, Steidl S, Tuncher A, et al. Nuclear translocation of the heterotrimeric CCAAT binding factor of Aspergillus oryzae is dependent on two redundant localising signals in a single subunit. Arch Microbiol. 2005;184:93–100. doi: 10.1007/s00203-005-0014-3. [DOI] [PubMed] [Google Scholar]
- Haas H. ) Iron – a key nexus in the virulence of Aspergillus fumigatus. Front Microbiol. 2012;3:28. doi: 10.3389/fmicb.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H, Schoeser M, Lesuisse E, Ernst JF, Parson W, Abt B, et al. Characterization of the Aspergillus nidulans transporters for the siderophores enterobactin and triacetylfusarinine C. Biochem J. 2003;371:505–513. doi: 10.1042/BJ20021685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas H, Eisendle M, Turgeon BG. Siderophores in fungal physiology and virulence. Annu Rev Phytopathol. 2008;46:149–187. doi: 10.1146/annurev.phyto.45.062806.094338. [DOI] [PubMed] [Google Scholar]
- Hettema EH, Motley AM. How peroxisomes multiply. J Cell Sci. 2009;122:2331–2336. doi: 10.1242/jcs.034363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissen AH, Wan AN, Warwas ML, Pinto LJ, Moore MM. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding L-ornithine N5-oxygenase, is required for virulence. Infect Immun. 2005;73:5493–5503. doi: 10.1128/IAI.73.9.5493-5503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoepfner D, Schildknegt D, Braakman I, Philippsen P, Tabak HF. Contribution of the endoplasmic reticulum to peroxisome formation. Cell. 2005;122:85–95. doi: 10.1016/j.cell.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Hynes MJ, Murray SL, Khew GS, Davis MA. Genetic analysis of the role of peroxisomes in the utilization of acetate and fatty acids in Aspergillus nidulans. Genetics. 2008;178:1355–1369. doi: 10.1534/genetics.107.085795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imazaki A, Tanaka A, Harimoto Y, Yamamoto M, Akimitsu K, Park P, Tsuge T. Contribution of peroxisomes to secondary metabolism and pathogenicity in the fungal plant pathogen Alternaria alternata. Eukaryot Cell. 2010;9:682–694. doi: 10.1128/EC.00369-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan CD, Kaplan J. Iron acquisition and transcriptional regulation. Chem Rev. 2009;109:4536–4552. doi: 10.1021/cr9001676. [DOI] [PubMed] [Google Scholar]
- Kiel JA, Veenhuis M, van der Klei IJ. PEX genes in fungal genomes: common, rare or redundant. Traffic. 2006;7:1291–1303. doi: 10.1111/j.1600-0854.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- Kiel JA, van den Berg MA, Fusetti F, Poolman B, Bovenberg RA, Veenhuis M, van der Klei IJ. Matching the proteome to the genome: the microbody of penicillin-producing Penicillium chrysogenum cells. Funct Integr Genomics. 2009;9:167–184. doi: 10.1007/s10142-009-0110-6. [DOI] [PubMed] [Google Scholar]
- Koek A, Komori M, Veenhuis M, van der Klei IJ. A comparative study of peroxisomal structures in Hansenula polymorpha pex mutants. FEMS Yeast Res. 2007;7:1126–1133. doi: 10.1111/j.1567-1364.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- Kragl C, Schrettl M, Abt B, Sarg B, Lindner HH, Haas H. EstB-mediated hydrolysis of the siderophore triacetylfusarinine C optimizes iron uptake of Aspergillus fumigatus. Eukaryot Cell. 2007;6:1278–1285. doi: 10.1128/EC.00066-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubodera T, Yamashita N, Nishimura A. Pyrithiamine resistance gene (ptrA) of Aspergillus oryzae: cloning, characterization and application as a dominant selectable marker for transformation. Biosci Biotechnol Biochem. 2000;64:1416–1421. doi: 10.1271/bbb.64.1416. [DOI] [PubMed] [Google Scholar]
- Langfelder K, Philippe B, Jahn B, Latge JP, Brakhage AA. Differential expression of the Aspergillus fumigatus pksP gene detected in vitro and in vivo with green fluorescent protein. Infect Immun. 2001;69:6411–6418. doi: 10.1128/IAI.69.10.6411-6418.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio-Hall LA, Keller NP. Mitochondrial beta-oxidation in Aspergillus nidulans. Mol Microbiol. 2004;54:1173–1185. doi: 10.1111/j.1365-2958.2004.04340.x. [DOI] [PubMed] [Google Scholar]
- Magliano P, Flipphi M, Arpat BA, Delessert S, Poirier Y. Contributions of the peroxisome and beta-oxidation cycle to biotin synthesis in fungi. J Biol Chem. 2011;286:42133–42140. doi: 10.1074/jbc.M111.279687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzanke BF, Bill E, Trautwein AX, Winkelmann G. Role of siderophores in iron storage in spores of Neurospora crassa and Aspergillus ochraceus. J Bacteriol. 1987;169:5873–5876. doi: 10.1128/jb.169.12.5873-5876.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WH, Bovenberg RA, Groothuis MH, Kattevilder F, Smaal EB, Van der Voort LH, Verkleij AJ. Involvement of microbodies in penicillin biosynthesis. Biochim Biophys Acta. 1992;1116:210–213. doi: 10.1016/0304-4165(92)90118-e. [DOI] [PubMed] [Google Scholar]
- Neuberger G, Maurer-Stroh S, Eisenhaber B, Hartig A, Eisenhaber F. Prediction of peroxisomal targeting signal 1 containing proteins from amino acid sequence. J Mol Biol. 2003;328:581–592. doi: 10.1016/s0022-2836(03)00319-x. [DOI] [PubMed] [Google Scholar]
- Oberegger H, Schoeser M, Zadra I, Abt B, Haas H. SREA is involved in regulation of siderophore biosynthesis, utilization and uptake in Aspergillus nidulans. Mol Microbiol. 2001;41:1077–1089. doi: 10.1046/j.1365-2958.2001.02586.x. [DOI] [PubMed] [Google Scholar]
- Oberegger H, Eisendle M, Schrettl M, Graessle S, Haas H. 4′-Phosphopantetheinyl transferase-encoding npgA is essential for siderophore biosynthesis in Aspergillus nidulans. Curr Genet. 2003;44:211–215. doi: 10.1007/s00294-003-0434-z. [DOI] [PubMed] [Google Scholar]
- Olivier LM, Krisans SK. Peroxisomal protein targeting and identification of peroxisomal targeting signals in cholesterol biosynthetic enzymes. Biochim Biophys Acta. 2000;1529:89–102. doi: 10.1016/s1388-1981(00)00139-6. [DOI] [PubMed] [Google Scholar]
- Olucha J, Meneely KM, Chilton AS, Lamb AL. Two structures of an N-hydroxylating flavoprotein monooxygenase: ornithine hydroxylase from Pseudomonas aeruginosa. J Biol Chem. 2011;286:31789–31798. doi: 10.1074/jbc.M111.265876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri L, Rottensteiner H, Girzalsky W, Scarcia P, Palmieri F, Erdmann R. Identification and functional reconstitution of the yeast peroxisomal adenine nucleotide transporter. EMBO J. 2001;20:5049–5059. doi: 10.1093/emboj/20.18.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott CC, Protchenko O. Response to iron deprivation in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7:20–27. doi: 10.1128/EC.00354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontecorvo G, Roper JA, Hemmons LM, Macdonald KD, Bufton AW. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- Punt PJ, Oliver RP, Dingemanse MA, Pouwels PH, van den Hondel CA. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene. 1987;56:117–124. doi: 10.1016/0378-1119(87)90164-8. [DOI] [PubMed] [Google Scholar]
- Reiser K, Davis MA, Hynes MJ. Aspergillus nidulans contains six possible fatty acyl-CoA synthetases with FaaB being the major synthetase for fatty acid degradation. Arch Microbiol. 2010;192:373–382. doi: 10.1007/s00203-010-0565-9. [DOI] [PubMed] [Google Scholar]
- van Roermund CW, Drissen R, van Den Berg M, Ijlst L, Hettema EH, Tabak HF, et al. Identification of a peroxisomal ATP carrier required for medium-chain fatty acid beta-oxidation and normal peroxisome proliferation in Saccharomyces cerevisiae. Mol Cell Biol. 2001;21:4321–4329. doi: 10.1128/MCB.21.13.4321-4329.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Schrader M, Fahimi HD. The peroxisome: still a mysterious organelle. Histochem Cell Biol. 2008;129:421–440. doi: 10.1007/s00418-008-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrettl M, Bignell E, Kragl C, Joechl C, Rogers T, Arst HN, Jr, et al. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J Exp Med. 2004;200:1213–1219. doi: 10.1084/jem.20041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrettl M, Bignell E, Kragl C, Sabiha Y, Loss O, Eisendle M, et al. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 2007;3:1195–1207. doi: 10.1371/journal.ppat.0030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrettl M, Kim HS, Eisendle M, Kragl C, Nierman WC, Heinekamp T, et al. SreA-mediated iron regulation in Aspergillus fumigatus. Mol Microbiol. 2008;70:27–43. doi: 10.1111/j.1365-2958.2008.06376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprote P, Brakhage AA, Hynes MJ. Contribution of peroxisomes to penicillin biosynthesis in Aspergillus nidulans. Eukaryot Cell. 2009;8:421–423. doi: 10.1128/EC.00374-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe Y, Maruyama J, Yamaoka S, Yahagi D, Matsuo I, Tsutsumi N, Kitamoto K. Peroxisomes are involved in biotin biosynthesis in Aspergillus and Arabidopsis. J Biol Chem. 2011;286:30455–30461. doi: 10.1074/jbc.M111.247338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekaia F, Latge JP. Aspergillus fumigatus: saprophyte or pathogen? Curr Opin Microbiol. 2005;8:385–392. doi: 10.1016/j.mib.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Tilburn J, Sarkar S, Widdick DA, Espeso EA, Orejas M, Mungroo J, et al. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 1995;14:779–790. doi: 10.1002/j.1460-2075.1995.tb07056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenciano S, Lucas JR, Pedregosa A, Monistrol IF, Laborda F. Induction of beta-oxidation enzymes and microbody proliferation in Aspergillus nidulans. Arch Microbiol. 1996;166:336–341. doi: 10.1007/s002030050392. [DOI] [PubMed] [Google Scholar]
- Vogel H. A convenient growth medium for Neurospora. Microb Genet Bull. 1956;13:42–46. [Google Scholar]
- Wallner A, Blatzer M, Schrettl M, Sarg B, Lindner H, Haas H. Ferricrocin, a siderophore involved in intra- and transcellular iron distribution in Aspergillus fumigatus. Appl Environ Microbiol. 2009;75:4194–4196. doi: 10.1128/AEM.00479-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg ED. Iron availability and infection. Biochim Biophys Acta. 2009;1790:600–605. doi: 10.1016/j.bbagen.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Winterberg B, Uhlmann S, Linne U, Lessing F, Marahiel MA, Eichhorn H, et al. Elucidation of the complete ferrichrome A biosynthetic pathway in Ustilago maydis. Mol Microbiol. 2010;75:1260–1271. doi: 10.1111/j.1365-2958.2010.07048.x. [DOI] [PubMed] [Google Scholar]
- Yasmin S, Alcazar-Fuoli L, Grundlinger M, Puempel T, Cairns T, Blatzer M, et al. Mevalonate governs interdependency of ergosterol and siderophore biosyntheses in the fungal pathogen Aspergillus fumigatus. Proc Natl Acad Sci USA. 2011;109:E497–E504. doi: 10.1073/pnas.1106399108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


