Abstract
The loss of cell volume, termed apoptotic volume decrease (AVD) has been a hallmark feature of apoptosis. However the role of this characteristic attribute of programmed cell death has always been questioned as to whether it plays an active or passive factor during apoptosis. Here we review studies that suggest that AVD plays an active role during apoptosis and the underlying flux of ions that results in this morphological event regulates the programmed cell death process.
Key Words: Cell Volume Regulation, Apoptotic Resistance, Osmotic stress, RVI
Lymphocyte Apoptosis
Apoptosis is a physiological series of cellular processes initiated by stimuli or signals that ultimately results in the death of individual cells within particular tissues. These apoptotic events permit the selective deletion of cells, leaving neighboring cells intact. This process is complementary, but opposing, to cell proliferation in the regulation of mammalian cell number homeostasis [1]. Apoptosis is characterized by a distinct set of morphological and biochemical characteristics that includes cell shrinkage, nuclear condensation, and internucleosomal DNA fragmentation [1, 2]. Additional features such as externalization of membrane phosphatidylserine, caspase activation, and mitochondrial membrane depolarization, along with the loss of cytochrome c from the mitochondria, have also been used to define and characterize this mode of cell death. During apoptosis, energy levels in the cell remain high and portions of the cell bud off in what has been termed apoptotic bodies. These apoptotic bodies are subsequently phagocytized by resident macrophages and/or neighboring cells permitting an efficient way for the body to rid itself of harmful cellular fragments in the absence of an inflammatory response. While it is thought that cells undergoing apoptosis follow a conserved, central pathway culminating in death, many of these apoptotic characteristics have been shown to be either stimulus or cell-type specific. However a remarkably constant feature in the apoptotic process is the loss of cell volume or cell shrinkage.
In vitro, and most likely in vivo, apoptosis occurs in both a time- and concentration-dependent manner. Individual cell types respond to diverse apoptotic stimuli in kinetically different ways in regards to the activation of the cell death program. For example, S49 mouse lymphoma cells were shown to undergo apoptosis with a 50% loss of cell viability within 10 hours upon increasing intracellular calcium using either A23187 or ionomycin [3]. In contrast, treatment of S49 cells with dexamethasone, a synthetic glucocorticoid, resulted in a drastically slower rate of cell death, achieving 50% viability only after 36 hours of treatment [3]. Despite the difference in response times to these stimuli, both the increase in intracellular calcium and glucocorticoid treatment of S49 cells resulted in the occurrence of internucleosomal DNA fragmentation [3]. Examination of signaling pathways involved in glucocorticoid-induced and survival factor withdrawal (spontaneous) apoptosis of primary rat thymocytes demonstrated that glucocorticoid-induced death required macromolecular synthesis along with caspase activity [4]. In contrast, cells deprived of survival factors did not require new gene expression or protein synthesis to die. These data illustrate the stochastic nature of apoptosis, where death occurs one cell at a time, and suggests that cells contain all the necessary machinery to undergo apoptosis without the need for additional RNA or protein synthesis.
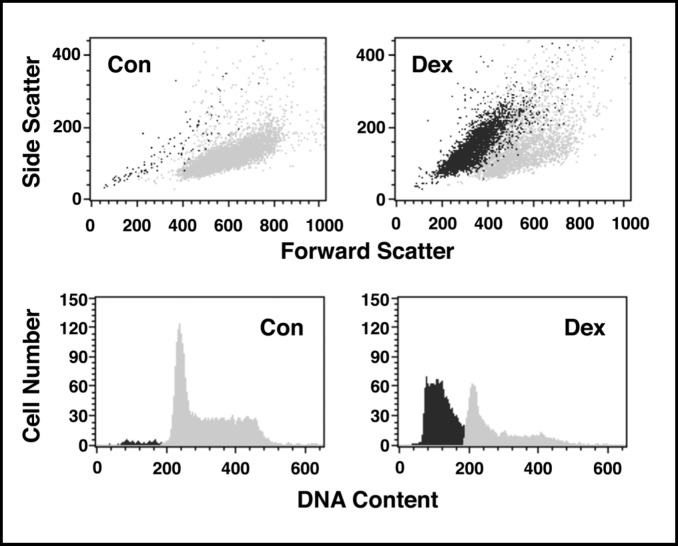
One of the most effective techniques to study this stochastic and dynamic apoptotic process is flow cytometry. Flow cytometry examines cells at the single cell level, one cell at a time. Moveover, flow cytometry couples the simultaneous examination of multiple characteristics of a single cell with the ability to study thousands of cells in a very short period of time. Our group was the first to use this technology to investigate glucocorticoid-induced apoptosis in thymocytes [5]. A quantitative reduction in acridine orange binding to DNA was observed in a subpopulation of dexamethasone-treated primary rat thymocytes suggesting DNA degradation occurs only in the fraction of cells undergoing apoptosis. This initial investigation was extended several years later when we showed by flow cytometry that DNA degradation during apoptosis occurs only in the shrunken population of cells [6]. This concept is illustrated in Figure 1 where using the light scattering properties of cells, in combination with the DNA content parameter using propidium iodide, flow cytometric studies show that the smaller, shrunken population of cells had degraded DNA, while the larger sized population of cells had a normal complement of DNA. Thus, flow cytometry has become a powerful tool in the study of apoptosis because of its ability to examine multiple cellular characteristics at the single cell level [7].
Fig. 1.
The shrunken population of cells has degraded DNA. S49 Neo cells were treated with dexamethasone (dex; 2.5 × 10-7 M) for 48 hours. Cells were fixed in 70% cold EtOH overnight, washed in 1X PBS, and stained with propidium iodide for DNA content. Control cells show a single population of cells on a forward-scatter versus side-scatter dot plot that have a normal DNA content. In contrast, cells treated with dex had two distinct populations of cells, with the smaller or shrunken cells having degraded their DNA.
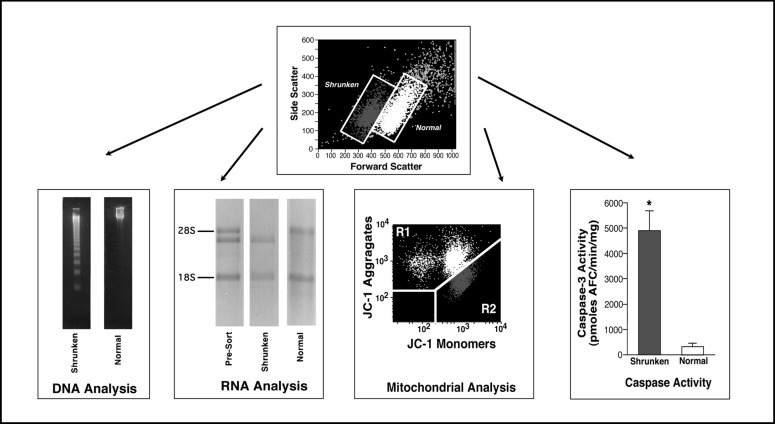
The loss of cell volume, or cell shrinkage, is a hallmark of apoptosis. Initially the term “shrinkage necrosis” was used to describe this physiological cell death based solely on cellular morphology [8, 9, 10]. The loss of cell volume as observed during apoptosis occurs under normotonic conditions. In 2000, Okada and colleagues [11] coined the term Apoptotic Volume Decrease (AVD) to distinguish the apoptotic loss of cell volume from cell shrinkage induced by anisotonic conditions. Many of the characteristics that occur during apoptosis are restricted to the shrunken population of cells. As illustrated in Figure 2, the relationship between the loss of cell volume and degraded DNA was confirmed by physically sorting the normal and shrunken populations of cells by flow cytometry and examining their DNA integrity by agarose gel eletrophoresis. Only the shrunken population of cells showed the ladder-like pattern of 180 to 200 base pair fragments, characteristic of apoptosis. Similarly, RNA degradation, specifically the cleavage of the 28S ribosomal RNA, is restricted to the shrunken population of apoptotic cells (Fig. 2). Additionally, analysis of the mitochondrial membrane potential with the dye JC-1 shows the loss of the mitochondrial membrane potential only in the shrunken population of cells (Fig. 2). Finally, caspase activity as assessed using a fluorometric assay on sorted normal and shrunken apoptotic cell populations also show that only the shrunken cells have activated caspases (Fig. 2). These data and other studies suggest that the characteristics of apoptosis are associated with the loss of cell volume [11, 12, 13].
Fig. 2.
Relationship between the apoptotic volume decrease and various characteristics associated with the apoptotic process. Physically sorted normal and shrunken populations of dex treated S49 Neo cells were examined for their DNA integrity by agarose gel eletrophoresis. Shrunken cells showed the presence of degraded DNA in the ladder like pattern of 180 to 200 bp fragments characteristic of apoptosis. In contrast, the normal or non-shrunken population of cells had not degraded their DNA. Specific cleavage of the 28S ribosomal RNA also occurs solely in the shrunken population of cells. Pre-sorted cells show both the intact and cleaved 28S ribosomal RNA, however only the sorted shrunken population of apoptotic cells has the cleaved RNA product, while the normal population of cells has an intact 28S ribosomal band. Furthermore, changes in the mitochondrial membrane potential were restricted to the shrunken, apoptotic cells. When the mitochondrial membrane potential dye (JC-1) is loaded into normal, healthy cells it forms aggregates with a high fluorescent emission in an aggregate state, shown as the light population of cells. When the mitochondrial membrane potential is lost, these aggregates form monomers, thus a loss of the aggregate fluorescence and an increase in the monomeric fluorescence is observed, shown as the dark population of cells. When this change in mitochondrial membrane potential is compared to the change in cell size, the shrunken, apoptotic cells have a decrease in the aggregate fluorescence and an increase in the monomeric fluorescence, suggesting that only the shrunken population of cells have lost their mitochondrial membrane potential. Finally, physically sorting the normal and shrunken cells and individually examining these cell populations for caspase activity using a fluorometric caspase assay again showed that an increase in caspase-3-like activity was restricted to the shrunken cells.
The loss of cell volume during apoptosis is thought to facilitate the breakdown of unwanted cells to form apoptotic bodies, aiding in the eventual engulfment of the dead or dying cells, as a smaller or shrunken cell would be easier to phagocytize than a larger cell. However, the question has always persisted as to whether cell shrinkage during apoptosis is an “active” or “passive” event. Specifically, is the loss of cell volume, or AVD, essential to the overall cell death program or is it a consequence of the apoptotic process when the cells are eliminated by resident macrophages? We have argued that cell shrinkage plays an active and critical role during apoptosis. Our current understanding of why and how a cell shrinks during apoptosis suggests that this simple morphological phenomenon plays an important role in both the activation and execution of the overall cell death program. Interestingly, it is not the morphological process of cell shrinkage that is important, but the underlying movement of intracellular ions that results in the observed loss of cell volume and coordination of the apoptotic killing machinery.
Cells are well known to compensate or acclimate to changes in their cell size through various volume regulatory mechanisms [14]. The majority of studies investigating inherent cell volume regulatory mechanisms use anisotonic conditions, either hypertonic or hypotonic medium, to evoke a change in cell size. Interestingly, during apoptosis the loss of cell volume occurs from an isotonic environment, without any osmotic change in the extracellular surroundings. Most cells have a high concentration of intracellular potassium and a low concentration of intracellular sodium. This condition is in contrast to the extracellular environment where there is a low concentration of potassium and a high concentration of sodium. This imposing imbalance of ions across the cell membrane, while setting up a concentration gradient for the loss of intracellular potassium and the gain of intracellular sodium, is maintained through the constitutive activity of various ionic transporters, primarily the Na+/ K+-ATPase [15]. This ionic gradient across the cell membrane aids in setting the plasma membrane potential and plays a critical role for the transport of various metabolites such as sugars and amino acids across the cell membrane.
Our understanding of how cells shrink during apoptosis stems from studies examining changes in the intracellular ionic composition of a cell. Over a decade ago, we showed using flow cytometry that a loss of both intracellular potassium and sodium occurs during apoptosis, prior to the loss of membrane integrity [16]. Additionally, preventing the loss of intracellular potassium using high extracellular potassium resulted in an inhibition of apoptosis, while depleting the cells of intracellular potassium enhanced the cell death program [16]. We further demonstrated that not only is there a loss of intracellular ions during apoptosis, but that this ionic change regulates the apoptotic process by controlling the activation of caspase 3 by cytochrome c and ATP [17]. Our studies revealed that both apoptotic nuclease activity and caspase activation were inhibited at normal, physiological levels of intracellular potassium. Subsequently, Cain et al [18] showed that oligomerization of Apaf-1 and the assembly of the apoptosome consisting of cytochrome c and caspase-9 are suppressed at normal levels of intracellular potassium, thus preventing the activation of caspase-9 during apoptosis. They also reported that elevated extracellular potassium inhibited apoptosis induced through either the intrinsic or extrinsic pathways, and prevented not only caspase activation and DNA degradation, but also the externalization of phosphatidylserine, mitochondrial depolarization, and cytochrome c release [19]. Since this time, many studies have supported the notion that a loss of intracellular ions regulates the apoptotic machinery during the cell death process [reviewed in 20-22].
While it is well established that the loss of intracellular ions, especially potassium, plays an important role during apoptosis, an additional ion flux was found to participate in the orchestration of the cell death program. Prior to the dramatic decrease in intracellular ions, a rapid increase in intracellular sodium was observed that resulted in a depolarization of the plasma membrane [23]. In a subsequent study, it was shown that this increase in intracellular sodium in apoptotic cells approaches approximately 114 mM early in the cell death process [24]. Interestingly in cells with an increase in intracellular sodium, a decrease in intracellular potassium to approximately 30 mM was also observed, suggesting an initial reversal of the normal intracellular cationic gradient in a dying cell [24]. Additionally, inhibition of the Na+/K+-ATPase was shown to occurr early during apoptosis, as a decrease in the alpha subunit and cleavage of the beta subunit of this protein were observed in anti-Fas treated Jurkat cells [23], suggesting that this transporter is an early target for inactivation. Furthermore, ouabain, a cardiac glycoside inhibitor of the Na+/K+-ATPase, potentiated Fas-induced apoptosis in Jurkat cells [23]. This marked inhibition of the Na+/K+-ATPase would account in part for an inability of Jurkat cells to repolarize in response to the increase in intracellular sodium. However, inhibition of this transporter could not completely account for the observed increase in intracellular sodium in apoptotic cells. In an earlier study, we showed that saxitoxin, a sodium channel blocker, could prevent the early increase in intracellular sodium [25], suggesting that the rise in sodium during apoptosis may occur by a combination of increased sodium channel activity and transporter inhibition. Additionally, inhibition of the Na+/ K+-ATPase during apoptosis would not only account for the observed increase in intracellular sodium and cellular depolarization, but also have an effect on potassium, since the exchange of sodium for potassium would not occur, eventually resulting in a decrease of intracellular potassium. Similar observations of plasma membrane depolarization during cell death were made in studies of glucocorticoid-induced apoptosis in primary rat thymocytes [26], where a specific role of plasma membrane depolarization as a signal for apoptosis was revealed. Subsequently, an early inhibition of the Na+/ K+-ATPase was shown in glucocorticoid and survival factor withdrawal induced apoptosis of primary rat thymocytes, an action that was enhanced by ouabain [27]. Again in these studies, cellular depolarization and loss of the Na+/K+-ATPase protein were restricted to the shrunken population of apoptotic cells.
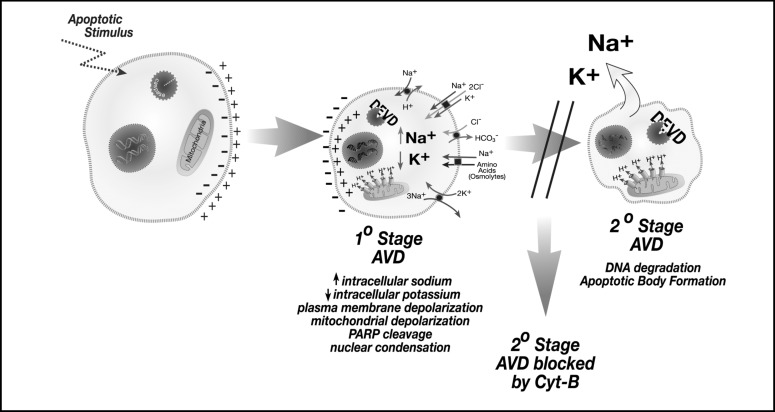
AVD is known to vary depending on both the apoptotic stimulus and cell type that is studied. For example, a 40% loss of water and 60% loss of intracellular potassium were shown to occur after a 2-hour staurosporine treatment of human lens epithelial cells [28]. However, a 6-hour cisplatin treatment of Ehrlich ascites tumor cells resulted in only a 30% loss of water [13]. Additionally, human eosinophils upon cytokine withdrawal required 48 hours to achieve a 63% reduction in cell volume [29]. Therefore, this variation in AVD during apoptosis may likely depend on the diverse ionic pathways that are employed in a stimulus and cell type specific manner. Insights into the relationship between AVD and intracellular ions were shown in a study examining the effect of cytoskeletal disruption in the apoptotic process [24]. In particular AVD was found to occur in two distinct stages as illustrated in Figure 3 [24]. The primary stage of AVD was characterized by the reversal of the normal intracellular sodium and potassium concentrations, externalization of phosphatidylserine, loss of mitochondrial membrane potential, caspase activation, and nuclear condensation. This primary stage of AVD resulted in a 20 to 40% decrease in cell volume. The secondary stage of AVD was characterized by the loss of intracellular sodium and potassium, DNA degradation, and apoptotic body formation that results in an 80 to 85% decrease in cell volume. The identification of discrete stages of AVD and their association with various apoptotic characteristics suggests that individual apoptotic events are linked to the unique ion fluxes that occur during the cell death process. Poulsen et al. [13] have also shown distinct stages of AVD in Ehrlich ascites tumor cells treated with cisplatin: an early AVD stage associated with 30% loss of cell water; a transition AVD stage where the cell volume is partly recovered; then a secondary or final AVD stage where the cell volume was further reduced. Additionally, early studies also suggested stages of AVD where a 25% decrease in cell size was observed in radiation-induced cell death of rat thymocytes, followed by a slower reduction in cell volume to 57% over time [30].
Fig. 3.
AV D occurs in distinct stages with specific apoptotic characteristics associated with each stage. During the primary stage of AVD, there is an increase in intracellular sodium coupled to a decrease in intracellular potassium, resulting in a 20 to 40% decrease in cell volume. During this stage, plasma membrane and mitochondrial depolarization is observed, along with caspase activity, and nuclear condensation. During the secondary stage of AVD, a loss of both intracellular sodium and potassium coupled to an 80 to 85% decrease in cell volume is observed. At this stage, DNA fragmentation and apoptotic body formation is observed. Disruption of the actin cytoskeleton with cytochalasin B prevents the secondary stage of AVD.
The work outlined above supports the idea that the loss of cell volume or AVD is an active participant in the cell death process. Thus, we hypothesize that the normal physiological concentrations of intracellular ions, specifically sodium and potassium, can act as a barrier to the initiation of apoptosis, preventing the unwanted activation of cell death program until a cell truly needs to die. For example, high concentrations of sodium inhibited in vitro apoptotic nuclease activity from glucocorticoid treated thymocyte extracts [31]. Additionally, repressed or latent internucleosomal cleavage activity was present in hepatocyte nuclear extracts, suggesting that the enzymes required for apoptosis are present in a cell and only need to be activated. Furthermore, physiological concentrations of potassium prevented caspase activation and subsequent apoptotic nuclease activity [17], and high extracellular potassium can block apoptosis by impeding the loss of this intracellular ion [16]. Cain and colleagues [18] suggested that the normal intracellular concentration of potassium also acts to safeguard cells from inappropriate formation of the apoptosome complex that may result from an inadvertent release of small amounts of cytochrome c. Moreover, a 5-fold increase in calcium was shown to be necessary to induce DNA degradation in the presence of physiological concentrations of potassium [32]. These data indicate that a normal ionic environment in healthy cells acts to repress the apoptotic machinery, suggesting an active role for AVD in the cell death process.
Although it is well established that many characteristics of apoptosis occur only in the shrunken population of apoptotic cells, the contribution of apoptotic proteases, caspases, to the AVD process has also been evaluated. Analysis of the relationship between cell shrinkage and ion efflux, along with changes in the mitochondria membrane potential, showed that these apoptotic events could be largely independent of caspase activity depending on the particular cell death signal [26]. For example, pan-caspase inhibition prevented all characteristics of apoptosis in Fas ligand treated Jurkat cells, however death induced by thapsigargin or the calcium ionophore A23187 resulted in cell shrinkage, potassium efflux, and loss of the mitochondrial membrane potential regardless of the inhibition of caspases [33]. Examination of the effects of UV-C-induced cell death in Jurkat cells showed that pan-caspase inhibition also blocked apoptosis, however specific caspase inhibitors were shown only to prevent caspase 3 and 8 processing, Bid cleavage, along with apoptotic DNA degradation [34]. This data in lymphocytes also revealed that both intrinsic and extrinsic apoptotic pathways are activated upon UV-induced cell death, and this redundancy ensures the death of a cell even during selective protease inhibition. Additionally, using lymphoid cells genetically deficient for caspase 8 or stably transfected with a dominant-negative mutant of caspase 9, helped distinguish that caspase 9 was critically important for the intrinsic pathway, while caspase 8 was necessary for the extrinsic pathway [35]. However, both cell types could undergo apoptosis with a loss of cell volume and efflux of intracellular potassium when given an appropriate signal. Together, these data suggest that AVD is highly dependent on the death-inducing stimulus, regardless of the presence or absence of various caspases.
Similar to the discriminating role of caspases in regards to AVD and the efflux of intracellular ions, chloride as a counter ion to sodium and potassium has been shown to play a critical, but selective role during apoptosis. Ion currents characteristic of volume-sensitive outwardly rectifying chloride channels were shown to be rapidly activated during both staurosporine and Fas ligand induced apoptosis in HeLa cells [36]. Induction of apoptosis via UV-C, but not Fas ligand, has also been linked to chloride flux where inhibition of chloride channels using SITS or reduced chloride medium resulted in diminished downstream apoptotic characteristics in Jurkat cells upon UV-C or intrinsic apoptotic stimuli, but not with extrinsic stimuli such as Fas ligand [37]. In a study of doxorubicin-induced AVD in cardiomyocytes, chloride channel inhibitors NPPB and IAA-94 were shown to prevent apoptosis, suggesting a critical role for anion conductance during programmed cell death [38]. Furthermore, NS3728, a high-affinity inhibitor of both volume-regulated anion channels and calcium-activated chloride channels, was shown to abolish AVD, ionic flux, and caspase-3 activation in cisplatin-induced Ehrlich ascites tumor cells [13].
The flux of ions, specifically chloride, has also been shown to play a role in other signaling cascades. For example, chloride ion flux specifically regulated UV-C induced activation of the kinase-signaling cascade in Jurkat cells, upstream of the mitochondria as phosphorylation of JNK and MKK4 were observed [37]. This phosphorylation of JNK and MKK4 were inhibited by the chloride channel blocker SITS and under the condition of reduced chloride. While chloride channel blockers can regulate the kinase signaling that occurs upon intrinsically induced cell death, it was shown that PKC stimulation provided protection during extrinsically induced apoptosis in Jurkat T-cells [39]. Activation of PKC with phorbol esters prevented not only cell shrinkage and potassium efflux in anti-Fas treated Jurkat cells, but also the caspase-dependent isotypic-specific cleavage of PKC [39]. Overall these studies define the complex signaling networks that are apoptotic pathway specific. However en mass, these data also support the idea that AVD is an intricate part of the cell death program.
As outlined above, the intracellular ionic environment plays a pivotal role in the progression of apoptosis. However, additional changes in the cytosolic milieu of cells, such as changes in the redox potential, can also play an important role in setting up a permissive apoptotic environment [40]. Glutathione (GSH) loss has been known to be an early hallmark in the progression of apoptosis in response to a variety of apoptotic stimuli [41, 42, 43]. GSH is the predominant low molecular weight thiol in animal cells that participates in many cellular reactions including antioxidant defense, drug detoxification, and cell signaling. Therefore, GSH is an essential molecule for cell survival that is largely depleted during the programmed cell death. This reduction of GSH is thought to be an active process, being transported out of the cell rather than depleted through the eventual loss of plasma membrane integrity. Only recently has our understanding of GSH loss from the cell come to light. Franco and Cidlowski [44] showed that GSH export in Fas ligand stimulated Jurkat cells occurs via the organic anion-transporting polypeptide proteins (OATP) encoded by SLCO genes. Interestingly, high extracellular GSH protected cells from undergoing apoptosis, and subsequent data revealed that GSH export occurred independent of the generation of reactive oxygen species [45].
As GSH loss was known to be an important component of apoptosis [41, 42, 43], the question remained as to its relationship to AVD and ion flux. In the apoptotic model of Fas ligand treated Jurkat cells, GSH depletion was paralleled by distinct degrees (or stages) of cell shrinkage [46]. These stages of AVD were similar to those defined in a previous study on the effects of cytoskeleton disruption and apoptosis [24]. In this regard, GSH depletion was shown to be necessary for the ionic imbalances observed during apoptosis [46]. High extracellular GSH prevented Fas ligand induced potassium loss, while stimulation of GSH export resulted in accelerated potassium depletion. These findings indicate that GSH efflux modulates the progression of the execution phase of apoptosis, in part through its ability to modulate potassium flux during the cell death process. While the exact mechanism regulating GSH loss, specifically in regards to OATP activation during apoptosis is currently unknown, the ionic imbalance that results in AVD may play a critical role in regulating this important thiol in animal cells.
Thus AVD is not simply the loss of cell volume, but a critical underlying flux of ions that results in the morphological change in cell size. For example, when the major sodium constituents of the extracellular medium were substituted for non-permeable, non-sodium components, cells induced to undergo apoptosis actually swelled during the cell death process [24]. This cellular swelling has been traditionally a characteristic of the cell death process known as necrosis. However, these swollen cells displayed many classical features of apoptosis including chromatin condensation, externalization of phosphatidylserine, caspase activity, and internucleosomal DNA degradation. These swollen cells also showed a marked decrease in intracellular potassium, and inhibition of this potassium loss completely blocked apoptosis. Provocatively, reintroduction of sodium ions to the cells reversed this cellular swelling, as a loss of cell volume and the characteristic apoptotic morphology were observed [24]. This data suggests that sodium influx controls the change in cell size and the activation of apoptosis, while potassium loss controls the progression of the cell death process.
Hindering our knowledge of AVD and cell volume regulation during apoptosis has been the lack of genetic models to understand the interplay between these events. As outlined above, we and others have shown the importance of regulatory cell volume mechanisms in protecting cells from adverse effects resulting in a change in cell size [6, 47, 48, 49, 50]. However, how a cell perceives and responds to a change in cell size in an anisotonic environment is not understood. Additionally, a complete understanding of how a cell shrinks from an isotonic environment as occurs during apoptosis is equally unclear. While it is understood that increased permeabilities for sodium, potassium, and chloride occur during apoptosis, the specific ionic transport mechanisms that are involved for each cell type and apoptotic stimulus are not known. To address this issue, we have recently developed a model system taking advantage of the fact that lymphocytes, specifically T-cells, are devoid of an initial regulatory volume increase (RVI) response. While these cells do not respond with an RVI upon hyperosmotic stress, it has been shown that they contain the necessary machinery to undergo this response. If T-cells initially acclimated in a hypotonic environment via a regulatory volume decrease (RVD) response, are then transferred back to an isotonic condition, the cells now sense this change as a hypertonic condition where they will shrink and volume regulate, via an RVI, back to a near normal cell size [51]. This RVI after RVD is a common response mechanism in cells that do not have a direct RVI response [11]. Murine immature T-cells (S49 Neo) undergo rapid apoptosis upon hyperosmotic stress [6]. These S49 Neo cells not only undergo a rapid death response to this osmotic stress, but the response is very synchronous compared to the stochastic nature of most cells to other apoptotic stimuli (unpublished data).
Using the S49 (Neo) cells as a model, we exposed these cells to multiple rounds of hyperosmotic stress, followed by a period of recovery under normal culture conditions (unpublished data). During these rounds of osmotic stress, greater than 95% of the cells died, however a small percentage survived and could be recovered as a viable population of cells. After 15 rounds of treatment and recovery, we obtained cells that were resistant to apoptosis induced by the intrinsic cell death pathway, while remaining sensitive to stimuli that acted through the extrinsic or death-receptor pathway. Interestingly, these cells acquired an inherent RVI response to acute hyperosmotic stress, and inhibition of this response sensitized these cells to undergo apoptosis (unpublished data). This investigation not only illustrates the protective nature of cell volume regulatory mechanisms in apoptosis, but also provides the first genetic model to study AVD and cell volume regulation.
Thus the initial observation by Kerr in 1971 [9] of “shrinkage necrosis” has evolved over the years into an entire field of study known as apoptosis. While many of the biochemical and signaling pathways have been elucidated defining this programmed cell death process, the idea that the cells shrink from an isotonic environment has remained a defining characteristic of apoptosis. Studies over the past 10 to 15 years have shown that AVD is not just a passive or secondary event during apoptosis, but plays an essential role in orchestrating the cell death process. The underlying flux of ions that results in this visual and distinctive characteristic has been shown to allow for a permissive environment that coordinates the apoptotic machinery. However, the impact on apoptosis of a cells ability to combat a change in cell size through inherent volume regulatory mechanisms can lead to the failure of cell death and the lingering existence of unwanted cells. There are still many questions to answer, but it is clear that volume regulation is an important component of life and death decisions.
Acknowledgements
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
References
- 1.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Cruchten S, Van Den Broeck W. Morphological and biochemical aspects of apoptosis, oncosis and necrosis. Anat Histol Embryol. 2002;31:214–223. doi: 10.1046/j.1439-0264.2002.00398.x. [DOI] [PubMed] [Google Scholar]
- 3.Caron-Leslie L-AM, Cidlowski JA. Similar actions of glucocorticoids and calcium on the regulation of apoptosis in S49 cells. Mol Endocrinol. 1991;5:1169–1179. doi: 10.1210/mend-5-8-1169. [DOI] [PubMed] [Google Scholar]
- 4.Mann CL, Hughes FM, Jr, Cidlowski JA. Delination of the signaling pathways involved in glucocorticoid-induced and spontaneous apoptosis of rat thymocytes. Endocrinol. 2000;141:528–538. doi: 10.1210/endo.141.2.7314. [DOI] [PubMed] [Google Scholar]
- 5.Compton MM, Haskill JS, Cidlowski JA. Analysis of glucocorticoid actions on rat thymocyte deoxyribonucleic acid by fluorescence-activated flow cytometry. Endocrinol. 1988;122:2158–2164. doi: 10.1210/endo-122-5-2158. [DOI] [PubMed] [Google Scholar]
- 6.Bortner CD, Cidlowski JA. Absence of volume regulatory mechanisms contributes to the rapid activation of apoptosis in thymocytes. Am J Physiol. 1996;271:C950–C961. doi: 10.1152/ajpcell.1996.271.3.C950. [DOI] [PubMed] [Google Scholar]
- 7.Telford WG, Komoriya A, Packard BZ, Bagwell CB. Multiparametric analysis of apoptosis by flow cytometry. Methods Mol Biol. 2011;699:203–227. doi: 10.1007/978-1-61737-950-5_10. [DOI] [PubMed] [Google Scholar]
- 8.Kerr JFR. A histochemical study of hypertrophy and ischemic injury of rat liver with special reference to changes in lysosomes. J Path Bact. 1967;90:419–435. doi: 10.1002/path.1700900210. [DOI] [PubMed] [Google Scholar]
- 9.Kerr JFR. Shrinkage necrosis: a distinct mode of cellular death. J Path. 1971;105:13–20. doi: 10.1002/path.1711050103. [DOI] [PubMed] [Google Scholar]
- 10.Kerr JFR. Shrinkage necrosis of adrenal cortical cells. J Path. 1972;107:217–219. doi: 10.1002/path.1711070309. [DOI] [PubMed] [Google Scholar]
- 11.Maeno E, Ishizaki Y, Kanaseki T, Hazama A, Okada Y. Normotonic cell shrinkage because of disordered volume regulation is an early prerequisite to apoptosis. Proc Natl Acad Sci USA. 2000;97:9487–9492. doi: 10.1073/pnas.140216197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ernest NJ, Habela CW, Sontheimer H. Cytoplasmic condensation is both necessary and sufficient to induce apoptotic cell death. J Cell Sci. 2008;121:290–297. doi: 10.1242/jcs.017343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poulsen KA, Andersen EC, Hansen CF, Klausen TK, Hougaard C, Lambert IH, Hoffmann EK. Deregulation o apoptotic volume decrease and ionic movements in multidrug-resistant tumor cells: role of chloride channels. Am J Physiol. 2010;298:C14–C25. doi: 10.1152/ajpcell.00654.2008. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann EK, Lambert IH, Pedersen SF. Physiology of cell volume regulation in vertebrates. Physiol Rev. 2009;89:193–277. doi: 10.1152/physrev.00037.2007. [DOI] [PubMed] [Google Scholar]
- 15.Panayiotidis MI, Bortner CD, Cidlowski JA. On the mechanisms of ionic regulation of apoptosis: would the Na+/ K+-ATPase please stand up? Acta Physiol. 2006;187:205–215. doi: 10.1111/j.1748-1716.2006.01562.x. [DOI] [PubMed] [Google Scholar]
- 16.Bortner CD, Hughes FM, Jr, Cidlowski JA. A primary role for K+ and Na+ efflux in the activation of apoptosis. J Biol Chem. 1997;272:32436–32442. doi: 10.1074/jbc.272.51.32436. [DOI] [PubMed] [Google Scholar]
- 17.Hughes FM, Jr, Bortner CD, Purdy GD, Cidlowski JA. Intracellular K+ suppresses the activation of apoptosis in lymphocytes. J Biol Chem. 1997;272:30567–30576. doi: 10.1074/jbc.272.48.30567. [DOI] [PubMed] [Google Scholar]
- 18.Cain K, Langlais C, Sun XM, Brown DG, Cohen GM. Physiological concentrations of K+ inhibit cytochrome c-dependent formation of the apoptosome. J Biol Chem. 2001;276:41985–41990. doi: 10.1074/jbc.M107419200. [DOI] [PubMed] [Google Scholar]
- 19.Thompson GJ, Langlais C, Cain K, Conley EC, Cohen GM. Elevated extracellular [K+] inhibits death-receptor-and chemical-mediated apoptosis prior to caspase activation and cytochrome c release. Biochem J. 2001;357:137–145. doi: 10.1042/0264-6021:3570137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu SP, Canzoniero LM, Choi DW. Ion homeostasis and apoptosis. Curr Opin Cell Biol. 2001;13:405–411. doi: 10.1016/s0955-0674(00)00228-3. [DOI] [PubMed] [Google Scholar]
- 21.Lang F, Shumilina E, Ritter M, Gulbins E, Vereninov A, Huber SM. Ion channels and cell volume in the regulation of cell proliferation and apoptotic cell death. Contrib Nephrol. 2006;152:142–160. doi: 10.1159/000096321. [DOI] [PubMed] [Google Scholar]
- 22.Bortner CD, Cidlowski JA. Cell shrinkage and monovalent cation fluxes: role in apoptosis. Arch Biochem Biophys. 2007;462:176–188. doi: 10.1016/j.abb.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bortner CD, Gomez-Angelats M, Cidlowski JA. Plasma membrane depolarization with repolarization in an early molecular event in anti-Fas-induced apoptosis. J Biol Chem. 2001;276:4304–4314. doi: 10.1074/jbc.M005171200. [DOI] [PubMed] [Google Scholar]
- 24.Bortner CD, Sifre MI, Cidlowski JA. Cationic gradient reversal and cytoskeleton-independent volume regulatory pathways define and early stage of apoptosis. J Biol Chem. 2008;283:7219–7229. doi: 10.1074/jbc.M707809200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bortner CD, Cidlowski JA. Uncoupling cell shrinkage from apoptosis reveals that Na+ influx is required for volume loss during programmed cell death. J Biol Chem. 2003;278:39176–39184. doi: 10.1074/jbc.M303516200. [DOI] [PubMed] [Google Scholar]
- 26.Mann CL, Cidlowski JA. Glucocorticoids regulate plasma membrane potential during rat thymocyte apoptosis in vivo and in vitro. Endocrinol. 2001;142:421–429. doi: 10.1210/endo.142.1.7904. [DOI] [PubMed] [Google Scholar]
- 27.Mann CL, Bortner CD, Jewell CM, Cidlowski JA. Glucocorticoid-induced plasma membrane depolarization during thymocyte apoptosis: Association with cell shrinkage and degradation of the Na+/ K+-Adenosine Triphosphatase. Endocrinol. 2001;142:5059–5068. doi: 10.1210/endo.142.12.8516. [DOI] [PubMed] [Google Scholar]
- 28.Chimote AA, Adragna NC, Lauf PK. Ion transport in a human lens epithelial cell line exposed to hyposmotic and apoptotic stress. J Cell Physiol. 2010;223:110–122. doi: 10.1002/jcp.22015. [DOI] [PubMed] [Google Scholar]
- 29.Beauvais F, Michel L, Dubertret L. Human eosinophils in culture undergo a striking and rapid shrinkage during apoptotis. Role of K+ channels. J Leukoc Biol. 1995;57:851–855. doi: 10.1002/jlb.57.6.851. [DOI] [PubMed] [Google Scholar]
- 30.Klassen NV, Walker PR, Ross CK, Cygler J, Lach B. Two-stage cell shrinkage and the OER for radiation-induced apoptosis of rat thymocytes. Int J Radiat Biol. 1993;64:571–581. doi: 10.1080/09553009314551791. [DOI] [PubMed] [Google Scholar]
- 31.Schwartzman RA, Cidlowski JA. Mechanism of tissue-specific induction of internucleosomal deoxyribonucleic acid cleavage activity and apoptosis by glucocorticoids. Endocrinol. 1993;133:591–599. doi: 10.1210/endo.133.2.8393769. [DOI] [PubMed] [Google Scholar]
- 32.Ajiro K, Bortner CD, Westmoreland J, Cidlowski JA. An endogenous calcium-dependent, caspase-independent intranuclear degradation pathway in thymocyte nuclei: antagonism by physiological concentration of K+ ions. Exp Cell Res. 2008;314:1237–1249. doi: 10.1016/j.yexcr.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bortner CD, Cidlowski JA. Caspase independent/dependent regulation of K+, cell shrinkage, and mitochondrial membrane potential during lymphocyte apoptosis. J Biol Chem. 1999;274:21953–21962. doi: 10.1074/jbc.274.31.21953. [DOI] [PubMed] [Google Scholar]
- 34.Scoltock AB, Cidlowski JA. Activation of intrinsic and extrinsic pathways in apoptotic signaling during UV-C-induced death of Jurkat cells: the role of caspase inhibition. Exp Cell Res. 2004;297:212–223. doi: 10.1016/j.yexcr.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 35.Vu CCQ, Bortner CD, Cidlowski JA. Differential involvement of initiator caspases in apoptotic volume decrease and potassium efflux during Fas- and UV-induced cell death. J Biol Chem. 2001;276:37602–37611. doi: 10.1074/jbc.M104810200. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu T, Numata T, Okada Y. A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl-channel. Proc Natl Acad Sci USA. 2004;101:6770–6773. doi: 10.1073/pnas.0401604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heimlich G, Cidlowski JA. Selective role of intracellular chloride in the regulation of the intrinsic but not extrinsic pathway of apoptosis in Jurkat T-cells. J Biol Chem. 2006;281:2232–2241. doi: 10.1074/jbc.M507367200. [DOI] [PubMed] [Google Scholar]
- 38.d’ Anglemont de Tassigny A, Souktani R, Henry P, Ghaleh B, Berdeaux A. Volumesensitive chloride channels (ICl, vol) mediate doxorubincin-induced apoptosis through apoptotic volume decrease in cardiomyocytes. Fundam Clin Pharmacol. 2004;18:531–538. doi: 10.1111/j.1472-8206.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Angelats M, Bortner CD, Cidlowski JA. Protein kinase C (PKC) inhibits Fas receptor-induced apoptosis through modulation of the loss of K+ and cell shrinkage. J Biol Chem. 2001;276:44944–44952. doi: 10.1074/jbc.M909563199. [DOI] [PubMed] [Google Scholar]
- 40.Franco R, Cidlowski JA. Apoptosis and glutathione: beyond and antioxidant. Cell Death Differ. 2009;16:1303–1314. doi: 10.1038/cdd.2009.107. [DOI] [PubMed] [Google Scholar]
- 41.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Circu ML, Aw TY. Glutathione and apoptosis. Free Radic Res. 2008;42:689–706. doi: 10.1080/10715760802317663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franco R, Schoneveld OJ, Pappa A, Panayiotidis MI. The central role of glutathione in the pathophysiology of human diseases. Arch Physiol Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 44.Franco R, Cidlowski JA. SLCO/OATP-like transport of glutathione in FasL-induced apoptosis. J Biol Chem. 2006;281:29542–29557. doi: 10.1074/jbc.M602500200. [DOI] [PubMed] [Google Scholar]
- 45.Franco R, Panayiotidis MI, Cidlowski JA. Glutathione depletion is necessary for apoptosis in lymphoid cell independent of reactive oxygen species formation. J Biol Chem. 2007;282:30452–30465. doi: 10.1074/jbc.M703091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franco R, DeHaven WI, Sifre MI, Bortner CD, Cidlowski JA. Glutathione depletion and disruption of intracellular ionic homeostasis regulate lymphoid cell apoptosis. J Biol Chem. 2008;283:36071–36087. doi: 10.1074/jbc.M807061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 48.Okada Y. Ion channels and transporters involved in cell volume regulation and sensor mechanisms. Cell Biochem Biophys. 2004;41:233–258. doi: 10.1385/CBB:41:2:233. [DOI] [PubMed] [Google Scholar]
- 49.Maeno E, Takahashi N, Okada Y. Dysfunction of regulatory volume increase is a key component of apoptosis. FEBS Lett. 2006;580:6513–6517. doi: 10.1016/j.febslet.2006.10.074. [DOI] [PubMed] [Google Scholar]
- 50.Subramanyam M, Takahashi N, Hasegawa Y, Mohri T, Okada Y. Inhibition of protein kinase Akt1 by apoptosis signal-regulating kinase-1 (ASK1) is involved in apoptotic inhibition of regulatory volume increase. J Biol Chem. 2010;285:6109–6117. doi: 10.1074/jbc.M109.072785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grinstein S, Clarke CA, Rothstein A. Activation of Na+/H+ exchange in lymphocytes by osmotically induced volume changes and by cytoplasmic acidification. J Gen Physiol. 1983;82:619–638. doi: 10.1085/jgp.82.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]