Abstract
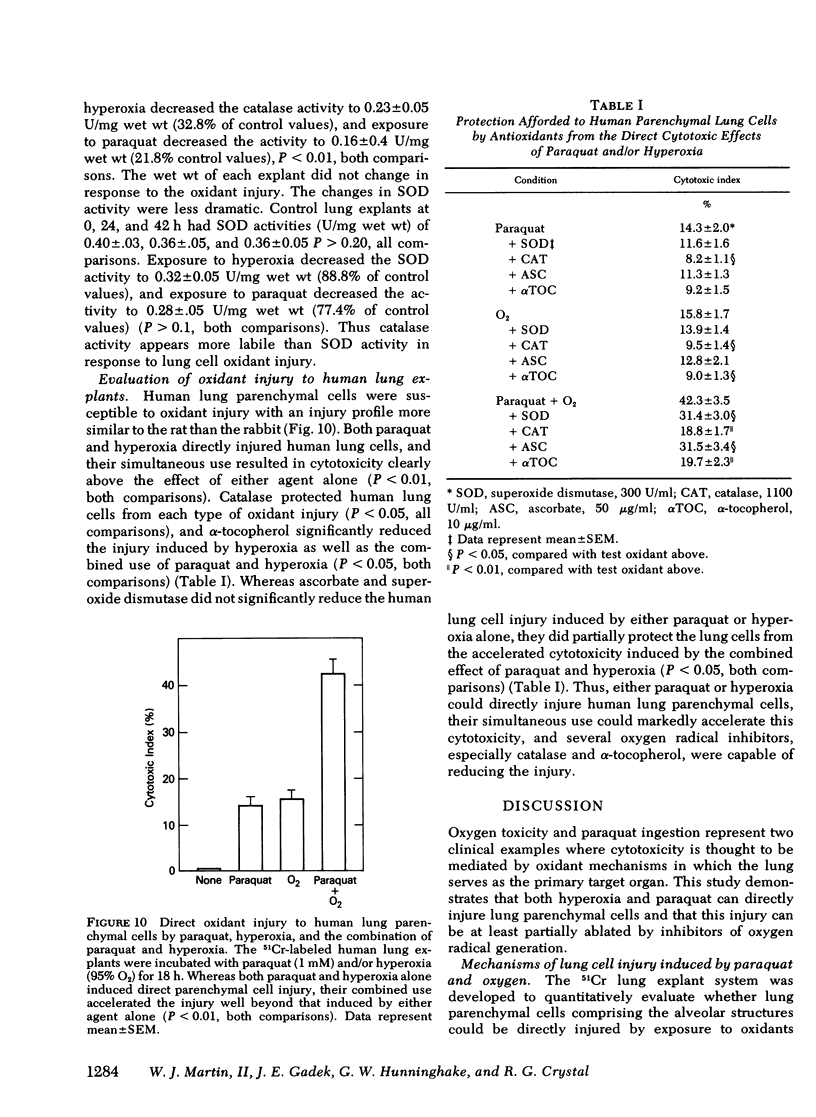
Hyperoxia and paraquat ingestion are two clinical examples of lung injury thought to be mediated by oxidant mechanisms. An in vitro cytotoxicity assay using freshly explanted 51Cr-labeled lung tissue as the target was used to quantify the ability of hyperoxia and paraquat to directly injure lung parenchymal cells in an environment where indirect mechanisms such as recruitment of inflammatory cells were not possible. There are clear species differences in the susceptibility of lung parenchyma to direct injury by hyperoxia (95% O2) and paraquat (10 microM--10 mM) for 18 h at 37 degrees C, with human and rat lung being more sensitive than rabbit lung. Oxygen radical inhibitors, particularly catalase (1,100 U/ml) and alpha-tocopherol (10 micrograms/ml), reduced hyperoxia and paraquat-induced lung injury, although their ability to do so depended on the oxidant and the species. The simultaneous use of hyperoxia and paraquat accelerated the in vitro lung parenchymal cell injury in each species tested. These studies demonstrate that both oxygen and paraquat can directly injure the cells of the lower respiratory tract without enlisting the aid of additional blood-derived inflammatory cells. In addition, the 51Cr-labeled lung explant assay used for these studies allows for the quantitative assessment of direct lung cell injury and thus may prove useful as an in vitro model by which to investigate lung injury of other etiologies.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., Kipnes R. S. Biological defense mechanisms. Evidence for the participation of superoxide in bacterial killing by xanthine oxidase. J Lab Clin Med. 1975 Feb;85(2):235–244. [PubMed] [Google Scholar]
- Bienkowski R. S., Cowan M. J., McDonald J. A., Crystal R. G. Degradation of newly synthesized collagen. J Biol Chem. 1978 Jun 25;253(12):4356–4363. [PubMed] [Google Scholar]
- Bradley K. H., McConnell S. D., Crystal R. G. Lung collagen composition and synthesis. Characterization and changes with age. J Biol Chem. 1974 May 10;249(9):2674–2683. [PubMed] [Google Scholar]
- Bus J. S., Aust S. D., Gibson J. E. Superoxide- and singlet oxygen-catalyzed lipid peroxidation as a possible mechanism for paraquat (methyl viologen) toxicity. Biochem Biophys Res Commun. 1974 Jun 4;58(3):749–755. doi: 10.1016/s0006-291x(74)80481-x. [DOI] [PubMed] [Google Scholar]
- Butler C., 2nd, Kleinerman J. Paraquat in the rabbit. Br J Ind Med. 1971 Jan;28(1):67–71. doi: 10.1136/oem.28.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. G., McElligott T. F., Hurst E. W. The toxicity of paraquat. Br J Ind Med. 1966 Apr;23(2):126–132. doi: 10.1136/oem.23.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. M., Lambertsen C. J. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971 Jun;23(2):37–133. [PubMed] [Google Scholar]
- Crapo J. D., Barry B. E., Foscue H. A., Shelburne J. Structural and biochemical changes in rat lungs occurring during exposures to lethal and adaptive doses of oxygen. Am Rev Respir Dis. 1980 Jul;122(1):123–143. doi: 10.1164/arrd.1980.122.1.123. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Sjostrom K., Drew R. T. Tolerance and cross-tolerance using NO2 and O2. I. Toxicology and biochemistry. J Appl Physiol Respir Environ Exerc Physiol. 1978 Mar;44(3):364–369. doi: 10.1152/jappl.1978.44.3.364. [DOI] [PubMed] [Google Scholar]
- Crapo J. D., Tierney D. F. Superoxide dismutase and pulmonary oxygen toxicity. Am J Physiol. 1974 Jun;226(6):1401–1407. doi: 10.1152/ajplegacy.1974.226.6.1401. [DOI] [PubMed] [Google Scholar]
- Drew W. L., Mintz L., Hoo R., Finley T. N. Growth of herpes simplex and cytomegalovirus in cultured human alveolar macrophages. Am Rev Respir Dis. 1979 Feb;119(2):287–291. doi: 10.1164/arrd.1979.119.2.287. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz R. A., Ablow R. C., Warshaw J. B. Prevention of bronchopulmonary dysplasia with vitamin E administration during the acute stages of respiratory distress syndrome. J Pediatr. 1979 Nov;95(5 Pt 2):873–878. doi: 10.1016/s0022-3476(79)80457-6. [DOI] [PubMed] [Google Scholar]
- Ehrenkranz R. A., Bonta B. W., Ablow R. C., Warshaw J. B. Amelioration of bronchopulmonary dysplasia after vitamin E administration. A preliminary report. N Engl J Med. 1978 Sep 14;299(11):564–569. doi: 10.1056/NEJM197809142991102. [DOI] [PubMed] [Google Scholar]
- Farrell P. M., Bieri J. G. Megavitamin E supplementation in man. Am J Clin Nutr. 1975 Dec;28(12):1381–1386. doi: 10.1093/ajcn/28.12.1381. [DOI] [PubMed] [Google Scholar]
- Feeney L., Berman E. R. Oxygen toxicity: membrane damage by free radicals. Invest Ophthalmol. 1976 Oct;15(10):789–792. [PubMed] [Google Scholar]
- Fisher H. K., Clements J. A., Wright R. R. Enhancement of oxygen toxicity by the herbicide paraquat. Am Rev Respir Dis. 1973 Feb;107(2):246–252. doi: 10.1164/arrd.1973.107.2.246. [DOI] [PubMed] [Google Scholar]
- Frank L., Bucher J. R., Roberts R. J. Oxygen toxicity in neonatal and adult animals of various species. J Appl Physiol Respir Environ Exerc Physiol. 1978 Nov;45(5):699–704. doi: 10.1152/jappl.1978.45.5.699. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Byers L. A., Finley T. N. Proliferative capacity of human alveolar macrophage. Nature. 1974 Feb 8;247(5440):373–375. doi: 10.1038/247373a0. [DOI] [PubMed] [Google Scholar]
- Golde D. W., Finley T. N., Cline M. J. The pulmonary macrophage in acute leukemia. N Engl J Med. 1974 Apr 18;290(16):875–878. doi: 10.1056/NEJM197404182901603. [DOI] [PubMed] [Google Scholar]
- Guerrero R. R., Rounds D. E., Booher J. An improved organ culture method for adult mammalian lung. In Vitro. 1977 Aug;13(8):517–524. doi: 10.1007/BF02615145. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Biochemical mechanisms accounting for the toxic action of oxygen on living organisms: the key role of superoxide dismutase. Cell Biol Int Rep. 1978 Mar;2(2):113–128. doi: 10.1016/0309-1651(78)90032-2. [DOI] [PubMed] [Google Scholar]
- Harris M. J., Herp A., Pigman W. Depolymerization of polysaccharides through the generation of free radicals at a platinum surface: a novel procedure for the controlled production of free-radical oxidations. Arch Biochem Biophys. 1971 Feb;142(2):615–622. doi: 10.1016/0003-9861(71)90526-1. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Paraquat and Escherichia coli. Mechanism of production of extracellular superoxide radical. J Biol Chem. 1979 Nov 10;254(21):10846–10852. [PubMed] [Google Scholar]
- Heikkila R. E., Cohen G. 6-Hydroxydopamine: evidence for superoxide radical as an oxidative intermediate. Science. 1973 Aug 3;181(4098):456–457. doi: 10.1126/science.181.4098.456. [DOI] [PubMed] [Google Scholar]
- Hoidal J. R., Beall G. D., Repine J. E. Production of hydroxyl radical by human alveolar macrophages. Infect Immun. 1979 Dec;26(3):1088–1092. doi: 10.1128/iai.26.3.1088-1092.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitt M. K. Therapeutic uses of vitamin E in medicine. Nutr Rev. 1980 Mar;38(3):105–113. doi: 10.1111/j.1753-4887.1980.tb05860.x. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Immunological reactivity of the lung II. Cytotoxic effector function of pulmonary mononuclear cell subpopulations. Cell Immunol. 1976 Sep;26(1):98–104. doi: 10.1016/0008-8749(76)90351-8. [DOI] [PubMed] [Google Scholar]
- Johnson L., Schaffer D., Boggs T. R., Jr The premature infant, vitamin E deficiency and retrolental fibroplasia. Am J Clin Nutr. 1974 Oct;27(10):1158–1173. doi: 10.1093/ajcn/27.8.1158. [DOI] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. 1977 Oct 10;252(19):6721–6728. [PubMed] [Google Scholar]
- Kimball R. E., Reddy K., Peirce T. H., Schwartz L. W., Mustafa M. G., Cross C. E. Oxygen toxicity: augmentation of antioxidant defense mechanisms in rat lung. Am J Physiol. 1976 May;230(5):1425–1431. doi: 10.1152/ajplegacy.1976.230.5.1425. [DOI] [PubMed] [Google Scholar]
- Lucy J. A. Functional and structural aspects of biological membranes: a suggested structural role for vitamin E in the control of membrane permeability and stability. Ann N Y Acad Sci. 1972 Dec 18;203:4–11. doi: 10.1111/j.1749-6632.1972.tb27849.x. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Day E. D., Jr Superoxide-dependent production of hydroxyl radical catalyzed by iron-EDTA complex. FEBS Lett. 1978 Feb 1;86(1):139–142. doi: 10.1016/0014-5793(78)80116-1. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The biology and pathology of oxygen radicals. Ann Intern Med. 1978 Jul;89(1):122–127. doi: 10.7326/0003-4819-89-1-122. [DOI] [PubMed] [Google Scholar]
- Menzel D. B., Roehm J. N., Lee S. D. Vitamin E: the biological and environmental antioxidant. J Agric Food Chem. 1972 May-Jun;20(3):481–486. doi: 10.1021/jf60181a039. [DOI] [PubMed] [Google Scholar]
- Misra H. P. Generation of superoxide free radical during the autoxidation of thiols. J Biol Chem. 1974 Apr 10;249(7):2151–2155. [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med. 1979 Oct 1;150(4):938–949. doi: 10.1084/jem.150.4.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa M. G., Tierney D. F. Biochemical and metabolic changes in the lung with oxygen, ozone, and nitrogen dioxide toxicity. Am Rev Respir Dis. 1978 Dec;118(6):1061–1090. doi: 10.1164/arrd.1978.118.6.1061. [DOI] [PubMed] [Google Scholar]
- Raffin T. A., Simon L. M., Braun D., Theodore J., Robin E. D. Impairment of phagocytosis by moderate hyperoxia (40 to 60 per cent oxygen) in lung macrophages. Lab Invest. 1980 Jun;42(6):622–626. [PubMed] [Google Scholar]
- Roehm J. N., Hadley J. G., Menzel D. B. Antioxidants vs lung disease. Arch Intern Med. 1971 Jul;128(1):88–93. [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L. M., Axline S. G., Robin E. D. The effect of hyperoxia on phagocytosis and pinocytosis in isolated pulmonary macrophages. Lab Invest. 1978 Dec;39(6):541–546. [PubMed] [Google Scholar]
- Steffen C., Netter K. J. On the mechanism of paraquat action on microsomal oxygen reduction and its relation to lipid peroxidation. Toxicol Appl Pharmacol. 1979 Mar 15;47(3):593–602. doi: 10.1016/0041-008x(79)90529-5. [DOI] [PubMed] [Google Scholar]
- Tappel A. L. Vitamin E and free radical peroxidation of lipids. Ann N Y Acad Sci. 1972 Dec 18;203:12–28. doi: 10.1111/j.1749-6632.1972.tb27851.x. [DOI] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- White H. L., White J. R. Interaction of streptonigrin with DNA in vitro. Biochim Biophys Acta. 1966 Sep;123(3):648–651. doi: 10.1016/0005-2787(66)90241-3. [DOI] [PubMed] [Google Scholar]





