Abstract
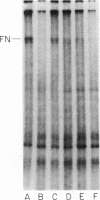
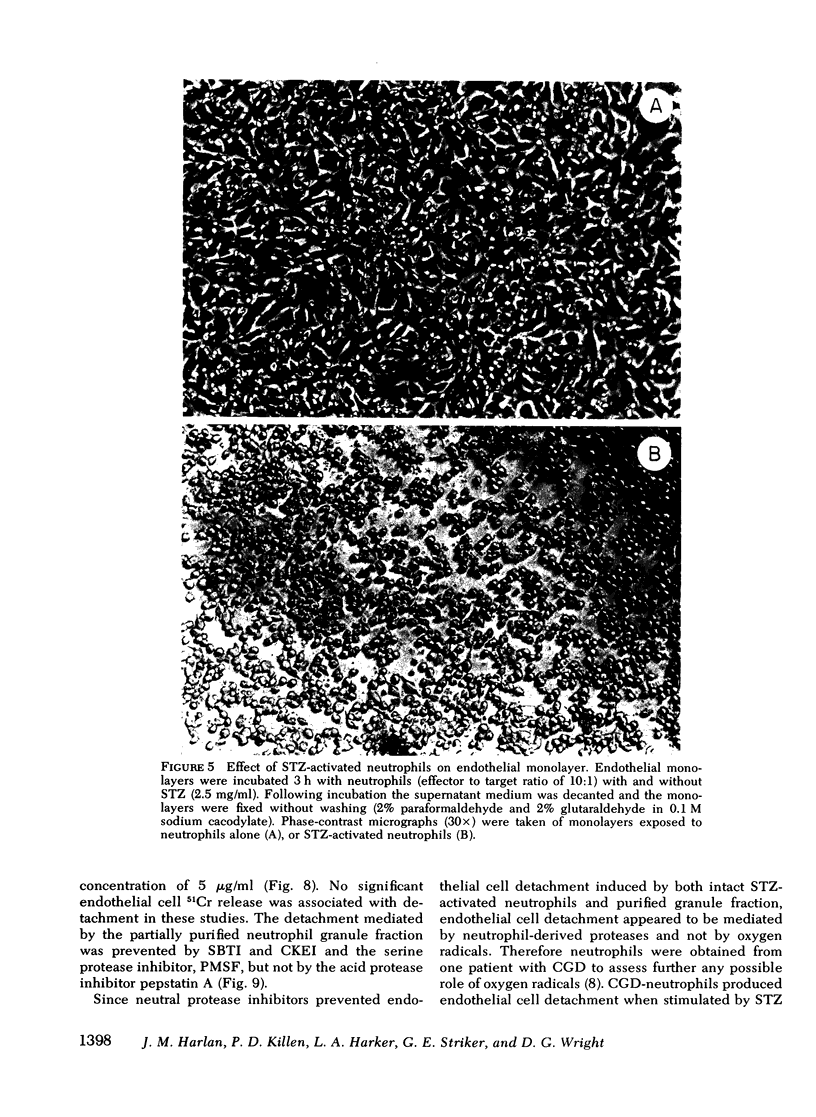
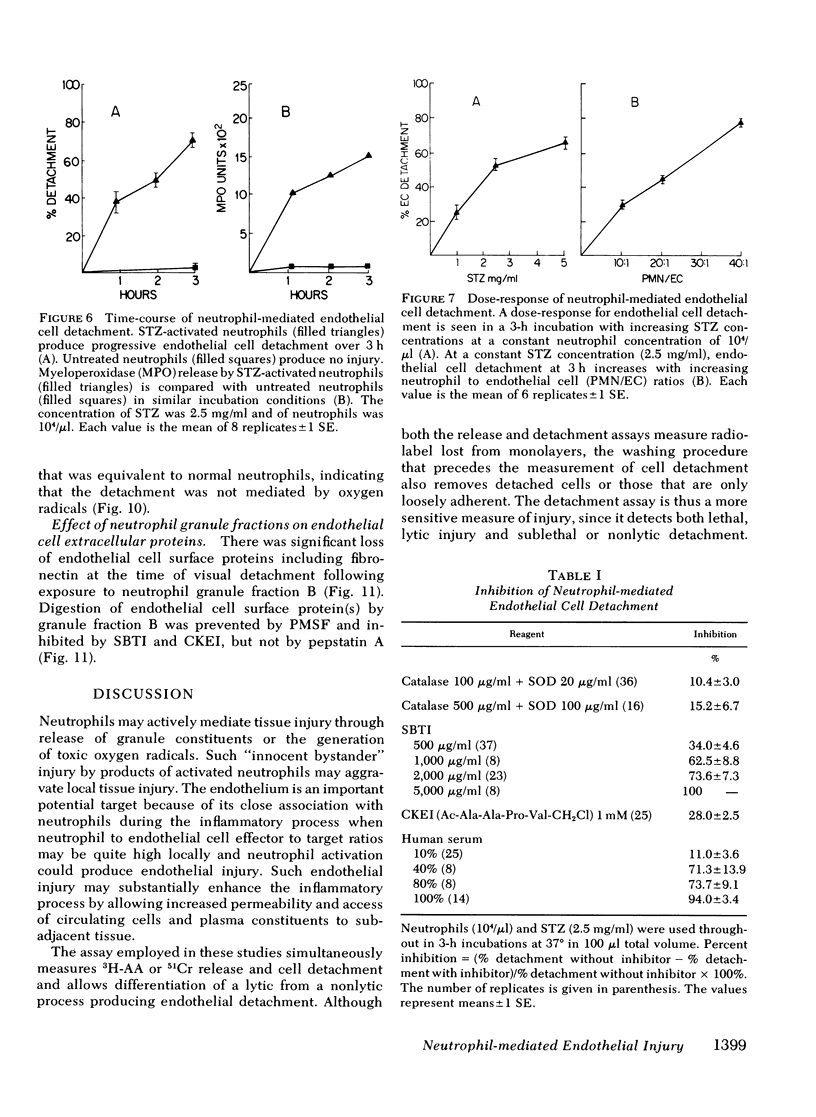
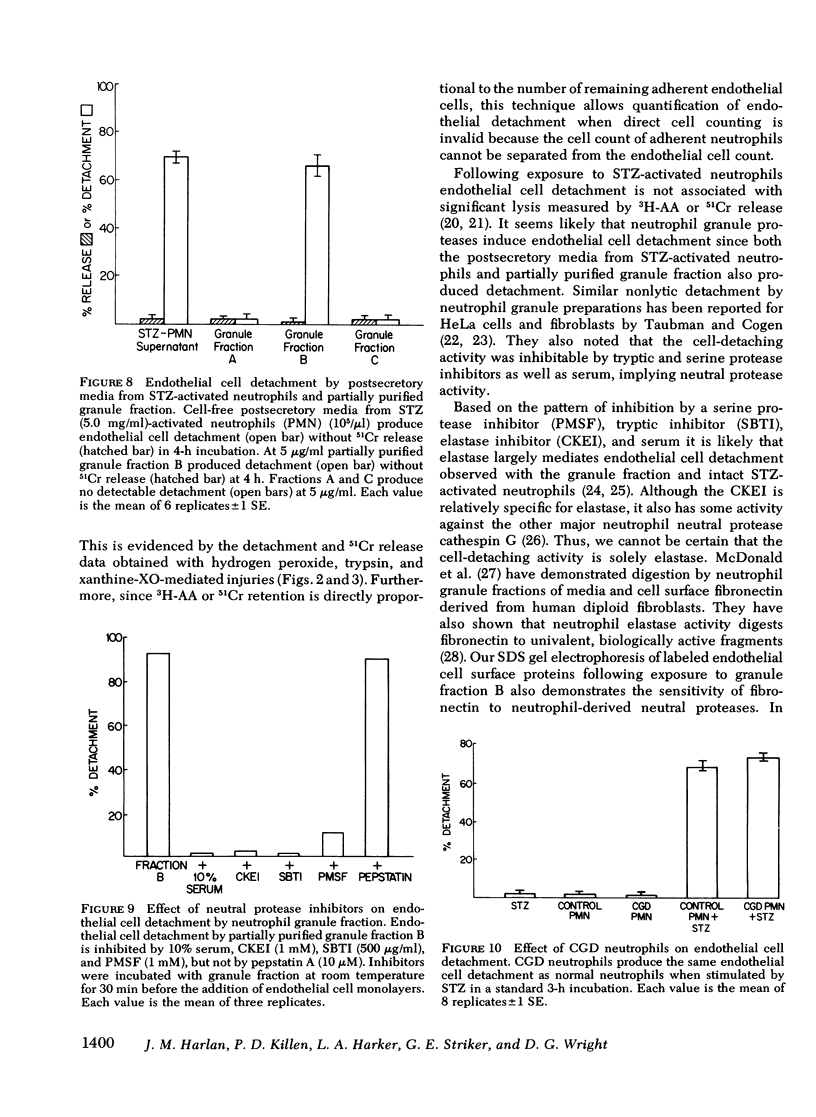
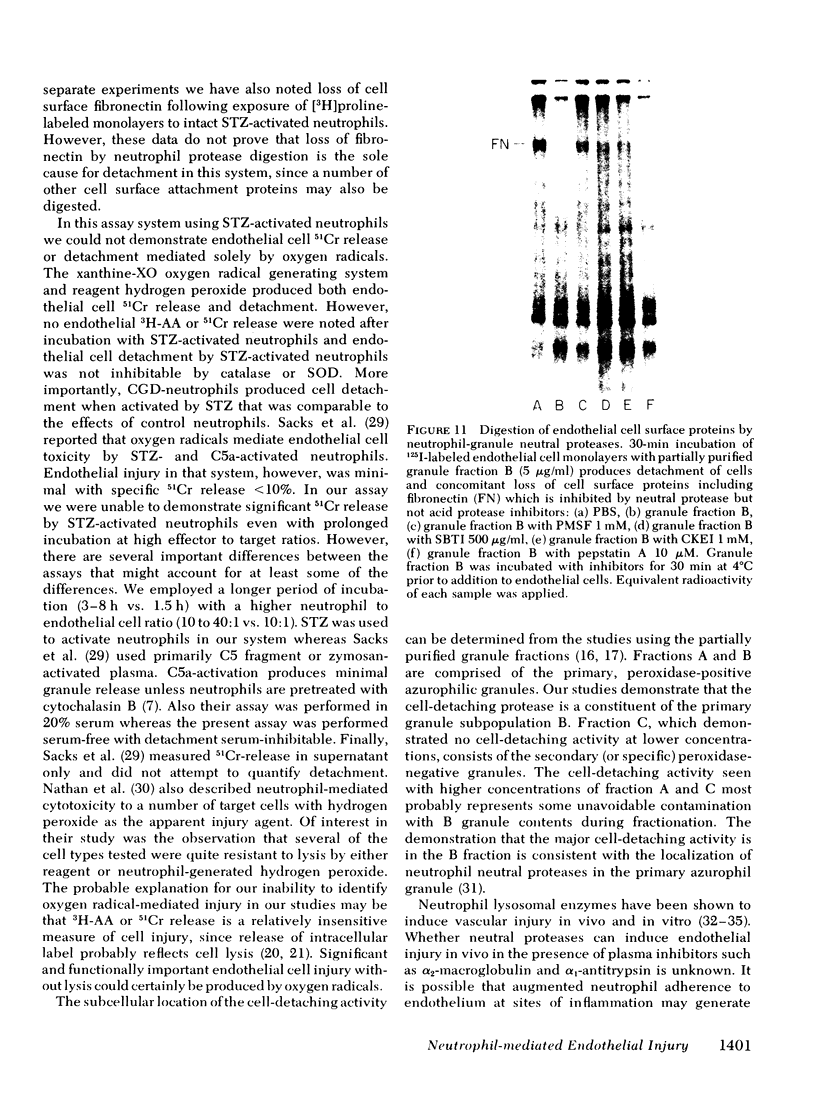
Neutrophil-mediated endothelial injury was assessed in vitro using assays of cell lysis and cell detachment. Activation of human peripheral blood neutrophils adherent to human umbilical vein endothelial cell monolayers by serum-treated zymosan produced dose-dependent endothelial cell detachment without concomitant cell lysis. This injury was inhibited by neutral protease inhibitors, but not by catalase or superoxide dismutase. Neutrophils from a patient with chronic granulomatous disease also produced endothelial cell detachment when activated by serum-treated zymosan similar to normal neutrophils. Endothelial detachment was also produced by cell-free postsecretory media from activated neutrophils or by partially purified human neutrophil granule fraction and was inhibitable by tryptic, elastase, and serine protease inhibitors, but not by an acid protease inhibitor. Analysis of iodinated endothelial cell surface proteins that had been exposed to partially purified neutrophil granule fraction showed complete loss of proteins migrating in the region of fibronectin by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. This result was prevented in the presence of neutral protease inhibitors. We conclude that neutrophil-derived neutral proteases mediate endothelial cell detachment in vitro through digestion of endothelial cell surface proteins including fibronectin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton A., Born G. V. Quantitative investigations of the adhesiveness of circulating polymorphonuclear leucocytes to blood vessel walls. J Physiol. 1972 Apr;222(2):447–474. doi: 10.1113/jphysiol.1972.sp009808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., Kipnes R. S. Biological defense mechanisms. Evidence for the participation of superoxide in bacterial killing by xanthine oxidase. J Lab Clin Med. 1975 Feb;85(2):235–244. [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Baumstark J. S., Lee C. T., Luby R. J. Rapid inactivation of alpha1-protease inhibitor (alpha1-antitrypsin) by elastase. Biochim Biophys Acta. 1977 Jun 10;482(2):400–411. doi: 10.1016/0005-2744(77)90254-6. [DOI] [PubMed] [Google Scholar]
- Birdwell C. R., Gospodarowicz D., Nicolson G. L. Identification, localization, and role of fibronectin in cultured bovine endothelial cells. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3273–3277. doi: 10.1073/pnas.75.7.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carp H., Janoff A. In vitro suppression of serum elastase-inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest. 1979 Apr;63(4):793–797. doi: 10.1172/JCI109364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Role of the myeloperoxidase-H2O2-halide system in concanavalin A-induced tumor cell killing by human neutrophils. J Immunol. 1979 Jun;122(6):2605–2610. [PubMed] [Google Scholar]
- Cochrane C. G., Aikin B. S. Polymorphonuclear leukocytes in immunologic reactions. The destruction of vascular basement membrane in vivo and in vitro. J Exp Med. 1966 Oct 1;124(4):733–752. doi: 10.1084/jem.124.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Goldstein I. M., Lind S., Hoffstein S., Weissmann G. Influence of local anesthetics upon human polymorphonuclear leukocyte function in vitro. Reduction of lysosomal enzyme release and superoxide anion production. J Exp Med. 1977 Aug 1;146(2):483–494. doi: 10.1084/jem.146.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M. Polymorphonuclear Leukocyte lysosomes and immune tissue injury. Prog Allergy. 1976;20:301–340. [PubMed] [Google Scholar]
- Henney C. S. Studies on the mechanism of lymphocyte-mediated cytolysis. II. The use of various target cell markers to study cytolytic events. J Immunol. 1973 Jan;110(1):73–84. [PubMed] [Google Scholar]
- Himmelhoch S. R., Evans W. H., Mage M. G., Peterson E. A. Purification of myeloperoxidases from the bone marrow of the guinea pig. Biochemistry. 1969 Mar;8(3):914–921. doi: 10.1021/bi00831a022. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3170–3174. doi: 10.1073/pnas.70.11.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Mosher D. F. Synthesis of fibronectin by cultured human endothelial cells. J Exp Med. 1978 Jun 1;147(6):1779–1791. doi: 10.1084/jem.147.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Zeligs J. D. Vascular injury and lysis of basement membrane in vitro by neutral protease of human leukocytes. Science. 1968 Aug 16;161(3842):702–704. doi: 10.1126/science.161.3842.702. [DOI] [PubMed] [Google Scholar]
- Lee C. T., Fein A. M., Lippmann M., Holtzman H., Kimbel P., Weinbaum G. Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory-distress syndrome. N Engl J Med. 1981 Jan 22;304(4):192–196. doi: 10.1056/NEJM198101223040402. [DOI] [PubMed] [Google Scholar]
- Lentnek A. L., Schreiber A. D., MacGregor R. R. The induction of augmented granulocyte adherence by inflammation. Mediation by a plasma factor. J Clin Invest. 1976 Apr;57(4):1098–1103. doi: 10.1172/JCI108354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C. Fibronectin: a function at the junction. Nature. 1979 Jun 7;279(5713):473–474. doi: 10.1038/279473a0. [DOI] [PubMed] [Google Scholar]
- MARCHESI V. T., FLOREY H. W. Electron micrographic observations on the emigration of leucocytes. Q J Exp Physiol Cogn Med Sci. 1960 Oct;45:343–348. doi: 10.1113/expphysiol.1960.sp001489. [DOI] [PubMed] [Google Scholar]
- Macarak E. J., Kirby E., Kirk T., Kefalides N. A. Synthesis of cold-insoluble globulin by cultured calf endothelial cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2621–2625. doi: 10.1073/pnas.75.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. A., Baum B. J., Rosenberg D. M., Kelman J. A., Brin S. C., Crystal R. G. Destruction of a major extracellular adhesive glycoprotein (fibronectin) of human fibroblasts by neutral proteases from polymorphonuclear leukocyte granules. Lab Invest. 1979 Mar;40(3):350–357. [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G. Degradation of fibronectin by human leukocyte elastase. Release of biologically active fragments. J Biol Chem. 1980 Sep 25;255(18):8848–8858. [PubMed] [Google Scholar]
- Nathan C. F., Brukner L. H., Silverstein S. C., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. I. Pharmacologic triggering of effector cells and the release of hydrogen peroxide. J Exp Med. 1979 Jan 1;149(1):84–99. doi: 10.1084/jem.149.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers J. C., Gupton B. F., Harley A. D., Nishino N., Whitley R. J. Specificity of porcine pancreatic elastase, human leukocyte elastase and cathepsin G. Inhibition with peptide chloromethyl ketones. Biochim Biophys Acta. 1977 Nov 23;485(1):156–166. doi: 10.1016/0005-2744(77)90203-0. [DOI] [PubMed] [Google Scholar]
- Sacks T., Moldow C. F., Craddock P. R., Bowers T. K., Jacob H. S. Oxygen radicals mediate endothelial cell damage by complement-stimulated granulocytes. An in vitro model of immune vascular damage. J Clin Invest. 1978 May;61(5):1161–1167. doi: 10.1172/JCI109031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson C. J. The mechanism of T cell mediated cytotoxicity. I. The release of different cell components. Proc R Soc Lond B Biol Sci. 1976 Jan 20;192(1107):221–239. doi: 10.1098/rspb.1976.0010. [DOI] [PubMed] [Google Scholar]
- Schumacher H. R., Agudelo C. A. Intravascular degranulation of neutrophils: an important factor in inflammation? Science. 1972 Mar 10;175(4026):1139–1140. doi: 10.1126/science.175.4026.1139. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Phillips J. K., Mergenhagen S. E. Biological activity of complement in vivo. Role of C5 in the accumulation of polymorphonuclear leukocytes in inflammatory exudates. J Exp Med. 1971 Nov 1;134(5):1131–1143. doi: 10.1084/jem.134.5.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taubman S. B., Cogen R. B. Cell-detaching activity mediated by an enzyme(s) obtained from human leukocyte granules. Lab Invest. 1975 Apr;32(4):555–560. [PubMed] [Google Scholar]
- Taubman S. B., Cogen R. B., Lepow I. H. Granule enzymes from human leukocytes: their effect on HeLa cells. Proc Soc Exp Biol Med. 1974 Mar;145(3):952–957. doi: 10.3181/00379727-145-37931. [DOI] [PubMed] [Google Scholar]
- WARD P. A., COCHRANE C. G., MUELLER-EBERHARD H. J. THE ROLE OF SERUM COMPLEMENT IN CHEMOTAXIS OF LEUKOCYTES IN VITRO. J Exp Med. 1965 Aug 1;122:327–346. doi: 10.1084/jem.122.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
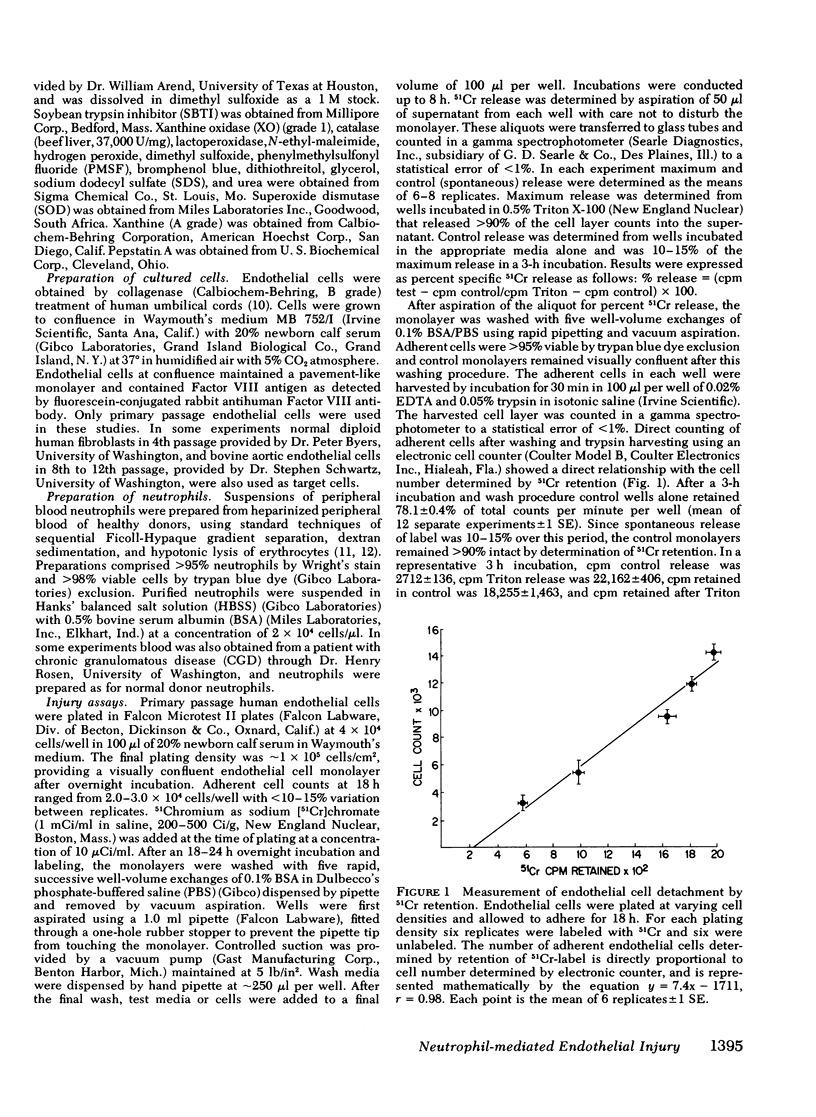
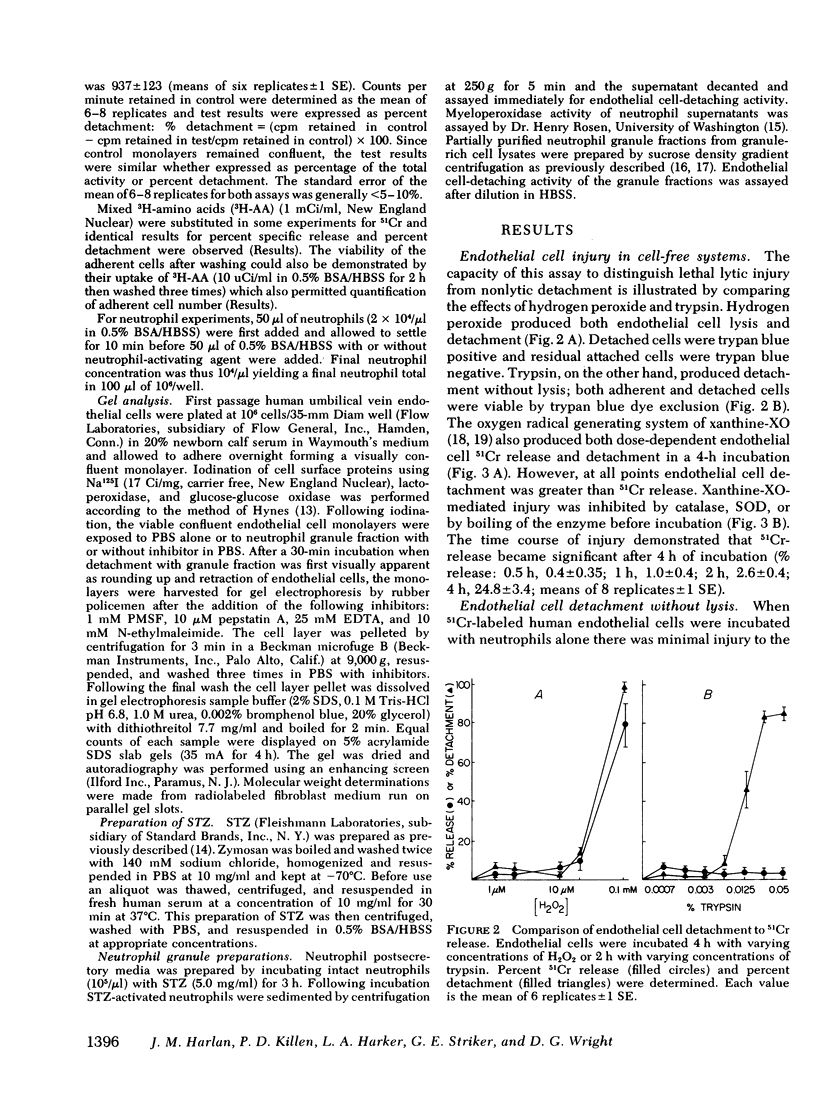
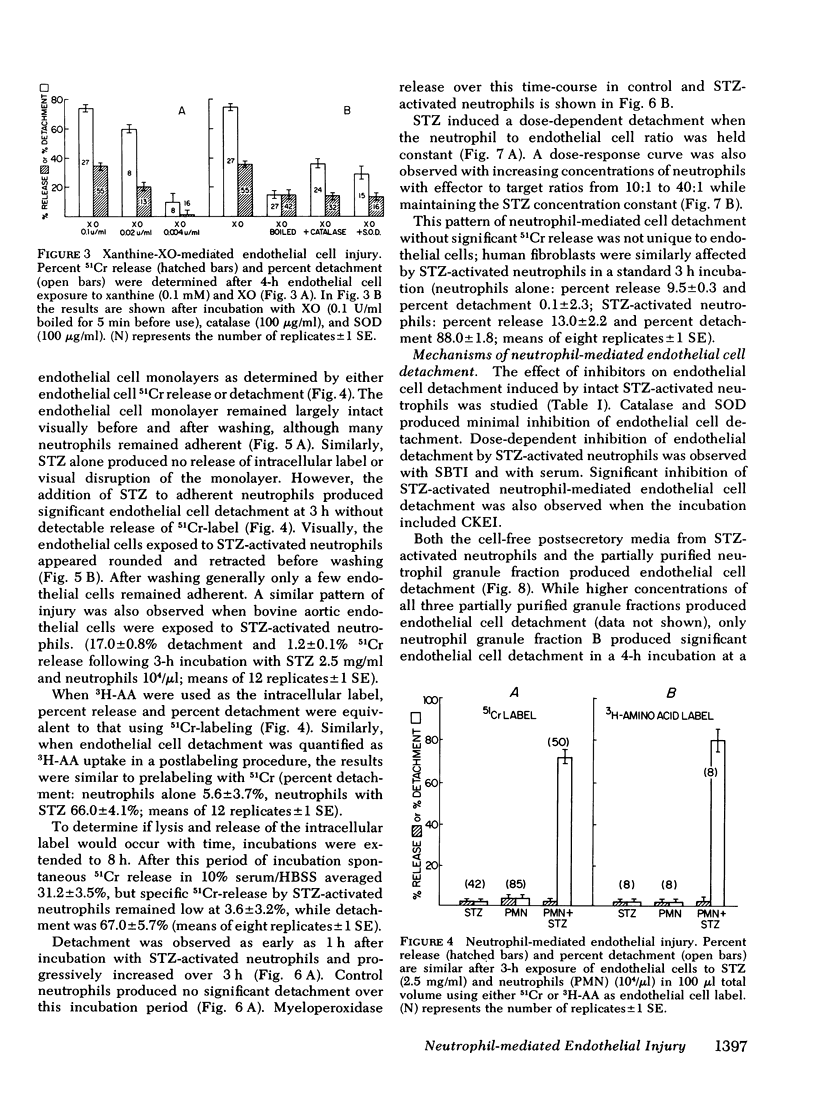
- Wall R. T., Harker L. A., Quadracci L. J., Striker G. E. Factors influencing endothelial cell proliferation in vitro. J Cell Physiol. 1978 Aug;96(2):203–213. doi: 10.1002/jcp.1040960209. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Smolen J. E., Korchak H. M. Release of inflammatory mediators from stimulated neutrophils. N Engl J Med. 1980 Jul 3;303(1):27–34. doi: 10.1056/NEJM198007033030109. [DOI] [PubMed] [Google Scholar]
- Wright D. G., Bralove D. A., Gallin J. I. The differential mobilization of human neutrophil granules. Effects of phorbol myristate acetate and ionophore A23187. Am J Pathol. 1977 May;87(2):273–284. [PMC free article] [PubMed] [Google Scholar]
- Wright D. G., Gallin J. I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979 Jul;123(1):285–294. [PubMed] [Google Scholar]
- Yamada K. M., Olden K. Fibronectins--adhesive glycoproteins of cell surface and blood. Nature. 1978 Sep 21;275(5677):179–184. doi: 10.1038/275179a0. [DOI] [PubMed] [Google Scholar]