Abstract
Reperfusion injury following hemorrhagic shock is accompanied by the development of a systemic inflammatory state that may lead to organ failure. C-peptide has been shown to exert anti-inflammatory effects in sepsis and myocardial ischemia-reperfusion injury, and to ameliorate renal dysfunction in diabetic animals. Hence, we investigated the effect of C-peptide on kidney injury following hemorrhagic shock. We hypothesized that C-peptide would exert reno-protective effects by blunting inflammation. Hemorrhagic shock was induced in male rats (3–4 months old) by withdrawing blood from the femoral artery to a mean arterial pressure of 50 mmHg. Animals were kept in shock for 3h at which time they were rapidly resuscitated by returning their shed blood. At the time of resuscitation and every hour thereafter, one group of animals received C-peptide (280 nmol/kg intravenously) while another group received vehicle. Hemorrhagic shock resulted in significant rise in plasma levels of creatinine and elevated kidney neutrophil infiltration as evaluated by myeloperoxidase (MPO) activity in vehicle-treated rats in comparison with sham rats, thus suggesting kidney injury. Treatment with C-peptide significantly attenuated the rise in creatinine and kidney MPO activity when compared to vehicle group. At a molecular level these effects of C-peptide were associated with reduced expression of the c-Fos subunit and reduced activation of the pro-inflammatory kinases, extracellular signal-regulated kinase (ERK 1/2) and c-Jun N-terminal kinase (JNK) and subsequently, reduced DNA binding of activator protein-1 (AP-1) in the kidney. Thus, our data suggest that C-peptide may exert reno-protective effects following hemorrhagic shock by modulating AP-1 signaling.
Keywords: C-peptide, Creatinine, Kidney Injury, Hemorrhagic Shock, AP-1
INTRODUCTION
Reperfusion injury is a common consequence of resuscitation following hemorrhagic shock. Reperfusion is characterized by the development of oxidative stress that in turn leads to development of a systemic inflammatory state which may contribute to organ injury (1). This systemic inflammatory state is orchestrated by activation of multiple signaling pathways involving mitogen activated protein kinases (MAPKs) leading to production of inflammatory mediators such as cytokines and chemokines. Among these kinases, ERK 1/2 and JNK have been reported to play a major role in inflammation. JNK activation leads to phosphorylation of c-Jun facilitating its hetero-dimerization with c-Fos to form the AP-1 (2). AP-1 then can facilitate the transcription of numerous genes involved in the production of inflammatory mediators such as cytokines and adhesion molecules.
Insulin connecting peptide (C-peptide) is a 31-amino-acid peptide that is cleaved from pro-insulin during insulin synthesis (3). Initially thought to be inert, it has become apparent that it may possess biologic activity and modulate intracellular signaling MAPKs (4), through binding of a G protein coupled receptor (5–6). There has been a specific interest in the role of this peptide in modulating the renal effects of diabetes. C-peptide has been shown to reduce glomerular hyperfiltration, microalbuminuria and reduce glomerular hypertrophy thereby ameliorating diabetic nephropathy (7). Furthermore, C-peptide has been shown to exert anti-apoptotic effects in kidney proximal tubular cells by reducing TNF-α induced apoptosis (6). Taken together it appears that C-peptide may exert reno-protective effects in diabetic animals.
In non-diabetic animal models C-peptide may also possess anti-inflammatory properties. Specifically, it may blunt the inflammatory response by mediating leukocyte adhesion and infiltration possibly by modulating NO production (8–9). In the setting of myocardial ischemia-reperfusion it may reduce neutrophil mediated cardiac contractile dysfunction (9). Our recent work suggests that it may also attenuate lung damage and reduce neutrophil infiltration in the setting of endotoxemia (10). Mechanistically this effect was associated with its ability to inhibit the activation of ERK 1/2. However, the spectrum of its anti-inflammatory effects remains unclear.
Since acute kidney injury is a common consequence of hemorrhagic shock, we investigated the biological effects of C-peptide treatment in a rodent model of hemorrhagic shock. We hypothesized that C-peptide would exert reno-protective effects by blunting inflammation through modulation of MAPKs and AP-1 signaling.
MATERIALS AND METHODS
Rodent Model of Hemorrhagic Shock
The investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996) and commenced with the approval of the institutional Animal Care and Use Committee. Male Wistar rats (3–4 months, Charles River Laboratories, Wilmington MA) were anesthetized with pentobarbital (80 mg/kg) intraperitoneally (IP). The right femoral artery and left common carotid artery were cannulated (PE-50 tubing) and used for drawing blood and measuring mean arterial pressure (MAP), respectively. The trachea was cannulated and the animals were placed on a rodent ventilator (Harvard Apparatus, Holliston, MA) with similar settings, tidal volume 2 ml, rate 60 breaths/min and FiO2 of 0.4. Animals underwent hemorrhage by drawing blood from the femoral artery as previously described (11). The animals were kept at a MAP of 50 mmHg for 3h by withdrawing or re-injecting blood through the femoral artery. At 3h rats were resuscitated over 10 min with their own shed blood supplemented with Ringer Lactate solution to a final volume equal to total shed blood and monitored for another 3h. Heart rates (HR) and MAP were measured using a pressure transducer and digitized using a Maclab A/D converter. This data was analyzed using Chart 5 software (AD Instruments Colorado Springs, CO) at 30 min intervals during the entire experiment.
Experimental Groups
Rats were assigned to 3 groups- Sham (n=5), Vehicle (n=5) and C-Peptide (n=5). Rats in the sham group underwent the surgical procedure, but were not bled. Rats in the vehicle and C-peptide groups underwent hemorrhagic shock followed by resuscitation and were treated with either vehicle (0.1% acetic acid) or C-peptide (280 nmol/Kg) intra-arterially at resuscitation and hourly thereafter. The dose of C-peptide was chosen on the basis of prior work in an in vivo model of endotoxemia (10). Acetic acid was the vehicle used to reconstitute C-peptide and was used as the vehicle. Animals were euthanized 3h after resuscitation and kidneys and plasma samples were collected and stored at −70°C.
Plasma Creatinine
Plasma levels of creatinine were evaluated using a commercial kit (Genzyme Diagnostics, Framingham MA).
Myeloperoxidase Activity
Myeloperoxidase activity was determined as an index of neutrophil accumulation in kidney tissues collected 3 h after. Kidney tissue was homogenized in a solution containing 0.5% hexa-decyl-trimethyl-ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) and centrifuged for 30 min at 4000 g a 4°C. An aliquot of the supernatant was allowed to react with a solution of tetra-methyl-benzidine (1.6 mM) and 0.1 mM hydrogen peroxide. The rate of change in absorbance was measured by spectrophotometry at 650 nm. Myeloperoxidase activity was defined as the quantity of enzyme degrading 1 µmol of hydrogen peroxide per min at 37°C and expressed in units per 100-mg weight of tissue.
Histopathological analysis
Frozen kidney tissue sections were stained with hematoxylin eosin stain. These sections were evaluated by light microscopy for cellular infiltration and injury by a pathologist.
Subcellular Fractionation and Protein extraction
Kidney samples were homogenized in a buffer containing 0.32 M sucrose, 10 mM Tris-HCl (pH 7.4), 1 mM ethylene glycol Bis-2-aminoethyl ether-N,N’,N”,n’-tetraacetic acid, 2mM EDTA, 5 mM sodium azide, 10 mM β-mercaptoethanol, 20 µM leupeptin EGTA, 0.15 µM pepstatin A, 0.2 mM phenylmethanesulfonyl fluoride, 50 Mm sodium fluoride, 1 mM sodium orthovanadate, 0.4 nM microcystin. The homogenates were centrifuged (1,000×g, 10 min), and the supernatant (cytosol plus membrane extract) was collected. The pellets were solubilized in Triton buffer (1% Triton X-100, 150 mM NaCl, 10 mM Tris-HCl (pH 7.4), 1 mM EGTA, 1 mM EDTA, 0.2 mM sodium orthovanadate, 20 µM leupeptin A, and 0.2 mM PMSF). The lysates were centrifuged (15,000×g, 30 min, 4°C), and the supernatant (nuclear extract) was collected.
Western Blot Analysis
The content of pERK 1/2, ERK 1/2, pJNK, JNK, cFos, p-cJun, cJun and β-actin were determined by immunoblot analysis using primary antibody against pERK 1/2, ERK 1/2, pJNK, JNK, p-cJun, cJun and β-actin. Cytosol and nuclear extracts were boiled in loading buffer (125 mM Tris-HCl pH 6.8, 4% sodium dodecyl sulfate, 20% glycerol and 10% 2-mercaptoethanol) and 50 µg of protein were loaded per lane on an 8% to 16% Tris-glycine gradient gel. Proteins were separated electrophoretically and transferred to nitrocellulose membranes. For immunoblotting, membranes were blocked with 5% nonfat dried milk in Tris-buffered saline for 1 h and then incubated with primary antibody as mentioned above for 1 h. The membranes were washed in Tris-buffered saline with 0.1% Tween 20 and incubated with secondary peroxidase-conjugated antibody. Immunoreaction was visualized by chemiluminescence on a photographic film. Densitometric analysis of blots was performed using ImageQuant (Molecular Dynamics, Sunnyvale, CA).
Electrophoretic Mobility Shift Assay (EMSA)
EMSA was performed using oligonucleotide probes corresponding to AP-1 consensus sequence (5’-CGC TTG ATG ACT CAG CCG GAA -3’). Oligonucleotide probe was labeled with γ-(32P)ATP using T4 polynucleotide kinase and purified in Bio-Spin chromatography columns (BioRad, Hercules, Calif). Ten micrograms of nuclear protein was incubated with EMSA buffer (12 mM N-2-hydroxyethylpiperazine-N’- 2-ethanesulfonic acid pH 7.9, 4 mM Tris-HCl pH 7.9, 25 mM potassium chloride, 5 mM magnesium chloride, 1 mM EDTA, 1 mM dithiothreitol, 50 ng/mL poly(d(I-C)), 12% glycerol vol/vol, and 0.2 mM phenylmethanesulfonyl fluoride) and radiolabeled oligonucleotide. The specificity of the binding reactions was determined by coincubating duplicate nuclear extract samples with 100-fold molar excess of respective unlabeled oligonucleotides (competitor assays). Protein-nucleic acid complexes were then resolved using a nondenaturing polyacrylamide gel and run in 0.5X Tris-HCl (45 mM), boric acid (45 mM), and EDTA (1 mM) for 1 h at constant current (30 mA). Gels were transferred to Whatman 3M paper, dried under a vacuum at 80°C for 1 h, and exposed to photographic film at −70°C with an intensifying screen. Densitometric analysis was performed using ImageQuant (Molecular Dynamics, Sunnyvale, CA).
Materials
The primary antibodies directed at pERK 1/2, ERK 1/2, pJNK, JNK, p-cJun, cJun, β-actin and the oligonucleotides for AP-1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). C-peptide was obtained from Sigma-Aldrich (St. Louis, MO).
Data analysis
Data was analyzed using SigmaStat for Windows Version 3.10 (SysStat Software, San Jose, CA). All values in the figures and text are expressed as mean ± SE of n observations. Parametric data was analyzed by ANOVA, while non-parametric data was analyzed by Kruskall-Wallis ANOVA on ranks. Multiple comparisons were done utilizing the Student-Newman-Keuls method. A value of p < 0.05 was considered significant.
RESULTS
Effect of C-peptide-treatment on Blood Pressure and Kidney Function
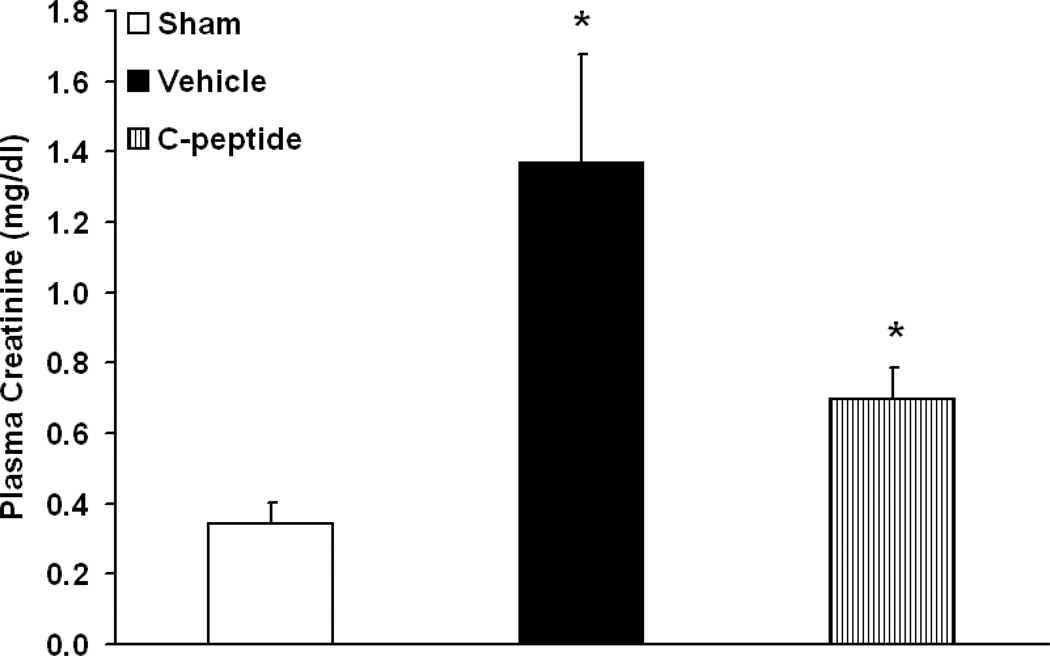
Mean arterial pressure levels in both C-peptide and vehicle-treated groups were similar prior to and during hemorrhagic shock (Table. 1). Following resuscitation, C-peptide-treated rats had significantly higher values of MAP towards the end of the experiment when compared to the vehicle-treated group (Table. 1). We further assessed kidney function by measuring plasma creatinine levels at baseline and following hemorrhagic shock and resuscitation. Rats undergoing hemorrhagic shock had a significant increase in plasma creatinine level, when compared to sham rats (3±0.2 mg/dl vs. 0.3±0.06 mg/dl, p<0.05). At 3h following resuscitation vehicle-treated rats had a reduction in creatinine levels (1.37±0.31 mg/dl), however these were still significantly elevated compared to basal levels in sham rats (p<0.05) (Fig. 1). Treatment with C-peptide further reduced creatinine levels to 0.70±0.09 mg/dl, representing a 50% reduction in level compared to vehicle-treatment (Fig. 1). Hence, this data suggests an amelioration of kidney function in C-peptide treated rats following resuscitation.
Table 1.
Effect of in vivo treatment with Vehicle or C-peptide on Mean Arterial Pressure (MAP) during Hemorrhagic shock and after Resuscitation
| Vehicle Mean Arterial Pressure (mmHg) |
C-Peptide Mean Arterial Pressure (mmHg) |
|
|---|---|---|
| Basal | 132.5±9.1 | 136.9±4.2 |
| Shock 1h | 52.8±0.4a | 52±0.2a |
| Shock 2h | 51.1±0.4a | 52.7±0.7a |
| Shock 3h | 51.2±0.7a | 49.5±0.3a |
| Resuscitation 1h | 96.1±7.1 | 106.4±4.8 |
| Resuscitation 2h | 87.6±7.2 | 110.9±4.6b |
| Resuscitation 3h | 77.6±6 | 110.6±7.8b |
Basal MAP was recorded in both groups following preparatory surgery but prior to induction of hemorrhage. Each MAP value represents mean ± SE of 5 individual rats in each group.
p < 0.05 vs. basal MAP in the respective group,
p < 0.05 vs. vehicle-treated group following resuscitation.
Figure 1.
Effect of in vivo treatment with vehicle or C-peptide on Plasma Creatinine level after hemorrhagic shock and resuscitation. Each data point represents mean ± SE of 5 individual rats in each group. *p < 0.05 vs. sham rats.
Effect of C-peptide-treatment on Neutrophil infiltration and Kidney Histology
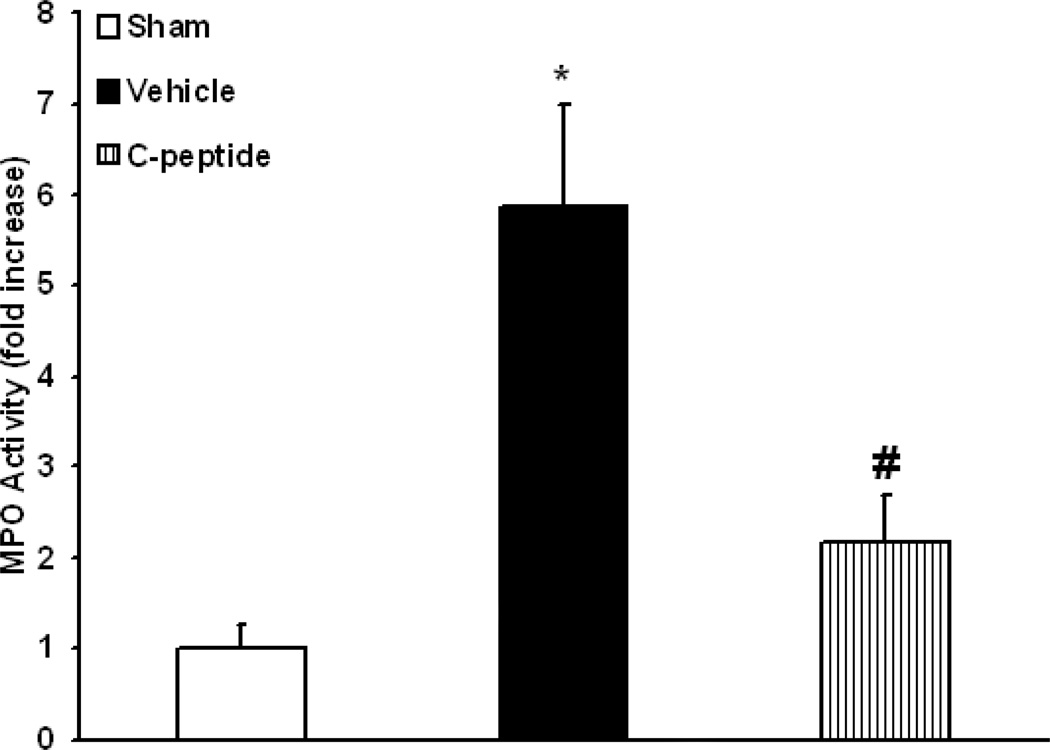
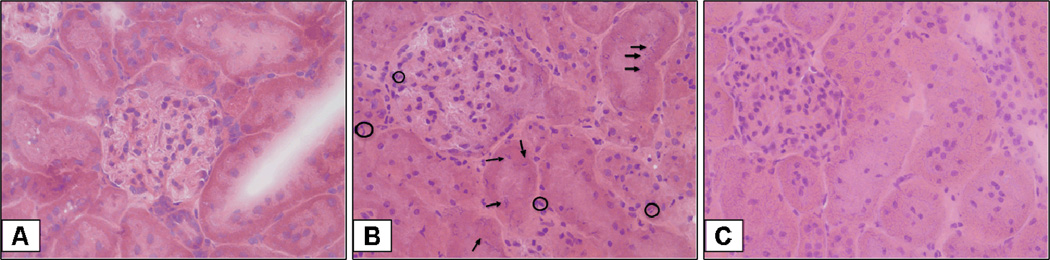
Since neutrophil infiltration in the kidney is a common occurrence following ischemia-reperfusion (12), we assessed kidney myeloperoxidase activity. Vehicle-treated rats had a significant increase in kidney neutrophil infiltration, nearly 6 fold when compared to basal levels in sham rats (p<0.05) (Fig. 2). C-peptide-treated rats had a significant reduction in neutrophil infiltration when compared to vehicle-treated rats (p<0.05) (Fig. 2). Specifically, in C-peptide treated rats neutrophil infiltration was only two fold over basal levels of sham rats at the end of resuscitation (Fig. 2). On histologic examination vehicle-treated rats had findings suggestive of early tubular damage demonstrated by nuclear condensation and pyknosis of tubular cells; furthermore, there was an increase in neutrophil infiltration (Fig. 3). These histologic findings were attenuated with C-peptide treatment (Fig. 3). Taken together our data suggests that C-peptide-treatment reduced kidney neutrophil infiltration and tubular damage following resuscitation.
Figure 2.
Effect of in vivo treatment with vehicle or C-peptide on Kidney Myeloperoxidase (MPO) activity after hemorrhagic shock and resuscitation. Each data point represents the mean ± SE of 5 individual rats in each group. *p < 0.05 vs. sham rats, # p < 0.05 vs. vehicle-treated rats.
Figure 3.
Effect of in vivo treatment with vehicle or C-peptide on Kidney Histology after hemorrhagic shock and resuscitation. (A), Representative photomicrograph of histology showing normal glomerular and tubular architecture from a sham rat (magnification 400×). (B), Representative photomicrograph of histology showing cellular infiltration (neutrophils delineated with clear circles) with nuclear condensation and pyknosis (arrows) suggestive of early tubular damage from a vehicle-treated rat (magnification 400×). (C), Representative photomicrograph of histology showing attenuated cellular infiltration and preservation of tubular cell nuclei suggestive of reduction in tubular damage from a C-peptide-treated rat (magnification 400×). Similar histologic changes were seen in different tissue sections ( n=5) in each experimental group.
Effect of C-peptide-treatment on ERK1/2 and JNK Activation
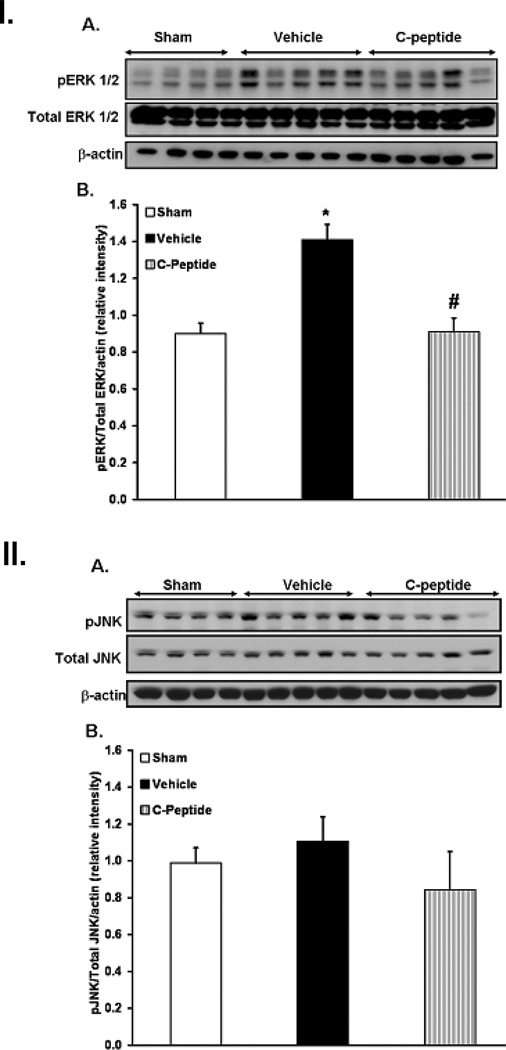
To gain further insights into the cellular mechanism of action of C-peptide we evaluated ERK 1/2 and JNK activation in kidney tissues. Since ERK 1/2 and JNK activation is characterized by phosphorylation, western blotting of the phosphorylated forms of these kinases was assessed in kidney extracts. At the end of resuscitation vehicle-treated rats had a significant increase in the phosphorylated form of ERK 1/2 in the cytosol when compared to sham rats (Fig. 4I). Similarly vehicle-treated rats appeared to have a moderate increase in JNK phosphorylation in the nucleus when compared to sham rats (Fig. 4II). In contrast, C-peptide treatment significantly reduced ERK 1/2 phosphorylation when compared to vehicle treatment (Fig. 4I). However, its effect on JNK phosphorylation was less notable (Fig. 4II). Hence, our data suggests that C-peptide may inhibit hemorrhage induced MAPKs activation in the kidney, notably ERK 1/2 phosphorylation.
Figure 4.
Effect of in vivo treatment with vehicle or C-peptide on phosphorylated form of ERK 1/2 in kidney and JNK in kidney extracts after hemorrhagic shock. I. (A), Western blot of phosphorylated form of ERK 1/2, total ERK 1/2 and β-actin as loading control in kidney cytosol extracts, (B), Densitometric analysis of pERK 1/2 /ERK 1/2 as determined by Western blotting, data normalized to β-actin and presented as fold change compared to sham. II. (A) Western blot of phosphorylated form of JNK, total JNK and actin as loading control in kidney nuclear extracts. (B), Densitometric analysis of JNK/Total JNK as determined by Western blotting, data normalized to β-actin and presented as fold change compared to sham.
Each data point represents the mean ± SE of 4 individual rats in sham group and 5 individual rats in vehicle and C-peptide treated group. *p < 0.05 vs. sham rats; # p < 0.05 vs. vehicle-treated rats.
Effect of C-peptide on AP-1 Activation and c-Fos Expression
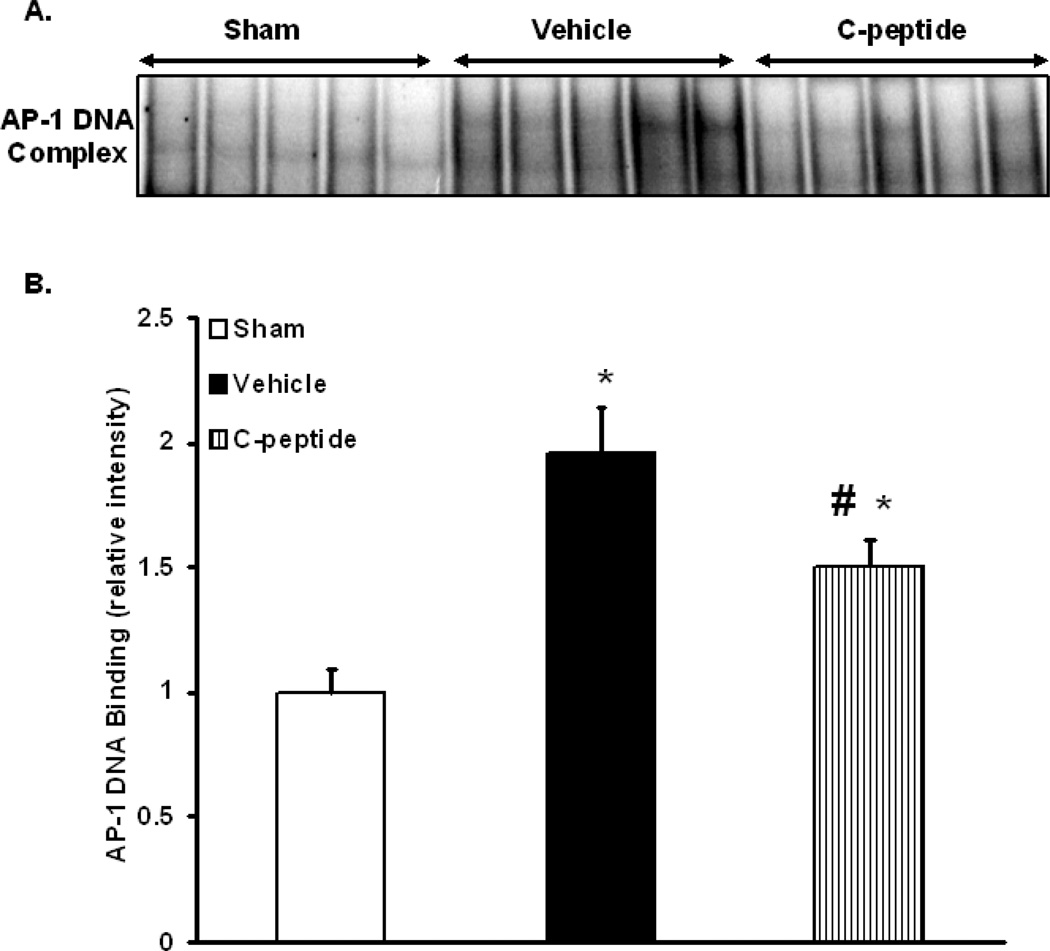
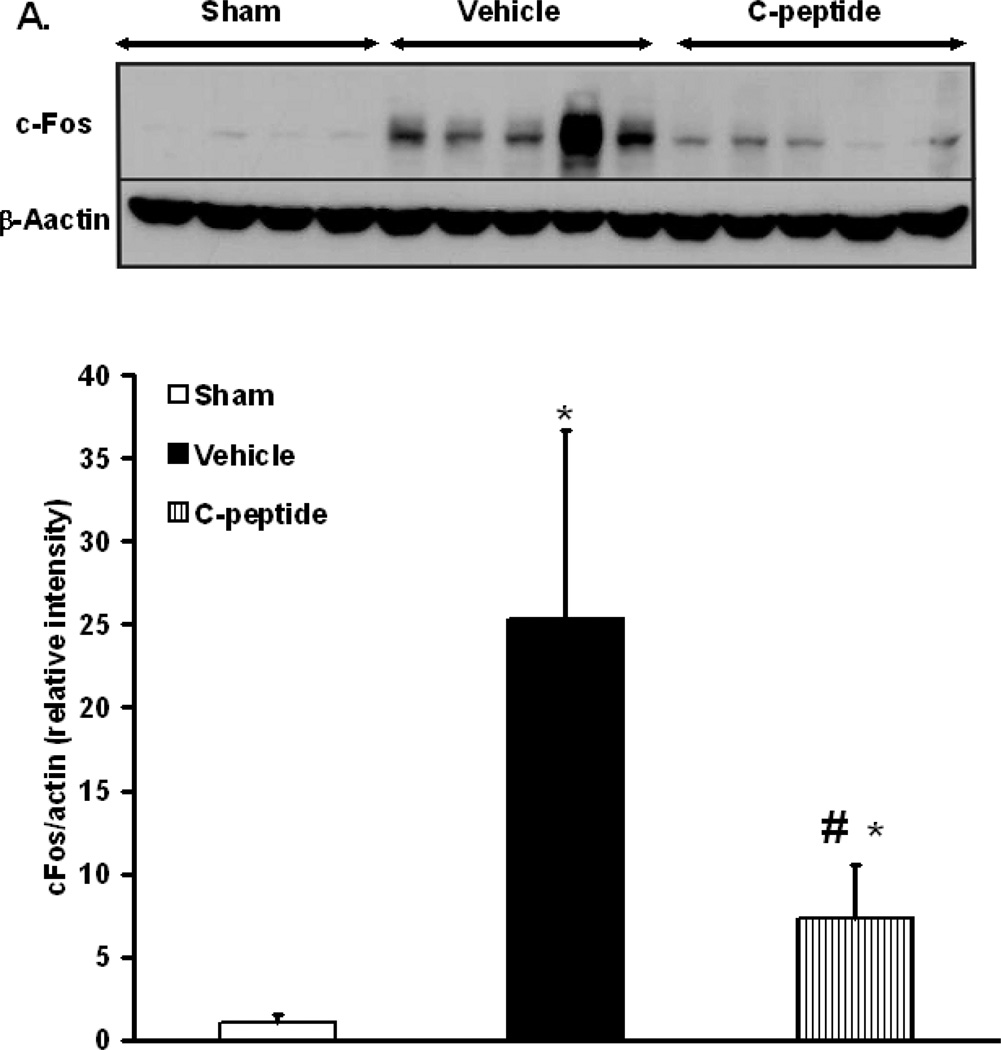
AP-1 is a major transcription factor that is activated following oxidative stress downstream of intracellular activation of the kinases JNK and ERK 1/2. Hence, we assessed AP-1 activation by measuring DNA binding by EMSA. Vehicle-treated rats had a significant increase in AP-1 DNA binding when compared to sham rats (p<0.05) (Fig. 5). In contrast, C-peptide treatment significantly reduced AP-1 DNA binding when compared to vehicle-treated rats (p<0.05) (Fig. 5). Since AP-1 activation may also be dependent on subunit availability (13), we determined the nuclear content of the AP-1 subunit c-Fos by western blot. At the end of resuscitation vehicle-treated rats had a significant increase in the nuclear content of c-Fos when compared to sham rats (p<0.05) (Fig. 5). C-peptide treatment on the other hand significantly reduced c-Fos content when compared to vehicle treatment (p<0.05) (Fig. 6). This data together suggests that C-peptide administration is associated with down-regulation of AP-1 activity in the kidney, likely as a consequence of limited availability of the c-Fos subunit.
Figure 5.
Effect of in vivo treatment with vehicle or C-peptide on AP-1 DNA binding in kidney nuclear extracts after hemorrhagic shock and resuscitation. (A), Representative autoradiograph of EMSA for AP-1; (B), image analysis of AP-1 DNA binding determined by densitometry. The fold increase was calculated vs. sham value which was set at 1.0. Each data point represents the mean ± SE of 5 individual rats in each group. *p < 0.05 vs. sham rats; # p < 0.05 vs. vehicle-treated rats.
Figure 6.
Effect of in vivo treatment with vehicle or C-peptide on c-Fos subunit expression in kidney nuclear extracts after hemorrhagic shock and resuscitation. (A), Western blot of c-Fos subunit expression. (B), densitometric analysis of nuclear content of c-Fos. Data normalized to β-actin and presented as fold change compared to sham. Each data point represents the mean ± SE of 4 individual rats in sham group and 5 individual rats in vehicle and C-peptide treated group. *p < 0.05 vs. sham rats; # p < 0.05 vs. vehicle-treated rats.
DISCUSSION
In this work we have explored the effects of C-peptide on kidney injury and inflammation following hemorrhagic shock. We have shown that C-peptide administered during re-perfusion following hemorrhagic shock was associated with a reduction in kidney injury and inflammation. This reno-protective effect was associated with a reduction in neutrophil infiltration and reduced activation of the kinases ERK 1/2, JNK and pro-inflammatory transcription factor AP-1.
In this study, C-peptide treated rats had significant increase in blood pressure at later time-points when compared to vehicle-treated rats. We have observed similar beneficial effects of C-peptide treatment on blood pressure following shock and resuscitation wherein C-peptide treatment has been associated with a blunted systemic inflammatory response following shock and resuscitation (author’s unpublished data). Additionally, C-peptide has been demonstrated to improve left ventricular contractility in the setting of myocardial ischemia-reperfusion (9). Though we did not assess cardiac contractility, it is possible that this could have contributed to an amelioration of the hypotensive state.
Acute kidney injury as seen following hemorrhagic shock is associated with the development of an inflammatory state characterized by activation of the innate immune system (14). This response can be detrimental to the host leading to kidney failure. Neutrophils may play an important role in the pathogenesis of kidney injury in the setting of ischemia-reperfusion (14–15). Prior work by our group has demonstrated that C-peptide treatment is associated with a reduction in lung neutrophil infiltration following endotoxemia (10). Similarly, our current data demonstrates that C-peptide-treated rats had a significant reduction in neutrophil infiltration when compared to vehicle-treated rats following hemorrhage and resuscitation. The exact mechanism by which C-peptide modulates neutrophil infiltration is unclear. C-peptide may reduce leukocyte rolling and adherence by modulating expression of endothelial cell adhesion molecules such as P-selectin, ICAM-1 and VCAM-1 (8). In our current work reduction in neutrophil infiltration in C-peptide treated rats was associated with a reduction in plasma creatinine level when compared with vehicle-treated rats. Our data are in agreement with previous reports demonstrating that blunting neutrophil infiltration affords protection following kidney ischemia-reperfusion (16). Additionally, since both vehicle and C-peptide treated rats were able to maintain blood pressure above the lower limit of renal auto regulation, it is likely that the lower creatinine levels in C-peptide treated rats reflect an attenuation of kidney injury, independent of its effect on blood pressure. Therefore, we, may hypothesize that the improvement in kidney function seen in our experiment may be a consequence of the ability of this peptide to reduce neutrophil infiltration and kidney injury.
In an attempt to define the anti-inflammatory molecular mechanisms of C-peptide we investigated the role of MAPKs signaling. Previous in vitro experiments have shown that C-peptide may modulate the activation MAPKs. In an opossum kidney cell line C-peptide activates ERK 1/2 thereby modulating cell proliferation (17). Similarly, in vitro in human renal tubular cells C-peptide has been shown to stimulate ERK 1/2 and JNK in protein kinase C dependent manner (18). In our model of hemorrhagic shock, vehicle-treated rats had an increase in both ERK 1/2 and JNK phosphorylation in the kidney after reperfusion, thus suggesting activation of these signaling pathways. However, contrary to what was observed in the in vitro studies, in our model of hemorrhagic shock we observed that C-peptide treatment was associated with a significant reduction in ERK 1/2 phosphorylation when compared to vehicle-treated rats. These data are in agreement with our prior work in endotoxemia, where C-peptide administration resulted in a reduction of ERK 1/2 activation and a concomitant attenuation in lung injury (10).
To further de-lineate the effects of C-peptide in our work on MAPKs signaling, we investigated its effect on the transcription factor AP-1. AP-1 is an important transcription factor downstream from ERK 1/2 and JNK in the signaling cascade and has been shown to play a role in injury consequent to ischemia and reperfusion. It is a heterodimer composed of multiple protein subunits, namely, Jun, Fos and ATF (19). AP-1 activity may be dependent on availability of each subunit, differential transcription of genes for each subunit, post-translation modification or interactions between subunits and other co-factors (19). In our study, vehicle treated rats had a significant increase in AP-1 DNA binding in the kidney when compared to rats in the sham group. In contrast, C-peptide-treated rats had a significant reduction in AP-1 DNA binding when compared to vehicle-treated rats. Since subunit availability may be an important mechanism regulating AP-1 activation (13), we assessed the expression of the subunits c-Fos and c-Jun. While there was no difference in c-Jun expression or phosphorylation amongst the treated groups (data not shown), C-peptide treated rats had a significant reduction in the expression of the c-Fos subunit when compared to vehicle-treated rats. The regulation of c-Fos expression is dependent on transcriptional control elements in its promoter (20). Moreover, both ERK and JNK may phosphorylate and activate elements in the c-Fos promoter thereby resulting in enhanced expression of c-Fos (20). Hence, it is possible that during hemorrhagic shock C-peptide may regulate c-Fos expression in the kidney and thereby affect AP-1 activity. Whether this is directly as a result of an effect of C-peptide on the c-Fos promoter or indirectly as a consequence of its effect on upstream ERK 1/2 and JNK expression is difficult to discern from our work. Nevertheless, taken together these results suggest that C-peptide reduces AP-1 activation in the kidney following hemorrhagic shock and that this effect is likely a consequence of limited availability of the c-Fos subunit.
Our study has some limitations. Most notably, our study is limited to assessing organ injury in a single organ at a single time point following hemorrhagic shock and resuscitation. We chose to focus on the kidney as published data has suggested a role for C-peptide being reno-protective in animal models of diabetes. It is also unclear whether C-peptide would have similar effects at earlier and later time points on kidney injury following resuscitation. In our current work we chose to study the protective effects of this peptide at the 3h time point in light of our prior studies utilizing a similar model. Hence, we are currently performing experiments that will assess the effect of this peptide on other organ systems and at multiple time points following shock and resuscitation.
In conclusion, our study is the first to demonstrate that C-peptide reduces kidney injury following hemorrhagic shock. This reduction is associated with a reduction in neutrophil infiltration. Mechanistically, these reno-protective effects are accompanied by a reduction of AP-1 activation. Thus, C-peptide may represent a novel endogenous peptide that may have potential therapeutic benefits in limiting acute kidney injury and inflammation following shock states.
Acknowledgements
This study was supported by the Shock Society/Novo Nordisk grant to young investigators (RC) and National Institutes of Health K12 HD028827 (to RC) and R01 AG027990 (to BZ). We would like to acknowledge Keith F. Stringer M.D. and David P. Witte M.D. for help with kidney histology.
REFERENCES
- 1.Flaherty JT, Weisfeldt ML. Reperfusion injury. Free Radic Biol Med. 1988;5:409–419. doi: 10.1016/0891-5849(88)90115-3. [DOI] [PubMed] [Google Scholar]
- 2.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 3.Steiner DF, Cunningham D, Spigelman L, Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967;157:697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- 4.Hills CE, Brunskill NJ. Intracellular signalling by C-peptide. Exp Diabetes Res. 2008;2008:635158. doi: 10.1155/2008/635158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rigler R, Pramanik A, Jonasson P, Kratz G, Jansson OT, Nygren P, Stahl S, Ekberg K, Johansson B, Uhlen S, Uhlen M, Jornvall H, Wahren J. Specific binding of proinsulin C-peptide to human cell membranes. Proc Natl Acad Sci U S A. 1999;96:13318–13323. doi: 10.1073/pnas.96.23.13318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Rasheed NM, Willars GB, Brunskill NJ. C-peptide signals via Galpha i to protect against TNF-alpha-mediated apoptosis of opossum kidney proximal tubular cells. J Am Soc Nephrol. 2006;17:986–995. doi: 10.1681/ASN.2005080797. [DOI] [PubMed] [Google Scholar]
- 7.Hills CE, Brunskill NJ, Squires PE. C-peptide as a therapeutic tool in diabetic nephropathy. Am J Nephrol. 2010;31:389–397. doi: 10.1159/000289864. [DOI] [PubMed] [Google Scholar]
- 8.Scalia R, Coyle KM, Levine BJ, Booth G, Lefer AM. C-peptide inhibits leukocyte-endothelium interaction in the microcirculation during acute endothelial dysfunction. Faseb J. 2000;14:2357–2364. doi: 10.1096/fj.00-0183com. [DOI] [PubMed] [Google Scholar]
- 9.Young LH, Ikeda Y, Scalia R, Lefer AM. C-peptide exerts cardioprotective effects in myocardial ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2000;279:H1453–H1459. doi: 10.1152/ajpheart.2000.279.4.H1453. [DOI] [PubMed] [Google Scholar]
- 10.Vish MG, Mangeshkar P, Piraino G, Denenberg A, Hake PW, O'Connor M, Zingarelli B. Proinsulin c-peptide exerts beneficial effects in endotoxic shock in mice. Crit Care Med. 2007;35:1348–1355. doi: 10.1097/01.CCM.0000260245.61343.B3. [DOI] [PubMed] [Google Scholar]
- 11.Chima RS, Hake PW, Piraino G, Mangeshkar P, Denenberg A, Zingarelli B. Ciglitazone ameliorates lung inflammation by modulating the increased inhibitor kappaB protein kinase/nuclear factor-kappaB pathway after hemorrhagic shock. Crit Care Med. 2008 doi: 10.1097/ccm.0b013e318187810e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–491. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- 13.Karin M, Liu Z, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 14.Jang HR, Rabb H. The innate immune response in ischemic acute kidney injury. Clin Immunol. 2009;130:41–50. doi: 10.1016/j.clim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 16.Kelly KJ, Williams WW, Jr, Colvin RB, Meehan SM, Springer TA, Gutierrez-Ramos JC, Bonventre JV. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J Clin Invest. 1996;97:1056–1063. doi: 10.1172/JCI118498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Rasheed NM, Meakin F, Royal EL, Lewington AJ, Brown J, Willars GB, Brunskill NJ. Potent activation of multiple signalling pathways by C-peptide in opossum kidney proximal tubular cells. Diabetologia. 2004;47:987–997. doi: 10.1007/s00125-004-1404-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhong Z, Kotova O, Davidescu A, Ehren I, Ekberg K, Jornvall H, Wahren J, Chibalin AV. C-peptide stimulates Na+, K+-ATPase via activation of ERK1/2 MAP kinases in human renal tubular cells. Cell Mol Life Sci. 2004;61:2782–2790. doi: 10.1007/s00018-004-4258-x. [DOI] [PubMed] [Google Scholar]
- 19.Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- 20.Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]