Abstract
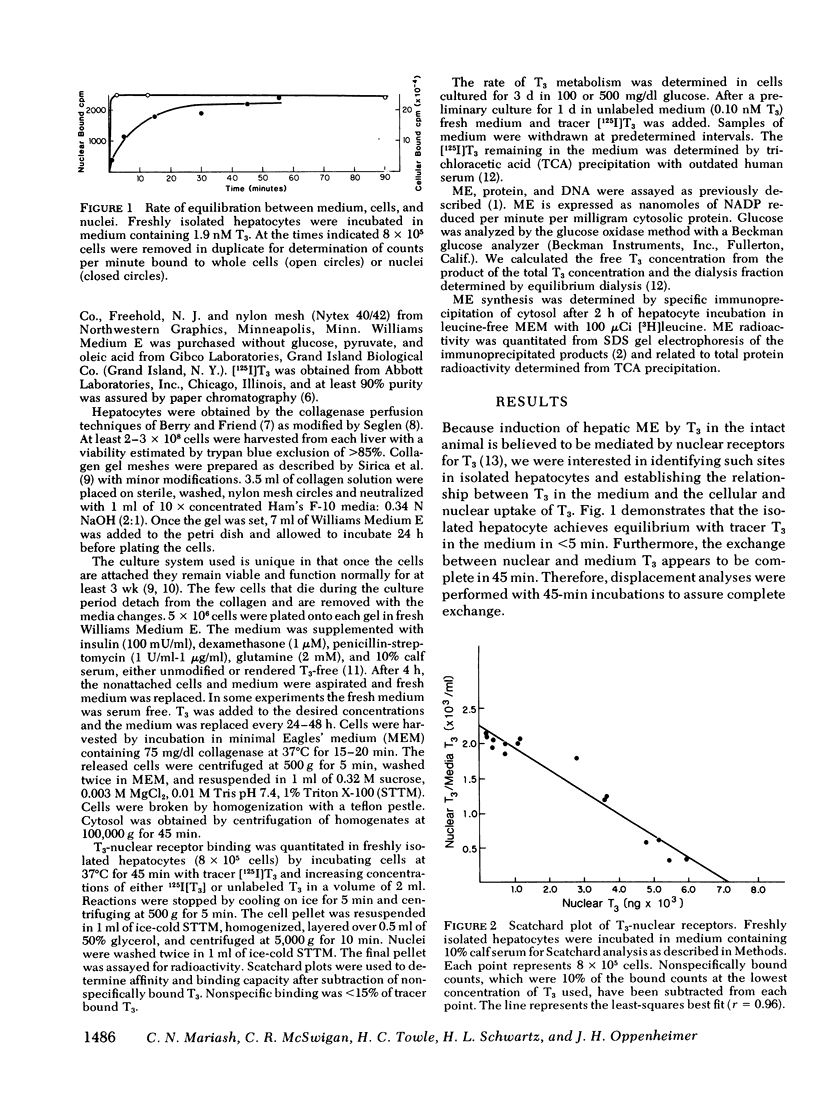
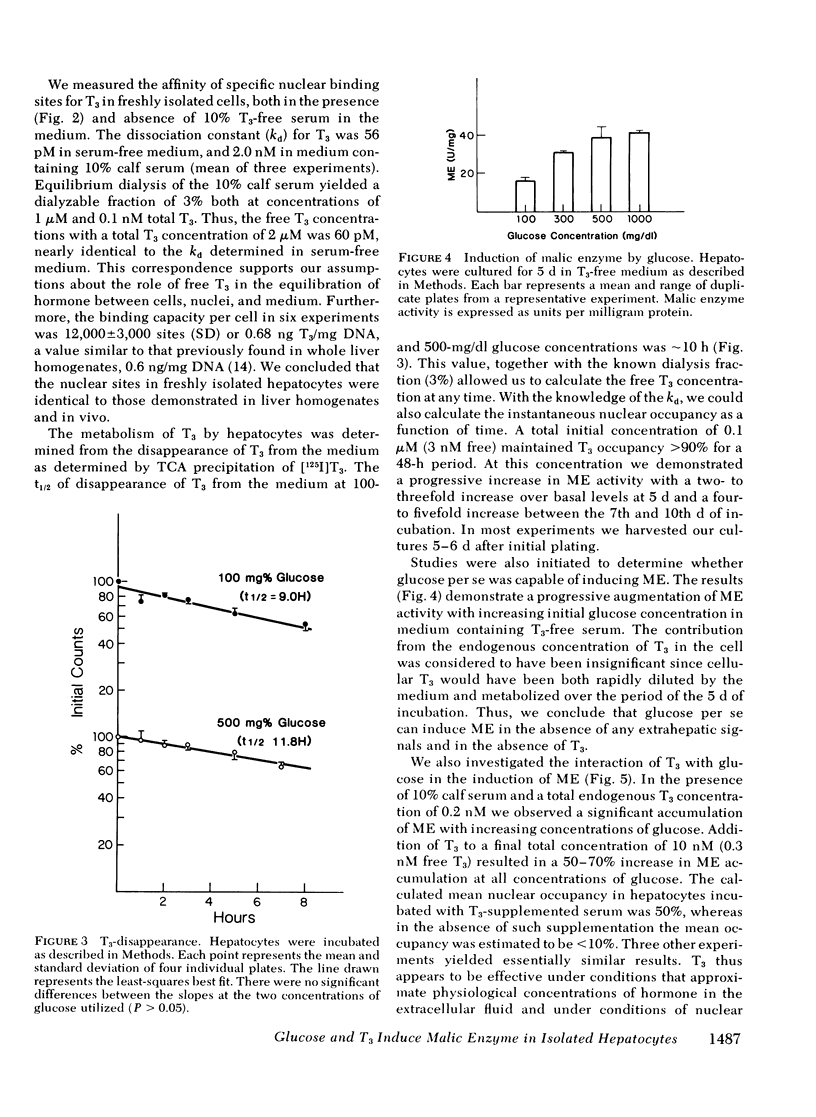
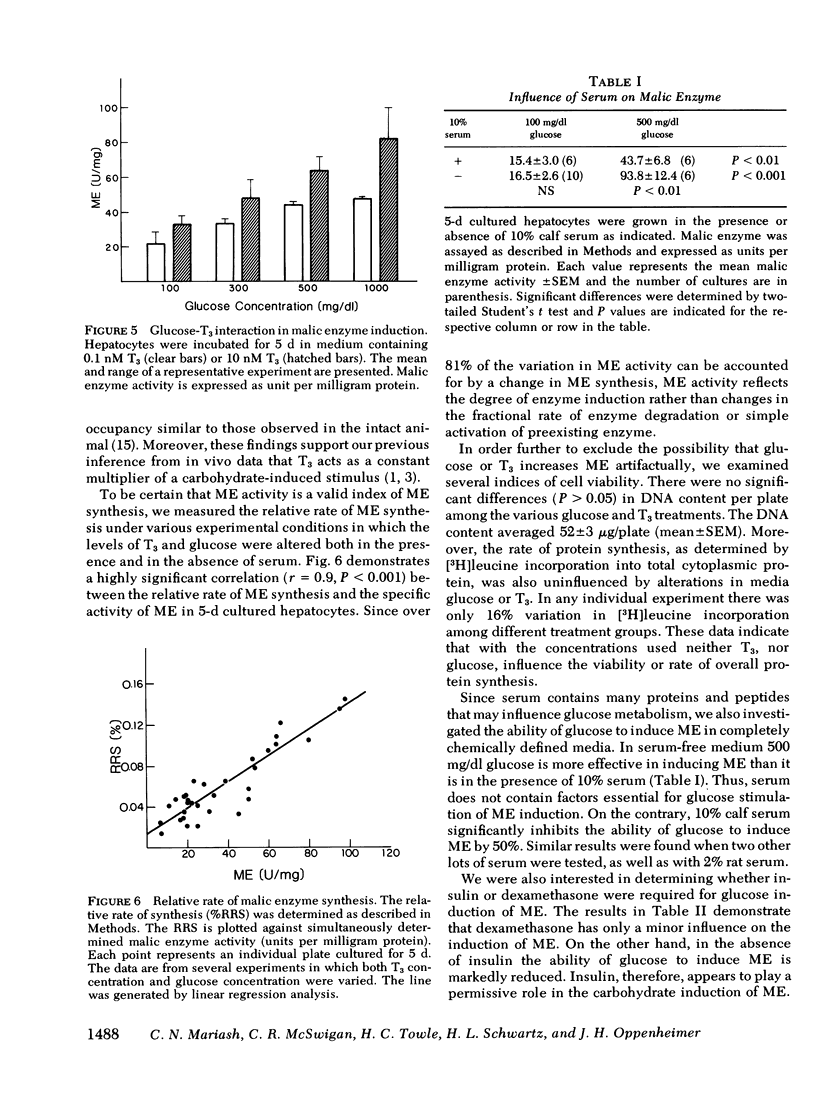
We have stimulated in a cultured hepatocyte system the synergistic interaction between triiodothyronine (T3) and dietary carbohydrate in the induction of malic enzyme (ME). Kinetic studies revealed that isolated hepatocytes equilibrate with media T3 within 5 min; nuclei equilibrate with media T3 by 45 min; and the half-time of T3 metabolism was 10 h in 10% serum. We demonstrated nuclear T3 receptors in isolated hepatocytes and the induction of ME by T3 in physiological concentrations. However, in the complete absence of T3 glucose could still induce ME. At all concentrations of glucose (100-1,000 mg/dl), T3 (0.3 nM free T3) resulted in a relatively constant (1.4- to 1.7-fold) increase in ME response. The augmentation in ME activity was paralleled by an enhanced rate of enzyme synthesis as determined by [3H]leucine incorporation into immunoprecipitable ME. Cells cultured in serum free media also demonstrated a glucose-dependent increase in ME. Insulin greatly stimulated the glucose induction of ME, whereas dexamethasone had very little influence on ME induction. These studies demonstrate the usefulness of an adult hepatocyte tissue culture model for the study of the effects of T3 on gene expression in cells that are not derived from tumor. They clearly demonstrate that well established effects of T3 can be simulated in such a system at levels of free hormone that approximate those in extracellular body fluids. Our results indicate that an increased concentration of glucose per se can induce the formation of ME in the absence of alterations in extrahepatic hormones or factors. Moreover, our findings confirm inferences from in vivo studies that T3 acts as a multiplier of a glucose-induced signal.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berdanier C. D., Wurdeman R., Tobin R. B. Further studies on the role of the adrenal hormones in responses of rats to meal-feeding. J Nutr. 1976 Dec;106(12):1791–1800. doi: 10.1093/jn/106.12.1791. [DOI] [PubMed] [Google Scholar]
- Bermudez F., Surks M. I., Oppenheimer J. H. High incidence of decreased serum triiodothyronine concentration in patients with nonthyroidal disease. J Clin Endocrinol Metab. 1975 Jul;41(1):27–40. doi: 10.1210/jcem-41-1-27. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forciea M. A., Schwartz H. L., Towle H. C., Mariash C. N., Kaiser F. E., Oppenheimer J. H. Thyroid hormone-carbohydrate interaction in the rat: correlation between age-related reductions in the inducibility of hepatic malic enzyme by triiodo-L-thyronine and a high carbohydrate, fat-free diet. J Clin Invest. 1981 Jun;67(6):1739–1747. doi: 10.1172/JCI110212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge A. G., Adelman T. G. Regulation of malic enzyme synthesis by insulin triiodothyronine, and glucagon in liver cells in culture. J Biol Chem. 1976 May 25;251(10):3027–3032. [PubMed] [Google Scholar]
- Kaiser F. E., Mariash C. N., Schwartz H. L., Oppenheimer J. H. Inhibition of malic enzyme induction by triiodothyronine in the diabetic rat: reversal by fructose feeding. Metabolism. 1980 Aug;29(8):767–772. doi: 10.1016/0026-0495(80)90201-2. [DOI] [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- Nace C. S., Szepesi B. Dietary fatty acids on the control of glucose-6-phosphate dehydrogenase and malic enzyme in the starved-refed rat. J Nutr. 1976 Feb;106(2):285–291. doi: 10.1093/jn/106.2.285. [DOI] [PubMed] [Google Scholar]
- OPPENHEIMER J. H., SQUEF R., SURKS M. I., HAUER H. BINDING OF THYROXINE BY SERUM PROTEINS EVALUATED BY EQUILIBRUM DIALYSIS AND ELECTROPHORETIC TECHNIQUES. ALTERATIONS IN NONTHYROIDAL ILLNESS. J Clin Invest. 1963 Nov;42:1769–1782. doi: 10.1172/JCI104862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Dillmann W. H., Schwartz H. L., Towle H. C. Nuclear receptors and thyroid hormone action: a progress report. Fed Proc. 1979 Jul;38(8):2154–2161. [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Koerner D., Surks M. I. Limited binding capacity sites for L-triiodothyronine in rat liver nuclei. Nuclear-cytoplasmic interrelation, binding constants, and cross-reactivity with L-thyroxine. J Clin Invest. 1974 Mar;53(3):768–777. doi: 10.1172/JCI107615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Surks M. I. Tissue differences in the concentration of triiodothyronine nuclear binding sites in the rat: liver, kidney, pituitary, heart, brain, spleen, and testis. Endocrinology. 1974 Sep;95(3):897–903. doi: 10.1210/endo-95-3-897. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Stanley F., Casanova J. Depletion of L-3,5,3'-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979 Jul;105(1):80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Schwartz H. L., Forciea M. A., Mariash C. N., Oppenheimer J. H. Age-related reduction in response of hepatic enzymes to 3,5,3'-triiodothyronine administration. Endocrinology. 1979 Jul;105(1):41–46. doi: 10.1210/endo-105-1-41. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Sirica A. E., Richards W., Tsukada Y., Sattler C. A., Pitot H. C. Fetal phenotypic expression by adult rat hepatocytes on collagen gel/nylon meshes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):283–287. doi: 10.1073/pnas.76.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle H. C., Mariash C. N., Oppenheimer J. H. Changes in the hepatic levels of messenger ribonucleic acid for malic enzyme during induction by thyroid hormone or diet. Biochemistry. 1980 Feb 5;19(3):579–585. doi: 10.1021/bi00544a029. [DOI] [PubMed] [Google Scholar]
- Volpe J. J., Vagelos P. R. Regulation of mammalian fatty-acid synthetase. The roles of carbohydrate and insulin. Proc Natl Acad Sci U S A. 1974 Mar;71(3):889–893. doi: 10.1073/pnas.71.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]


