Abstract
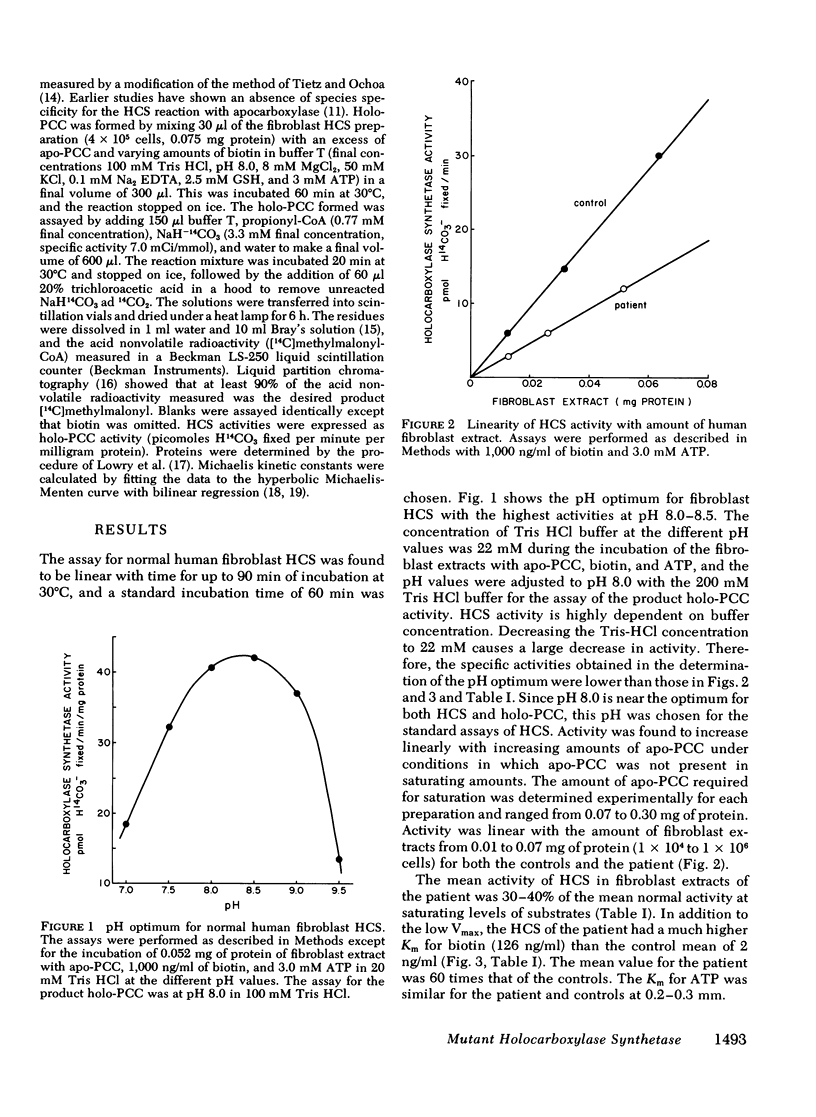
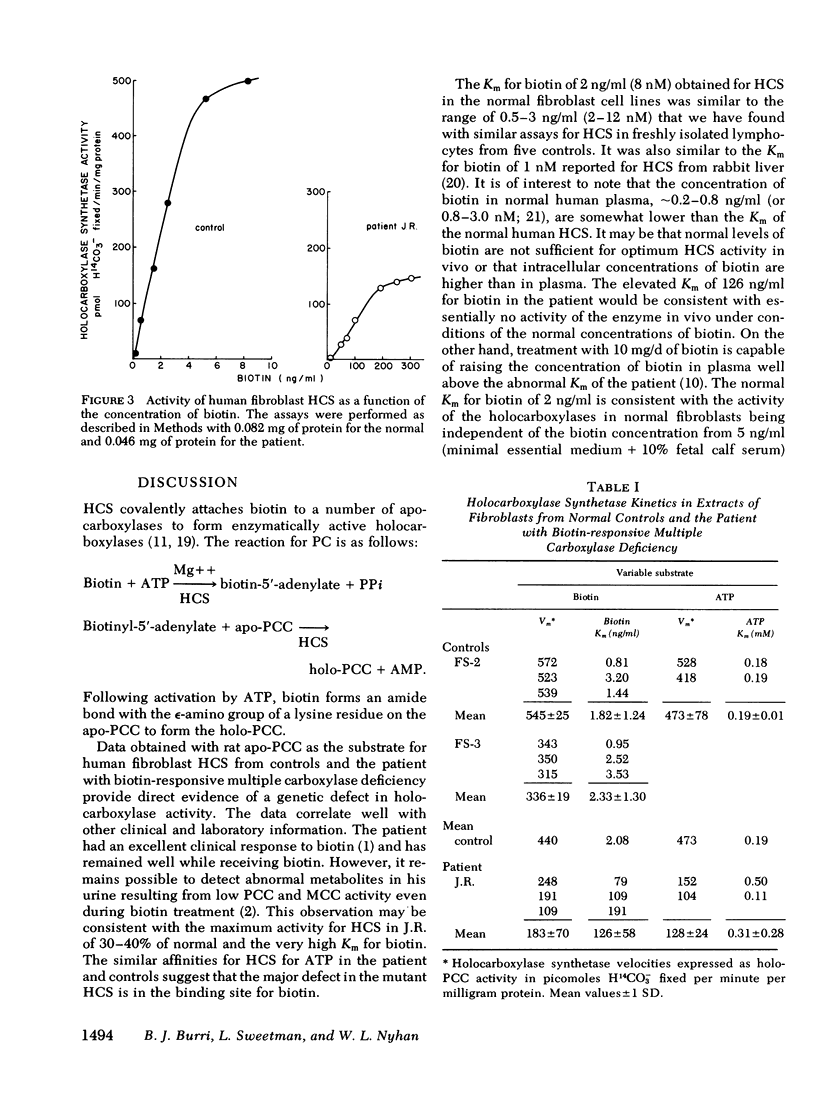
Biotin-responsive multiple carboxylase deficiency is an inherited disorder of organic acid metabolism in man in which there are deficiencies of propionyl-coenzyme A (CoA), 3-methylcrotonyl-CoA, and pyruvate carboxylases that can be corrected with large doses of biotin. It has been proposed that the basic defect in patients with the early infantile form of the disease is in holocarboxylase synthetase, the enzyme that covalently attaches biotin to the inactive apocarboxylases to form active holocarboxylases. We have developed an assay for holocarboxylase synthetase in extracts of human fibroblasts using as substrate apopropionyl-CoA carboxylase partially purified from livers of biotin-deficient rats. Fibroblasts from the initial patient with the infantile form of biotin-responsive multiple carboxylase deficiency were shown to have abnormal holocarboxylase synthetase activity with a maximum velocity about 30-40% of normal, a Km for ATP of 0.3 mM similar to the normal Km of 0.2 mM, and a highly elevated Km for biotin of 126 ng/ml, about 60 times the normal Km of 2 ng/ml. These results show that the primary defect in this patient is a mutation affecting holocarboxylase synthetase activity, and thus a genetic defect of the metabolism of biotin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER H., FRANK O., MATOVITCH V. B., PASHER I., AARONSON S., HUTNER S. H., SOBOTKA H. A new assay method for biotin in blood, serum, urine, and tissues. Anal Biochem. 1962 Jan;3:31–39. doi: 10.1016/0003-2697(62)90041-6. [DOI] [PubMed] [Google Scholar]
- Charles B. M., Hosking G., Green A., Pollitt R., Bartlett K., Taitz L. S. Biotin-responsive alopecia and developmental regression. Lancet. 1979 Jul 21;2(8134):118–120. doi: 10.1016/s0140-6736(79)90003-5. [DOI] [PubMed] [Google Scholar]
- Cowan M. J., Wara D. W., Packman S., Ammann A. J., Yoshino M., Sweetman L., Nyhan W. Multiple biotin-dependent carboxylase deficiencies associated with defects in T-cell and B-cell immunity. Lancet. 1979 Jul 21;2(8134):115–118. doi: 10.1016/s0140-6736(79)90002-3. [DOI] [PubMed] [Google Scholar]
- Gompertz D., Draffan G. H., Watts J. L., Hull D. Biotin-responsive beta-methylcrotonylglycinuria. Lancet. 1971 Jul 3;2(7714):22–24. doi: 10.1016/s0140-6736(71)90009-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Landman A. D., Landman N. N. The binding of biotin to sepharose-avidin column. Demonstration of the affinity chromatography technique. J Chem Educ. 1976 Sep;53(9):591–592. doi: 10.1021/ed053p591. [DOI] [PubMed] [Google Scholar]
- Munnich A., Saudubray J. M., Coude F. X., Charpentier C., Saurat J. H., Frezal J. Fatty-acid-responsive alopecia in multiple carboxylase deficiency. Lancet. 1980 May 17;1(8177):1080–1081. doi: 10.1016/s0140-6736(80)91518-4. [DOI] [PubMed] [Google Scholar]
- Roth K., Cohn R., Yandrasitz J., Preti G., Dodd P., Segal S. Beta-methylcrotonic aciduria associated with lactic acidosis. J Pediatr. 1976 Feb;88(2):229–235. doi: 10.1016/s0022-3476(76)80987-0. [DOI] [PubMed] [Google Scholar]
- SIEGEL L., FOOTE J. L., COON M. J. THE ENZYMATIC SYNTHESIS OF PROPIONYL COENZYME A HOLOCARBOXYLASE FROM D-BIOTINYL 5'-ADENYLATE AND THE APOCARBOXYLASE. J Biol Chem. 1965 Mar;240:1025–1031. [PubMed] [Google Scholar]
- Saunders M., Sweetman L., Robinson B., Roth K., Cohn R., Gravel R. A. Biotin-response organicaciduria. Multiple carboxylase defects and complementation studies with propionicacidemia in cultured fibroblasts. J Clin Invest. 1979 Dec;64(6):1695–1702. doi: 10.1172/JCI109632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman L., Bates S. P., Hull D., Nyhan W. L. Propionyl-CoA carboxylase deficiency in a patient with biotin-responsive 3-methylcrotonylglycinuria. Pediatr Res. 1977 Nov;11(11):1144–1147. doi: 10.1203/00006450-197711000-00006. [DOI] [PubMed] [Google Scholar]
- Thoene J., Baker H., Yoshino M., Sweetman L. Biotin-responsive carboxylase deficiency associated with subnormal plasma and urinary biotin. N Engl J Med. 1981 Apr 2;304(14):817–820. doi: 10.1056/NEJM198104023041404. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B., Hsia Y. E., Sweetman L., Feldman G., Boychuk R. B., Bart R. D., Crowell D. H., Di Mauro R. M., Nyhan W. L. Multiple carboxylase deficiency: clinical and biochemical improvement following neonatal biotin treatment. Pediatrics. 1981 Jul;68(1):113–118. [PubMed] [Google Scholar]


