Abstract
Rickettsia conorii, the causative agent of Mediterranean spotted fever, preferentially infects human microvascular endothelium and activates pro-inflammatory innate immune responses as evidenced by enhanced expression and secretion of cytokines and chemokines. Our recent studies reveal that human microvascular endothelial cells (HMECs) infected with R. conorii also launch ‘antiviral’ host defence mechanisms typically governed by type I interferons. To summarize, infected HMECs secrete IFN-β to activate STAT1 in an autocrine/paracrine manner and display increased expression of IFN-stimulated genes, for example ISG15, which in turn activate innate responses to interfere with intracellular replication of rickettsiae. We now present evidence that UBP43 and SOCS1, known negative regulators of JAK/STAT signalling, are also induced in R. conorii-infected HMECs, of which UBP43 but not SOCS1 functions to negatively regulate STAT1 activation. Interestingly, UBP43 induction is almost completely abolished in the presence of IFN-β-neutralizing antibody, implicating an important role for UBP43 as a feedback inhibitor for IFN-β-mediated STAT1 activation. In contrast, SOCS1 expression is only partially affected by IFN-β neutralization, implicating potential involvement of as-yet-unidentified IFN-independent mechanism(s) in SOCS1 induction during R. conorii infection. A number of IFN-stimulated genes, including ISG15, OAS1, MX1, IRF1, IRF9 and TAP1 are also induced in an IFN-β-dependent manner, whereas GBP1 remains unaffected by IFN-β neutralization. Increased STAT1 phosphorylation in HMECs subjected to UBP43 knockdown led to transcriptional activation of OAS1, MX1 and GBP1, confirming the negative regulatory role of UBP43. Although IRF1, IRF9 and TAP1 were induced by IFN-β, siRNA-mediated silencing of UBP43 or SOCS1 did not significantly affect their transcriptional activation. Expression of ISG15 was, however, increased in HMECs transfected with siRNA for UBP43 and SOCS1. Thus, unique regulatory patterns of induced expression of UBP43, SOCS1 and IFN-stimulated genes represent pathogen-specific responses underlying IFN-β-mediated host endothelial signalling during the pathogenesis of spotted fever group rickettsiosis.
Introduction
Rickettsia conorii, a Gram-negative, obligate intracellular α-proteobacterium known to cause Mediterranean spotted fever (MSF) in humans, represents one of the major spotted fever group (SFG) Rickettsia species. Typically transmitted to humans by infected ticks and characterized by visible skin lesions termed ‘tache-noire’ at the bite site, the disease symptoms include high fever, headache and body rash (Raoult et al., 1986; Sousa et al., 2003). Although MSF is traditionally considered a benign disease, significant morbidity and mortality are evident among people exposed to strains with higher levels of virulence and in cases with delayed diagnosis due to non-specific, initial flu-like symptoms and late intervention with doxycycline therapy. As a prototypic member of SFG rickettsiae, R. conorii preferentially infects the vascular endothelial monolayer lining small and medium-sized blood vessels, causing ‘endothelial activation’ as well as injury (George et al., 1993; Mansueto et al., 2012). Vascular endothelial cells infected with R. conorii acquire a pro-adhesive and pro-inflammatory phenotype characterized by increased expression of surface adhesion molecules and secretion of cytokines and chemokines such as interleukin (IL)-1α, IL-6, IL-8, monocyte chemoattractant protein (MCP)-1 and fractalkine (Kaplanski et al., 1995; Valbuena et al., 2003). In addition, endothelial cells stimulated with interferon (IFN)-γ and tumour necrosis factor-α are capable of killing intracellular R. conorii via nitric oxide-dependent mechanism(s) (Walker et al., 1997) and mice lacking IFN-γ exhibit more than 100-fold greater susceptibility to infection with Rickettsia australis (Walker et al., 2001).
Since their discovery, IFNs have generally been considered as cytokines secreted by virus-infected cells to induce an ‘antiviral state’ in neighbouring host cells through autocrine/paracrine signalling mechanism(s). Among these, a single IFN-β along with a number of IFN-α proteins are classified as type I IFNs, which interfere with viral replication by inducing host gene expression. Although some of these target genes also display anti-bacterial activity (Monroe et al., 2010), type I IFNs have traditionally been assigned a relatively minor role and consequently received much less attention in anti-bacterial host defence mechanisms. Recently, we have reported on the expression and secretion of IFN-β from cultured microvascular endothelial cells and demonstrated an important role for this type I IFN in modulating innate immune responses to inhibit intracellular growth of R. conorii. Intriguingly, as a component of the Janus kinase–signal transducer and activator of transcription (JAK/STAT) signalling pathway, phosphorylation/activation of transcription factor STAT1 primarily involves autocrine/paracrine effects of IFN-β and is indispensable for IFN-β’s anti-rickettsial activity in cultured human endothelium (Sahni et al., 2009; Colonne et al., 2011a). Our findings further suggest that R. conorii infection also induces the expression of an IFN-stimulated gene of 15 kDa (ISG15) via IFN-β-dependent JAK/STAT signalling in human endothelial cells. Moreover, intracellular levels of free ISG15 as well as ISG15 conjugated to other as yet unknown host cellular proteins are also increased during infection and participate in protective innate immune response by suppressing the intracellular rickettsial growth in infected host cells (Colonne et al., 2011b).
Activation of JAK/STAT signalling by IFNs is tightly regulated by specific negative regulators at multiple levels. Three families of proteins known as phospho-tyrosine phosphatases (PTPs), suppressors of cytokine signalling (SOCS), and protein inhibitors of activated STAT (PIAS) have been implicated in negative regulation of the JAK/STAT signalling pathway. Specifically, members of the PTP family proteins, namely SHP1 and SHP2, negatively regulate IFN signalling by dephosphorylating activated JAK1 and JAK2 proteins (Klingmüller et al., 1995; You et al., 1999). SH2 domains of SOCS proteins, on the other hand, inhibit IFN signalling by competing with STATs for the receptor binding sites, inhibiting JAKs by direct binding, or by targeting bound proteins for proteasomal degradation (Kamizono et al., 2001; Kile et al., 2002). SOCS1, a prototype member of the SOCS family, can interact with different cellular proteins such as JAKs, IRAK (interleukin-1-receptor-associated kinase) and NF-κB subunits p50/p65 to regulate a wide range of cellular functions, including proliferation, differentiation, apoptosis and immune responses in a cell-specific manner (Fujimoto & Naka, 2010). Negative regulators belonging to the PIAS family inhibit STAT-mediated gene activation. PIAS1 and PIAS3 bind to STAT1 and STAT3, respectively, to inhibit DNA-binding activity of phosphorylated STAT proteins thereby inhibiting transcriptional activation of IFN-stimulated genes (Chung et al., 1997; Liao et al., 2000). UBP43, a ubiquitin-specific protease, has also been identified as a negative regulator of type I IFN signalling. It associates with IFN-α receptor 2 subunit (IFNAR2), preventing its binding to JAK and thereby inhibiting downstream IFN-β signalling cascades (Malakhova et al., 2006).
Subversion of JAK/STAT signalling through negative regulatory proteins has emerged as an important survival strategy for intracellular microbes. As an example, influenza A and herpes simplex viruses interfere with IFN signalling by activating SOCS3 expression during host invasion (Pauli et al., 2008; Yokota et al., 2004). Although R. conorii infection augments IFN-β response during endothelial cell infection, the status of negative regulators of the JAK/STAT pathway remains completely unknown. To address this critical regulatory aspect of IFN signalling, we have investigated whether or not R. conorii infection alters the expression of SOCS1 and UBP43 and further determined the effects of such changes on IFN-β-dependent STAT1 activation and stimulation of responsive downstream genes in human endothelial cells. The presented results reveal that, although R. conorii infection induces the expression of both SOCS1 and UBP43 in endothelium, IFN-β-dependent STAT1 activation is selectively regulated by UBP43 but not SOCS1 protein. Moreover, we have also identified a specific subset of IFN-stimulated genes induced by R. conorii infection and evaluated the inhibitory effects of UBP43 and SOCS1 on these IFN-stimulated genes in R. conorii-infected endothelium.
Methods
Cell culture and infection.
Rickettsia conorii (Malish 7 strain) was propagated in Vero cells and stocks prepared by density-gradient centrifugation followed by plaque formation assay to estimate the infectivity titres were kept frozen as aliquots. An immortalized line of human dermal microvascular endothelial cells (HMEC-1) was grown under sterile culture conditions in MCDB 131 medium (Gibco), supplemented with FBS (10 % v/v; Aleken Biologicals), epidermal growth factor (10 ng ml−1; Becton Dickinson), hydrocortisone (1 µg ml−1; Sigma) and l-glutamine (10 mM; Gibco). At approximately 80 % confluence, the monolayers of HMECs were infected with 6×104 p.f.u. of R. conorii per cm2 of culture surface area according to our established protocols (Sporn et al., 1997; Colonne et al., 2011a). At 3 h post-infection, extracellular bacteria in the culture medium were removed by aspiration and gentle washing and infected cells were placed in fresh culture medium alone for the remaining duration of incubation. This protocol consistently results in the infection of 80 to 90 % of cells with a mean of 3 to 4 rickettsiae per cell at 6 h post-infection (Sporn et al., 1997; Rydkina et al., 2007; Colonne et al., 2011a). In each experiment, the viability of both mock- and R. conorii-infected host cells at different time points was ascertained microscopically.
Cell treatment.
To neutralize secreted IFN-β, culture medium was supplemented with anti-human IFN-β antibody (10 µg ml−1; R&D Systems) immediately after 3 h infection. To inhibit the metabolic activity of intracellular R. conorii, tetracycline (20 µg ml−1; Sigma) was added to the culture medium at 3 h post-infection. For treatment, recombinant human IFN-β (10 ng ml−1; PBL Interferon Source) was added to the cell culture medium.
Small interfering RNAs for SOCS1 and UBP43.
Specific ON-TARGETplus smart pools of siRNA for SOCS1 and UBP43 along with a control (scrambled) sequence were obtained from Thermo Scientific. HMECs at 80 % confluence were transfected with SOCS1-specific, UBP43-specific, or scrambled siRNAs (final concentration of 100 nM) using Lipofectamine 2000 (Invitrogen) according to our published protocols (Colonne et al., 2011a, b). After 6 h, transfection medium was replaced with fresh culture medium and the cells were allowed to recover for at least 12 h prior to infection with R. conorii.
Gene expression analysis by quantitative real-time PCR.
Total RNA isolated from mock- and R. conorii-infected endothelial cells using TRI-Reagent (Molecular Research Center) was further purified using an RNA purification kit (Qiagen) and quantified on a Nanodrop spectrophotometer (ND-1000, Thermo Scientific). Complementary DNA (cDNA) was then synthesized using the RT2 First Strand kit (Qiagen). Validated primers quantifying the expression of SOCS1, UBP43, ISG15, OAS1, TAP1, MX1, GBP1, IRF1, IRF9 and GAPDH were purchased from Qiagen. Quantitative PCRs were performed in a MyiQ thermal cycler (Bio-Rad) with RT2 Real-time SYBR Green Master mix (Qiagen) according to the manufacturer’s instructions. The levels of expression of target genes were normalized to GAPDH and relative expression was calculated by the ΔΔCt method.
Western blot analysis.
Monolayers of uninfected and R. conorii-infected HMECs were washed with PBS and disrupted by scraping and suspension in a cell lysis solution [Tris buffer (100 mM, pH 7.4), supplemented with a mixture of protease and phosphatase inhibitors and 0.2 % w/v SDS] followed by mild sonication. Total protein lysates thus prepared were separated on a 10 % w/v polyacrylamide gel. The proteins were then transferred onto a nitrocellulose membrane by wet blotting at 100 V for 90 min. The blots were probed with primary antibodies against pSTAT1 (Tyr701) and UBP43 (Cell Signaling Technology) and a compatible HRP-linked secondary antibody for chemiluminescence-based detection. To normalize for variations in the loading of samples on different lanes, the blots from all experiments were stripped and probed with a mouse anti-human α-tubulin antibody (Accurate Chemical & Scientific Corporation), followed by detection with an anti-mouse IgG-HRP (Santa Cruz). Protein–antibody complexes were revealed using the Western Lightning enhanced chemiluminescence detection system (PerkinElmer) and exposure to X-ray film.
Densitometric and statistical analysis.
Blots were scanned in grayscale mode at a resolution of 600 d.p.i. Band intensities were calculated using ImageJ software (version 1.42), normalized to the housekeeping gene α-tubulin, and assigned values relative to the corresponding uninfected control, which was given a value of 1 for ease of comparison. All experiments were performed at least in triplicate and statistical significance between control and experimental conditions was evaluated by Student’s t-test. Results were considered to be statistically significant at a threshold P-value of ≤0.05.
Results
R. conorii infection induces SOCS1 and UBP43 expression in human endothelial cells
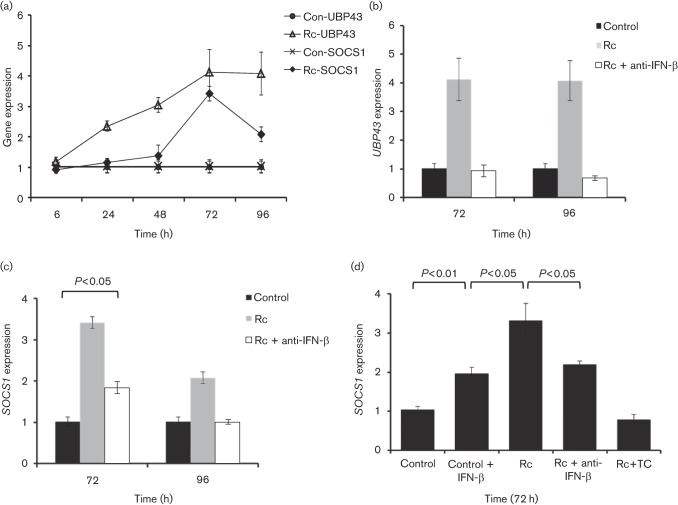
Human microvascular endothelial cells respond to R. conorii infection in vitro by secreting IFN-β, which is responsible for activating autocrine and/or paracrine innate immune responses via transcriptional activation of STAT1 to inhibit intracellular rickettsial replication (Colonne et al., 2011a). Because negative regulators of the JAK/STAT pathway represent an important arm of the signalling network involved in the regulation of expression of host IFN-responsive genes, we first determined which specific players are induced in R. conorii-infected HMECs. We found that expression of SOCS1 and UBP43, known inhibitors of IFN signalling, was induced during rickettsial infection (Fig. 1). UBP43 expression was significantly higher during R. conorii infection in comparison with the corresponding uninfected controls at 24 and 48 h, which was followed by the peak level of response at 72 h and then sustained through 96 h post-infection. SOCS1 expression, on the other hand, displayed only minimal changes early during the infection followed by significant increase of about 3.5-fold at 72 h post-infection and a subsequent decline to a mean of 2-fold induction at 96 h. These results demonstrate induced expression of SOCS1 and UBP43 and reveal clearly noticeable differences in the intensity and kinetics of such responses during R. conorii infection of host endothelial cells (Fig. 1a). Further, upregulation of UBP43 expression was attributable to IFN-β produced and secreted by endothelial cells since infection in the presence of an antibody capable of neutralizing IFN-β completely abolished this host cell response. This finding also implies the dependence of cellular UBP43 induction during infection predominantly on autocrine/paracrine effects of IFN-β and rules this response out as a consequence of pathogen invasion and/or intracellular replication (Fig. 1b). Interestingly, SOCS1 expression was only partially inhibited at 72 h and completely attenuated by neutralization of IFN-β at 96 h (Fig. 1c), implicating potential contributions from IFN-β-independent transcriptional activation mechanism(s), likely triggered by R. conorii invasion and intracellular multiplication. To this end, we further quantified the levels of SOCS1 mRNA expression in cells treated with recombinant IFN-β alone in comparison with those infected with R. conorii in the presence and absence of tetracycline and an IFN-β neutralizing antibody. As shown in Fig. 1d, SOCS1 expression during R. conorii infection was significantly higher than in HMECs subjected to IFN-β treatment alone and neutralization of IFN-β yielded only partial inhibition of SOCS1 expression in infected cells. Also, inhibition of rickettsial metabolic activity by tetracycline treatment completely attenuated SOCS1 expression, indicating that infection with viable intracellular rickettsiae is essential for SOCS1 induction. Taken together, these data suggest that R. conorii infection induces the expression of SOCS1 and UBP43, known negative regulators of IFN signalling, in host HMECs.
Fig. 1.
R. conorii infection induces SOCS1 and UBP43 expression in human microvascular endothelial cells. (a) Time-course analysis of SOCS1 and UBP43 expression during R. conorii infection. The levels of SOCS1 and UBP43 expression were determined relative to the housekeeping gene GAPDH by quantitative RT-PCR. (b) Inclusion of an IFN-β-neutralizing antibody during R. conorii infection inhibits UBP43 expression in HMECs. Infection was carried out in the absence (Rc) or presence of an antibody capable of neutralizing soluble IFN-β (Rc+anti-IFN-β) in comparison with the corresponding uninfected cells (Con). (c) Partial dependence of SOCS1 expression on R. conorii-induced IFN-β at 72 and 96 h post-infection. SOCS1 expression was determined in RNA preparations from uninfected (Con) and R. conorii-infected (Rc) HMECs and those infected in the presence of an IFN-β-neutralizing antibody (Rc+anti-IFN-β) as mentioned above. (d) Further analysis of IFN-β-independent expression of SOCS1. The expression of SOCS1 transcript was quantified in HMECs subjected to the following experimental conditions: uninfected/untreated (Control), recombinant human IFN-β-treated (Control+IFN-β), R. conorii-infected (Rc), infection in the presence of an anti-IFN-β antibody (Rc+anti-IFN-β) and infection in the presence of tetracycline as described in Methods (Rc+TC). The datasets represent the mean±se of the mean from a minimum of three independent experiments performed in duplicate.
Inhibitory effect of UBP43, but not SOCS1, on R. conorii-induced STAT1 activation
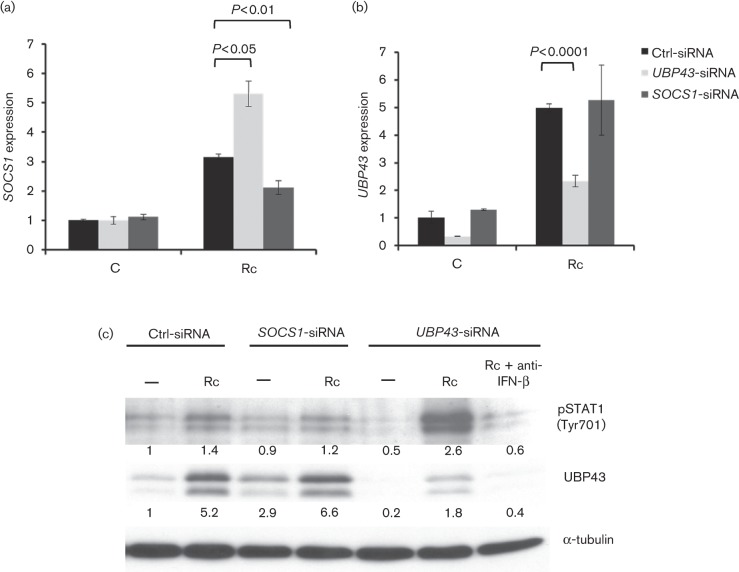
Since both UBP43 and SOCS1 have been implicated in the negative regulation of the STAT1 activation response, we next investigated whether IFN-β-dependent STAT1 phosphorylation during R. conorii infection of HMECs is regulated by SOCS1 or UBP43. To investigate this, we utilized the RNA interference approach to transiently knockdown the expression of UBP43 or SOCS1 in HMECs prior to infection with R. conorii. As expected, infection stimulated the expression of both SOCS1 and UBP43 by a mean of 3- and 5-fold, respectively, in HMECs transfected with a scrambled siRNA sequence used as a negative control. Also, transcriptional activation of both of these genes following infection was significantly inhibited in HMECs subjected to introduction of specific ON-target siRNA for SOCS1 (Fig. 2a) and UBP43 (Fig. 2b), respectively. Interestingly, siRNA-mediated knockdown of SOCS1 did not have a significant impact on the transcriptional activation of UBP43, but HMECs transfected with UBP43-specific siRNA and subsequently infected with R. conorii had significantly higher levels of SOCS1 expression (Fig. 2a, b). Although analysis of protein expression further ascertained that SOCS1 knockdown did not adversely affect the expression of both full-length protein as well as a truncated isoform (splice variant) of UBP43 in infected endothelial cells (Fig. 2c), we were not able to determine the levels of SOCS1 protein by Western blotting owing to relatively very low abundance in HMECs. R. conorii infection resulted in the stimulation of STAT1 Tyr701 phosphorylation in HMECs transfected with both scrambled sequence (control) and SOCS1-specific siRNA, the intensity of which was not significantly different (Fig. 2c). Thus, interference with SOCS1 expression did not affect the status of STAT1 phosphorylation, indicating that IFN-β-dependent STAT1 activation due to R. conorii infection is not regulated by SOCS1. In contrast, depletion of UBP43 led to the potentiation of STAT1 phosphorylation and this response was completely blocked by the presence of IFN-β-neutralizing antibody in the culture medium. Together, these data show that UBP43, but not SOCS1, functions as a feedback inhibitor to regulate IFN-β-dependent STAT1 activation in infected endothelium.
Fig. 2.
Effect of UBP43 and SOCS1 silencing with small interfering RNA on the status of STAT1 activation in R. conorii-infected HMECs. Human microvascular endothelial cells were transfected with siRNA sequences against SOCS1 (SOCS1-siRNA) or UBP43 (UBP43-siRNA) and then infected with R. conorii for 72 h. The levels of expression were determined relative to the housekeeping gene GAPDH. (a) Effect of UBP43 knockdown on SOCS1 expression. UBP43-siRNA transfected cells were either left uninfected (C) or infected with R. conorii (Rc) and SOCS1 expression was analysed by quantitative RT-PCR. UBP43 expression was also measured to confirm gene knockdown. Cells transfected with non-specific, scrambled sequences were used as controls (Ctrl-siRNA). (b) Effect of SOCS1 knockdown on UBP43 expression in HMECs. Cells were transfected with SOCS1-specific siRNA and infected with R. conorii followed by quantitative RT-PCR measurements to measure UBP43 expression. The data are presented as the mean ± se of three independent experiments. (c) Effects of SOCS1 and UBP43 siRNA on STAT1 phosphorylation induced by R. conorii infection. Total proteins were extracted at 72 h post-infection and processed for Western detection using antibodies directed against pSTAT1 (Tyr701) and UBP43. Primary antibody used in this experiment is capable of binding with both the full-length and the truncated isoform of UBP43 protein. The band intensities for pSTAT1 and UBP43 were determined by quantitative densitometry, for which the levels of α-tubulin were used to account for any variations in the loading of samples.
Transcriptional activation of IFN-stimulated genes in R. conorii-infected endothelium
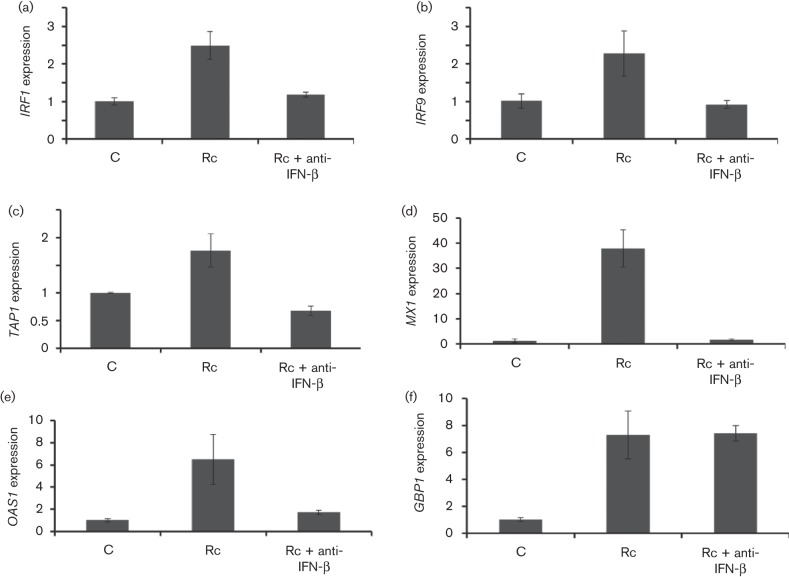
Previous studies from our laboratory have shown that secreted IFN-β from R. conorii-infected HMECs induces an IFN-stimulated gene ISG15 to activate innate host defence to inhibit intracellular growth of the pathogen (Colonne et.al, 2011b). Using quantitative PCR, we have further identified a series of IFN-stimulated genes whose mRNA expression is significantly upregulated in endothelial cells infected with R. conorii. As shown, transcriptional activation of IFN regulatory factor-1 (IRF1), IRF9, transporter of antigen peptides-1 (TAP1), myxovirus resistance protein (MX1) and oligoadenylate synthetase-1 (OAS1) was clearly evident and occurred in an IFN-β-dependent manner, because presence of an antibody capable of neutralizing the activity of IFN-β effectively curtailed the expression of these genes (Fig. 3a–e). IFN-β neutralization, however, did not have an effect on the induction of guanylate binding protein-1 (GBP1) (Fig. 3f).
Fig. 3.
Transcriptional status of IFN-stimulated genes in HMECs after R. conorii infection. Endothelial cells were infected with R. conorii for 72 h in the absence (Rc) and presence of an antibody to neutralize the autocrine/paracrine effects of IFN-β (Rc+anti-IFN-β). Simultaneously processed uninfected cells (C) were used to analyse the basal level of expression. The bar diagrams depict the expression levels of IFN-stimulated genes, namely IRF1 (a), IRF9 (b), TAP1 (c), MX1 (d), OAS1 (e) and GBP1 (f), as determined by quantitative RT-PCR and normalized to GAPDH (housekeeping gene) by the ΔΔCt method. The data represent the mean±se from at least three separate observations in relation to baseline expression in uninfected controls which was given a value of 1 to facilitate comparison between experimental groups.
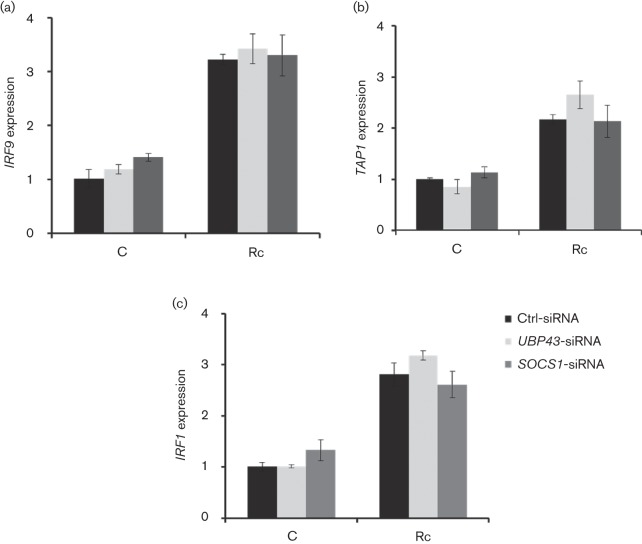
Role of SOCS1 and UBP43 in regulating IFN-stimulated genes in R. conorii-infected HMECs
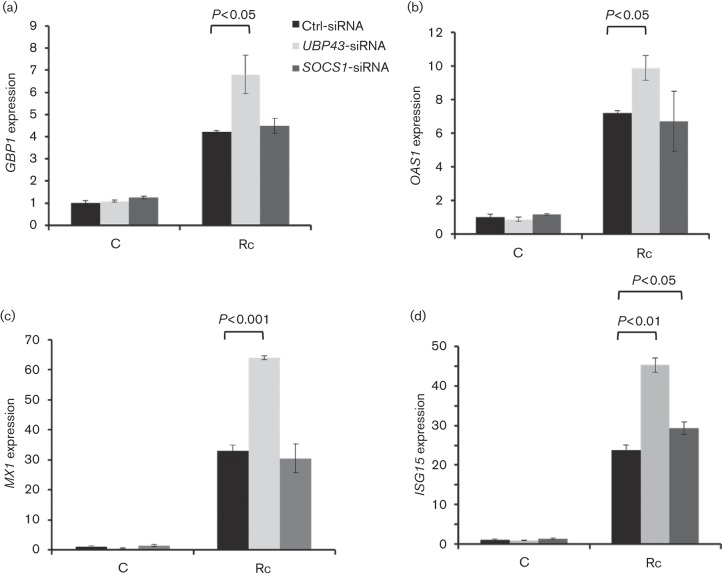
Because expression of IFN-stimulated downstream genes during inflammatory and microbial insults is subject to tight regulatory control in order to prevent severe immunopathology and damage to the host cells and our findings suggest increased expression of UBP43 and SOCS1 during R. conorii infection of HMECs, we reasoned that UBP43 and SOCS1 may have an important role in the regulation of transcriptional activation of IFN-stimulated genes. Therefore, HMECs transfected with either UBP43 or SOCS-1-specific siRNA along with a control scrambled sequence were infected with R. conorii prior to the analysis of expression of IFN-stimulated genes. As expected, R. conorii infection induced the expression of GBP1, OAS1, MX1, ISG15, IRF1, IRF9 and TAP1 in cells transfected with control siRNA (Figs 4 and 5). Further, siRNA-mediated knockdown of UBP43 led to significant increase in the expression of GBP1, OAS1 and MX1, whereas SOCS1 knockdown had no significant effect on these genes (Fig. 4a–c). ISG15 expression during infection was, however, induced further in host cells subjected to UBP43 as well as SOCS1 siRNA, although the effect was much more pronounced with the knockdown of UBP43 as compared with SOCS1 (Fig. 4d). On the other hand, the expression of IRF1, IRF9 and TAP1 was not significantly affected by silencing the expression of either SOCS1 or UBP43 (Fig. 5). These results thus suggest that UBP43 and SOCS1 not only are induced in response to R. conorii infection, but also selectively regulate the expression of specific IFN-stimulated genes in microvascular endothelial cells.
Fig. 4.
Effect of SOCS1 and UBP43 knockdown on transcriptional activation of IFN-stimulated genes in R. conorii-infected HMECs. Endothelial cells were transfected with gene-specific siRNA sequences directed against SOCS1 or UBP43 prior to infection with R. conorii for 72 h. The expression levels (relative to GAPDH) of GBP1 (a), OAS1 (b), MX1 (c) and ISG15 (d) in uninfected controls (C) and infected HMECs (Rc) were analysed by quantitative RT-PCR. Expression of all of these IFN-stimulated genes was negatively regulated by UBP43, with the exception of ISG15, which was negatively regulated by both UBP43 and SOCS1. The datasets represent mean±se from a minimum of three independent observations performed in duplicate.
Fig. 5.
Effect of SOCS1 and UBP43 knockdown on transcriptional activation of IFN-stimulated genes in R. conorii-infected HMECs. Endothelial cells were transfected with gene-specific siRNA sequences directed against SOCS1 or UBP43 and then infected with R. conorii for 72 h. Expression levels (relative to GAPDH) of IRF9 (a), TAP1 (b) and IRF1 (c) in uninfected controls (C) and infected HMECs (Rc) were analysed by quantitative RT-PCR. Notably, expression of none of these IFN-stimulated genes was significantly altered by either UBP43 or SOCS1 knockdown. The datasets represent mean±se from a minimum of three independent observations performed in duplicate.
Discussion
We have recently demonstrated the activation of host cell JAK/STAT signalling and an essential role for IFN-β, a type I IFN, in STAT1-mediated interference with intracellular replication of R. conorii in vascular endothelial cells in vitro (Sahni et al., 2009; Colonne et al., 2011a). Signalling mechanisms downstream of JAKs and STATs are controlled at many steps through a variety of distinct mechanisms, including key negative regulators such as the suppressor of cytokine signalling (SOCS) proteins. Microbial pathogens are known to subvert and subterfuge IFN-governed innate immune responses of the host via modulation of negative regulators of the JAK/STAT pathway, yet the potential importance of such mediators in determining IFN-β-mediated responses during host cell interactions with pathogenic Rickettsia species is not at all understood. In the present study, we have identified increased expression of UBP43 and SOCS1, proteins implicated in the feedback inhibitory loop of the JAK/STAT signalling pathway, during R. conorii infection of HMECs and report on their selective involvement in the regulation of downstream IFN-β-responsive genes.
UBP43, a member of the family of ubiquitin isopeptidases, specifically cleaves a ubiquitin-like molecule ISG15, which is induced by stress signals such as type I IFNs and viral infections and associates with other cellular proteins to form ISGylated complexes. Documented evidence suggests that UBP43 negatively regulates IFN signalling by blocking interactions between JAK kinase and the IFNAR2 receptor subunit, thereby interfering with STAT1 activation (Malakhova et al., 2006). Our findings are in agreement with such a role for UBP43, because neutralization of IFN-β during R. conorii infection results in complete attenuation of UBP43 induction, indicating that UBP43 likely functions as a feedback inhibitory mechanism to regulate STAT1 activation. Silencing of UBP43 expression also results in even stronger STAT1 phosphorylation in infected cells, yielding further support to the notion that activation of STAT1 is negatively regulated by UBP43. In addition to its role as an inhibitor for IFN signalling, UBP43 catalyses the deISGylation reaction by cleaving ISG15–protein complexes and in doing so regulates the extent of cytosolic protein ISGylation (Malakhov et al., 2002). Thus, UBP43 maintains the delicate cellular equilibrium between free cytosolic ISG15 and ISG15–protein conjugates, which are strongly increased after IFN stimulation. We have also demonstrated that isopeptidase activity of UBP43 leads to increased accumulation of ISG15–protein complexes in endothelial cells subjected to RNA interference during rickettsial infection and accumulation of free ISG15 and/or proteins conjugated to ISG15 exerts inhibitory effects on R. conorii replication (Colonne et al., 2011b). Therefore, UBP43 apparently performs a dual role as an ISG15 protease as well as a negative regulator for IFN-β signalling in R. conorii-infected host endothelium.
SOCS1 is one of the major prototypical proteins belonging to the suppressor of cytokine signalling family. It binds to JAK proteins via an SH2 domain and blocks the phosphorylation of STAT1, preventing its dimerization and nuclear translocation, a critically important step for IFN signalling (Dai et al., 2006; Qin et al., 2006). Intracellular pathogens specifically induce SOCS proteins during host invasion to antagonize IFN signalling, leading to the dampening of immune responses. For instance, hepatitis C virus infection has been shown to induce SOCS1 and SOCS3 expression, resulting in the inhibition of STAT1 and STAT3 phosphorylation and subsequent interference with T-cell functions (Yao et al., 2005). Our findings clearly demonstrate that R. conorii-infected endothelial cells display increased SOCS1 transcription and, interestingly, only partial inhibition of SOCS1 expression following IFN-β neutralization. It is well established that the promoter region of the SOCS1 gene contains putative STAT1 binding sites (Naka et al., 1997; Saito et al., 2000). Therefore, activated STAT1 apparently contributes to only partial induction of SOCS1 expression. This may further explain the increased transcriptional activation of SOCS1 in cells experiencing UBP43 knockdown because UBP43 depletion strongly increased STAT1 phosphorylation in infected cells. It is also possible that induced SOCS1 expression is a component of rickettsial strategy to subdue and subvert IFN signalling in host endothelium and that the levels of accumulation of SOCS1 in infected host cells may correlate with the virulence potential of the invading pathogen and the severity of resulting disease. Although the magnitude of SOCS1 induction independent of IFN-β is about two-fold, it is expected to have significant implications since SOCS proteins are potent, tightly regulated inhibitors known for their capacity to exert biological effects even in relatively small quantities (Chen et al., 2000). Considering that interference with SOCS1 yields no adverse effect on STAT1 phosphorylation, it apparently functions through a mechanism independent of inhibitory feedback regulation of STAT1 activation. SOCS1 may, however, negatively regulate a specific subset of host genes, for example ISG15, via other mechanism(s) independent of direct inhibition of IFN-β signalling in R. conorii-infected endothelium. Another important consideration in this context is that anti-rickettsial host responses activated by IFN-γ may also be adversely affected because SOCS1 is capable of inhibiting IFN-γ mediated immune responses (Alexander et al., 1999), which have been implicated in rickettsial clearance in vivo (Walker et al., 2001). Therefore, further detailed studies are currently being performed to investigate the potential impact of SOCS1 induction on the host immune responses and rickettsial replication in both in vitro and in vivo murine models relevant to the pathogenesis of human rickettsiosis. Also, the identity of pathogen-associated molecular patterns or rickettsial effectors responsible for the induction and/or subversion of host cell signalling mechanisms is the subject of further detailed investigations in our laboratory. Production of IFN-β and expression of downstream genes by host endothelium likely constitutes an essential first line of defence against invading and intracellular rickettsiae and offer a unique strategic opportunity to identify potential rickettsial effectors and/or pathogen recognition mechanisms involved in switching on the upstream signalling mechanisms leading to such responses.
In addition to UBP43 and SOCS1, this study also identifies transcriptional activation of OAS1, IRF1, IRF9, MX1 and TAP1, and provides evidence for the dependence of these genes on IFN-β production and resultant autocrine/paracrine signalling from infected cells. Expression of GBP1, on the other hand, seems to occur independently of the host IFN-β signalling as the presence of IFN-β-neutralizing antibody during the infection has no effect on its expression. GBP1 is an IFN-γ-inducible gene known for antimicrobial effects against intracellular bacteria, for example Chlamydia and Listeria infections (Tietzel et al., 2009; Kim et al., 2011). However, it can also be induced by type I IFNs, IL-1β and TNF (Decker et al., 1991; Guenzi et al., 2001, 2003). Although the detailed molecular mechanism of this activation is not yet established, IFN-β may indirectly regulate endothelial GBP1 activation, possibly via activated STAT1, because GBP1 expression displays significant increase after UBP43 knockdown, which is associated with increased STAT1 phosphorylation. Nevertheless, the potential involvement of GBP1 in anti-rickettsial activity has not been explored and requires detailed investigation.
Among other IFN-stimulated genes, OAS1 activates latent RNase, which degrades viral RNA, thereby inhibiting viral replication (Coccia et al., 1990; Lin et al., 2009). So far, there is no published evidence to suggest that OAS1 has anti-bacterial functions. Therefore, some of the IFN-stimulated genes may simply represent a somewhat generalized response to IFN-β, instead of specific cellular responses of the host to intracellular rickettsiae. Another downstream gene, MX1, codes for a member of the large GTPase family of proteins, which is mainly induced by IFNα/β during viral infections (Staeheli et al., 1986; Grimm et al., 2007). However, the exact mechanisms of MX1 protein function also remain largely unknown. In our system, the expression of both OAS1 and MX1 was negatively regulated by UBP43 but not by SOCS1, because increased STAT1 activation due to UBP43 interference enhances transcriptional activation of both OAS1 and MX1 genes. TAP1 facilitates translocation of peptides from cytosol into endoplasmic reticulum and participates in the expression of MHC class I molecules on the cell surface and antigen presentation to cytotoxic T lymphocytes during microbial infections (Van Kaer et al., 1992; de la Salle et al., 1994). Interestingly, the TAP1 gene can be induced by both IFN-γ and IFN-β via formation of STAT1 homodimers, which bind to GAS elements on the gene promoter (Min et al., 1998).
Initially characterized for its role in the transcriptional activation of type I IFN genes, IRF1 acts as a transcription factor by binding to the IFN-β gene promoter to regulate its transcription and that of several other downstream IFN-stimulated genes implicated in promoting anti-bacterial and antiviral innate immunity (Miyamoto et al., 1988; Harada et al., 1989). As another key transcription factor, IRF9, associates with phosphorylated STAT1/STAT2 heterodimers to form the ISGF3 complex, which translocates to the nucleus and binds to regulatory IFN-stimulated response elements (ISREs) to induce the expression of specific IFN-stimulated genes (Levy et al., 1989; Fu et al., 1992). We find that although IRF1, IRF9 and TAP1 are induced during R. conorii infection in an IFN-β dependent manner, the expression of these genes is refractory to interference with UBP43 or SOCS1 response. Thus, R. conorii-induced STAT1 activation is apparently sufficient to trigger the expression of IRF1, IRF9 and TAP1 genes, and potentiation of STAT1 phosphorylation as a consequence of UBP43 knockdown essentially has no effect on their transcriptional activation. On the contrary, ISG15 is negatively regulated by both SOCS1 and UBP43. Since ISG15 is a STAT1-dependent gene, increased STAT1 activation following UBP43 knockdown could trigger a strong transcriptional activation of ISG15 expression. Importantly, ISG15 represents the only IFN-stimulated gene identified in this study to be regulated by SOCS1, as evidenced by significant increase in its expression in cells subjected to SOCS1 knockdown. Since STAT1 activation remains unaffected by SOCS1 interference, the inhibitory effects of SOCS1 on ISG15 expression may be attributed to other as-yet-unidentified STAT1-independent mechanism(s).
To summarize, distinct patterns of the regulation of IFN-stimulated genes by SOCS1 and UBP43 may represent host cell-specific mechanisms of innate immune responses against Rickettsia infection. Elucidation of the roles of these newly identified IFN-stimulated genes and their negative regulators in anti-rickettsial host defence is an important area of future scientific enquiry. Considering the emergence of severe forms/strains of R. conorii with fatal outcomes despite treatment with antibiotics, understanding the pathogen-induced regulation of IFN signalling is expected to provide new insights into novel virulence mechanisms exploited by infectious rickettsiae during the onset and progression of resultant clinical syndromes.
Acknowledgements
This work was supported, in part, by extramural funds provided by the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA, through research project grants R01 AI067613, R21 AI 076697 and R56 AI091777.
Abbreviations:
- HEMC
human microvascular endothelial cell
References
- Alexander W. S., Starr R., Fenner J. E., Scott C. L., Handman E., Sprigg N. S., Corbin J. E., Cornish A. L., Darwiche R., et al. (1999). SOCS1 is a critical inhibitor of interferon γ signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98, 597–608 10.1016/S0092-8674(00)80047-1 [DOI] [PubMed] [Google Scholar]
- Chen X. P., Losman J. A., Rothman P. (2000). SOCS proteins, regulators of intracellular signaling. Immunity 13, 287–290 10.1016/S1074-7613(00)00028-5 [DOI] [PubMed] [Google Scholar]
- Chung C. D., Liao J., Liu B., Rao X., Jay P., Berta P., Shuai K. (1997). Specific inhibition of Stat3 signal transduction by PIAS3. Science 278, 1803–1805 10.1126/science.278.5344.1803 [DOI] [PubMed] [Google Scholar]
- Coccia E. M., Romeo G., Nissim A., Marziali G., Albertini R., Affabris E., Battistini A., Fiorucci G., Orsatti R., et al. (1990). A full-length murine 2-5A synthetase cDNA transfected in NIH-3T3 cells impairs EMCV but not VSV replication. Virology 179, 228–233 10.1016/0042-6822(90)90292-Y [DOI] [PubMed] [Google Scholar]
- Colonne P. M., Eremeeva M. E., Sahni S. K. (2011a). Beta interferon-mediated activation of signal transducer and activator of transcription protein 1 interferes with Rickettsia conorii replication in human endothelial cells. Infect Immun 79, 3733–3743 10.1128/IAI.05008-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonne P. M., Sahni A., Sahni S. K. (2011b). Rickettsia conorii infection stimulates the expression of ISG15 and ISG15 protease UBP43 in human microvascular endothelial cells. Biochem Biophys Res Commun 416, 153–158 10.1016/j.bbrc.2011.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X., Sayama K., Yamasaki K., Tohyama M., Shirakata Y., Hanakawa Y., Tokumaru S., Yahata Y., Yang L., et al. (2006). SOCS1-negative feedback of STAT1 activation is a key pathway in the dsRNA-induced innate immune response of human keratinocytes. J Invest Dermatol 126, 1574–1581 10.1038/sj.jid.5700294 [DOI] [PubMed] [Google Scholar]
- de la Salle H., Hanau D., Fricker D., Urlacher A., Kelly A., Salamero J., Powis S. H., Donato L., Bausinger H., et al. (1994). Homozygous human TAP peptide transporter mutation in HLA class I deficiency. Science 265, 237–241 10.1126/science.7517574 [DOI] [PubMed] [Google Scholar]
- Decker T., Lew D. J., Darnell J. E., Jr (1991). Two distinct alpha-interferon-dependent signal transduction pathways may contribute to activation of transcription of the guanylate-binding protein gene. Mol Cell Biol 11, 5147–5153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. Y., Schindler C., Improta T., Aebersold R., Darnell J. E., Jr (1992). The proteins of ISGF-3, the interferon alpha-induced transcriptional activator, define a gene family involved in signal transduction. Proc Natl Acad Sci U S A 89, 7840–7843 10.1073/pnas.89.16.7840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M., Naka T. (2010). SOCS1, a negative regulator of cytokine signals and TLR responses, in human liver diseases. Gastroenterol Res Pract 2010, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George F., Brouqui P., Boffa M. C., Mutin M., Drancourt M., Brisson C., Raoult D., Sampol J. (1993). Demonstration of Rickettsia conorii-induced endothelial injury in vivo by measuring circulating endothelial cells, thrombomodulin, and von Willebrand factor in patients with Mediterranean spotted fever. Blood 82, 2109–2116 [PubMed] [Google Scholar]
- Grimm D., Staeheli P., Hufbauer M., Koerner I., Martínez-Sobrido L., Solórzano A., García-Sastre A., Haller O., Kochs G. (2007). Replication fitness determines high virulence of influenza A virus in mice carrying functional Mx1 resistance gene. Proc Natl Acad Sci U S A 104, 6806–6811 10.1073/pnas.0701849104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenzi E., Töpolt K., Cornali E., Lubeseder-Martellato C., Jörg A., Matzen K., Zietz C., Kremmer E., Nappi F., et al. (2001). The helical domain of GBP-1 mediates the inhibition of endothelial cell proliferation by inflammatory cytokines. EMBO J 20, 5568–5577 10.1093/emboj/20.20.5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenzi E., Töpolt K., Lubeseder-Martellato C., Jörg A., Naschberger E., Benelli R., Albini A., Stürzl M. (2003). The guanylate binding protein-1 GTPase controls the invasive and angiogenic capability of endothelial cells through inhibition of MMP-1 expression. EMBO J 22, 3772–3782 10.1093/emboj/cdg382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada H., Fujita T., Miyamoto M., Kimura Y., Maruyama M., Furia A., Miyata T., Taniguchi T. (1989). Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell 58, 729–739 10.1016/0092-8674(89)90107-4 [DOI] [PubMed] [Google Scholar]
- Kamizono S., Hanada T., Yasukawa H., Minoguchi S., Kato R., Minoguchi M., Hattori K., Hatakeyama S., Yada M., et al. (2001). The SOCS box of SOCS-1 accelerates ubiquitin-dependent proteolysis of TEL-JAK2. J Biol Chem 276, 12530–12538 10.1074/jbc.M010074200 [DOI] [PubMed] [Google Scholar]
- Kaplanski G., Teysseire N., Farnarier C., Kaplanski S., Lissitzky J. C., Durand J. M., Soubeyrand J., Dinarello C. A., Bongrand P. (1995). IL-6 and IL-8 production from cultured human endothelial cells stimulated by infection with Rickettsia conorii via a cell-associated IL-1 alpha-dependent pathway. J Clin Invest 96, 2839–2844 10.1172/JCI118354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile B. T., Schulman B. A., Alexander W. S., Nicola N. A., Martin H. M., Hilton D. J. (2002). The SOCS box: a tale of destruction and degradation. Trends Biochem Sci 27, 235–241 10.1016/S0968-0004(02)02085-6 [DOI] [PubMed] [Google Scholar]
- Kim B. H., Shenoy A. R., Kumar P., Das R., Tiwari S., MacMicking J. D. (2011). A family of IFN-γ-inducible 65-kD GTPases protects against bacterial infection. Science 332, 717–721 10.1126/science.1201711 [DOI] [PubMed] [Google Scholar]
- Klingmüller U., Lorenz U., Cantley L. C., Neel B. G., Lodish H. F. (1995). Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell 80, 729–738 10.1016/0092-8674(95)90351-8 [DOI] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Darnell J. E., Jr (1989). Cytoplasmic activation of ISGF3, the positive regulator of interferon-alpha-stimulated transcription, reconstituted in vitro. Genes Dev 3, 1362–1371 10.1101/gad.3.9.1362 [DOI] [PubMed] [Google Scholar]
- Liao J., Fu Y., Shuai K. (2000). Distinct roles of the NH2- and COOH-terminal domains of the protein inhibitor of activated signal transducer and activator of transcription (STAT) 1 (PIAS1) in cytokine-induced PIAS1–Stat1 interaction. Proc Natl Acad Sci U S A 97, 5267–5272 10.1073/pnas.97.10.5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R. J., Yu H. P., Chang B. L., Tang W. C., Liao C. L., Lin Y. L. (2009). Distinct antiviral roles for human 2′,5′-oligoadenylate synthetase family members against dengue virus infection. J Immunol 183, 8035–8043 10.4049/jimmunol.0902728 [DOI] [PubMed] [Google Scholar]
- Malakhov M. P., Malakhova O. A., Kim K. I., Ritchie K. J., Zhang D. E. (2002). UBP43 (USP18) specifically removes ISG15 from conjugated proteins. J Biol Chem 277, 9976–9981 10.1074/jbc.M109078200 [DOI] [PubMed] [Google Scholar]
- Malakhova O. A., Kim K. I., Luo J. K., Zou W., Kumar K. G., Fuchs S. Y., Shuai K., Zhang D. E. (2006). UBP43 is a novel regulator of interferon signaling independent of its ISG15 isopeptidase activity. EMBO J 25, 2358–2367 10.1038/sj.emboj.7601149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansueto P., Vitale G., Cascio A., Seidita A., Pepe I., Carroccio A., di Rosa S., Rini G. B. , et al. (2012). New insight into immunity and immunopathology of rickettsial diseases. Clin Dev Immunol 2012, 967852 10.1155/2012/967852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min W., Pober J. S., Johnson D. R. (1998). Interferon induction of TAP1: the phosphatase SHP-1 regulates crossover between the IFN-α/β and the IFN-γ signal-transduction pathways. Circ Res 83, 815–823 10.1161/01.RES.83.8.815 [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. (1988). Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-β gene regulatory elements. Cell 54, 903–913 10.1016/S0092-8674(88)91307-4 [DOI] [PubMed] [Google Scholar]
- Monroe K. M., McWhirter S. M., Vance R. E. (2010). Induction of type I interferons by bacteria. Cell Microbiol 12, 881–890 10.1111/j.1462-5822.2010.01478.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka T., Narazaki M., Hirata M., Matsumoto T., Minamoto S., Aono A., Nishimoto N., Kajita T., Taga T., et al. (1997). Structure and function of a new STAT-induced STAT inhibitor. Nature 387, 924–929 10.1038/43219 [DOI] [PubMed] [Google Scholar]
- Pauli E. K., Schmolke M., Wolff T., Viemann D., Roth J., Bode J. G., Ludwig S. (2008). Influenza A virus inhibits type I IFN signaling via NF-κB-dependent induction of SOCS-3 expression. PLoS Pathog 4, e1000196 10.1371/journal.ppat.1000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H., Wilson C. A., Lee S. J., Benveniste E. N. (2006). IFN-β-induced SOCS-1 negatively regulates CD40 gene expression in macrophages and microglia. FASEB J 20, 985–987 10.1096/fj.05-5493fje [DOI] [PubMed] [Google Scholar]
- Raoult D., Weiller P. J., Chagnon A., Chaudet H., Gallais H., Casanova P. (1986). Mediterranean spotted fever: clinical, laboratory and epidemiological features of 199 cases. Am J Trop Med Hyg 35, 845–850 [DOI] [PubMed] [Google Scholar]
- Rydkina E., Sahni A., Silverman D. J., Sahni S. K. (2007). Comparative analysis of host-cell signalling mechanisms activated in response to infection with Rickettsia conorii and Rickettsia typhi. J Med Microbiol 56, 896–906 10.1099/jmm.0.47050-0 [DOI] [PubMed] [Google Scholar]
- Sahni S. K., Kiriakidi S., Colonne M. P., Sahni A., Silverman D. J. (2009). Selective activation of signal transducer and activator of transcription (STAT) proteins STAT1 and STAT3 in human endothelial cells infected with Rickettsia rickettsii. Clin Microbiol Infect 15 (Suppl. 2), 303–304 10.1111/j.1469-0691.2008.02248.x [DOI] [PubMed] [Google Scholar]
- Saito H., Morita Y., Fujimoto M., Narazaki M., Naka T., Kishimoto T. (2000). IFN regulatory factor-1-mediated transcriptional activation of mouse STAT-induced STAT inhibitor-1 gene promoter by IFN-gamma. J Immunol 164, 5833–5843 [DOI] [PubMed] [Google Scholar]
- Sousa Rd., Nóbrega S. D., Bacellar F., Torgal J. (2003). [Epidemiologic features of Mediterranean spotted fever in Portugal]. Acta Med Port 16, 429–436 (in Portuguese) [PubMed] [Google Scholar]
- Sporn L. A., Sahni S. K., Lerner N. B., Marder V. J., Silverman D. J., Turpin L. C., Schwab A. L. (1997). Rickettsia rickettsii infection of cultured human endothelial cells induces NF-κB activation. Infect Immun 65, 2786–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staeheli P., Haller O., Boll W., Lindenmann J., Weissmann C. (1986). Mx protein: constitutive expression in 3T3 cells transformed with cloned Mx cDNA confers selective resistance to influenza virus. Cell 44, 147–158 10.1016/0092-8674(86)90493-9 [DOI] [PubMed] [Google Scholar]
- Tietzel I., El-Haibi C., Carabeo R. A. (2009). Human guanylate binding proteins potentiate the anti-chlamydia effects of interferon-γ. PLoS ONE 4, e6499 10.1371/journal.pone.0006499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valbuena G., Bradford W., Walker D. H. (2003). Expression analysis of the T-cell-targeting chemokines CXCL9 and CXCL10 in mice and humans with endothelial infections caused by rickettsiae of the spotted fever group. Am J Pathol 163, 1357–1369 10.1016/S0002-9440(10)63494-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kaer L., Ashton-Rickardt P. G., Ploegh H. L., Tonegawa S. (1992). TAP1 mutant mice are deficient in antigen presentation, surface class I molecules, and CD4−8+ T cells. Cell 71, 1205–1214 10.1016/S0092-8674(05)80068-6 [DOI] [PubMed] [Google Scholar]
- Walker D. H., Popov V. L., Crocquet-Valdes P. A., Welsh C. J., Feng H. M. (1997). Cytokine-induced, nitric oxide-dependent, intracellular antirickettsial activity of mouse endothelial cells. Lab Invest 76, 129–138 [PubMed] [Google Scholar]
- Walker D. H., Olano J. P., Feng H. M. (2001). Critical role of cytotoxic T lymphocytes in immune clearance of rickettsial infection. Infect Immun 69, 1841–1846 10.1128/IAI.69.3.1841-1846.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z. Q., Waggoner S. N., Cruise M. W., Hall C., Xie X., Oldach D. W., Hahn Y. S. (2005). SOCS1 and SOCS3 are targeted by hepatitis C virus core/gC1qR ligation to inhibit T-cell function. J Virol 79, 15417–15429; 80, 8287 10.1128/JVI.79.24.15417-15429.2005 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yokota S., Yokosawa N., Okabayashi T., Suzutani T., Miura S., Jimbow K., Fujii N. (2004). Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 contributes to inhibition of the interferon signaling pathway. J Virol 78, 6282–6286 10.1128/JVI.78.12.6282-6286.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You M., Yu D. H., Feng G. S. (1999). Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol Cell Biol 19, 2416–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]