Abstract
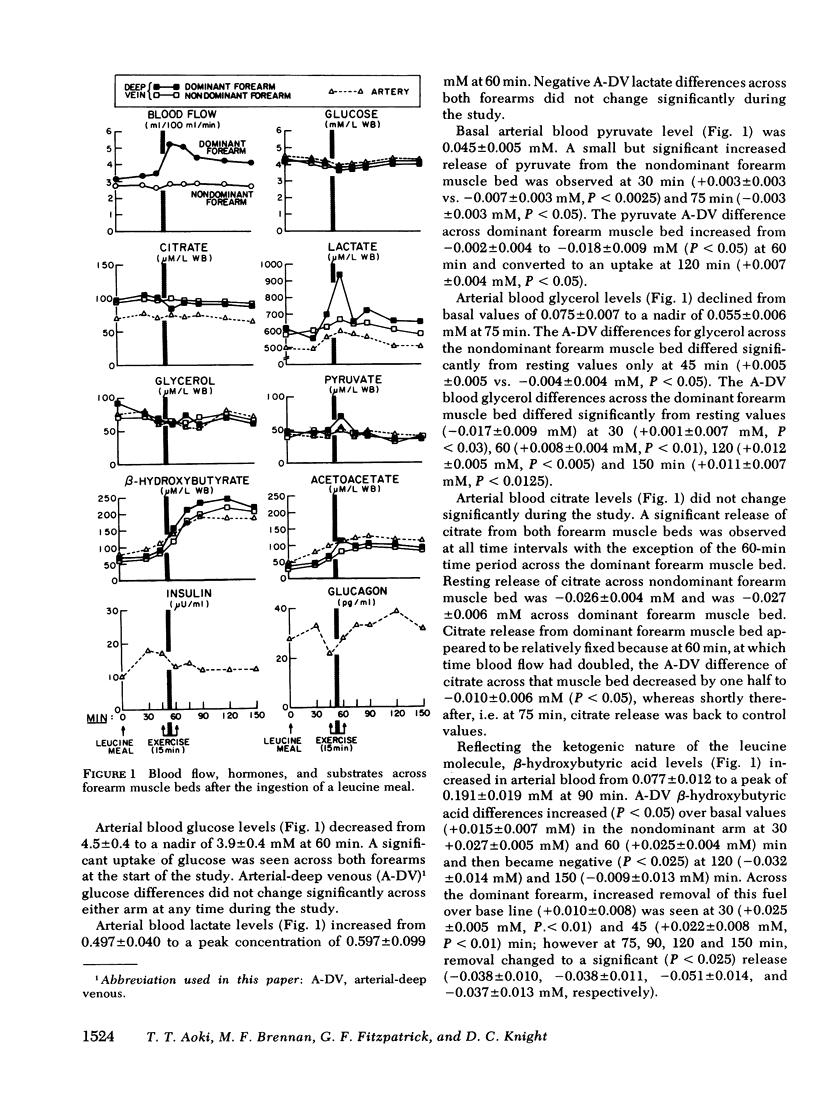
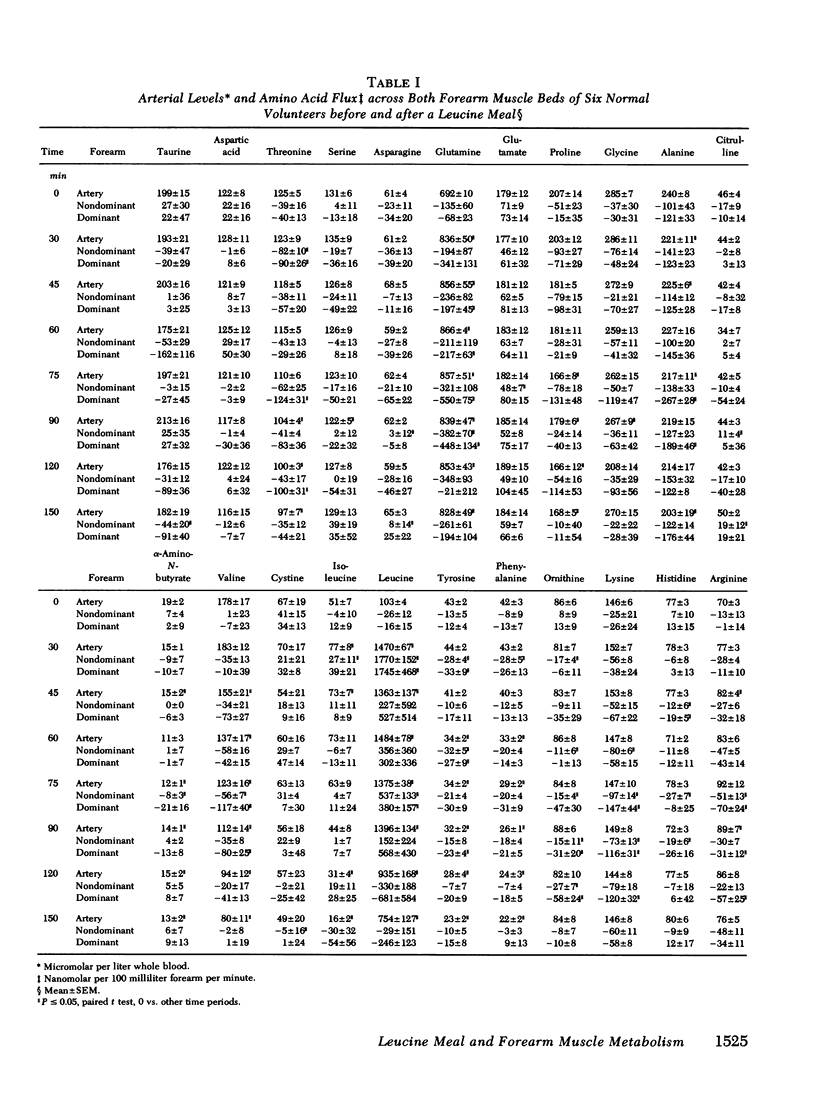
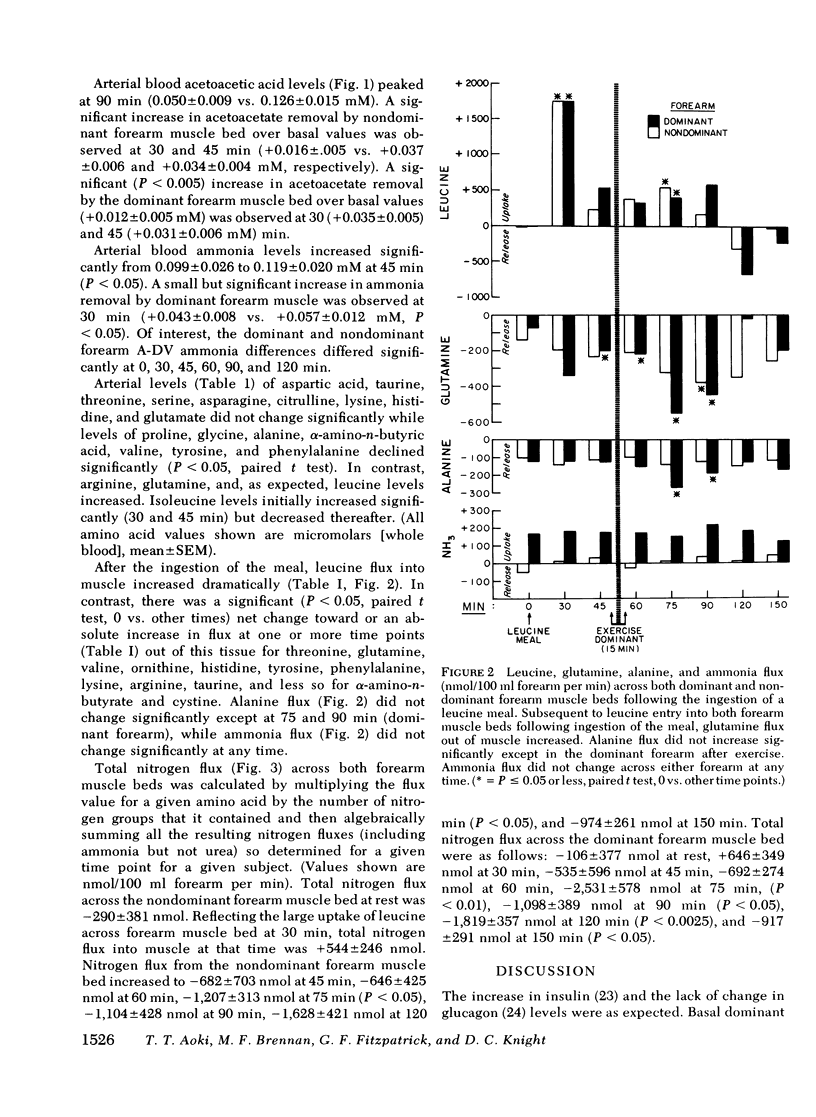
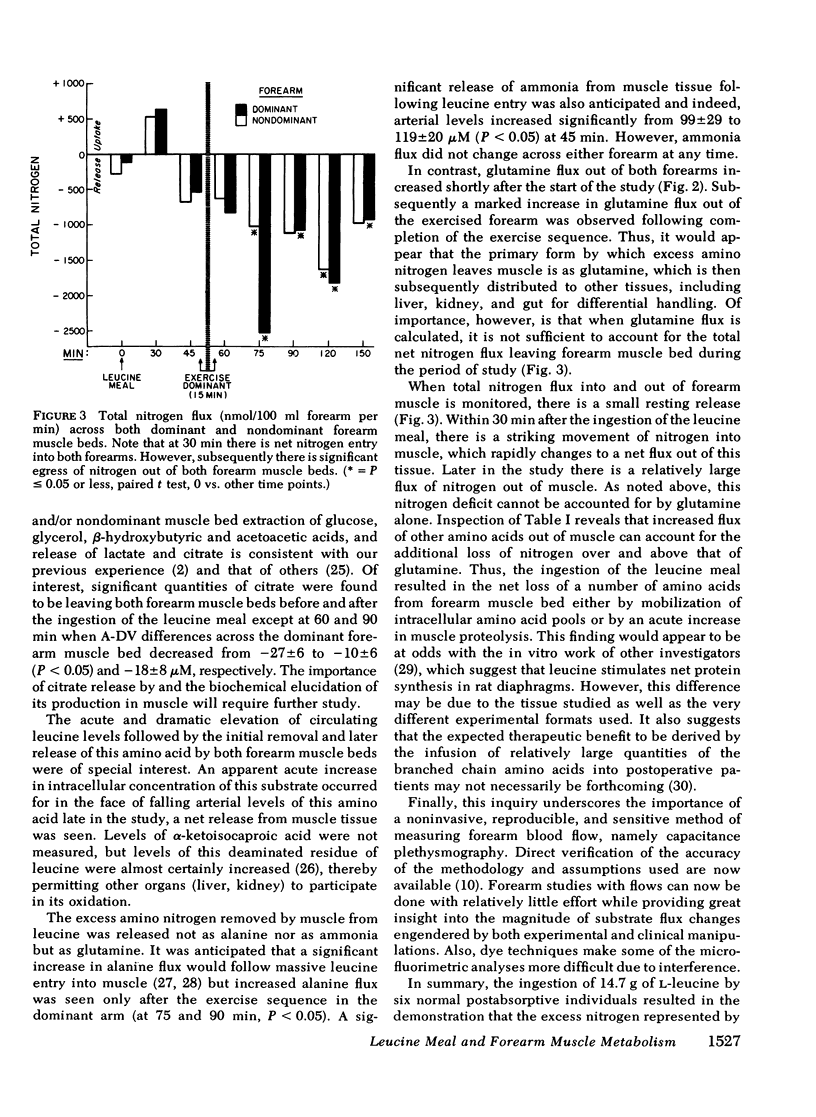
To assess the consequences of elevated branched chain amino acid levels on alanine, glutamine, and ammonia metabolism in muscle, L-leucine meals (14.7 g) were consumed by six normal postabsorptive individuals. Bilateral forearm studies were performed, and the dominant arm was subjected to 15 min of light exercise, using a calibrated dynamometer, beginning 45 min after the ingestion of the meal. Large uptakes of leucine were seen across both forearm muscle beds within 30 min of the meal. After exercise, blood flow in the dominant arm increased from 3.1 +/- 0.4 to 5.2 +/- 0.9 ml/100 ml forearm per minute (mean +/- SEM, P less than 0.005). Glutamine flux out of the dominant forearm increased threefold after the ingestion of the leucine meal and increased eightfold over base line after exercise. Less marked changes (significant only at 90 min) in the nonexercised, nondominant arm were also seen. Alanine flux out of the dominant forearm muscle bed increased modestly at 75 and 90 min. No significant change in ammonia flux across either forearm muscle bed was noted. Unexpectedly, large and significant net nitrogen loss from both forearm muscle beds was documented. Thus, following the ingestion of a leucine meal and light exercise, the primary means by which excess nitrogen is routed out of muscle is via glutamine formation and release with alanine and ammonia pathways playing relatively minor roles. More importantly, the ingestion of significant amounts of leucine by normal subjects, presumably in optimal nitrogen balance, results in a net loss of nitrogen from muscle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar-Parada E., Eisentraut A. M., Unger R. H. Effects of starvation on plasma pancreatic glucagon in normal man. Diabetes. 1969 Nov;18(11):717–723. doi: 10.2337/diab.18.11.717. [DOI] [PubMed] [Google Scholar]
- Aoki T. T., Brennan M. F., Müller W. A., Soeldner J. S., Alpert J. S., Saltz S. B., Kaufmann R. L., Tan M. H., Cahill G. F., Jr Amino acid levels across normal forearm muscle and splanchnic bed after a protein meal. Am J Clin Nutr. 1976 Apr;29(4):340–350. doi: 10.1093/ajcn/29.4.340. [DOI] [PubMed] [Google Scholar]
- Aoki T. T., Muller W. A., Brennan M. F., Cahill G. F., Jr Blood cell and plasma amino acid levels across forearm muscle during a protein meal. Diabetes. 1973 Oct;22(10):768–775. doi: 10.2337/diab.22.10.768. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Reid S. S. Leucine. A possible regulator of protein turnover in muscle. J Clin Invest. 1975 Nov;56(5):1250–1261. doi: 10.1172/JCI108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLOYD J. C., Jr, FAJANS S. S., KNOPF R. F., CONN J. W. EVIDENCE THAT INSULIN RELEASE IS THE MECHANISM FOR EXPERIMENTALLY INDUCED LEUCINE HYPOGLYCEMIA IN MAN. J Clin Invest. 1963 Nov;42:1714–1719. doi: 10.1172/JCI104857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Pozefsky T., Marliss E., Cahill G. F., Jr Alanine: key role in gluconeogenesis. Science. 1970 Feb 13;167(3920):1003–1004. doi: 10.1126/science.167.3920.1003. [DOI] [PubMed] [Google Scholar]
- Freund H., Hoover H. C., Jr, Atamian S., Fischer J. E. Infusion of the branched chain amino acids in postoperative patients. Anticatabolic properties. Ann Surg. 1979 Jul;190(1):18–23. doi: 10.1097/00000658-197907000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson S. M., Cree T. C., Harper A. E. Regulation of leucine and alpha-ketoisocaproate metabolism in skeletal muscle. J Biol Chem. 1978 Nov 25;253(22):8126–8133. [PubMed] [Google Scholar]
- Longhurst J., Capone R. J., Mason D. T., Zelis R. Comparison of blood flow measured by plethysmograph and flowmeter during steady state forearm exercise. Circulation. 1974 Mar;49(3):535–540. doi: 10.1161/01.cir.49.3.535. [DOI] [PubMed] [Google Scholar]
- Odessey R., Khairallah E. A., Goldberg A. L. Origin and possible significance of alanine production by skeletal muscle. J Biol Chem. 1974 Dec 10;249(23):7623–7629. [PubMed] [Google Scholar]
- Owen O. E., Reichard G. A., Jr Human forearm metabolism during progressive starvation. J Clin Invest. 1971 Jul;50(7):1536–1545. doi: 10.1172/JCI106639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozefsky T., Felig P., Tobin J. D., Soeldner J. S., Cahill G. F., Jr Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. J Clin Invest. 1969 Dec;48(12):2273–2282. doi: 10.1172/JCI106193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha D. M., Faloona G. R., Unger R. H. Glucagon-stimulating activity of 20 amino acids in dogs. J Clin Invest. 1972 Sep;51(9):2346–2351. doi: 10.1172/JCI107046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin R. S. Effect of starvation on the turnover and metabolic response to leucine. J Clin Invest. 1978 Jun;61(6):1471–1481. doi: 10.1172/JCI109067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976 Apr;57(4):987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir G. C., Turner R. C., Martin D. B. Glucagon radioimmunoassay using antiserum 30K: interference by plasma. Horm Metab Res. 1973 Jul;5(4):241–244. doi: 10.1055/s-0028-1093958. [DOI] [PubMed] [Google Scholar]
- Wenger C. B., Roberts M. F., Stolwijk J. A., Nadel E. R. Forearm blood flow during body temperature transients produced by leg exercise. J Appl Physiol. 1975 Jan;38(1):58–63. doi: 10.1152/jappl.1975.38.1.58. [DOI] [PubMed] [Google Scholar]
- Wood J. R., Hyman C. A direct reading capacitance plethysmograph. Med Biol Eng. 1970 Jan;8(1):59–70. doi: 10.1007/BF02551750. [DOI] [PubMed] [Google Scholar]


