Summary
Preferentially expressed antigen in melanoma (PRAME) and prostate-specific membrane antigen (PSMA) are tumor-associated antigens implicated in cellular differentiation, genetic stability, and angiogenesis. MKC1106-PP is an immunotherapeutic regimen cotargeting PRAME and PSMA, comprised of a recombinant plasmid (pPRA-PSM encoding fragments derived from both antigens) and 2 peptides (E-PRA and E-PSM derived from PRAME and PSMA, respectively). This multicenter study evaluated MKC1106-PP with a fixed plasmid dose and 2 different peptide doses, administered by intralymph node injection in a prime-boost sequence in human leukocyte antigen-A*0201 and tumor-antigen-positive patients with progressing metastatic solid tumors who had failed standard therapy. Immune monitoring was done by tetramer and enzymatic-linked immune spot analysis. The treatment was well tolerated, with no significant differences in safety, immune response, and clinical outcome relative to peptide doses. Fifteen of 24 evaluable patients showed an immune response, as defined by the expansion of PRAME-specific or PSMA-specific T cells in the blood. There were no partial or complete responses by the Response Evaluation Criteria in Solid Tumors. Seven patients showed stable disease (SD) for 6 months or longer, or prostate specific antigen decline: 4 of 10 with prostate carcinoma, 2 of 2 with renal clear cell carcinoma, and 1 of 10 with metastatic melanoma. In addition, there was an association between the induction and persistence of antigen-specific T cells in blood above baseline levels and disease control, defined as SD for 6 months or longer. These results support further development of MKC1106-PP in specific clinical indications.
Keywords: active immunotherapy, PRAME, PSMA, solid tumor, intralymph node vaccination
After decades of rather modest clinical evidence for the benefit of cancer vaccines,1,2 there is renewed interest and effort in active immunotherapy for cancer, exemplified by the recent US Food and Drug Administration approval of a personalized, autologous cell-based “vaccine” for prostate carcinoma (Sipuleucel-T, Provenge). The primary mechanism of action of cancer vaccines likely involves the generation of tumor-associated antigen (TAA)-specific T cells. These cells have the potential to suppress tumor growth, or eliminate tumor cells that otherwise have the capability to establish metastatic lesions.3,4
Two broad classes of cancer vaccines are in development: autologous vaccines, generally containing tumor or antigen-presenting cells from individual patients and intended for use in the donors; and standardized or “off the shelf” vaccines that are synthetic and applicable to broader patient populations.5 Although Sipuleucel-T, a personalized, cell-based vaccine, has successfully translated the cancer vaccine concept from bench to clinic and marketplace, more potent but less cumbersome technologies, easier to develop, manufacture, and implement, are likely to have a broader impact.
We focused on 2 TAAs applicable across a range of tumor types: preferentially expressed antigen in melanoma (PRAME), a regulator of the retinoic acid receptor6,7 and prostate-specific membrane antigen (PSMA), a dihydrofolate reductase with several other functions.8,9 Notably, PRAME is overexpressed by a variety of cancers of epithelial, neuroectodermal, and bone marrow origin,10 has a role in tumor progression by inhibiting cellular differentiation through blockade of retinoic acid receptor signaling in cancer cells,11 and contains defined human leukocyte antigen (HLA) A*0201-restricted epitopes.12 PRAME has not been explored yet as a therapeutic target in the clinic.
PSMA is expressed by the neovasculature in diverse solid tumors13 and mediates angiogenesis through integrin regulation.10 In prostate carcinoma, PSMA is substantially overexpressed in hormone-resistant cancer cells and triggers genome instability during cellular proliferation.14 PSMA also contains a number of immunogenic HLA-A*0201-restricted epitopes.15 Although there have been significant efforts to evaluate immune interventions against PSMA, comprising both antibodies and active immunotherapies,9 only 1 PSMA-related product has been approved to date, an antibody-based diagnostic test.16
MKC1106-PP is a 3-component investigational vaccine with several novel features. First, it was designed to cotarget cancer cells and tumor-associated neovasculature through PRAME and PSMA, respectively. Second, it exploits direct intralymph node administration of a DNA prime, dual-peptide boost immunization regimen to maximize antitumor immunity. This strategy is based on several preclinical findings. For example, in the case of nonreplicating vaccine vectors, intranodal administration has been generally shown to be superior compared with intramuscular, intradermal, or subcutaneous administration, yielding higher magnitude T-cell responses.17,18 In addition, coupled with intranodal administration, heterologous prime boosting can result in further elevation of immune responses.19 Notably, intralymph node DNA priming elicited a population of specific T cells with central memory phenotype, expressing low levels of PD-1 and other immune-inhibitory receptors.20 Heterologous peptide boosting further amplified and converted plasmid DNA-primed T cells into antitumor effector cells.20 Moreover, previous clinical evidence in patients with malignant melanoma supports the safety and feasibility of direct intralymph node administration of DNA plasmid vectors, with only mild or moderate therapy-related adverse events (AEs) such as a flu-like syndrome.21,22 Although modest levels of immunogenicity were demonstrated in these early trials, objective responses were not observed.
In addition to a dual antigen-expressing DNA plasmid for priming, MKC1106-PP contains 2 synthetic peptides for boosting that are analogs which correspond to defined PRAME and PSMA epitopes restricted by HLA-A*0201, the most prevalent HLA allele in the human population.19 The trial was carried out in a patient population with diverse tumor types (hormone refractory prostate carcinoma, malignant melanoma, renal clear cell carcinoma, and others), to characterize the overall safety and immune response profile and to evaluate and document any evidence of clinical benefit afforded by this immune intervention.
PATIENTS AND METHODS
Study Population
Twenty-six patients were enrolled at 6 cancer centers in the US: USC Norris Comprehensive Cancer Center, Los Angeles, California; Moffitt Cancer Center, Tampa, Florida; Nevada Cancer Institute, Las Vegas, Nevada; Arizona Cancer Center, Tucson, Arizona; Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire; and Lombardi Comprehensive Cancer Center, Georgetown, District of Columbia. Eligible patients had progressing, advanced, and/or refractory cancer with measurable disease per the Response Evaluation Criteria in Solid Tumors (RECIST), or a minimal of 2 documented rising prostate specific antigen (PSA) at least 1 week apart, for patients with prostate carcinoma. Patients were required to be HLA-A*0201 positive and to have progressed after at least 1 standard systemic treatment, radiation, or relapsed after surgery. They had an Eastern Cooperative Oncology Group performance status of 0, 1, or 2, and an anticipated survival of at least 3 months. At enrollment, patients were required to have paraffin-embedded tumor specimen from prior biopsies or surgical resection, for immunohistochemical analysis of PRAME, PSMA, and β2-microglobulin expression. The expression level of both antigens and β2-microglobulin required for enrollment was at least 10% of tumor cells or in case of PSMA, any vascular endothelial expression as applicable. Patients were also required to render informed consent using documents approved by the National Institutes of Health Office of Biotechnology Activities and the local institutional review boards.
Investigational Agent
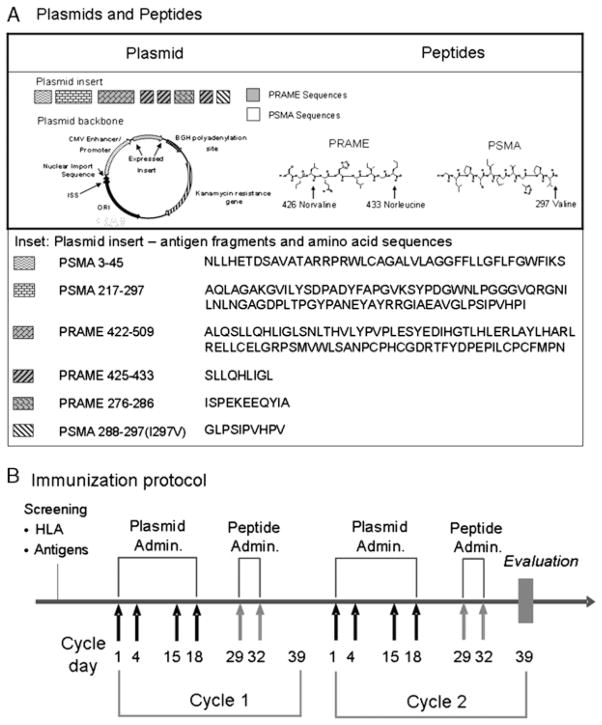
The MKC1106-PP vaccine consists of 3 components: a DNA plasmid (pPRA-PSM) and 2 peptides E-PRA and E-PSM (Fig. 1A). pPRA-PSM is a recombinant plasmid expressing segments of the 2 target antigens, PRAME and PSMA, formulated as a sterile aqueous solution at a concentration of 4.0 mg/mL. The plasmid insert was engineered as a “string-of-beads” to yield the epitopes PRAME 425–433 and PSMA 288–297 (I297V) (Fig. 1A and inset). In addition, the plasmid contained the sequences corresponding to PRAME antigen fragment 422–509 and PSMA antigen fragments 3–45 and 217–297 (Fig. 1A and inset). To facilitate antigen processing, several processing enhancing sequences were utilized between the epitope “beads.” Preclinical evaluation of plasmid immunogenicity in HLA-A2 HHD-1 transgenic mice showed induction of T-cell immunity by plasmid immunization, against both the PRAME 425–433 and PSMA 288–297 epitopes in direct support of processing and presentation of these epitopes in vivo (Suppl. Table1 http://links.lww.com/JIT/A119).
FIGURE 1.
A, Schematic representation of the plasmid (pPRA-PSM) with the location and sequences of PRAME and PSMA antigen fragments within the open reading frame. On the right side: the 2 boosting peptide analogs E-PRA and E-PSM, with the amino acid residue changes indicated. B, The immunization schema, with timing of priming and boosting injections indicated. Clinical evaluation and decision to continue on treatment was made every other therapeutic cycle or whenever there was an indication of clinical progression. BGH indicates bovine growth hormone; CMV, cytomegalovirus; ISS, immune stimulating sequence; ORI, origin of replication; PRAME, preferentially expressed antigen in melanoma; PSMA, prostate-specific membrane antigen.
The E-PRA and E-PSM are peptides corresponding to the HLA-A*0201-restricted epitopes PRAME 425–433 and PSMA 288–297, respectively. These 2 epitopes were immunogenic in vitro as they could expand and activate human-specific T cells, resulting in specific cytotoxicity of target cells [Suppl. Fig. 1 (supplemental digital content 1 http://links.lww.com/JIT/A115) and article in preparation]. The sequence of E-PRA is Ser-Nva*-Leu-Gln-His-Leu-Ile-Gly-Nle* with non-natural norvaline (Nva) and norleucine (Nle) amino acid substitutions at major histocompatibility complex (MHC) class I-anchor positions (indicated by *). E-PSM carries an Ile to Val substitution at the C-terminal MHC class I-anchor position and has the sequence of Gly-Leu-Pro-Ser-Ile-Pro-Val-His-Pro-Val* (* indicates amino acid substitution). The target epitopes were selected based on a range of criteria, including HLA-A*0201 binding and stability, presentation on tumor cell lines, in vitro immunogenicity utilizing human blood cells and testing in a preclinical model expressing HLA-A*0201 compared with other PSMA and PRAME peptides such as those described in Refs.12,15 (article in preparation). The amino acid substitutions of the select epitopes modestly improved the already substantial binding, yet significantly enhanced the half-life of MHC class I-peptide complexes—the latter being important for the stability of immunological synapses and consequent immunity.23 More specifically, the I297V substitution in PSMA 288–297 increased the peptide-MHC half-life from about 7–17 hours; similarly, the double substitution L426Nva and L433Nle in PRAME 425–433, increased the half-life of the complex from about 12.5 to 17 hours (article in preparation). Both peptides were formulated as sterile aqueous solutions at concentrations of 0.5 mg/mL for E-PRA and 1.0mg/mL for E-PSM.
Treatment
Eligible patients received a repeat prime-boost regimen of MKC1106-PP (Fig. 1B) with a fixed priming dose of plasmid (2400 μg/dose). The peptide doses used were: low dose E-PSM (30 μg) and E-PRA (22.5 μg); and high dose E-PSM (300 μg) and E-PRA (150 μg). Each component was injected separately into the subcapsular space of clinically uninvolved superficial inguinal or axillary lymph nodes that were visualized by ultrasonography. Vaccine components were administered by bolus injection using unibody polypropylene hypodermic syringes (0.3 mL) with a fixed 25-gauge echogenic needle (1.5″ echogenic canula) (Ultimed, De Smet, SD) to ensure appropriate intralymph node administration under ultrasound guidance. Each treatment cycle included 4 plasmid and 2 peptide administrations. Bilateral plasmid injections (1200μg/node) were carried out on days 1, 4, 15 and 18, followed by peptide injection on days 29 and 32 (Fig. 1B). Peptides were administrated on the same day, into contralateral lymph node groups (eg, E-PRA in an inguinal lymph node on the right side and E-PSM in an inguinal lymph node on the left side).
Clinical evaluation including computed tomography/magnetic resonance imaging was performed after every 2 treatment cycles. Protocol treatment was continued as long as the patients were determined to be nonprogressors per protocol, for up to 6 cycles (9 mo). New cycles were initiated within 10 days after the completion of a prior cycle (Fig. 1B).
Immunohistochemistry
The immunohistochemical (IHC) analysis was performed at Genzyme Genetic Analytic Services (Los Angeles, CA) under good laboratory practice (GLP). In brief, before immunization, paraffin-embedded tumor specimens from patients were deparaffinized in xylene and rehydrated. Endogeneous peroxidase activity was quenched with 0.3% hydrogen peroxide in methyl alcohol for 10 minutes. Heat-induced epitope retrieval was performed at 125°C for 30 seconds with a Diva device (Biocare Medical, Concord, CA), in a decloaking chamber (Biocare) on all slides except for the ones for PRAME, which were treated with Borg (Biocare) in 95°C water bath for 40 minutes. After cooling and washing in Tris-Buffered Saline with Tween-20 buffer 10× (Dako), slides were incubated with the primary antibodies (for β2-microglobulin, Dako #A0072; and for PSMA, Dako # M3620) in antibody diluent (Dako) at an appropriate concentration at room temperature, for 60 minutes. Slides for PRAME staining were incubated with anti-PRAME monoclonal antibody (MannKind Corp., ATCC # PTA-11101) overnight at 4°C. This was followed by incubation with horseradish peroxidase polymer-labeled secondary antibody (Nemesis Polymer HRP kit, Biocare) for 25 minutes. After rinsing with Tris-Buffered Saline with Tween-20, Betazoid DAB (Biocare) substrate was added for 5 minutes to generate visible staining. Slides were counterstained with Harris hematoxylin for 1 minute. Negative and positive tissue control samples were included in all stains. All stained slides were evaluated in a blinded manner by a pathologist for intensity, pattern, and fraction (%) of cells stained for each of the markers. Slide digitization, imaging, and quantitative analysis were not performed.
Immune Response Assessments
A flow cytometric assay using MHC class I-peptide tetramers was used to enumerate PRAME and PSMA epitope-specific CD8+ cytolytic T lymphocyte (CTL) within peripheral blood mononuclear cells (PBMCs), freshly harvested and without any prior in vitro stimulation. Whole blood was collected in potassium ethylenediaminetetraacetic acid-treated specimen collection tubes before immunization and on days 29 and 39 of each treatment cycle. Within 48 hours, the samples were sent to Genzyme Genetic Analytical Service (Los Angeles, CA), a Clinical Laboratory Improvement Amendments (CLIA) certified independent laboratory, for processing and analysis under GLP. In brief, PBMCs were isolated by Ficoll gradient centrifugation, incubated for 30 minutes at room temperature in dark with 7AAD, anti-CD3, and anti-CD8 IgGs (BD Biosciences, San Jose, CA) and tetramer reagents (Beckman Coulter, Brea, CA) for the native epitope peptides PRAME 425–433 (SLLQHLIGL) and PSMA 288–297 (GLPSIPVHPI), respectively. Cells were then washed and analyzed on a BD FACS Canto II flow cytometer using FACS Diva software (BD Biosciences, San Jose, CA). As part of the gating strategy, dying or dead cells were gated out and the analysis focused on CD3+ cells within the forward scatter/side scatter region, corresponding to mononuclear cells of small-to-moderate sizes. The quadrant limits were established and applied consistently, utilizing appropriate negative controls. A minimum of 500,000 events per sample were acquired.
The lower limit of detection (LLD) of this assay was established using epitope-specific T cell lines generated by repeat in vitro stimulation of white blood cells from healthy human donors. The LLD was 0.03% (CD8+ tetramer+ T cells/total CD8+ T cells ×100). A positive tetramer response was defined as greater than a 2-fold increase in the number of tetramer+ T cells at any time point after starting immunization compared with a preimmunization value, with the postimmunization value also significantly different from the background (>2×LLD).
A direct, ex vivo interferon-γ enzymatic-linked immune spot (ELISPOT) assay was performed on blood samples from all treated patients. Whole blood samples at screening, on days 29 and 39 of each treatment cycle were collected at clinical sites and shipped to Cellular Technologies Limited (CTL, Cleveland, OH) within 24 hours of sample collection. After processing and cryopreservation, the samples were tested simultaneously. In brief, Ficoll gradient-separated cells were cultured in proprietary CTL serum-free test medium with 1% L-glutamine (CTL, Cleveland, OH) and stimulated with 1.25, 2.5, or 5 mg/mL of phytohemagglutinin (Sigma) as control, or with 5 μg/mL of PRAME 425–433 peptide or PSMA 288–297 peptide. The stimulation was done in 96-well ELISPOT plates with polyvinylidene fluoride membrane (Millipore, Billerica, MA), in triplicate wells. Cells were cultured for 24 hours and the analysis carried out with a CTL ImmunoSpot Analyzer equipped with the SpotMap software (CTL, Cleveland, OH). Assay validation and execution were executed in compliance with CTL’s Standard Operation Protocols and US Food and Drug Administration’s GLP. The results were expressed as the total number of spot forming colonies per 106 PBMCs, if the ELISPOT readout was >1 spot per 105 cells, after subtracting the assay background. A positive ELISPOT response was defined as >10 spots per 105 cells and at least a 3-fold increase over the baseline values and 3 times the standard deviation.
Serum cytokine levels were measured using commercial ELISA kits from R&D Systems (Minneapolis, MN) [interleukin (IL)-2, IL-6, and interferon-γ] and PBL Biomedical Laboratories (Piscataway, NJ) (interferon-α) according to manufacturer’s instructions.
Quantitative Polymerase Chain Reaction of Plasmid
Total DNA was extracted from whole blood using a DNA blood Mini Kit (Qiagen, Valencia, CA). Real-time polymerase chain reaction based on 5′ nuclease reaction was performed with MmpliTaq Gold polymerase (ABI #4311806) using the ABI 7900 thermocycler with the following conditions: 50°C (2 min), 95°C (15 min), followed by 45 cycles of 94°C for 30 seconds and 60°C for 60 seconds. The sequences of primers and the probe used are: sense primer—CCCGGAGAAGGAAGAGCAGTAT; antisense primer—TAGAAGGCACAGTCGAGGCTGA; probe—FAM-TCAGCGCCTTTAAACGGGCC-Blackhole quencher1 (BioSearch Technologies). A standard curve used to estimate the amount of plasmid present in a given blood sample, was generated by plotting threshold cycles against known amounts of plasmid. The results were expressed as copy numbers of plasmid per microgram of genomic DNA.
Safety and Clinical Response Evaluation
AEs were recorded at visits that occurred during every treatment cycle and an end-of-study visit. AEs were graded for severity according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE Version 3.0).
Detailed clinical evaluation of patients was carried out at baseline and after every other treatment cycle, including physical examination, disease evaluation, and laboratory tests. Objective responses were categorized using the RECIST 1.0.
In patients with prostate cancer, serum PSA levels were measured during screening and at the end of every treatment cycle. A PSA response was a decrease of 50% or greater based on 2 measurements performed 4 or more weeks apart; otherwise, it was documented as a PSA decline, as applicable.
Overall, in light of the progressing nature of disease before immunization, disease control was defined as stable disease for 6 months, or longer. This was an arbitrary cut-off, as clinical response was not a primary objective of this phase 1 trial.
Statistical Analysis
Data management and statistical analysis were performed independently by Theradex (Princeton, NJ) under good clinical practice compliance using SAS software (version 8.01). For continuous variables, data were presented as means and medians, with range and standard deviations. Discrete variables were expressed as frequencies or proportions and analyzed by the 2-tailed Fisher exact test.
RESULTS
Demographics
Twenty-six patients were treated on this trial, evenly distributed in the low and high dose peptide cohorts. The low and high dose peptide cohorts were also balanced with respect to ethnic origin, sex, and age (Table 1). In both cohorts, the majority of patients were male (92.3% and 84.6%, respectively), and white (84.6% and 100.0%), with a mean age of 68.6 years and 68.8 years, respectively. A greater percentage of patients with the Eastern Cooperative Oncology Group performance status of 1 was present in the low dose (69.2%) compared with the high peptide dose group (38.5%). The most frequent tumor types in both cohorts were prostate cancer (23.1% and 53.8%, respectively) and melanoma (46.2% and 30.8%) (Table 1). The mean duration of disease as primary diagnosis was the same in both cohorts (68.7 mo). In the low and high peptide dose cohorts, 61.5% and 46.2% of patients received prior chemotherapy; 38.5% and 7.7% received immunotherapy; 23.1% and 46.2% had hormonal therapy; and 46.2% and 76.9% received prior radiation therapy.
TABLE 1.
Demographic Characteristics and Tumor Types
| Demographic Characteristics | Assigned Dose Cohort
|
||
|---|---|---|---|
| Low | High | Total | |
| No. Patients | 13 | 13 | 26 |
| Age (y)*† | |||
| N | 13 | 13 | 26 |
| Mean | 68.6 | 68.8 | 68.7 |
| SD | 11.72 | 7.65 | 9.70 |
| Median | 72.0 | 70.0 | 71.5 |
| Minimum | 46 | 56 | 46 |
| Maximum | 88 | 79 | 88 |
| 18 to 64 | 4 (30.8%) | 4 (30.8%) | 8 (30.8%) |
| 65+ | 9 (69.2%) | 9 (69.2%) | 18 (69.2%) |
| Sex* | |||
| Male | 12 (92.3%) | 11 (84.6%) | 23 (88.5%) |
| Female | 1 (7.7%) | 2 (15.4%) | 3 (11.5%) |
| Ethnic Origin* | |||
| White | 11 (84.6%) | 13 (100.0%) | 24 (92.3%) |
| Black or African American | 2 (15.4%) | 0 | 2 (7.7%) |
| ECOG Performance Status* | |||
| 0 | 3 (23.1%) | 6 (46.2%) | 9 (34.6%) |
| 1 | 9 (69.2%) | 5 (38.5%) | 14 (53.8%) |
| 2 | 1 (7.7%) | 2 (15.4%) | 3 (11.5%) |
| Tumor types | |||
| Prostate carcinoma | 3 | 7 | 10 |
| Melanoma | 6 | 4 | 10 |
| Renal cell carcinoma | 1 | 1 | 2 |
| Mesothelioma | 1 | 1 | 2 |
| Esophageal carcinoma | 1 | 0 | 1 |
| Basal cell carcinoma | 1 | 0 | 1 |
The total number of patients was used as denominator to calculate percentages.
Age at enrollment.
ECOG indicates Eastern Cooperative Oncology Group; SD, standard deviation.
Target Antigen Expression by Different Tumor Types
Tumor biopsies from a total of 55 patients who expressed HLA-A*0201, were evaluated by IHC for antigen and β2-microglobulin expression. Utilizing prospective empirical criteria for positive staining (>10% of cancer cells for PRAME or PSMA, or endothelial cell expression for PSMA), we showed that virtually all prostate, renal, colorectal carcinoma and melanomas expressed PRAME within the tumor (Table 2), with patterns ranging from diffuse to focal or scattered expression by cancerous cells. The expression of PSMA was also frequent (Table 2), but confined to vascular endothelial cells (scattered) with the exception of prostate carcinoma, where it also showed an intense, diffuse staining of cancerous cells. Other tumor types showed coexpression of the target antigens but the data set was very limited. Specifically, the rate of coexpression of the target antigens within tumor biopsies was: 11 of 12 in prostate carcinoma, 4 of 6 in renal cell carcinoma, and 11 of 16 in melanoma. Beta2-microglobulin, a marker of membrane MHC class I, was expressed homogenously or heterogeneously in the tumors of all patients, with only 2 of 55 patients showing an expression of <10% of cells. There were no obvious differences in regards to disease stage and antigen expression, between screened patients who were subsequently treated or non-treated respectively, as all had advanced, progressing disease refractory to standard of care.
TABLE 2.
Tumor Antigen Expression by Immunohistochemistry in Patients Screened for Treatment Eligibility
| Tumor Type | PRAME | PSMA | PRAME and PSMA | β2-microglobulin |
|---|---|---|---|---|
| Melanoma | 14/16* | 12/16 | 11/16 | 16/16 |
| Prostate | 11/12 | 12/12 | 11/12 | 12/12 |
| Colorectal | 5/5 | 2/5 | 2/5 | 5/5 |
| Renal | 6/6 | 4/6 | 4/6 | 5/6 |
| Esophageal | 1/1 | 1/1 | 1/1 | 1/1 |
| Adrenal cortical | 1/1 | 1/1 | 1/1 | 1/1 |
| Squamous cell | 1/1 | 1/1 | 1/1 | 1/1 |
| Mesothelioma | 2/3 | 2/3 | 2/3 | 3/3 |
| Breast | 1/2 | 0/2 | 0/2 | 2/2 |
| Sarcoma | 1/1 | 1/1 | 1/1 | 1/1 |
| Other | 3/3 | 3/3 | 3/3 | 3/3 |
| Overall | 38/55 | 39/55 | 36/55 | 54/55 |
| 84%† | 71% | 65% | 98% |
The number of patients showing positive staining in the biopsy per total number of patients with a select tumor type, as part of the process of evaluating eligibility criteria.
The percentage was calculated based on the total number of HLA-A2+ patients screened, with biopsied tissue available for IHC.
HLA indicates human leukocyte antigen; IHC, immunohistochemistry.
These data are in agreement with a separate analysis on archived tumor samples, by qRT-polymerase chain reaction and IHC, showing that the expression of PRAME and PSMA was frequent in prostate, renal clear cell carcinoma, and melanoma, in addition to breast, ovarian, lung, pancreatic carcinoma, and glioblastoma multiforme (Suppl. Table http://links.lww.com/JIT/A120, Suppl. Fig. 2 http://links.lww.com/JIT/A116).
Safety Profile and Measurement of Plasmid and Cytokine Levels in Blood
The safety and AE profiles of MKC1106-PP were evaluated for all patients dosed with MKC1106-PP (Table 3). Repeat administration of the 3-component regimen into inguinal lymph nodes was well tolerated and associated with over 90% compliance. No dose-limiting toxicities were reported, and no grade 3 to 4 AEs were related to the study regimen. One patient experienced a serious AE unrelated to the investigational agent, and thereafter discontinued from the study. Modest or moderate short-lived fatigue and injection site pain were reported as the most frequent regimen-related and treatment-emergent AEs, not related to tumor type or peptide dose (Table 4). In conclusion, repeat intranodal administration of MKC1106-PP to patients with advanced cancer was safe, feasible, and well tolerated, without dose-limiting toxicities.
TABLE 3.
Exposure of Patients to the Investigational Agent
| Dose Cohort | Tumor Type | Patient No. | Total No. Treatment Cycles* |
|---|---|---|---|
| Low peptide dose | Melanoma | 1048 | 1 |
| 1000 | 2 | ||
| 1002 | 2 | ||
| 1058 | 2 | ||
| 1038 | 2 | ||
| 1049 | 3 | ||
| 1024† | 6 | ||
| Prostate | 1044 | 2 | |
| 1057 | 2 | ||
| 1006 | 4 | ||
| Kidney | 1019 | 4 | |
| Esophageal | 1028 | 2 | |
| Mesothelioma | 1065 | 1 | |
| High peptide dose | Melanoma | 1112 | 1 |
| 1118 | 2 | ||
| 1142 | 2 | ||
| 5037 | 2 | ||
| Prostate | 1105 | N/A‡ | |
| 5056 | 2 | ||
| 1100 | 2 | ||
| 1104 | 3 | ||
| 1063 | 3 | ||
| 1119 | 6 | ||
| 5083 | 6 | ||
| 1091 | 6 | ||
| Kidney | 5075 | 7 | |
| Mesothelioma | 1087 | 1 |
A treatment cycle lasted for about 6 weeks. Patients were clinically evaluated after every 2 cycles and continued on treatment if there was no evidence of disease progression.
Patients who underwent at least 4 and up to 6 cycles of therapy were shaded in yellow; those who underwent at least 6 therapeutic cycles (9 mo or longer) were shaded in green.
N/A—This patient was enrolled in the trial but was never dosed with plasmid or peptide due to a very rapid disease progression.
TABLE 4.
Summary of Treatment-Related Adverse Events in Evaluable Patients
| Assigned Peptide Dose Cohort
|
|||
|---|---|---|---|
| Low | High | Total | |
| No. Patients | 13 | 13 | 26 |
| Number and percentage of patients with any treatment-related adverse events* | 10 (76.9%) | 6 (46.2%) | 16 (61.5%) |
| Fatigue | 3 (23.1%) | 1 (7.7%) | 4 (15.4%) |
| Injection site pain | 4 (30.8%) | 2 (15.4%) | 6 (23.1%) |
| Chills | 2 (15.4%) | 0 | 2 (7.7%) |
| Injection site reaction | 0 | 2 (15.4%) | 2 (7.7%) |
| Dizziness | 2 (15.4%) | 0 | 2 (7.7%) |
| Headache | 1 (7.7%) | 1 (7.7%) | 2 (7.7%) |
| Rash | 1 (7.7%) | 1 (7.7%) | 2 (7.7%) |
The percentage was calculated based on total number of patients in a given dose group. The treatment-related adverse events were all mild or moderate, grades 1 and 2.
In several patients upon dosing, pPRA-PSM plasmid levels were transiently detectable in the blood (data not shown). Plasmid was not detected in any blood samples at follow-up visits after treatment completion. These results indicate that there is no systemic accumulation of the plasmid (probably due to degradation), consistent with the general safety profile.
Serum cytokine levels were evaluated to assess the release of acute phase reactants or a possible cytokine storm syndrome, as a result of injection of relatively large amounts of plasmid or peptides. Levels of interferon-α, interferon-γ, and IL-2 in serum samples collected from patients at screening and on day 39 of each treatment cycle were constantly below the LLD of standard enzyme-linked immunosorbent assays. IL-6 levels varied in samples from each patient, ranging from below the LLD (<4 pg/mL) to about 40 ng/mL, with no distinct trend related to clinical or immune outcome (data not shown). This suggests various degrees of preexisting inflammation in patients with cancer, or a limited and transient acute phase reaction to the investigational agent. None of the patients experienced AEs suggestive of a cytokine storm syndrome or septic shock, such as hypotension, fever or rigor.
Overall, these results demonstrate that intralymph node administration of the prime-boost investigational vaccine MKC1106-PP results in limited systemic exposure to the plasmid and minimal-to-modest toxicities.
T-Cell Immune Responses
The immune responses to PRAME and PSMA were measured by tetramer analysis utilizing flow cytometry and by ELISPOT analysis of PBMCs, as described in Methods section. The immune response criteria were defined prospectively: a tetramer response was defined as greater than a 2-fold increase in the number of tetramer+ T cells at any time point after starting immunization; an ELISPOT response was defined as at least a 3-fold increase over the baseline value and 3 times the standard deviation. Twenty-four of 26 patients had PBMC samples available for immune analysis before, during, and after therapy.
A majority of the evaluable patients (15 of 24; 63%) showed de novo induction or an increased frequency of PRAME-specific and PSMA-specific T cells at 1 or multiple-time points during treatment (Fig. 2, Suppl. Fig. 3 http://links.lww.com/JIT/A117). There were 3 patterns of immunity observed (Fig. 2): (i) induction of persisting levels of specific T cells over the duration of treatment (4 of 24 evaluable patients); (ii) transient induction or enhancement of specific T cells followed by decline below the LLD (11 of 24 patients); and (iii) no elevation compared with pretreatment values (9 of 24 patients). There was no apparent difference in immune response between the low and high peptide dose cohorts, or across tumor types. Strikingly, the immunogenicity of PRAME and PSMA components of the MKC1106-PP vaccine generally paralleled each other. Detailed analysis of the immune response showed that plasmid priming during the first treatment cycle induced an immune response in a majority of patients. The effect of peptide boosting was heterogenous, ranging from an enhancement to an apparent diminution, of the antigen-specific T-cell frequency in the peripheral blood (Suppl. Fig. 3). In contrast to the substantial number of immune responders by tetramer-flow cytometric assay, only 1 patient showed an interferon-γ ELISPOT response, in parallel with a tetramer response (Suppl. Fig. 4 http://links.lww.com/JIT/A118).
FIGURE 2.
Immune response patterns for PRAME and PSMA. Patients were categorized by immune response status in 3 groups (induction of persisting immunity; transient/modest immunity, and no significant induction of immunity). The tumor type and clinical outcome are represented together with the immune response status along with the percentage of tetramer+ CD8+ T cells for each antigen. The LLD was 0.03% tetramer+ CD8+ T cells/CD8+ T cells. The cut-off for a positive response was at least 2-fold increase over baseline values and >2×LLD (in red). Other measured data points, within the range of background or baseline, are shown in blue. ESO indicates esophageal carcinoma; MEL, melanoma; NED, no evidence of disease; PC, prostate carcinoma; RC, renal carcinoma; PRAME, preferentially expressed antigen in melanoma; PSMA, prostate-specific membrane antigen.
An interesting observation was the detection of PRAME-specific and PSMA-specific T cells in blood in a significant proportion of patients before the initiation of treatment (15 of 24 evaluable patients as defined by >0.1% tetramer+ CD8+ T cells which is >3×LLD of the assay). The presence of PRAME 425–433-specific T cells before treatment was accompanied in most cases by measurable PSMA 288–297 T cells, indicating that both antigens were processed and epitopes presented in unvaccinated cancer patients. There was an inverse and statistically significant correlation between the immune response elicited by MKC1106-PP and the presence at baseline of T cells, specific for each antigen, respectively (P<0.05; Suppl. Table 3 http://links.lww.com/JIT/A121).
Clinical Outcome
There were no partial or complete responses by RECIST criteria. However, 10 patients (38.5%) had documented SD after 2 cycles of treatment (12 wk after treatment initiation), evenly distributed between the low and high peptide dose cohort (Table 5). Seven of those 10 patients showed disease control, defined as SD for 6 months or longer or a PSA decline: 3 patients in the low peptide dose and 4 patients in the high peptide dose group, respectively.
TABLE 5.
Clinical Outcome Summary
| Total No. Patients | Low Dose | High Dose | Overall |
|---|---|---|---|
|
| |||
| 13 | 13 | 26 | |
| Best response | |||
| PD | 7 | 8 | 15 |
| SD (Duration on treatment) | 5 (3, 3, 6, 6*, 9 mo) | 5 (3, 9, 9, 9*, 11 mo) | 10 |
| Not evaluable | 1 | 0 | 1 |
| Length of treatment (wk) | |||
| Median (range) | 13 (5–36) | 14 (2–52) | 13.5 (2–52) |
Patients with a PSA decline.
Within the prostate carcinoma subpopulation comprising 10 evaluable patients, there were 4 patients with disease control or a PSA decline (Tables 5, 6). Notably, patient PC5083 in the high peptide dose group, with castration-resistant prostate carcinoma and metastatic disease affecting pelvic lymph nodes and lungs, showed tumor regression in the pelvic lymph nodes 36 weeks after treatment initiation (corresponding to 6 therapeutic cycles). This patient also showed a decline in PSA: −30% and −20% relative to baseline, after 2 and 3 cycles of therapy, respectively. The other 3 prostate carcinoma patients with advancing disease and detectable or rising PSA levels at the start of treatment, showed SD for 6 to 9 months, accompanied in 1 case by a decrease in PSA levels (patient 1006, showing a −60% and −50% PSA decline relative to baseline, after 2 or 4 cycles of therapy, qualifying as a PSA response).
TABLE 6.
Best Clinical Outcomes by Tumor Type*
| Tumor Type | Sex/Age/Patient No. | Site of Disease | Prior Treatment | Best Outcome | Duration of SD (mo) |
|---|---|---|---|---|---|
| Prostate carcinoma | M/72 1006 | Bone, LN | Casodex/Lupron Docetaxel Mitoxantrone |
SD† | 6 |
| M/56 1119 | Lung | Casodex/Zoladex | SD†‡ | 9 | |
| M/79 5083 | Lung, LN | Flutamide/Zoladex Ketoconazole Hydrocortisone |
SD | 9+ | |
| M/79 1091 | LN | No prior treatment | SD | 9 | |
| Kidney cancer | M/46 1019 | Skin mets. | IL-2 | SD§ | 18+ |
| M/67 5075 | Lung | Sutent Nexavar | SD | 11 | |
| Melanoma | M/88 1024 | Lung/liver/LN | No prior treatment | SD | 18+ |
Patients with SD of 6 months or better. Shaded table cells correspond to patients in high peptide dose group.
PSA decline.
This patient showed objective tumor regression in the pelvic lymph nodes by computed tomographic scan at 36 weeks in treatment (but has not met the PR criteria under RECIST).
This patient had no evidence of disease (NED) at +18 months after resection of metastatic tumors after completion of 4 cycles of therapy (neoadjuvant setting).
LN indicates lymph nodes.
Both patients with metastatic renal clear cell carcinoma showed evidence of disease control (Table 6). Patient RC1019 previously treated with IL-2 and presenting with rapidly advancing cutaneous metastases, had evidence of disease stabilization on treatment with MKC1106-PP. On completion of 4 cycles of treatment, the patient underwent a successful surgical resection of all metastases, with subsequent durable remission (ongoing at +18 mo). The other kidney cancer patient, RC5075, previously treated with sunitinib and sorafenib, presented with progressive lung metastases. On treatment with MKC1106-PP, his disease stabilized for 11 months (Table 6).
One of 10 patients with malignant melanoma had durable SD lasting at least 18 months. Notably, this patient who presented with ocular melanoma, had progressive stage IV M1c disease, with metastases to lymph nodes, lungs, and liver.
Association Between Immunity and Clinical Outcome
An analysis of immunity against the 2 target epitopes PRAME 425–433 and PSMA 288–297 versus disease control yielded several patterns. First, patients with evidence of disease control (SD for 6 mo or better) generally had lower or no detectable antigen-specific T cells at baseline, yet, almost invariably, mounted an immune response against both antigens (6 of 7 patients) (Fig. 3). In contrast, only 7 of 16 patients in the group of patients with rapidly progressing disease while on treatment, showed an elevation of T cells against either antigen on immunization, with a majority of patients having preexisting antigen-specific T cells.
FIGURE 3.

Peak changes (post-versus-pre immunization) in the frequency of antigen-specific T cells in patients with evidence of disease control, defined as SD for 6 months or longer (A), compared with patients with rapid disease progression (B). The values are the percentage of tetramer+ CD8+ T cells in blood at baseline and the peak level attained at any time after the initiation of immunization, for individual patients, against each antigen. The numbers embedded within diagrams represent the median of percentage of tetramer+ CD8+ T cells for each group. Green lines: immune responders according to prespecified criteria. Red lines: nonresponders.
The patients in this trial could be grouped into 3 different immune response patterns: persisting, transient, and negligible immune response (Fig. 2 and Suppl. Fig. 3). It is interesting to note that, 4 of 7 patients with evidence of disease control, developed an immune response against both antigens by the first clinical evaluation (after 2 cycles) that persisted throughout additional cycles (Fig. 2). Conversely, only 1 of 17 patients who progressed clinically within the first 2 cycles of therapy, showed an immune response against both antigens measurable at the first clinical evaluation (after 2 cycles). The association between induction of persistent immunity against both antigens and disease control was statistically significant (Table 7); however, there was no association between the peak magnitude of immunity and disease progression. In addition, there was no apparent association between clinical evolution (progression vs. durable SD) and other parameters including frequency of total CD3+, CD8+ T cells, or nonspecific polyclonal T-cell reactivity measured by an interferon-γ ELISPOT/mitogen assay (data not shown). These results argue against the concept that the immune response to MKC1106-PP is a bystander phenomenon, simply a reflection of the overall immune status and devoid of clinical impact. In prostate cancer, the induction of immune response preceded an eventual PSA decline (eg, patient PC1091; Suppl. Fig. 4). Nevertheless, not all patients with disease control showed an expansion of specific T cells in the blood (most notably patient RC5075). In this case, we could not rule out a very transient response or localized immune response confined to the tumor tissue, as the measurement of immune response in this trial was done only on PBMCs.
TABLE 7.
Association Between Immune Response and Clinical Outcome
| Outcome* | Immune Responders† | Percent T cells at Baseline‡ Median (Range)
|
Peak percent T Cells After Immunization§ Median (Range)
|
Patients With Coinduction of T Cells at End of Cycle 2||
|
||
|---|---|---|---|---|---|---|
| PRAME | PSMA | PRAME | PSMA | PRAME & PSMA | ||
| Rapid tumor progression | 7/14 | 0.17 (0–0.62) | 0.19 (0–0.43) | 0.23 (0.08–1.54) | 0.22 (0.13–1.29) | 1/14 |
| Disease control | 6/7 | 0.07 (0–0.48) | 0.05 (0–0.44) | 0.43 (0.16–0.84) | 0.51 (0.16–1.67) | 4/7 |
Patients were categorized retrospectively in 2 clinical outcome groups: with SD for 6 months or longer (disease control), or with rapidly progressing disease.
Immune responders according to preset criteria/total number of patients in that clinical outcome group. P value of 2-tailed Fisher exact test comparing the 2 groups was 0.1736.
Percent tetramer+ CD8+ T cells in peripheral blood, at baseline.
Percent tetramer+ CD8+ T cells in peripheral blood, after immunization.
Number of patients who showed measurable immune responses against both antigens at the completion of second cycle, per total number of patients in that clinical outcome group. P value of 2-tailed Fisher exact test comparing the 2 groups was 0.0251.
DISCUSSION
In this study, we describe “first-in-human” results with a novel cancer vaccine MKC1106-PP, directed against PRAME and PSMA. This vaccine has several new features relative to other investigational immunotherapies24–27: (1) it cotargets both cancer cells and tumor vasculature; (2) it is directly administered into clinically uninvolved lymph nodes; and (3) it utilizes a heterologous plasmid prime/peptide boost strategy. These vaccine attributes are all designed to maximize an antitumor immunity which should translate to clinical benefit, manifested by durable disease control.
Our data indicate that PRAME and PSMA are overexpressed in a variety of solid tumors (Table 2), including prostate carcinoma (11 of 12 patients, 91%), melanoma (11 of 16 patients, 69%) and kidney cancer (4 of 6 patients, 67%). Further, these target antigens are expressed in various other tumor types (Suppl. Table 2, Suppl. Fig. 2). As the available sample size is still limited, the results suggest that this vaccine is potentially applicable to many cancers that express the TAAs of interest. This expands the ultimate potential target population for MKC1106-PP, unlike other more disease-focused vaccines.
Although PRAME was expressed relatively homogenously by cancerous cells, PSMA expression was limited to endothelial vascular cells in all cancers except prostate carcinoma, where it was intensely expressed by tumor cells in virtually all patients (Suppl. Fig. 2). The expression profile reflects the roles of PRAME and PSMA in tumor biology6–8,11,28; the former being a regulator of cellular differentiation through the retinoic acid receptor6 and the latter, a highly pleiotropic molecule with enzymatic activity and roles in angiogenesis8,10 and genome stability.14
This study accomplished its primary objectives. First, repeat intralymph node immunization with MKC1106-PP (alternating plasmid and peptides), was safe and feasible. There were no significant systemic reactions, or plasmid accumulation. In addition, MKC1106-PP administration resulted in immune responses against both PRAME and PSMA in half of immunized patients with advanced, progressive cancer. The T-cell immune response fit 3 patterns: persistent immunity, transitory or negligible responses. There was a patient subset, those with mimimal or no preexisting immunity to the target antigens, more likely to develop persistent immunity against both antigens, together with evidence of disease control (Fig. 2, Suppl. Fig. 3). There were no partial or complete responses as per RECIST criteria. Nevertheless, 7 patients showed evidence of disease control defined as SD for 6 months or longer and in 2 instances, PSA declines. Although these results warrant subsequent evaluation of this particular regimen, a direct comparison between our dual targeting regimen (PRAME and PSMA) and similar regimens separately targeting PRAME or PSMA has yet to be done.
Quite frequently, cancer patients had measurable epitope-specific T cells in their peripheral blood in the absence of prior vaccination against PRAME and PSMA (Fig. 3, Suppl. Table 3); such cells were not found in healthy donors (data not shown). This finding suggests that in some patients with tumors coexpressing PRAME and PSMA, the immune system has already been exposed to the antigens. Furthermore, the high levels of T cells against PRAME 425–433 and PSMA 288–297 epitopes at baseline, in some patients reaching 0.4% to 0.5%, indicate that these tumor-derived epitopes are effectively processed and are presented in vivo. Unexpectedly and in contrast to evidence on different antigens,29 patients with PRAME-specific and PSMA-specific T cells at baseline were less likely to show further T-cell expansion and disease control (Suppl. Table 3). This finding suggests that those preexisting PRAME-specific and PSMA-specific T cells induced by the tumor, are at least partially anergic or disabled through various mechanisms. In contrast, immunization of patients with limited or no measurable baseline immunity led to induction or expansion of functional T cells against both antigens; a majority of patients who showed disease control fit this particular pattern (Fig. 3). This hypothesis remains to be tested through larger trials and more extensive immune response analysis. Finally, as we measured several cytokines in blood to assess or rule out a possible acute phase response or cytokine storm syndrome, there was no evident relationship between blood cytokine levels and outcome in terms of immune or clinical response.
Several findings were discordant with earlier preclinical results19,20 employing a similar prime-boost strategy in a murine model: (1) a relatively low frequency of post-vaccine antigen-specific T cells in the blood in man; (2) a limited impact of peptide boosting on antigen-specific immunity in most patients; (3) a decrease of TAA-specific T cell frequency in the peripheral blood of several patients upon continued immunization; and (4) a lack of correlation between the magnitude of immunity (peak values) and clinical outcome. In addition, the lack of IFN-γ ELISPOT reactivity of PBMCs (with the exception of 1 patient) may be a reflection of the low frequency of IFN-γ producing cells as the assay was a direct ELISPOT without prior in vitro stimulation. Alternatively, the antigen-specific T cells in blood could be producing a different range of immune mediators. Furthermore, the intratumoral immunity does not necessarily mirror the immune status in blood.30–33 Yet, another explanation could be a subdominant nature of the evaluated epitopes in man, although the presence or frequency at baseline of PRAME 425–433 and PSMA 288–297 specific T cells argues against this possibility. Preclinical studies also suggest an explanation for some of these discrepancies. The lower immune response encountered in patients may reflect a relatively limited immunogenicity of this immunization regimen in man, in the face of a possible presence of Treg cells and tumor or treatment-related immune suppressing mechanisms.19 A positive impact of plasmid priming and peptide boosting may occur at different levels, such as generation of PD-1low effector T cells, relatively refractory to immune inhibiting mechanisms,20 and therefore quite functional within the tumor microenvironment. Further studies are warranted to elucidate this hypothesis and whether the observed synergistic effect between priming and boosting agents seen in preclinical models also occurs in a clinical setting. Similarly, the translation of the preclinical finding that intralymph node administration is a very immunogenic route17–20 awaits a resolution as other clinical studies showed only a moderate response to intralymph node administration of dendritic cells.34–36 Nevertheless, in all these cases, dendritic cells were prepared by utilizing PBMCs manipulated ex vivo. As by directly injecting synthetic antigen into the lymph nodes, the number and nature of antigen presenting cells that are deployed is different, an appropriate comparison between distinct routes of immunization in man is still warranted.
This phase 1 clinical trial was designed to provide patients with advanced, progressing disease, with at least 2 cycles of therapy before clinical evaluation. Patients determined to be nonprogressors remained onstudy and received treatment for up to 6 cycles. Although an assessment of overall survival and progression-free survival were outside the scope of this phase 1 trial, such endpoints will be included in subsequent trials to determine efficacy, particularly in light of a mechanism of action of this vaccine approach that could be cytostatic rather than cytoreductive.37,38 Given that these patients with progressive disease were refractory to other therapies, a surprising number continued on treatment beyond 2 cycles without disease progression. Seven patients showed evidence of disease control (SD for 6 mo or longer), with 2 patients showing a decrease in PSA levels and 1 patient demonstrating tumor reduction (Tables 5, 6). Although there was an association between the immune response (induction and persistence of antigen-specific T cells measured in blood) and disease control, there were exceptions. Thus, based on these data and independent studies,31,32 mere enumeration of antigen-specific cells in peripheral blood may not be an appropriate immune monitoring strategy for active immunotherapy in cancer. This could be due to the increasingly acknowledged impact of the functional profile of TAA-specific T cells,39 along with their trafficking capability. The latter is supported by the discrepancy between the presence and number of specific T cells in circulation, lymphoid organs, and tumor site, as documented in independent studies.30–33 On the basis of such considerations, we plan to include enumeration and analysis of tumor-infiltrating T cells for immune monitoring in future studies of MKC1106-PP.
Altogether, these results support further investigation of PRAME and PSMA as targets for active immunotherapy in tumor types amenable to immune intervention.40–42 In particular, more extensive evaluation to assess the clinical effect of MKC1106-PP in specific tumor types such as prostate carcinoma and kidney cancer, is warranted.
Supplementary Material
Footnotes
All remaining authors have declared that there are no conflicts of interest in regards of this study.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.immunotherapy-journal.com.
References
- 1.Jones D. Rethinking therapeutic cancer vaccines. Nat Rev Drug Discov. 2009;8:S685–S686. doi: 10.1038/nrd2994. [DOI] [PubMed] [Google Scholar]
- 2.Morse MA, Whelan M. A year of successful cancer vaccines points to a path forward. Curr Opin Mol Ther. 2010;12:11–13. [PubMed] [Google Scholar]
- 3.Rosenberg SA. Shedding light on immunotherapy for cancer. N Engl J Med. 2004;350:1461–1463. doi: 10.1056/NEJMcibr045001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brichard VG, Lejeune D. Cancer immunotherapy targeting tumour-specific antigens: towards a new therapy for minimal residual disease. Expert Opin Biol Ther. 2008;8:951–968. doi: 10.1517/14712598.8.7.951. [DOI] [PubMed] [Google Scholar]
- 5.Old LJ. Cancer vaccines: an overview. Cancer Immun. 2008;8:S1. [PubMed] [Google Scholar]
- 6.Epping MT, Wang L, Edel MJ, et al. The human tumor antigen PRAME is a dominant repressor of retinoic acid receptor signaling. Cell. 2005;122:835–847. doi: 10.1016/j.cell.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Paydas S. Is everything known in all faces of iceberg in PRAME? Leuk Res. 2008;32:1356–1357. doi: 10.1016/j.leukres.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Rajasekaran AK, Anilkumar G, Christiansen JJ. Is prostate-specific membrane antigen a multifunctional protein? Am J Physiol Cell Physiol. 2005;288:C975–C981. doi: 10.1152/ajpcell.00506.2004. [DOI] [PubMed] [Google Scholar]
- 9.Slovin SF. Targeting novel antigens for prostate cancer treatment: focus on prostate-specific membrane antigen. Expert Opin Ther Targets. 2005;9:561–570. doi: 10.1517/14728222.9.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conway RE, Petrovic N, Li Z, et al. Prostate-specific membrane antigen regulates angiogenesis by modulating integrin signal transduction. Mol Cell Biol. 2006;26:5310–5324. doi: 10.1128/MCB.00084-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oehler VG, Guthrie KA, Cummings CL, et al. The preferentially expressed antigen in melanoma (PRAME) inhibits myeloid differentiation in normal hematopoietic and leukemic progenitor cells. Blood. 2009;114:3299–3308. doi: 10.1182/blood-2008-07-170282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffioen M, Kessler JH, Borghi M, et al. Detection and functional analysis of CD8+T cells specific for PRAME: a target for T-cell therapy. Clin Cancer Res. 2006;12:3130–3136. doi: 10.1158/1078-0432.CCR-05-2578. [DOI] [PubMed] [Google Scholar]
- 13.Murphy GP, Elgamal AA, Su SL, et al. Current evaluation of the tissue localization and diagnostic utility of prostate specific membrane antigen. Cancer. 1998;83:2259–2269. [PubMed] [Google Scholar]
- 14.Rajasekaran SA, Christiansen JJ, Schmid I, et al. Prostate-specific membrane antigen associates with anaphase-promoting complex and induces chromosomal instability. Mol Cancer Ther. 2008;7:2142–2151. doi: 10.1158/1535-7163.MCT-08-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu J, Celis E. Recognition of prostate tumor cells by cytotoxic T lymphocytes specific for prostate-specific membrane antigen. Cancer Res. 2002;62:5807–5812. [PubMed] [Google Scholar]
- 16.Manyak MJ. Indium-111 capromab pendetide in the management of recurrent prostate cancer. Expert Rev Anticancer Ther. 2008;8:175–181. doi: 10.1586/14737140.8.2.175. [DOI] [PubMed] [Google Scholar]
- 17.Maloy KJ, Erdmann I, Basch V, et al. Intralymphatic immunization enhances DNA vaccination. Proc Natl Acad Sci U S A. 2001;98:3299–3303. doi: 10.1073/pnas.051630798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansen P, Häffner AC, Koch F, et al. Direct intralymphatic injection of peptide vaccines enhances immunogenicity. Eur J Immunol. 2005;35:568–574. doi: 10.1002/eji.200425599. [DOI] [PubMed] [Google Scholar]
- 19.Smith KA, Tam VL, Wong RM, et al. Enhancing DNA vaccination by sequential injection of lymph nodes with plasmid vectors and peptides. Vaccine. 2009;27:2603–2615. doi: 10.1016/j.vaccine.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 20.Smith KA, Qiu Z, Wong R, et al. Multivalent immunity targeting tumor-associated antigens by intra-lymph node DNA-prime, peptide-boost vaccination. Can Gene Ther. 2011;18:63–76. doi: 10.1038/cgt.2010.45. [DOI] [PubMed] [Google Scholar]
- 21.Tagawa ST, Lee P, Snively J, et al. Phase 1 study of intranodal delivery of a plasmid DNA vaccine for subjects with stage IV melanoma. Cancer. 2003;1:144–154. doi: 10.1002/cncr.11462. [DOI] [PubMed] [Google Scholar]
- 22.Weber J, Boswell W, Smith J, et al. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J Immunother. 2008;31:215–223. doi: 10.1097/CJI.0b013e3181611420. [DOI] [PubMed] [Google Scholar]
- 23.Micheletti F, Bazzaro M, Canella A, et al. The lifespan of major histocompatibility complex class I/peptide complexes determines the efficiency of cytotoxic T-lymphocyte responses. Immunology. 1999;96:411–415. doi: 10.1046/j.1365-2567.1999.00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elsässer-Beile U, Bühler P, Wolf P. Targeted therapies for prostate cancer against the prostate specific membrane antigen. Curr Drug Targets. 2009;10:118–125. doi: 10.2174/138945009787354601. [DOI] [PubMed] [Google Scholar]
- 25.Olson WC, Heston WD, Rajasekaran AK. Clinical trials of cancer therapies targeting prostate-specific membrane antigen. Rev Recent Clin Trials. 2007;2:182–190. doi: 10.2174/157488707781662724. [DOI] [PubMed] [Google Scholar]
- 26.Todorova K, Ignatova I, Tchakarov S. Humoral immune response in prostate cancer patients after immunization with gene-based vaccines that encode for a protein that is proteasomally degraded. Cancer Immun. 2005;5:1. [PubMed] [Google Scholar]
- 27.Low L, Mander A, McCann K. DNA vaccination with electroporation induces increased antibody responses in patients with prostate cancer. Hum Gene Ther. 2009;20:1269–1278. doi: 10.1089/hum.2009.067. [DOI] [PubMed] [Google Scholar]
- 28.Matsushita M, Yamazaki R, Ikeda H, et al. Preferentially expressed antigen of melanoma (PRAME) in the development of diagnostic and therapeutic methods for hematological malignancies. Leuk Lymphoma. 2003;44:439–444. doi: 10.1080/1042819021000035725. [DOI] [PubMed] [Google Scholar]
- 29.Tsuji T, Altorki NK, Ritter G, et al. Characterization of preexisting MAGE-A3-specific CD4+ T cells in cancer patients and healthy individuals and their activation by protein vaccination. J Immunol. 2009;183:4800–4808. doi: 10.4049/jimmunol.0900903. [DOI] [PubMed] [Google Scholar]
- 30.Ribas A, Weber JS, Chmielowski B, et al. Intra-Lymph node prime-boost vaccination against Melan A and Tyrosinase for the treatment of metastatic melanoma: results of a phase 1 clinical trial. Clin Cancer Res. 2011;17:2987–2996. doi: 10.1158/1078-0432.CCR-10-3272. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen MB, Marincola FM. Melanoma vaccines: the paradox of T cell activation without clinical response. Cancer Chemother Pharmacol. 2000;46(Suppl):S62–S66. doi: 10.1007/pl00014052. [DOI] [PubMed] [Google Scholar]
- 32.Lee KH, Panelli MC, Kim CJ, et al. Functional dissociation between local and systemic immune response during anti-melanoma peptide vaccination. J Immunol. 1998;161:4183–4194. [PubMed] [Google Scholar]
- 33.Kochenderfer JN, Gress RE. A comparison and critical analysis of preclinical anticancer vaccination strategies. Exp Biol Med (Maywood) 2007;232:1130–1141. doi: 10.3181/0702-MR-42. [DOI] [PubMed] [Google Scholar]
- 34.Fong L, Brockstedt D, Benike C, et al. Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J Immunol. 2001;166:4254–4259. doi: 10.4049/jimmunol.167.12.7150. [DOI] [PubMed] [Google Scholar]
- 35.Lesimple T, Neidhard EM, Vignard V, et al. Immunologic and clinical effects of injecting mature peptide-loaded dendritic cells by intralymphatic and intranodal routes in metastatic melanoma patients. Clin Cancer Res. 2006;12:7380–7388. doi: 10.1158/1078-0432.CCR-06-1879. [DOI] [PubMed] [Google Scholar]
- 36.Verdijk P, de Vries Figdor, et al. Limited amounts of dendritic cells migrate into the T-cell area of lymph nodes but have high immune activating potential in melanoma patients. Clin Cancer Res. 2009;15:2531–2540. doi: 10.1158/1078-0432.CCR-08-2729. [DOI] [PubMed] [Google Scholar]
- 37.Gimotty PA, Guerry D, Flaherty K. Using benchmarks based on historical survival rates for screening new therapies for stage IV melanoma patients. J Clin Oncol. 2008;26:517–518. doi: 10.1200/JCO.2007.14.3156. [DOI] [PubMed] [Google Scholar]
- 38.Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 39.Lin Y, Gallardo HF, Ku GY, et al. Optimization and validation of a robust human T-cell culture method for monitoring phenotypic and polyfunctional antigen-specific CD4 and CD8 T-cell responses. Cytotherapy. 2009;11:1–11. doi: 10.3109/14653240903136987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Antonarakis ES, Drake CG. Current status of immunological therapies for prostate cancer. Curr Opin Urol. 2010;20:241–246. doi: 10.1097/MOU.0b013e3283381793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biswas S, Eisen T. Immunotherapeutic strategies in kidney cancer: when TKIs are not enough. Nat Rev Clin Oncol. 2009;6:478–487. doi: 10.1038/nrclinonc.2009.91. [DOI] [PubMed] [Google Scholar]
- 42.Heine A, Holderried TA, Brossart P. Immunotherapy in renal cell carcinoma. Immunotherapy. 2009;1:97–107. doi: 10.2217/1750743X.1.1.97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.