Abstract
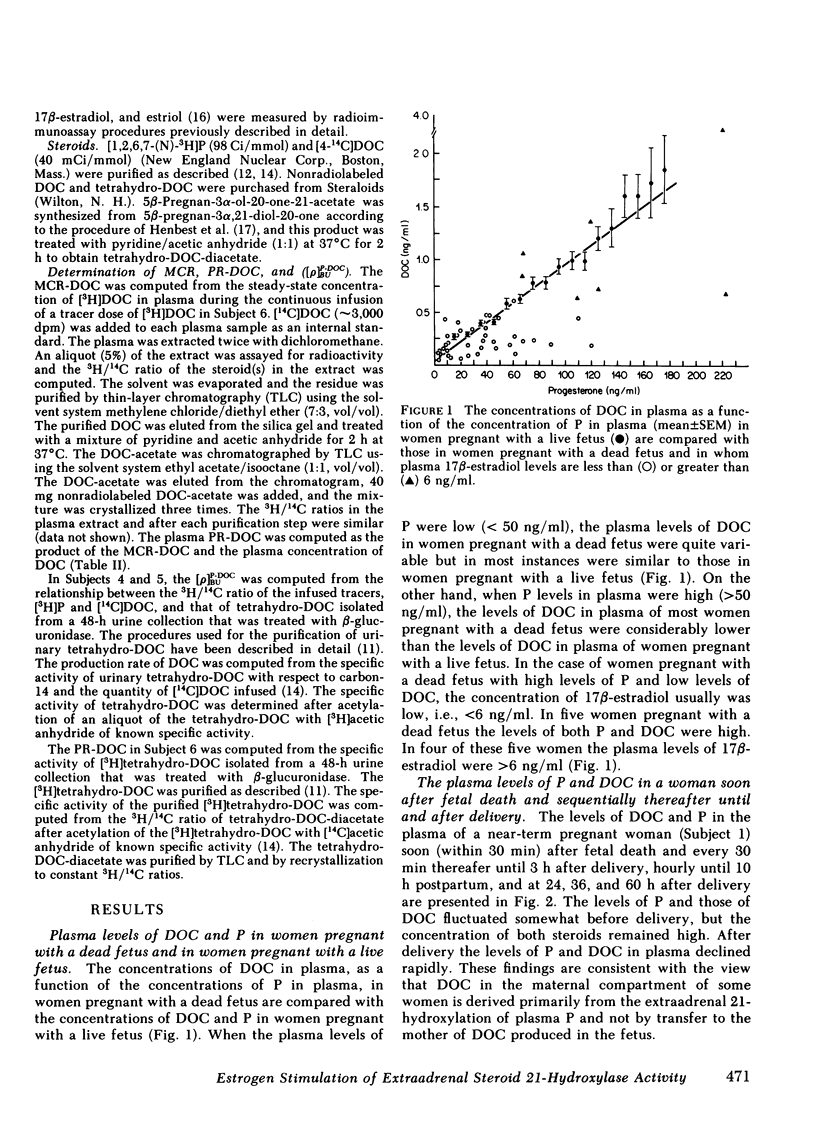
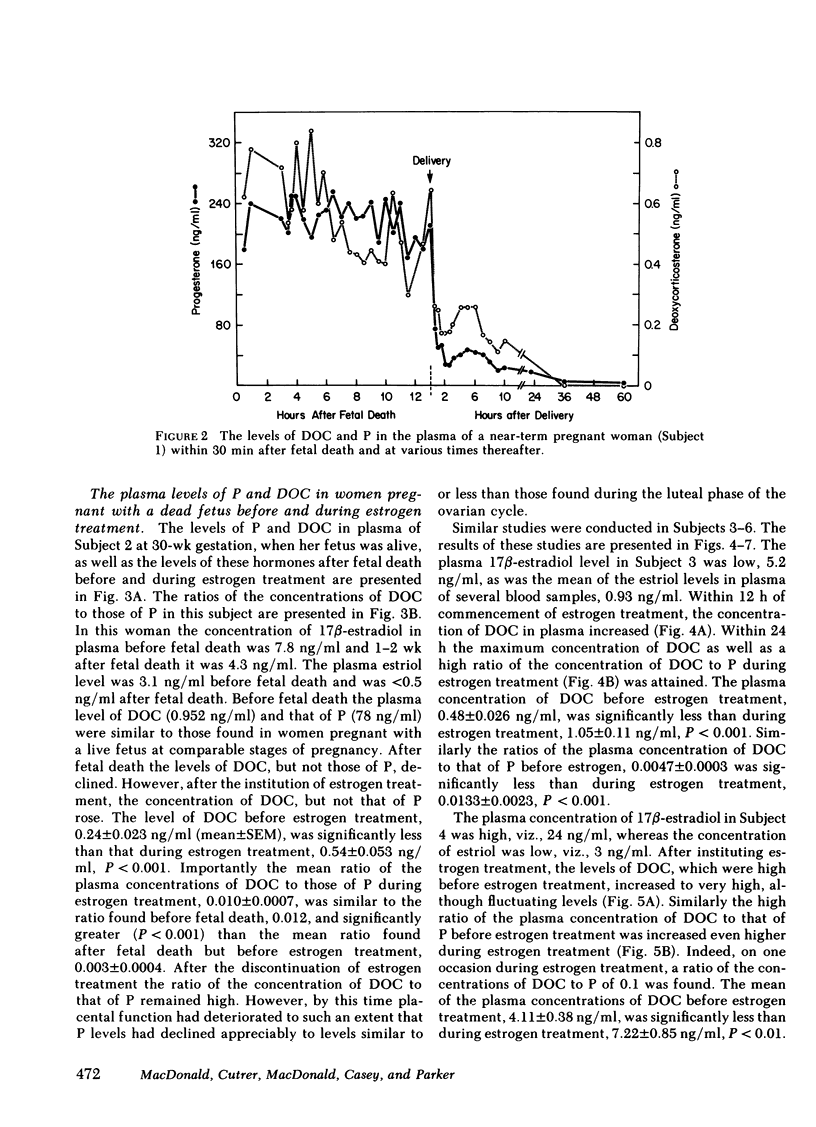
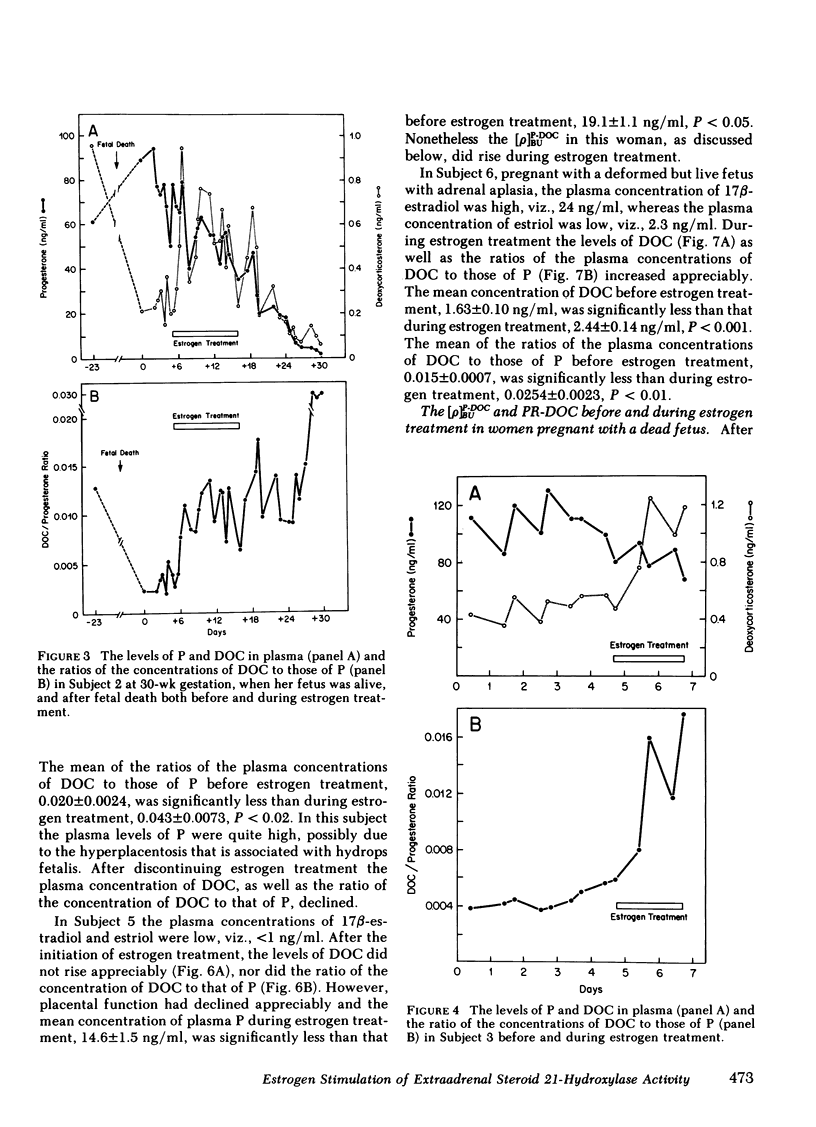
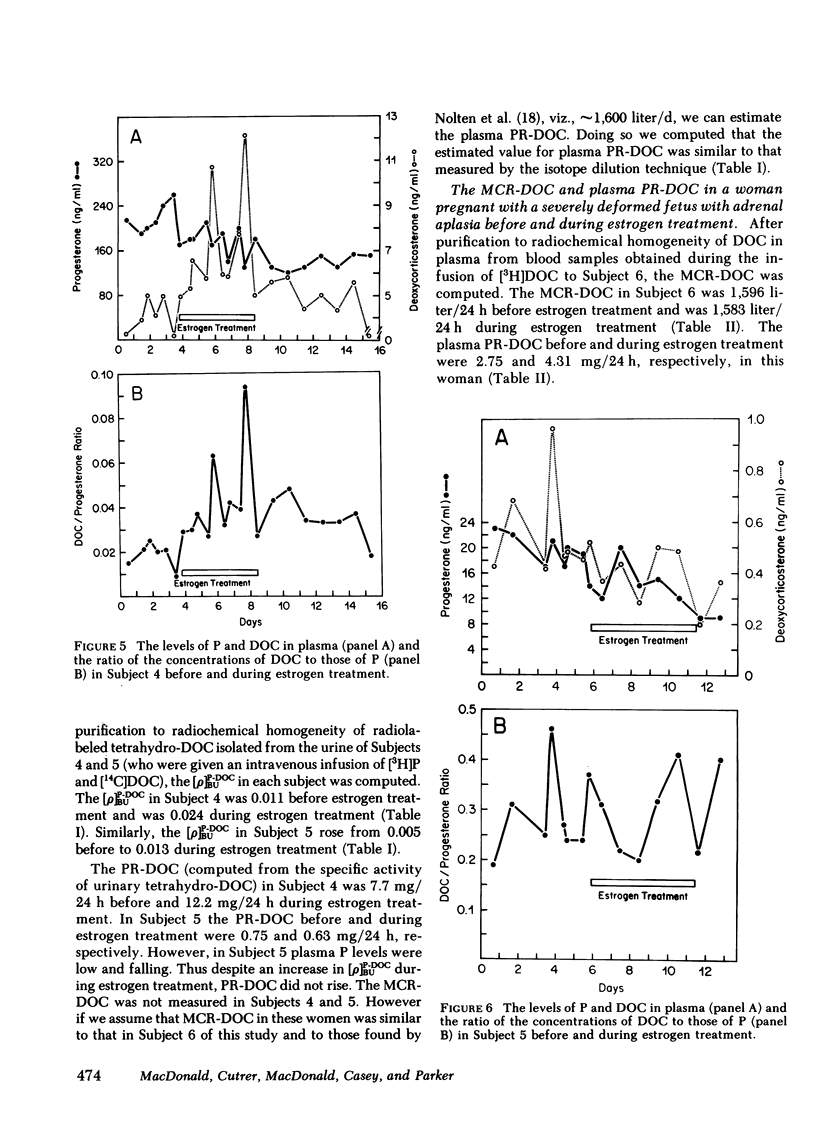
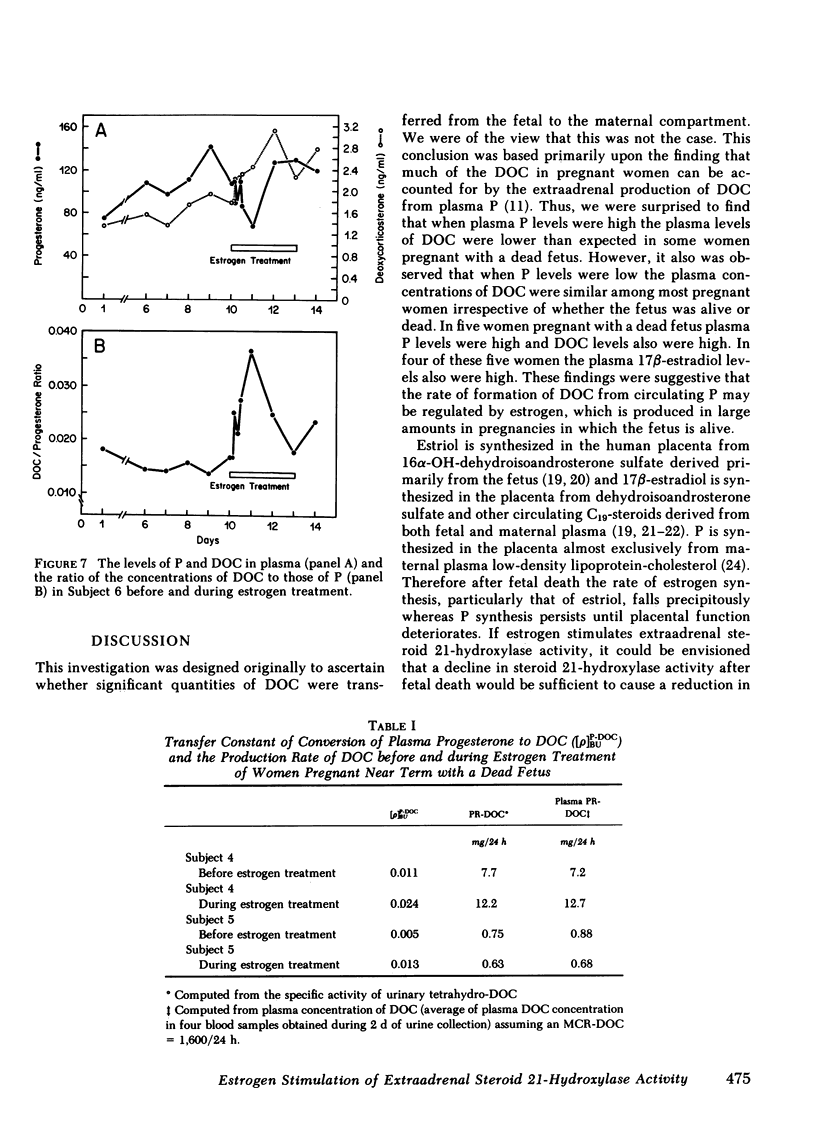
We measured deoxycorticosterone (DOC) and progesterone (P) in plasma of 47 women pregnant with a dead fetus and sequentially throughout gestation in 35 women pregnant with a live fetus. When P levels in plasma were low, the plasma levels of DOC in women pregnant with a dead fetus varied but usually were similar to those in women pregnant with a live fetus. However, when P levels were high, the levels of DOC in some women pregnant with a dead fetus were considerably lower than those in women pregnant with a live fetus. To test whether this finding was due to loss of transfer of DOC from fetus to mother or else loss of extraadrenal steroid 21-hydroxylase activity in the mother after death of the fetus, we conducted several studies. The levels of P and DOC in plasma of one woman remained constant from 30 min after fetal death until delivery occurred 13 h later. Estrogen treatment of four women pregnant with a dead fetus brought about an increase in plasma levels of DOC in three of the women. In one woman the ratio of plasma DOC to P was 0.015, a value similar to that found before fetal death, but was 0.003 after fetal death but before estrogen treatment. In two women pregnant with a dead fetus the transfer constants of conversion of plasma P to DOC were 0.011 and 0.005 before, and 0.024 and 0.013, respectively, during estrogen treatment. In one woman pregnant with a deformed fetus with adrenal agenesis, the metabolic clearance rates of DOC before and during estrogen treatment were similar, whereas the plasma production rates of DOC were 2.75 before and 4.31 mg/24 h during estrogen treatment. We suggest that (a) the DOC in plasma of near-term pregnant women arises in part by extraadrenal 21-hydroxylation of plasma P and (b) estrogen stimulates steroid 21-hydroxylase activity in extraadrenal tissues.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bibbo M., Haenszel W. M., Wied G. L., Hubby M., Herbst A. L. A twenty-five-year follow-up study of women exposed to diethylstilbestrol during pregnancy. N Engl J Med. 1978 Apr 6;298(14):763–767. doi: 10.1056/NEJM197804062981403. [DOI] [PubMed] [Google Scholar]
- Bolté E., Wiqvist N., Diczfalusy E. Metabolism of dehydroepiandrosterone and dehydroepiandrosterone sulphate by the human foetus at midpregnancy. Acta Endocrinol (Copenh) 1966 Aug;52(4):583–597. doi: 10.1530/acta.0.0520583. [DOI] [PubMed] [Google Scholar]
- Branchaud C., Schweitzer M., Giroud C. J. Characterization of the 21-yl sulfates of 11-beta, 17-alpha,21-trihydroxypregn-4-ene-3,20-dione; 17-alpha,21-dihydroxypregn-4-ene-3, 11,20-trione; 11-beta,21-dihydroxyregn-4-ene-3,20-dione; 21-hydroxypregn-4-ene-3, 11,20-trione and 21-hydroxypregn-4-ene,3,20-dione in human cord plasma. Steroids. 1969 Aug;14(2):179–190. doi: 10.1016/0039-128x(69)90032-4. [DOI] [PubMed] [Google Scholar]
- Brown R. D., Strott C. A., Liddle G. W. Plasma deoxycorticosterone in normal and abnormal pregnancy. J Clin Endocrinol Metab. 1972 Nov;35(5):736–742. doi: 10.1210/jcem-35-5-736. [DOI] [PubMed] [Google Scholar]
- Ghraf R., Lax E. R., Schriefers H. Regulation of 3alpha-hydroxysteroid-dehydrogenase activities in rat kidney cytosol: dependence of estrogenic induction on the endocrine status. Hoppe Seylers Z Physiol Chem. 1977 Jun;358(6):699–702. [PubMed] [Google Scholar]
- MACDONALD P. C., SITERI P. K. ORIGIN OF ESTROGEN IN WOMEN PREGNANT WITH AN ANENCEPHALIC FETUS. J Clin Invest. 1965 Mar;44:465–474. doi: 10.1172/JCI105160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewich L., Gomez-Sanchez C., Madden J. D., MacDonald P. C. Isolation and characterization of 5alpha-pregnane-3,20-dione and progesterone in pepipheral blood of pregnant women. measurement throughout pregnancy. Gynecol Invest. 1975;6(5):291–306. [PubMed] [Google Scholar]
- Nissenson R., Fluoret G., Hechter O. Opposing effects of estradiol and progesterone on oxytocin receptors in rabbit uterus. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2044–2048. doi: 10.1073/pnas.75.4.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolten W. E., Holt L. H., Rueckert P. A. Desoxycorticosterone in normal pregnancy. III. Evidence of a fetal source of desoxycorticosterone. Am J Obstet Gynecol. 1981 Feb 15;139(4):477–482. doi: 10.1016/0002-9378(81)90328-8. [DOI] [PubMed] [Google Scholar]
- Nolten W. E., Lindheimer M. D., Oparil S., Ehrlich E. N. Desoxycorticosterone in normal pregnancy. I. Sequential studies of the secretory patterns of desoxycorticosterone, aldosterone, and cortisol. Am J Obstet Gynecol. 1978 Oct 15;132(4):414–420. [PubMed] [Google Scholar]
- Nolten W. E., Lindheimer M. D., Oparil S., Rueckert P. A., Ehrlich E. N. Desoxycorticosterone in normal pregnancy. II. Cortisol-dependent fluctuations in free plasma desoxycorticosterone. Am J Obstet Gynecol. 1979 Mar 15;133(6):644–648. doi: 10.1016/0002-9378(79)90012-7. [DOI] [PubMed] [Google Scholar]
- Nolten W., Vecsei P., Köhler M., Purjesz I., Wolff H. P. Untersuchungen über Sekretion und Stoffwechsel von Desoxycorticosteron an Gesunden und Kranken. Verh Dtsch Ges Inn Med. 1968;74:1218–1221. [PubMed] [Google Scholar]
- Parker C. R., Jr, Everett R. B., Whalley P. J., Quirk J. G., Jr, Gant N. F., MacDonald P. C. Hormone production during pregnancy in the primigravid patient. II. Plasma levels of deoxycorticosterone throughout pregnancy of normal women and women who developed pregnancy-induced hypertension. Am J Obstet Gynecol. 1980 Nov 15;138(6):626–631. [PubMed] [Google Scholar]
- Schweitzer M., Branchaud C., Giroud C. J. Maternal and umbilical cord plasma concentrations of steroids of the pregn-4-ene C-21-yl sulfate series at term. Steroids. 1969 Nov;14(5):519–532. doi: 10.1016/s0039-128x(69)80045-0. [DOI] [PubMed] [Google Scholar]
- Siiteri P. K., MacDonald P. C. Placental estrogen biosynthesis during human pregnancy. J Clin Endocrinol Metab. 1966 Jul;26(7):751–761. doi: 10.1210/jcem-26-7-751. [DOI] [PubMed] [Google Scholar]
- Sippell W. G., Becker H., Versmold H. T., Bidlingmaier F., Knorr D. Longitudinal studies of plasma aldosterone, corticosterone, deoxycorticosterone, progesterone, 17-hydroxyprogesterone, cortisol, and cortisone determined simultaneously in mother and child at birth and during the early neonatal period. I. Spontaneous delivery. J Clin Endocrinol Metab. 1978 Jun;46(6):971–985. doi: 10.1210/jcem-46-6-971. [DOI] [PubMed] [Google Scholar]
- Soloff M. S. Uterine receptor for oxytocin: effects of estrogen. Biochem Biophys Res Commun. 1975 Jul 8;65(1):205–212. doi: 10.1016/s0006-291x(75)80080-5. [DOI] [PubMed] [Google Scholar]
- Winkel C. A., Casey M. L., Simpson E. R., MacDonald P. C. Deoxycorticosterone biosynthesis from progesterone in kidney tissue of the human fetus. J Clin Endocrinol Metab. 1981 Jul;53(1):10–15. doi: 10.1210/jcem-53-1-10. [DOI] [PubMed] [Google Scholar]
- Winkel C. A., Milewich L., Parker C. R., Jr, Gant N. F., Simpson E. R., MacDonald P. C. Conversion of plasma progesterone to deoxycorticosterone in men, nonpregnant and pregnant women, and adrenalectomized subjects. J Clin Invest. 1980 Oct;66(4):803–812. doi: 10.1172/JCI109918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel C. A., Parker C. R., Jr, Simpson E. R., MacDonald P. C. Production rate of deoxycorticosterone in women during the follicular and luteal phases of the ovarian cycle: the role of extraadrenal 21-hydroxylation of circulating progesterone in deoxycorticosterone production. J Clin Endocrinol Metab. 1980 Dec;51(6):1354–1358. doi: 10.1210/jcem-51-6-1354. [DOI] [PubMed] [Google Scholar]
- Winkel C. A., Simpson E. R., Milewich L., MacDonald P. C. Deoxycorticosterone biosynthesis in human kidney: potential for formation of a potent mineralocorticosteroid in its site of action. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7069–7073. doi: 10.1073/pnas.77.12.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel C. A., Snyder J. M., MacDonald P. C., Simpson E. R. Regulation of cholesterol and progesterone synthesis in human placental cells in culture by serum lipoproteins. Endocrinology. 1980 Apr;106(4):1054–1060. doi: 10.1210/endo-106-4-1054. [DOI] [PubMed] [Google Scholar]
- Wright K., Collins D. C., Preedy J. R. Comparative specificity of antisera raised against estrone, estradiol-17 and estriol using 6-0-carboxy-methyloxime bovine serum albumin derivatives. Steroids. 1973 May;21(5):755–769. doi: 10.1016/0039-128x(73)90140-2. [DOI] [PubMed] [Google Scholar]


