Abstract
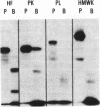
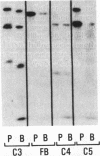
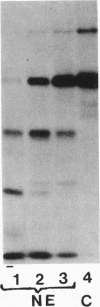
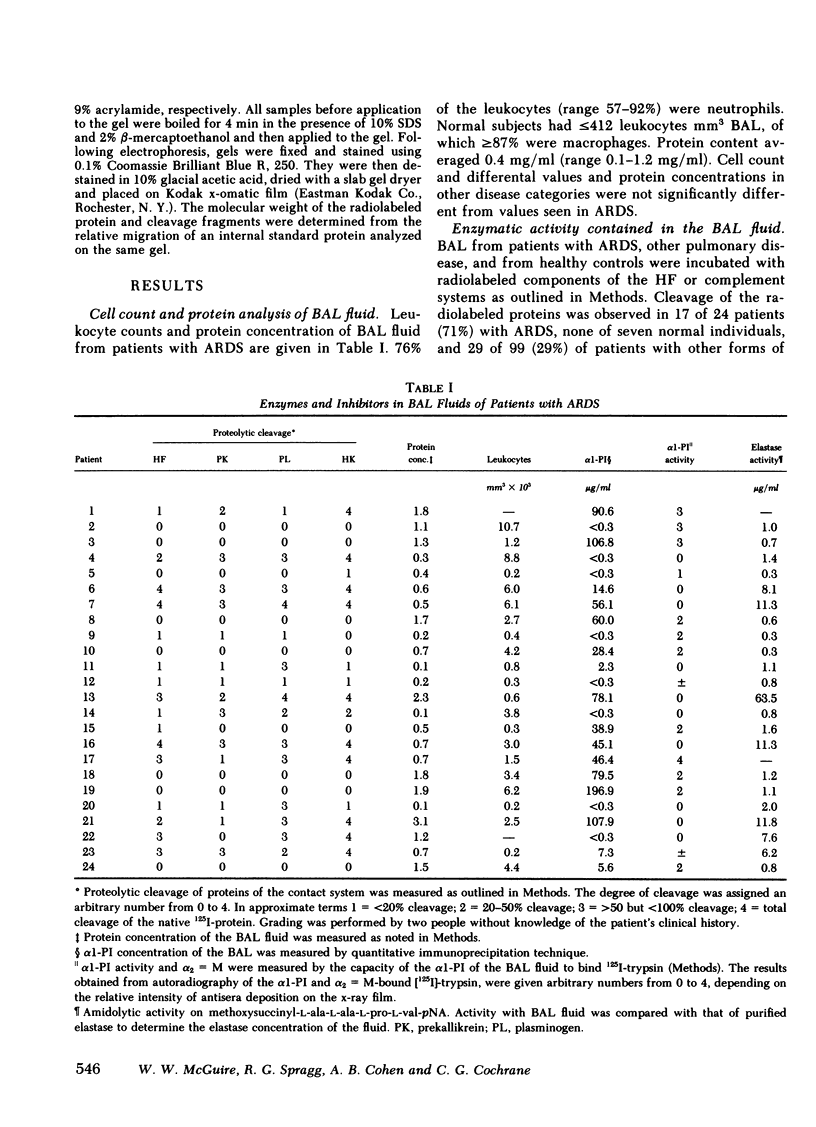
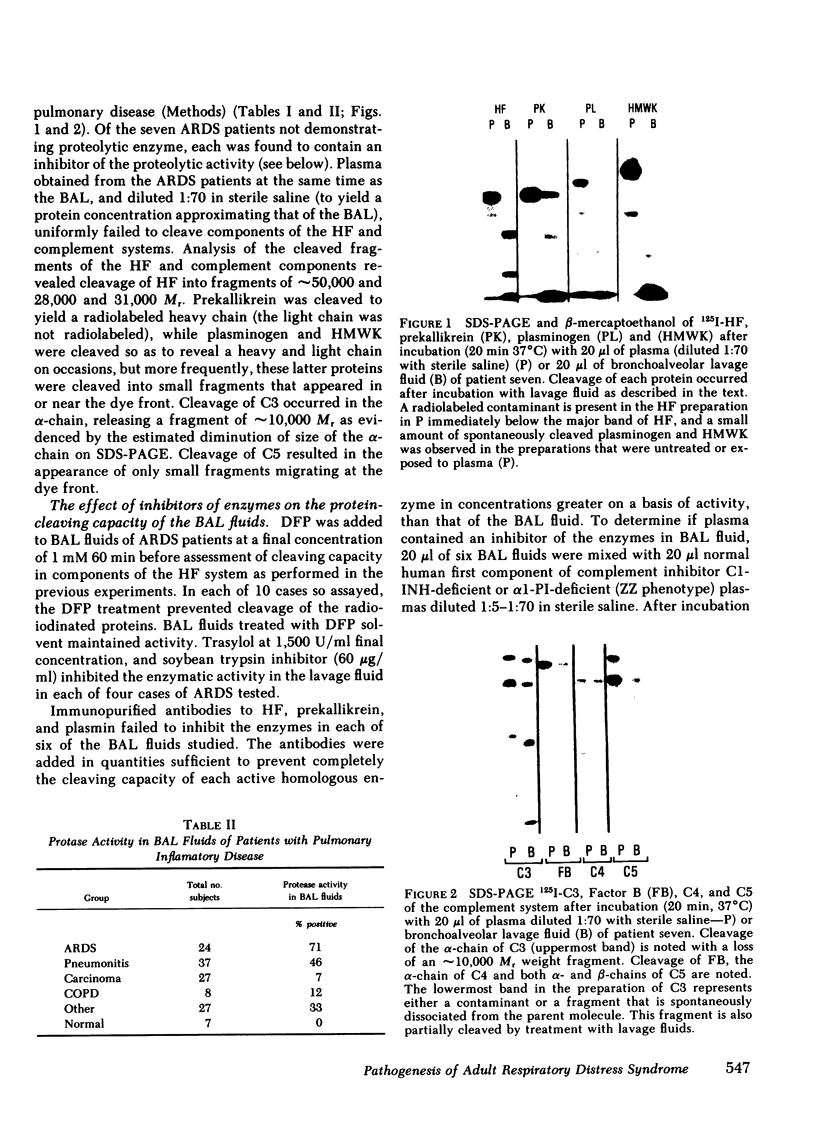
Bronchoalveolar lavage (BAL) fluid was obtained from 24 sequentially studied patients with adult respiratory distress syndrome (ARDS) for assessment of potential activating and mediating factors. Proteolytic activity of the fluids was observed by measuring cleavage of radiolabeled proteins of the contact (Hageman factor) and complement systems. Proteolytic activity was observed in 17 of 24 (71%) patients with ARDS, and BAL fluid of the 7 ARDS patients without demonstrable, active, enzyme exhibited inhibitory activity for the proteolytic activity. The enzymes cleaved Hageman factor, prekallikrein, plasminogen, high molecular weight kininogen, C4, C3, C5, and Factor B of the complement system. Cleavage of the contact system proteins producted fragments similar or identical in size to the fragments observed during activation of these molecules, although continued incubation invariably reduced the protein to small peptide fragments. None of 7 normal individuals, and 29 of 99 patients (29%) with other forms of pulmonary disease contained measurable enzymes.
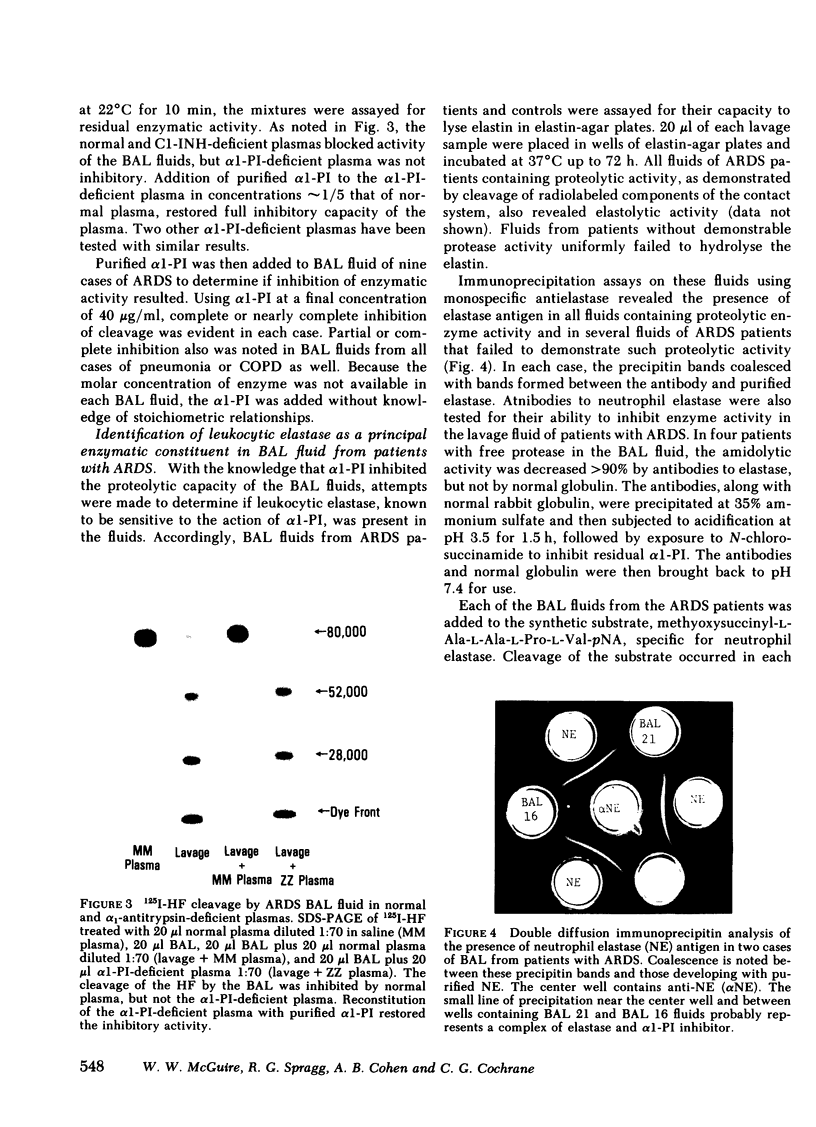
The proteolytic activity in BAL fluid of ARDS patients was blocked by diisopropylphosphofluoridate (0.1 mM), Trasylol, soybean trypsin inhibitor, and normal plasma, or plasma deficient in inhibition of the first component of complement. Alpha1-proteinase inhibitor (α1-PI)-deficient plasma failed to inhibit the proteolytic activity and addition of α1-PI to the deficient plasma reconstituted the inhibition.
Much of the proteolytic activity of the BAL fluid from ARDS patients was identified as neutrophil elastase: the fluids cleaved elastin and synthetic peptide substrate of neutrophil elastase, neutrophil elastase antigen was present in the BAL fluids as determined immunologically using antineutrophil elastase, α1-PI was the major inhibitor in plasma, and the enzyme was inhibited by diisopropylphosphofluoridate but not chelation. In addition, purified neutrophil elastase produced cleavage fragments of proteins of the contact system similar to those of the BAL fluids.
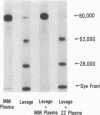
In each of the seven BAL fluids of ARDS patients that did not reveal active elastase, α1-PI was present in active form (as determined by 125I-trypsin binding). In 9 of the 17 patients with active elastase in the BAL fluid, α1-PI antigen was present in the fluid, but was inactive (no binding of 125I-trypsin). Immunoelectrophoretic analysis of elastase and α1-PI throughout proteins in these BAL fluids revealed the presence of both elastase and α1-PI that migrated with the same Rf, suggesting the presence of an enzyme-inhibitor complex. Free, inactive α1-PI was also observed in these fluids.
The data reveal that in BAL fluids from all 24 patients with ARDS, leukocytic elastase and/or α1-PI exist. A complex of elastase and α1-PI was observed in BAL fluids, and in some cases where active enzyme and α1-PI coexisted, free, but inactive α1-PI was present.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. R., Holliday R. L., Driedger A. A., Lefcoe M., Reid B., Sibbald W. J. Documentation of pulmonary capillary permeability in the adult respiratory distress syndrome accompanying human sepsis. Am Rev Respir Dis. 1979 Jun;119(6):869–877. doi: 10.1164/arrd.1979.119.6.869. [DOI] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Baumstark J. S. Studies on the elastase-serum protein interaction. I. Molecular identity of the inhibitors in human serum and direct demonstration of inhibitor-elastase complexes by zone and immunoelectrophoresis. Arch Biochem Biophys. 1967 Mar 20;118(3):619–630. doi: 10.1016/0003-9861(67)90397-9. [DOI] [PubMed] [Google Scholar]
- Bone R. C., Francis P. B., Pierce A. K. Intravascular coagulation associated with the adult respiratory distress syndrome. Am J Med. 1976 Nov;61(5):585–589. doi: 10.1016/0002-9343(76)90135-2. [DOI] [PubMed] [Google Scholar]
- Carp H., Janoff A. Possible mechanisms of emphysema in smokers. In vitro suppression of serum elastase-inhibitory capacity by fresh cigarette smoke and its prevention by antioxidants. Am Rev Respir Dis. 1978 Sep;118(3):617–621. doi: 10.1164/arrd.1978.118.3.617. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Aikin B. S. Polymorphonuclear leukocytes in immunologic reactions. The destruction of vascular basement membrane in vivo and in vitro. J Exp Med. 1966 Oct 1;124(4):733–752. doi: 10.1084/jem.124.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Griffin J. H. Molecular assembly in the contact phase of the Hageman factor system. Am J Med. 1979 Oct;67(4):657–664. doi: 10.1016/0002-9343(79)90253-5. [DOI] [PubMed] [Google Scholar]
- Cohen A. B. The effects in vivo and in vitro of oxidative damage to purified alpha1-antitrypsin and to the enzyme-inhibiting activity of plasma. Am Rev Respir Dis. 1979 Jun;119(6):953–960. doi: 10.1164/arrd.1979.119.6.953. [DOI] [PubMed] [Google Scholar]
- Conference report: Mechanisms of acute respiratory failure. Am Rev Respir Dis. 1977 Jun;115(6):1071–1078. doi: 10.1164/arrd.1977.115.6.1071. [DOI] [PubMed] [Google Scholar]
- Craddock P. R., Fehr J., Brigham K. L., Kronenberg R. S., Jacob H. S. Complement and leukocyte-mediated pulmonary dysfunction in hemodialysis. N Engl J Med. 1977 Apr 7;296(14):769–774. doi: 10.1056/NEJM197704072961401. [DOI] [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Fells G. A., Crystal R. G. Cigarette smoking induces functional antiprotease deficiency in the lower respiratory tract of humans. Science. 1979 Dec 14;206(4424):1315–1316. doi: 10.1126/science.316188. [DOI] [PubMed] [Google Scholar]
- Griffin J. H., Cochrane C. G. Human factor XII (Hageman factor). Methods Enzymol. 1976;45:56–65. doi: 10.1016/s0076-6879(76)45009-7. [DOI] [PubMed] [Google Scholar]
- Haynes J. B., Hyers T. M., Giclas P. C., Franks J. J., Petty T. L. Elevated fibrin(ogen) degradation products in the adult respiratory distress syndrome. Am Rev Respir Dis. 1980 Dec;122(6):841–847. doi: 10.1164/arrd.1980.122.6.841. [DOI] [PubMed] [Google Scholar]
- Hinman L. M., Stevens C. A., Matthay R. A., Gee J. B. Elastase and lysozyme activities in human alveolar macrophages. Effects of cigarette smoking. Am Rev Respir Dis. 1980 Feb;121(2):263–271. doi: 10.1164/arrd.1980.121.2.263. [DOI] [PubMed] [Google Scholar]
- Horsfield K., Cumming G. Morphology of the bronchial tree in man. J Appl Physiol. 1968 Mar;24(3):373–383. doi: 10.1152/jappl.1968.24.3.373. [DOI] [PubMed] [Google Scholar]
- Janoff A., Carp H., Lee D. K., Drew R. T. Cigarette smoke inhalation decreases alpha 1-antitrypsin activity in rat lung. Science. 1979 Dec 14;206(4424):1313–1314. doi: 10.1126/science.316187. [DOI] [PubMed] [Google Scholar]
- Janoff A. Mediators of tissue damage in leukocyte lysosomes. X. Further studies on human granulocyte elastase. Lab Invest. 1970 Mar;22(3):228–236. [PubMed] [Google Scholar]
- Janoff A., Sandhaus R. A., Hospelhorn V. D., Rosenberg R. Digestion of lung proteins by human leukocyte granules in vitro. Proc Soc Exp Biol Med. 1972 Jun;140(2):516–519. doi: 10.3181/00379727-140-36493. [DOI] [PubMed] [Google Scholar]
- Janoff A., Scherer J. Mediators of inflammation in leukocyte lysosomes. IX. Elastinolytic activity in granules of human polymorphonuclear leukocytes. J Exp Med. 1968 Nov 1;128(5):1137–1155. doi: 10.1084/jem.128.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Sloan B., Weinbaum G., Damiano V., Sandhaus R. A., Elias J., Kimbel P. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis. 1977 Mar;115(3):461–478. doi: 10.1164/arrd.1977.115.3.461. [DOI] [PubMed] [Google Scholar]
- Johnson D., Travis J. Structural evidence for methionine at the reactive site of human alpha-1-proteinase inhibitor. J Biol Chem. 1978 Oct 25;253(20):7142–7144. [PubMed] [Google Scholar]
- Johnson K. J., Fantone J. C., 3rd, Kaplan J., Ward P. A. In vivo damage of rat lungs by oxygen metabolites. J Clin Invest. 1981 Apr;67(4):983–993. doi: 10.1172/JCI110149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbiriou D. M., Griffin J. H. Human high molecular weight kininogen. Studies of structure-function relationships and of proteolysis of the molecule occurring during contact activation of plasma. J Biol Chem. 1979 Dec 10;254(23):12020–12027. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. T., Fein A. M., Lippmann M., Holtzman H., Kimbel P., Weinbaum G. Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory-distress syndrome. N Engl J Med. 1981 Jan 22;304(4):192–196. doi: 10.1056/NEJM198101223040402. [DOI] [PubMed] [Google Scholar]
- Luterman A., Manwaring D., Curreri P. W. The role of fibrinogen degradation products in the pathogenesis of the respiratory distress syndrome. Surgery. 1977 Nov;82(5):703–709. [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Schuyler M., Travis J. Interaction of human alpha-1-proteinase inhibitor with neutrophil myeloperoxidase. Biochemistry. 1981 Jan 20;20(2):331–336. doi: 10.1021/bi00505a016. [DOI] [PubMed] [Google Scholar]
- Matheson N. R., Wong P. S., Travis J. Enzymatic inactivation of human alpha-1-proteinase inhibitor by neutrophil myeloperoxidase. Biochem Biophys Res Commun. 1979 May 28;88(2):402–409. doi: 10.1016/0006-291x(79)92062-x. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Cochrane C. G. Direct evidence for Hageman factor (factor XII) activation by bacterial lipopolysaccharides (endotoxins). J Exp Med. 1974 Sep 1;140(3):797–811. doi: 10.1084/jem.140.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewiarowski S., Bańkowski E., Fiedoruk T. Adsorption of Hageman factor (Factor XII) on collagen. Experientia. 1964 Jul 15;20(7):367–368. doi: 10.1007/BF02147961. [DOI] [PubMed] [Google Scholar]
- Niewiarowski S., Bańkowski E., Rogowicka I. Studies on the adsorption and activation of the Hageman factor (factor XII) by collagen and elastin. Thromb Diath Haemorrh. 1965 Nov 15;14(3-4):387–400. [PubMed] [Google Scholar]
- Reynolds H. Y., Fulmer J. D., Kazmierowski J. A., Roberts W. C., Frank M. M., Crystal R. G. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977 Jan;59(1):165–175. doi: 10.1172/JCI108615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saba T. M., Blumenstock F. A., Scovill W. A., Bernard H. Cryoprecipitate reversal of opsonic alpha2-surface binding glycoprotein deficiency in septic surgical and trauma patients. Science. 1978 Aug 18;201(4356):622–624. doi: 10.1126/science.675246. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Tegner H., Kuhn C., Ohlsson K., Starcher B. C., Pierce J. A. The induction of pulmonary emphysema with human leukocyte elastase. Am Rev Respir Dis. 1977 Sep;116(3):469–475. doi: 10.1164/arrd.1977.116.3.469. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Burnstein Y., Gertler A. Effect of oxidation of methionine residues in chicken ovoinhibitor on its inhibitory activities against trypsin, chymotrypsin, and elastase. Biochemistry. 1977 Mar 8;16(5):992–997. doi: 10.1021/bi00624a029. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Burstein Y., Patchornik A. Selective oxidation of methionine residues in proteins. Biochemistry. 1975 Oct 7;14(20):4497–4503. doi: 10.1021/bi00691a025. [DOI] [PubMed] [Google Scholar]
- Twumasi D. Y., Liener I. E. Proteases from purulent sputum. Purification and properties of the elastase and chymotrypsin-like enzymes. J Biol Chem. 1977 Mar 25;252(6):1917–1926. [PubMed] [Google Scholar]
- Ward P. A., Hill J. H. C5 chemotactic fragments produced by an enzyme in lysosomal granules of neutrophils. J Immunol. 1970 Mar;104(3):535–543. [PubMed] [Google Scholar]
- White R. R., Norby D., Janoff A., Dearing R. Partial purification and characterization of mouse peritoneal exudative macrophage elastase. Biochim Biophys Acta. 1980 Mar 14;612(1):233–244. doi: 10.1016/0005-2744(80)90297-1. [DOI] [PubMed] [Google Scholar]
- Wiggins R. C., Loskutoff D. J., Cochrane C. G., Griffin J. H., Edgington T. S. Activation of rabbit Hageman factor by homogenates of cultured rabbit endothelial cells. J Clin Invest. 1980 Jan;65(1):197–206. doi: 10.1172/JCI109651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilner G. D., Nossel H. L., LeRoy E. C. Activation of Hageman factor by collagen. J Clin Invest. 1968 Dec;47(12):2608–2615. doi: 10.1172/JCI105943. [DOI] [PMC free article] [PubMed] [Google Scholar]