Patients who are younger, have better ECOG performance status, or have higher grade tumors are more likely to undergo recurrence score testing.
Abstract
Purpose:
Oncotype Dx 21-gene assay recurrence score (RS) predicts recurrence of early-stage breast cancer (ESBC). We investigated whether patient, tumor, or practice characteristics drive its use and explored Oncotype DX RS and chemotherapy use in subgroups.
Methods:
Patients with ESBC with documented estrogen receptor–positive, lymph node–negative, human epidermal growth factor receptor 2–negative tumors registered within McKesson Specialty Health's iKnowMed electronic health record were included. Patient and practice characteristics by region and size were analyzed. The association between Oncotype DX RS value and use of chemotherapy were assessed.
Results:
The study included 6,229 patients. Of these, 1,822 (29%) had an Oncotype DX RS result. Test use was 36%, 38%, 34%, 25%, and 6%, respectively, in patients age ≤ 45, 46-55, 56-65, 66-75, and ≥ 76 years; 33%, 25%, and 9% in patients with Eastern Cooperative Oncology Group performance status of 0, 1, and ≥ 2; 7%, 9%, 25%, 38%, 27%, and 10% in T1mic, T1a, T1b, T1c, T2, and T3 tumors; and 26%, 32%, and 33% for grades 1, 2, and 3 tumors. Of the 1,822 patients with available Oncotype DX RS, adjuvant chemotherapy use was 6%, 42%, and 84% in the low-, intermediate-, and high-risk groups.
Conclusion:
Patients who were younger, had better ECOG performance status, or had higher grade tumors were more likely to undergo RS testing. It appears that the RS test may have influenced the decision about whether to administer adjuvant chemotherapy: a low RS score was associated with lower chemotherapy use and a high RS score was associated with higher chemotherapy use.
Introduction
The development of genomic assays prognostic for the risk of recurrence of early-stage breast cancer (ESBC) and predictive of the likelihood of a patient benefiting from chemotherapy has helped refine treatment decisions in patients with ESBC. Genomic assays build on previous efforts to individualize treatment, such as receptor assays, tumor size, tumor grade, and others. Such assays help personalize treatment so that each patient will receive the most beneficial treatment for his or her individual cancer scenario. This might entail receiving more or less chemotherapy, depending in what has been determined to be the most appropriate for the individual. Therefore, physician and patient adjuvant treatment decision making is positively affected by the use of genomic assays.1
Historically, chemotherapy has been recommended in the United States and Europe for most women with primary breast cancer tumor size > 1 cm, with no consideration given as to whether axillary lymph nodes are involved.2–5 Chemotherapy is used in approximately 75% of women less than 50 years of age, in 30% of women between 50 and 69, and in approximately 5% of women ≥ 70.6
Adjuvant hormonal therapy is adequate treatment for approximately 85% of women with early-stage estrogen receptor–positive (ER+) breast cancer.7 Fewer than 20% of women with lymph node–negative (LN-), ER+ breast cancer treated with tamoxifen will have a recurrence of cancer within 10 years.8 Because many women can be cured by local and/or hormone therapy, many may undergo chemotherapy unnecessarily.
To better discern which patients will benefit from chemotherapy and which can safely avoid it, the Oncotype Dx assay (Genomic Health, Redwood, CA) was developed. It is a 21-gene assay capable of providing an individualized prediction of the benefit of chemotherapy and the rate of cancer recurrence at 10 years. With this test, cancer treatment can be planned that will be most effective for an individual, on the basis of a genomic analysis of the person's tumor tissue. The test is a clinically validated9 genomic test that provides a recurrence score (RS), which can be used to predict the chance of recurrence in patients with newly diagnosed early stage LN–, ER+ breast cancer and in postmenopausal women with lymph node–positive (LN+), hormone receptor–positive (HR+) invasive breast cancer. It is a proven, multigene expression assay that is incorporated into ASCO10 and National Comprehensive Cancer Network11 guidelines.
The Oncotype DX RS is divided into low-, intermediate-, and high-risk categories. These categories help to provide a more accurate picture of which patients are more likely to benefit from chemotherapy. Studies12–20 have analyzed and validated the use of the RS for predicting distant and locoregional recurrence of breast cancer. By analyzing the RS results in 668 of 675 tumor blocks, Paik et al8 determined that patients could be categorized as being at low (51%), intermediate (22%), and high risk (27%) of distant recurrence of cancer. Kaplan-Meier analysis indicated that the risk of 10-year recurrence was 6.8% in the low-risk, 14.3% in the intermediate-risk, and 30.5% in the high-risk patients.8
By identifying low-risk patients unlikely to benefit from chemotherapy, the test helps to prevent unnecessary chemotherapy use; additional chemotherapy costs; and chemotherapy-associated adverse events including toxicity, hospitalization, reduced productivity and quality of life, and possibly even death. Meanwhile, high RS indicates a greater magnitude of benefit from chemotherapy.21,22
The goals of this study were to determine whether patient or disease characteristics drive Oncotype DX RS use, to evaluate the association between RS and chemotherapy use, and to determine the concordance between RS use and overall chemotherapy administration.
Methods
This was a retrospective observational cohort study that used the iKnowMed (iKM) electronic health record (EHR) system of McKesson Specialty Health (MSH) and The US Oncology Network (USON) to address research questions. Patients diagnosed between January 2008 and June 2009 with stages I-II, LN–, estrogen receptor–positive (ER+), or human epidermal growth factor receptor 2–negative (HER2-) breast cancer were included in the study.
ER+ status is notated in our health records on the basis of at least 1% staining of ER in the invasive component of the cancer. HER2– status is based on immunohistochemistry or fluorescence in situ hybridization and noted on the EHR by the treating physician. These criteria are clinician-validated information.
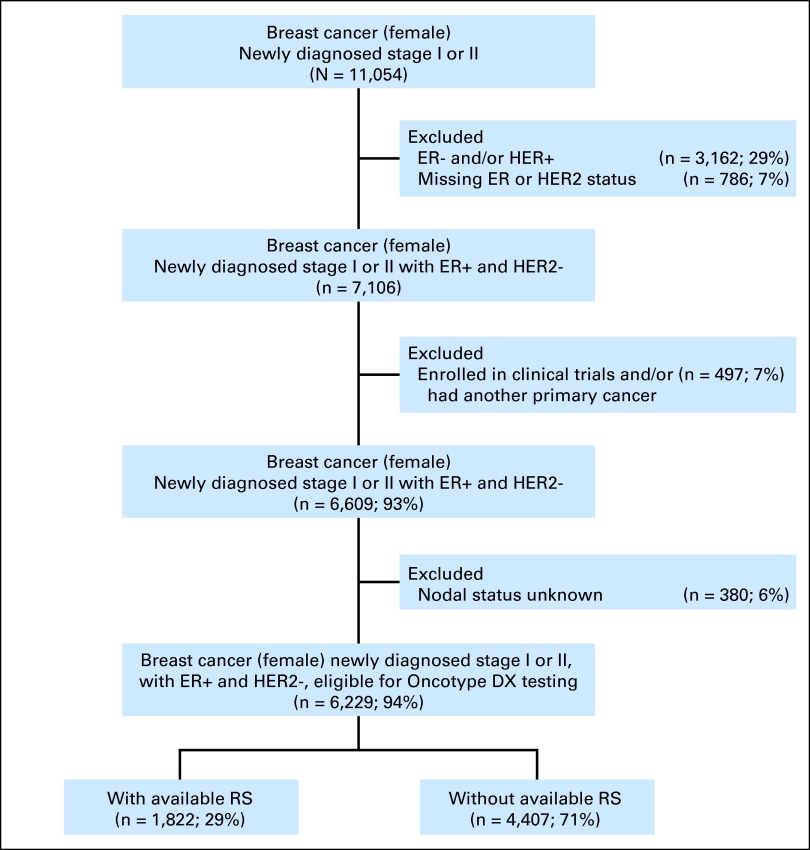
iKM is a proprietary oncology-specific EHR system that is currently adopted by more than 80% of USON-affiliated sites. It has been previously used as the basis of research analyses.23–26 All patients enrolled in clinical trials during the study period were excluded, as were patients treated for another cancer. Patients with missing ER and HER2 values and nodal status in their chart were also excluded (Figure 1).
Figure 1.
Patient flow diagram. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; RS, recurrence score.
Oncotype RS for breast cancer patients is documented in a standardized fashion in iKM. In the rare instances that patients have missing RS data, text mining with a key word search for “Oncotype” or “recurrence score” was conducted in free-text areas, such as physician notes or nurse notes, to determine whether an Oncotype DX was ordered but the RS was not reported. No additional RSs were identified in free-text notes. The majority of those with Oncotype or RS not identified in the free-text notes but with RS entered into iKM failed the RS test because of insufficient tissue, and a few failed for reasons that were not described.
RS documentation and results were abstracted via programmatic query of the iKM EHR system. RSs were segmented into low (<18), intermediate (18-30), and high (≥ 31) risk groups. Patients were further characterized with respect to age at diagnosis, baseline Eastern Cooperative Oncology Group performance status (ECOG PS), tumor size, tumor grade, geographic region, and receipt of chemotherapy during the study period. Age was segmented into five categories, ≤ 45, 46 to 55, 56 to 65, 66 to 75, and ≥ 76; tumor size was grouped into three categories, ≤ 2.0 cm, > 2.0 cm and ≤ 5.0 cm, and > 5.0 cm; and tumor grade was grouped into grades 1, 2, and 3. Patients were also divided by geographic region into midwest, northeast, south and west (the US Census Regions and Divisions). This study was conducted after it was approved by the institutional review boards of MSH and USON.
Patients were described at baseline regarding demographic and clinical characteristics overall and stratified by RS utilization (yes/no). To identify factors associated with RS use, comparisons were made between patients with and without a documented RS test. Among patients with available RS results, comparisons were made by chemotherapy status (yes/no) to evaluate the association between RS and the likelihood of receiving chemotherapy. To further explore factors associated with treatment decisions, we conducted stratified analyses of risk categories (ie, low, intermediate, high) by RS to determine whether other clinical factors (age, tumor grade, tumor size, and baseline ECOG PS) were associated with chemotherapy use in patients in the various risk categories.
The statistical significance of observed associations was evaluated using the χ2 test, and the Cochran-Mantel-Haenszel test. Homogeneity of association across risk groups was evaluated using the Breslow-Day test. Statistical analyses were conducted with SAS version 9.2. All statistical tests were interpreted at alpha = 0.05, two-tailed.
Results
In this study, 6,229 patients with EBC were identified who were eligible for RS testing. Of these, 1,822 (29%) had available RS results; 4,407 (71%) did not (Table 1; Figure 1). In patients who underwent RS testing, use was highest in those between 46 and 65 years of age (1,170; 64%), with better ECOG PS of 0 and 1 (1,632; 90%), and smaller tumor (1,462; 80%). Approximately 81% of patients (1,479) had tumor grade ≤ 2.
Table 1.
Patient Characteristics at Baseline
| Characteristics | Total (N = 6,229) No. | With Recurrence Score (n = 1,822) |
Without Recurrence Score (n = 4,407) |
P | ||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Age at diagnosis, years | ||||||
| ≤ 45 | 660 | 238 | 36 | 422 | 64 | |
| 46-55 | 1,491 | 569 | 38 | 922 | 62 | |
| 56-65 | 1,758 | 601 | 34 | 1,157 | 66 | |
| 66-75 | 1,402 | 355 | 25 | 1,047 | 75 | |
| ≥ 76 | 918 | 59 | 6 | 859 | 94 | < .001 |
| ECOG PS | ||||||
| 0 | 3,773 | 1,250 | 33 | 2,523 | 67 | |
| 1 | 1,533 | 382 | 25 | 1,151 | 75 | |
| ≥ 2 | 159 | 15 | 9 | 144 | 91 | < .001 |
| Missing | 764 | 175 | 23 | 589 | 77 | |
| Tumor size, original | ||||||
| 0 | 3 | — | — | 3 | 100 | |
| DCIS | 5 | — | — | 5 | 100 | |
| 1mic | 87 | 6 | 7 | 81 | 93 | |
| 1a | 505 | 46 | 9 | 459 | 91 | |
| 1b | 1,602 | 399 | 25 | 1,203 | 75 | |
| 1c | 2,626 | 1,011 | 38 | 1,615 | 62 | |
| 2 | 1,264 | 344 | 27 | 920 | 73 | |
| 3 | 124 | 12 | 10 | 112 | 90 | |
| 4a | 1 | — | — | 1 | 100 | |
| 4b | 1 | — | — | 1 | 100 | |
| Missing | 11 | 4 | 36 | 7 | 64 | — |
| Tumor size, grouped, cm | ||||||
| ≤ 2 cm | 4,828 | 1,462 | 30 | 3,366 | 70 | |
| 2-5 cm | 1,264 | 344 | 27 | 920 | 73 | |
| > 5 cm | 126 | 12 | 10 | 114 | 90 | < .001 |
| Missing | 11 | 4 | 36 | 7 | 64 | |
| Tumor grade | ||||||
| 1 | 2,067 | 535 | 26 | 1,532 | 74 | |
| 2 | 2,945 | 944 | 32 | 2,001 | 68 | |
| 3 | 829 | 277 | 33 | 552 | 67 | < .001 |
| Missing | 388 | 66 | 17 | 322 | 83 | |
| Region | ||||||
| Midwest | 817 | 236 | 29 | 581 | 71 | |
| Northeast | 335 | 93 | 28 | 242 | 72 | |
| South | 3,359 | 951 | 28 | 2,408 | 72 | |
| West | 1,718 | 542 | 32 | 1,176 | 68 | .1012 |
Abbreviations: DCIS, ductal carcinoma in situ; ECOG PS, Eastern Cooperative Oncology Group performance status.
With regard to tumor size, patients with a T1c tumor (2,626) more commonly underwent RS testing (1,011; 38%). RS use was greatest among patients with tumors on the borderline of chemotherapy choice, as dictated by guidelines.27 These include T1b, T1c, and T2 tumors. Use decreased in patients with very small (T1mic and T1a) and very large (T3) tumors, for whom decisions regarding chemotherapy were more likely to be based on size, given favorable pathology.
Of the 983 patients with a low RS, 6% (95% CI, 4% to 7%) received chemotherapy; of the 668 patients with an intermediate RS, 42% (95% CI, 38% to 46%) received chemotherapy; of the 171 patients with a high RS, 84% (95% CI, 77% to 89%) received chemotherapy. An association between RS and chemotherapy use was observed in our study (P < .001; Appendix Table A1, online only).
Patients age ≥ 76 years had decreased chemotherapy use across low-, intermediate-, and high-RS groups (0%, 20%, and 50%, respectively; Appendix Table A2, online only). Patients with smaller tumors had decreased chemotherapy use across low-, intermediate-, and high-RS groups (4%, 39%, and 84%, respectively), whereas those with larger tumors had increased chemotherapy use across low, intermediate, and high RS groups (38%, 67%, and 100%, respectively; Appendix Table A3, online only).
Patients with lower grade tumors had decreased chemotherapy use, whereas those with higher grade tumors had increased chemotherapy use in the low and intermediate RS groups. Among the patients with high RS, chemotherapy use was the same for those grades 2 and 3 tumors (84%), but higher for those with grade 1 tumors (90%). This may be because fewer patients were identified with high RS and lower grade tumors (Appendix Table A4, online only). When stratified by RS, ECOG PS and geographic region did not have a significant impact on the decision regarding chemotherapy utilization (Appendix Tables A5 and A6, only only).
Adjusted for other covariates (Table 2), for a patient with a low-risk RS, the odds of receiving chemotherapy were 23.2% (95% CI, 0.17% to 0.317%) of the odds for those without a valid RS test. Compared to patients without a valid RS, those with an intermediate-risk RS were 3.19 (95% CI, 2.59 to 3.93) times more likely to undergo chemotherapy, and those with a high-risk RS were 23.79 (95% CI, 14.36 to 39.41) times more likely to undergo chemotherapy.
Table 2.
Multivariable Logistic Regression Analysis
| Variable | Estimate | OR Estimate | 95% CI for OR Estimate | P |
|---|---|---|---|---|
| Recurrence score | ||||
| No test | — | — | — | |
| Low | −2.178 | 0.232 | 0.170 to 0.317 | |
| Intermediate | 0.443 | 3.190 | 2.588 to 3.932 | |
| High | 2.452 | 23.791 | 14.364 to 39.406 | < .001 |
| Age, years | ||||
| ≤ 45 | — | — | — | |
| 46-55 | 0.869 | 0.586 | 0.457 to 0.751 | |
| 56-65 | 0.319 | 0.338 | 0.263 to 0.435 | |
| 66-75 | −0.359 | 0.172 | 0.129 to 0.229 | |
| ≥ 76 | −2.233 | 0.026 | 0.015 to 0.046 | < .001 |
| ECOG PS | ||||
| 0 | — | — | — | |
| 1 | 0.572 | 1.138 | 0.943 to 1.373 | |
| ≥ 2 | −1.015 | 0.233 | 0.096 to 0.567 | .0017 |
| Tumor size | ||||
| < 2 cm | — | — | — | |
| 2-5 cm | 0.041 | 3.340 | 2.771 to 4.026 | |
| > 5 cm | 1.124 | 9.876 | 6.157 to 15.841 | < .001 |
| Tumor grade | ||||
| 1 | — | — | — | |
| 2 | −0.141 | 1.799 | 1.470 to 2.201 | |
| 3 | 0.869 | 4.936 | 3.862 to 6.308 | < .001 |
NOTE. One thousand eighty-seven patients with missing information (baseline ECOG PS, tumor size, or tumor grade) were excluded from the modeling. Five thousand one hundred forty-two patients were included in the multivariable logistic regression analysis.
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status; OR, odds ratio.
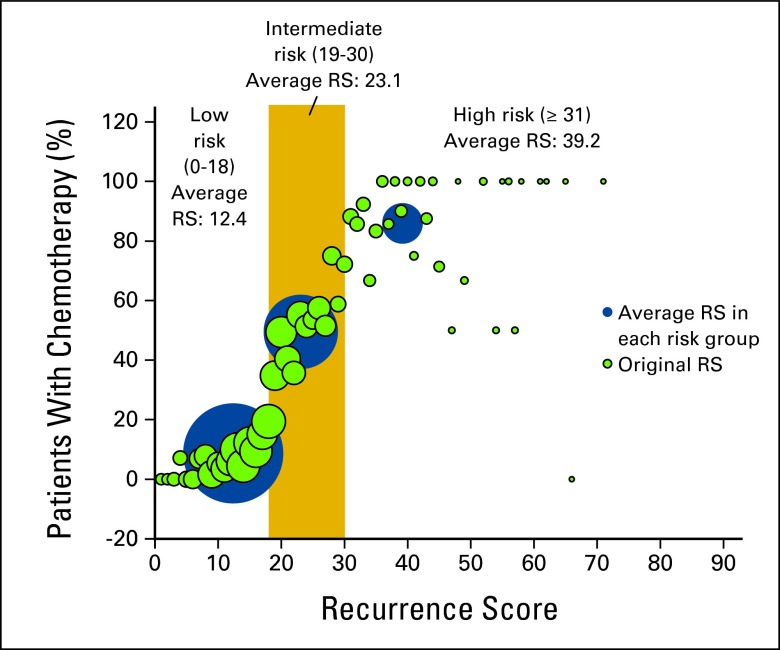
In Appendix Figure A1 (online only), green bubbles represent original continuous RS, and blue bubbles represent RS in each risk group. The size of the bubbles represents the sample size of each specific score. The significant increasing trends in chemotherapy use can be observed in both original RS and average RS, which demonstrate that RS was highly associated with chemotherapy use.
Discussion
The data suggest that the RS test is generally ordered to help physicians decide whether a patient should receive chemotherapy. However, in certain eligible patients, the decision whether to use chemotherapy may have been based on other factors, such as tumor type, tumor size, poor ECOG PS, patient age, or patient's or physician's treating preference, in which case the RS test may not be ordered, although a wide distribution of RSs has been reported across age, grade, and tumor size.28
Chemotherapy use is not always associated with Oncotype DX RS. The most obvious reason for this is that the decision whether to use chemotherapy is a choice made between a doctor and a patient on the basis of many variables. Despite being at low risk, a younger patient with a large tumor may perceive that she would be more comfortable receiving chemotherapy in hopes of risk reduction, despite the intent to follow the RS value in determining the treatment plan. Conversely, an older patient with significant comorbidity and a high RS might make an educated choice to forego chemotherapy despite the high RS. Of the 6,229 patients included in our study, RS results were obtained for 1,822 (29%) of these patients; 983, 668, and 171 patients had low, intermediate, and high RS, respectively. Of these, 6%, 42%, and 84% of patients in the low, intermediate, and high RS groups, respectively, received adjuvant chemotherapy. Three-way contingency tables demonstrate that chemotherapy administration for each category was higher among patients who were younger, had better ECOG PS, and had larger and higher grade tumors.29
The RS value was associated with the use of current chemotherapy regimens in USON; chemotherapy use was high in patients with a high RS and low in patients with a low RS. In the intermediate RS group, chemotherapy use was 42%. Practice patterns of chemotherapy use in the intermediate RS range are variable, and further insight regarding the benefit of chemotherapy use in this cohort should come from the Taylor-Rx trial, which should report results in 2015.
Results of our study provide evidence that RS influences the decision-making process of whether to incorporate chemotherapy into the care of patients with ESBC seen in the outpatient community setting. In a studyinvolving 17 medical oncologists, Lo et al1 found that RS had an impact on medical oncologists' adjuvant treatment recommendations. Eighty-nine (96%) of 93 patients completed questionnaires before and after undergoing RS testing. After the study, 24 (27%) of the patients changed their treatment decisions. Nine (10.1%) of these changed their decision from chemotherapy plus hormone therapy (CHT) before the test to hormone treatment (HT) alone after the test. For 20 patients, physicians recommended CHT treatment before the test, but after the test they changed their recommendation for all 20 patients to HT treatment only. As a result of RS testing, treatment recommendations changed for 22.5% of patients on the basis of individual test results. A recent meta-analysis of 912 patients conducted by Hornberger et al30 suggested a similar rate in change of treatment recommendations; 37% of decisions were changed after the RS was obtained. Of interest in this analysis is that 4% of patients were originally scheduled for HT only but were rated as high risk according to their RS. The result of the RS test changed the decision to include chemotherapy, because the test result indicated high risk for recurrence and that the tumors would respond favorably to chemotherapy. This is an important consideration, because the introduction of genomics may allow further consideration of which treatment is optimal based on tumor biology where there may be both certainty and uncertainty in clinical-pathological variables before treatment recommendations are made. For example, in the B-20 results,12 16% of patients with a tumor less than 1 cm had a high RS. Similar findings based on analysis of two clinical variables were found in the study by Leiberman et al.31 Analyses were conducted in a 2 × 2 format by age, size, and grade and suggested both low- and high-risk RS in patients for whom the results may have indicated a different approach.
Limitations of our study include the lack of capture of RS use if the sample for testing was obtained at a USON clinic that did not use the iKM EHR system or RS results were not documented in the patient's EHR. This may be the case when surgical oncologists order the test in advance of referral to the medical oncologist. Patients also may actually undergo the RS test; however, results might be sent to the physician's office via fax and saved in the iKM EHR as a PDF attachment, from which data are less easily retrievable. We conducted text mining of physicians' notes for the keywords “Oncotype” or “recurrence score (RS),” but no additional RS values were found. However, this does not mean that additional RS tests were not performed, only that they were not found using these keywords. Therefore, the use rate of the RS is likely underreported in this article. Another limitation is that there is a lack of data regarding the reliability and validity of the iKM database. In the future, as data are extracted, perhaps such data will emerge, as they have for such data sources as SEER. Last, the iKM data are collected with an intent-to-treat approach, meaning the data are not collected for research purposes but for clinical practice reasons. This may impede the standardization of the data collection methods and instruments and the reporting practices of the physician.
In conclusion, our study analysis using iKM revealed that only 29% of patients eligible for Oncotype Dx RS testing actually underwent the test. These patients were younger and had better ECOG PS. The data suggest that the RS score influenced the decision about whether to use chemotherapy. This finding is highly significant and demonstrates that the results of the RS test strongly influenced physician and patient decisions as to whether the patient should undergo adjuvant chemotherapy. Future studies should more fully evaluate the independent and interactive associations between patient and practice characteristics, Oncotype Dx RS use, and subsequent chemotherapy.
Acknowledgment
Supported by Genomic Health. Presented in part at the American College of Clinical Oncology Conference, Chicago, IL, June 3-7, 2011.
Appendix
Table A1.
Chemotherapy Decision by Recurrence Score
| Recurrence Score Value | n | Chemotherapy Use |
Proportion of Chemotherapy | 95% CI | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Low risk | 983 | 56 | 927 | 6 | 4 to 7 |
| Intermediate risk | 668 | 281 | 387 | 42 | 38 to 46 |
| High risk | 171 | 143 | 28 | 84 | 77 to 89 |
P < .001.
Table A2.
Chemotherapy Treatment Decision by Age Group and Recurrence Score
| Recurrence Score and Age Group | Total No. | With Chemotherapy |
||
|---|---|---|---|---|
| No. | % | 95% CI (%) | ||
| Low risk | ||||
| All | 983 | 56 | 6 | 4 to 7 |
| ≤ 45 | 123 | 11 | 9 | 5 to 15 |
| 46-55 | 318 | 27 | 8 | 6 to 12 |
| 56-65 | 321 | 16 | 5 | 3 to 8 |
| 66-75 | 190 | 2 | 1 | 0 to 4 |
| ≥ 76 | 31 | — | — | |
| Intermediate risk | ||||
| All | 668 | 281 | 42 | 38 to 46 |
| ≤ 45 | 92 | 61 | 66 | 56 to 76 |
| 46-55 | 210 | 95 | 45 | 38 to 52 |
| 56-65 | 217 | 83 | 38 | 32 to 45 |
| 66-75 | 129 | 38 | 29 | 22 to 38 |
| ≥ 76 | 20 | 4 | 20 | 6 to 44 |
| High risk | ||||
| All | 171 | 143 | 84 | 77 to 89 |
| ≤ 45 | 23 | 21 | 91 | 72 to 99 |
| 46-55 | 41 | 35 | 85 | 71 to 94 |
| 56-65 | 63 | 52 | 83 | 71 to 91 |
| 66-75 | 36 | 31 | 86 | 71 to 95 |
| ≥ 76 | 8 | 4 | 50 | 16 to 84 |
| No test | ||||
| All | 4,407 | 794 | 18 | 17 to 19 |
| ≤ 45 | 422 | 193 | 46 | 41 to 51 |
| 46-55 | 922 | 286 | 31 | 28 to 34 |
| 56-65 | 1,157 | 197 | 17 | 15 to 19 |
| 66-75 | 1,047 | 104 | 10 | 8 to 12 |
| ≥ 76 | 859 | 14 | 2 | 1 to 3 |
Table A3.
Chemotherapy Treatment Decision by Recurrence Score and Tumor Size
| Recurrence Score and Tumor Size | With Chemotherapy |
95% CI (%) | ||
|---|---|---|---|---|
| Total No. | No. | % | ||
| Low risk | ||||
| All | 982 | 56 | 6 | 4 to 7 |
| ≥ 2 cm | 811 | 35 | 4 | 3 to 6 |
| 2-5 cm | 163 | 18 | 11 | 7 to 17 |
| > 5 cm | 8 | 3 | 38 | 9 to 76 |
| Intermediate risk | ||||
| All | 665 | 281 | 42 | 38 to 46 |
| ≤ 2 cm | 530 | 209 | 39 | 35 to 44 |
| 2-5 cm | 132 | 70 | 53 | 44 to 62 |
| > 5 cm | 3 | 2 | 67 | 9 to 99 |
| High risk | ||||
| All | 171 | 143 | 84 | 77 to 89 |
| ≤ 2 cm | 121 | 102 | 84 | 77 to 90 |
| 2-5 cm | 49 | 40 | 82 | 68 to 91 |
| > 5 cm | 1 | 1 | 100 | 3 to 100 |
| No test | ||||
| All | 4,400 | 794 | 18 | 17 to 19 |
| ≤ 2 cm | 3,366 | 366 | 11 | 10 to 12 |
| 2-5 cm | 920 | 360 | 39 | 36 to 42 |
| > 5 cm | 114 | 68 | 60 | 50 to 69 |
NOTE. Eleven patients with missing tumor size (four with recurrence score, seven without recurrence score) were excluded.
Table A4.
Chemotherapy Treatment Decision by Recurrence Score and Tumor Grade
| Recurrence Score and Tumor Grade | Total No. | With Chemotherapy |
||
|---|---|---|---|---|
| No. | % | 95% CI (%) | ||
| Low risk | ||||
| All | 945 | 54 | 6 | 4 to 7 |
| 1 | 354 | 12 | 3 | 2 to 6 |
| 2 | 516 | 31 | 6 | 4 to 8 |
| 3 | 75 | 11 | 15 | 8 to 25 |
| Intermediate risk | ||||
| All | 646 | 273 | 42 | 38 to 46 |
| 1 | 171 | 60 | 35 | 28 to 43 |
| 2 | 359 | 150 | 42 | 37 to 47 |
| 3 | 116 | 63 | 54 | 45 to 64 |
| High risk | ||||
| All | 165 | 139 | 84 | 78 to 89 |
| 1 | 10 | 9 | 90 | 56 to 100 |
| 2 | 69 | 58 | 84 | 73 to 92 |
| 3 | 86 | 72 | 84 | 74 to 91 |
| No test | ||||
| All | 4,085 | 711 | 17 | 16 to 19 |
| 1 | 1,532 | 115 | 8 | 6 to 9 |
| 2 | 2,001 | 352 | 18 | 16 to 19 |
| 3 | 552 | 244 | 44 | 40 to 48 |
NOTE. Three hundred eighty-five patients with missing tumor grade (63 with recurrence score, 322 without) were excluded.
Table A5.
Treatment Decision by Recurrence Score and ECOG PS
| Recurrence Score and ECOG PS | Total No. | With Chemotherapy |
||
|---|---|---|---|---|
| No. | % | 95% CI (%) | ||
| Low risk | ||||
| All | 879 | 53 | 6 | 5 to 8 |
| 0 | 683 | 45 | 7 | 5 to 9 |
| 1 | 192 | 8 | 4 | 2 to 8 |
| ≥ 2 | 4 | — | — | |
| Intermediate risk | ||||
| All | 609 | 261 | 43 | 39 to 47 |
| 0 | 450 | 192 | 43 | 38 to 47 |
| 1 | 153 | 67 | 44 | 36 to 52 |
| ≥ 2 | 6 | 2 | 33 | 4 to 78 |
| High risk | ||||
| All | 159 | 134 | 84 | 78 to 90 |
| 0 | 117 | 99 | 85 | 77 to 91 |
| 1 | 37 | 31 | 84 | 68 to 94 |
| ≥ 2 | 5 | 4 | 80 | 28 to 99 |
| No test | ||||
| All | 3,818 | 707 | 19 | 18 to 20 |
| 0 | 2,523 | 491 | 19 | 18 to 21 |
| 1 | 1,151 | 213 | 19 | 16 to 21 |
| ≥ 2 | 144 | 3 | 2 | 0 to 6 |
NOTE. Seven hundred sixty-four patients with missing tumor grade (175 with recurrence schore, 589 without recurrence score) were excluded.
Abbreviation: ECOG PS, Eastern Cooperative Oncology Group performance status.
Table A6.
Treatment Decision by Recurrence Score and Region
| Recurrence Score and Region | Total | With Chemotherapy |
||
|---|---|---|---|---|
| No. | % | 95% CI (%) | ||
| Low risk | ||||
| All | 983 | 56 | 6 | 4 to 7 |
| Midwest | 134 | 11 | 8 | 4 to 14 |
| Northeast | 49 | 3 | 6 | 1 to 17 |
| South | 535 | 28 | 5 | 4 to 7 |
| West | 265 | 14 | 5 | 3 to 9 |
| Intermediate risk | ||||
| All | 668 | 281 | 42 | 38 to 46 |
| Midwest | 80 | 33 | 41 | 30 to 53 |
| Northeast | 35 | 12 | 34 | 19 to 52 |
| South | 328 | 134 | 41 | 35 to 46 |
| West | 225 | 102 | 45 | 39 to 52 |
| High risk | ||||
| All | 171 | 143 | 84 | 77 to 89 |
| Midwest | 22 | 15 | 68 | 45 to 86 |
| Northeast | 9 | 9 | 100 | 66 to 100 |
| South | 88 | 69 | 78 | 68 to 86 |
| West | 52 | 50 | 96 | 87 to 100 |
| No test | ||||
| All | 4,407 | 794 | 18 | 17 to 19 |
| Midwest | 581 | 110 | 19 | 16 to 22 |
| Northeast | 242 | 39 | 16 | 12 to 21 |
| South | 2,408 | 468 | 19 | 18 to 21 |
| West | 1,176 | 177 | 15 | 13 to 17 |
Figure A1.
Chemotherapy rate by recurrence score (RS). Green bubbles represent original continuous RS, and blue bubbles represent RS in each risk group. The size of bubbles represents the sample size of each specific score.
Authors' Disclosures of Potential Conflicts of Interest
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Debra A. Patt, US Oncology Network/McKesson Specialty Health (C) Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: None Expert Testimony: None Other Remuneration: None
Author Contributions
Conception and design: Clara Chen, Rahul Dhanda, Debra A. Patt
Administrative support: Rahul Dhanda, Michael Forsyth, Debra A. Patt
Provision of study materials or patients: Clara Chen
Collection and assembly of data: Clara Chen
Data analysis and interpretation: Clara Chen, Rahul Dhanda, Wan-Yu Tseng, Michael Forsyth, Debra A. Patt
Manuscript writing: Clara Chen, Rahul Dhanda, Wan-Yu Tseng, Debra A. Patt
Final approval of manuscript: All authors
References
- 1.Lo SS, Mumby PB, Norton J, et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol. 2010;28:1671–1676. doi: 10.1200/JCO.2008.20.2119. [DOI] [PubMed] [Google Scholar]
- 2.Bast RC, Jr, Ravdin P, Hayes DF, et al. 2000 update of recommendations for the use of tumor markers in breast and colorectal cancer: Clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1865–1878. doi: 10.1200/JCO.2001.19.6.1865. [DOI] [PubMed] [Google Scholar]
- 3.Eifel P, Axelson JA, Costa J, et al. National Institutes of Health Consensus Development Conference Statement: Adjuvant therapy for breast cancer, November 1-3, 2000. J Natl Cancer Inst. 2001;93:979–989. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 4.Carlson RW, Edge SB, Theriault RL. NCCN: Breast cancer. Cancer Contr. 2001;(suppl 2):854–861. [PubMed] [Google Scholar]
- 5.Goldhirsch A, Wood WC, Gelber RD, et al. Meeting highlights: Updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003;21:3357–3365. doi: 10.1200/JCO.2003.04.576. [DOI] [PubMed] [Google Scholar]
- 6.Sparano JA. TAILORx: Trial assigning individualized options for treatment (Rx) Clin Breast Cancer. 2006;7:347–350. doi: 10.3816/CBC.2006.n.051. [DOI] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: An overview of the randomized trials. Lancet. 1998;351:1451–1467. [PubMed] [Google Scholar]
- 8.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 9.Genomic Health. What is the Oncotype DX test? http://www.oncotypedx.com/Breast/PatientsCaregiversInvasive.
- 10.Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25:5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, breast cancer, (Version 2.2008) http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#breast. [DOI] [PubMed]
- 12.Mamounas EP, Tang G, Fisher B, et al. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: Results from NSAPB B-14 and NSABP B-20. J Clin Oncol. 2010;29:1677–1683. doi: 10.1200/JCO.2009.23.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Habel LA, Shak S, Jacobs MK, et al. A population-based study of tumor gene expression and risk of breast cancer death among lymph node-negative patients. Breast Cancer Res. 2006;8:R25. doi: 10.1186/bcr1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyman GH, Kuderer NM. Gene expression profile assays as predictors of recurrence-free survival in early-stage breast cancer: A metaanalysis. Clin Breast Can. 2006;7:372–379. doi: 10.3816/CBC.2006.n.053. [DOI] [PubMed] [Google Scholar]
- 15.Swain SM. A step in the right direction. J Clin Oncol. 2006;24:3717–3718. doi: 10.1200/JCO.2006.06.7025. [DOI] [PubMed] [Google Scholar]
- 16.Paik S. Development and clinical utility of a 21-gene recurrence score prognostic assay in patients with early breast cancer treated with tamoxifen. Oncologist. 2007;12:631–635. doi: 10.1634/theoncologist.12-6-631. [DOI] [PubMed] [Google Scholar]
- 17.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65. doi: 10.1016/S1470-2045(09)70314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oratz R, Paul D, Cohn AL, et al. Impact of a commercial reference laboratory test recurrence score on decision making in early-stage breast cancer. J Oncol Pract. 2007;3:182–186. doi: 10.1200/JOP.0742001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asad J, Jacobson AF, Estabrook A, et al. Does RS recurrence score affect the management of patients with early-stage breast cancer? Am J Surg. 2008;196:527–529. doi: 10.1016/j.amjsurg.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 20.Simon R. Roadmap for developing and validating therapeutically relevant genomic classifiers. J Clin Oncol. 2005;23:7332–7341. doi: 10.1200/JCO.2005.02.8712. [DOI] [PubMed] [Google Scholar]
- 21.Cartwright TH, Yim YM, Yu E, et al. Survival outcomes of bevacizumab beyond progression in metastatic colorectal cancer patients treated in US Community Oncology. Clin Colorectal Cancer. doi: 10.1016/j.clcc.2012.05.005. [epub ahead of print on June 21, 2012] [DOI] [PubMed] [Google Scholar]
- 22.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 23.Tang G, Shak S, Paik S, et al. Comparison of the prognostic and predictive utilities of the 21-gene recurrence score assay and Adjuvant! For women with node-negative, ER-positive breast cancer: Results from NSABP B-14 and NSABP B-20. Breast Cancer Res Treat. 2011;127:133–142. doi: 10.1007/s10549-010-1331-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muehlenbein CE, Hoverman JR, Gruschkus, et al. Evaluation of the reliability of electronic medical record data in identifying comorbid conditions among patients with advanced non-small cell lung cancer. J Clin Epidemiol. 2011;2011:983271. doi: 10.1155/2011/983271. Epub 2011 May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadler E, Yu E, Ravelo A, et al. Bevacizumab treatment to progression after chemotherapy: Outcomes from a U.S. community practice network. Oncologist. 2011;16:486–496. doi: 10.1634/theoncologist.2010-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beveridge R, Satram-Hoang S, Sail K, et al. Economic impact of disease progression in follicular non-Hodgkin lymphoma. Leuk Lymphoma. 2011;52:2117–2123. doi: 10.3109/10428194.2011.592623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson RW, Brown E, Burstein HJ, et al. NCCN Task Force report: Adjuvant therapy for breast cancer. J Natl Compr Canc Netw. 2006;4(suppl 1):S1–S26. [PubMed] [Google Scholar]
- 28.Klang N, Liebermann S, Rizel N, et al. The recurrence score and chemotherapy treatment in node-positive, ER+ early-stage breast cancer patients in Israel. J Clin Oncol. 2010;28(suppl):15s. abstr 6075. [Google Scholar]
- 29.Kazzaz DR, Chen C, Shankelton MT, et al. Evaluation of relation between Oncotype Dx recurrence score and adjuvant chemotherapy administration. J Clin Oncol. 2011;9(suppl) abstr e11105. [Google Scholar]
- 30.Hornberger J, Chien R, Krebs K, et al. US insurance program's experience with a multigene assay for early-stage breast cancer. J Oncol Pract. 2011;7(suppl 3):e38s–e45s. doi: 10.1200/JOP.2011.000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lieberman N, Baehner FL, Soussan-Gutman L, et al. Evaluation of recurrence score and traditional clinicopathologic assessments in a large ER-positive, lymph node-negative patient cohort. J Clin Oncol. 2011;29(suppl):78s. abstr 632. [Google Scholar]