Abstract
We tested two hypotheses relating to the sensory deficit that follows a unilateral superior laryngeal nerve (SLN) lesion in an infant animal model. We hypothesized that it would result in (1) a higher incidence of aspiration and (2) temporal changes in sucking and swallowing. We ligated the right-side SLN in six 2–3-week-old female pigs. Using videofluoroscopy, we recorded swallows in the same pre- and post-lesion infant pigs. We analyzed the incidence of aspiration and the duration and latency of suck and swallow cycles. After unilateral SLN lesioning, the incidence of silent aspiration during swallowing increased from 0.7 to 41.5 %. The durations of the suck containing the swallow, the suck immediately following the swallow, and the swallow itself were significantly longer in the post-lesion swallows, although the suck prior to the swallow was not different. The interval between the start of the suck containing a swallow and the subsequent epiglottal movement was longer in the post-lesion swallows. The number of sucks between swallows was significantly greater in post-lesion swallows compared to pre-lesion swallows. Unilateral SLN lesion increased the incidence of aspiration and changed the temporal relationships between sucking and swallowing. The longer transit time and the temporal coordinative dysfunction between suck and swallow cycles may contribute to aspiration. These results suggest that swallow dysfunction and silent aspiration are common and potentially overlooked sequelae of unilateral SLN injury. This validated animal model of aspiration has the potential for further dysphagia studies.
Keywords: Superior laryngeal nerve, Aspiration, Sucking, Swallowing, Videofluoroscopy, Deglutition, Deglutition disorders, Animal models, Nerve lesion, Sensorimotor
Introduction
Sensory innervation is critical to a normal swallow [1–3], suggesting that sensory disruption, even on one side, has the potential to produce movement pathology. The internal branch of the superior laryngeal nerve (iSLN) is the sensory branch of the superior laryngeal nerve (SLN) coming from the vagus nerve (CNX). The iSLN innervates the hypopharyngeal and supraglottic areas of the pharynx and larynx. The SLN is vulnerable to injury in a number of surgeries, including thyroidectomy, neck dissection, cricopharyngeal myotomy, anterior approaches to the cervical spine, carotid endarterectomy, and supraglottic laryngectomy [4]. Injury to this sensory nerve causes swallowing dysfunction which can lead to the subsequent development of aspiration pneumonia in patients [4]. It has been reported that bilateral SLN lesions result in a significantly high incidence of aspiration [5, 6] and that unilateral sensory loss of the laryngopharynx can cause aspiration as well [7]. Sasaki et al. [8] reported that a unilateral SLN lesion results in reduced force of glottic closure in the pig, and they implied that this could contribute to laryngeal aspiration. Using a different style of experiment, Amis et al. [9, 10] reported that stimulation of the external SLN (eSLN) in dogs activates the cricothyroid muscle and causes both pharyngeal dilation and glottis constriction. However, it is still unclear whether unilateral SLN lesions can directly result in aspiration and its mechanism.
The infant pig is a validated model of swallowing function [11–15]. In particular, infant pigs normally have several suck or intraoral transport cycles prior to each pharyngeal swallow. The advantage of studying these different kinds of cycles separately is that changes due to pathophysiology in oral function and intraoral transport can be separated from changes in pharyngeal function [13]. Thexton et al. [12] defined three cycle types in infant sucking behavior using electromyographic criteria: pure suck cycles, suck–swallow cycles, and post-swallow suck cycles. We used a similar scheme to permit evaluation of the temporal relationships between sucks and swallows when aspiration occurred after unilateral SLN lesion.
Our hypotheses were that the sensory deficit following unilateral SLN lesion could result in (1) a higher incidence of aspiration and (2) temporal changes in both sucking and swallowing. To test these hypotheses, using videofluoroscopy we recorded feeding sessions in infant pigs (Sus scrofa) before and after creation of the SLN lesion. Because radiation dosage in the pig does not raise the same level of concern as it does in human videofluoroscopic swallowing studies (VFSS), we were able to collect extensive data, including entire feeding sessions with over 50 swallows in individual animals.
Materials and Methods
Materials
Six intact female pigs were obtained from Tom Morris Farms (Reisterstown, MD). They were 2–3 weeks old and weighed 4–5 kg at the time of arrival at the vivarium of Johns Hopkins University. Five times a day each animal was hand-fed using a bottle with a “pig nipple” (Nasco Inc., Fort Atkinson, WI, USA) and containing a milk replacement formula (Land O Lakes Solustart Pig Milk Replacer, St. Paul, MN, USA). Vital signs were tested by the veterinarian and indicated that the pigs were healthy prior to surgery. All procedures on the living animals were approved by the Johns Hopkins IACUC (Protocol No. SW10M212).
Surgical Procedures: SLN Lesion
Anesthesia was induced with inhaled 5 % isoflurane via a face mask. The pig was then intubated by an experienced veterinarian technician using a flexible cuffed tube passed into the trachea to provide adequate ventilation. General anesthesia was maintained with 3.5–5 % isoflurane in 100 % O2 as a carrier gas. The isoflurane level was adjusted to maintain anesthesia with stable hemodynamics. Heart rate, femoral artery blood pressure, respiratory rate, and electrocardiographic data were continuously monitored throughout the two surgeries.
In the first surgery, the pig was placed in a supine position with a fully extended neck. Under sterile conditions, we made a vertical incision in the anterior midline of the neck, starting approximately 2 cm above the hyoid bone and continuing to 1 cm below the cricoid cartilage. A skin flap was retracted laterally in the subplatysma plane to expose the right side of the thyrohyoid membrane and the omohyoid muscle. With the aid of surgical loupes, the right-side SLN was found originating from the vagus (CNX) inside the carotid sheath. It ran caudally and medially on the surface of the thyrohyoid membrane beneath the omohyoid muscle. At this point, its internal sensory branch pierced the thyrohyoid membrane. We dissected the SLN close to its vagus origin and gently tied two loose silk sutures around the SLN for future identification. The SLN tracing procedures in the carotid triangle were performed with microsurgical instruments to avoid injury to the SLN and surrounding tissues. During this surgery, electromyography (EMG) electrodes and other markers were placed in various oral and pharyngeal muscles and structures, although that data will not be presented here. The skin was carefully approximated with 4-0 suture material (Ethicon Inc., Somerville, NJ, USA). Dressings and bandages were placed around the neck and the body. Using a rigid laryngoscope to visualize the epiglottis, a radiopaque surgical ligating Hemoclip (Teleflex Medical, Kenosha, WI, USA) was placed in the middle of the free tip of the epiglottis as a marker [12, 13]. These procedures followed those of German et al. [13] and Thexton et al. [12], and have been used successfully for 20 years with no damage to the animals. The animals were then permitted to wake and recover the ability to support themselves on all four limbs. Full recovery from anesthesia took between 20 min and 2 h. Two to 4 h after surgery, each pig was placed in the feeding box where it was bottle-fed. We collected both lateral and dorsoventral imaging data while also recording EMG activity. At this time the data obtained were of control (pre-lesion) feeding, as the SLN was intact.
A subsequent surgery to lesion the SLN was carried out 48–72 h later under general anesthesia identical to that of the first surgery. We opened a new window with a 2-cm vertical incision in the right carotid triangle in the plane of the thyrohyoid membrane. It was easy to find the previously marked right-side SLN given the two black loose sutures. We tied the sutures tightly, cut the SLN between the two knots, and displaced the two ends so that no potential reinnervation was possible. In general, the second surgical procedure was finished within 30 min. Closing, dressing, and recovery were identical to those of the first surgery. Data collected after this operation were the postlesion data.
Analgesics and antibiotics were administered daily, starting after the first surgery and continuing after the second to control pain and infection risk throughout the course of the experiments. We delivered buprenorphine (0.01 mg/kg) via intramuscular injection twice a day, at 8:00 and 17:00, and gave intramuscular injections of meloxicam (0.4 mg/kg) and amoxicillin (20 mg/kg) once a day at 17:00. The pigs showed no signs of distress or pain after the surgeries.
Data Collection
After the pig was totally awake and alert, generally 2–4 h after surgery, we started collecting imaging data every 3–4 h daily for up to 3 days. All six pigs underwent VFSS in both dorsoventral and lateral planes before SLN lesion (control swallows) and after SLN lesion (lesioned swallows). Each animal served as its own control, and the unit of analysis was a swallow. This paired design reduces the impact of between-individual variation. The VFSS was performed using a remote-controlled videofluoroscope (Allura FD20, Philips Healthcare, Best, The Netherlands) equipped with a high-resolution digital flat-panel detector (154 × 154 micron pixel-pitch, 30 × 40 cm). The 14-bit video images were recorded at a rate of 60 frames/s and exported in DICOM format. Some VFSS was performed using a Toshiba Infinix C-Arm imaging system (Infinix-I, Toshiba Corp., Tokyo, Japan) with a recording rate of 30 frames/s. The video images were then exported into AVI format for later analysis. All subjects were fed milk mixed with barium powder in a fixed ratio of 8 oz. milk to 1/3 cup powdered barium (E-Z-HD, E-Z-EM, Inc., Westbury, NY, USA). Each subject was fed in a Plexiglas feeding box once they started sucking and swallowing up until the time of satiation. We recorded the VFSS in both dorsoventral and lateral planes before and after unilateral SLN lesion. In this article we present only the data analyzed in the lateral plane. We recorded over 30 swallows per animal per condition. However, some swallows were not used because of alignment issues such as tilting of the head. The data included a total of 144 prelesion swallows (36, 25, 20, 13, 28, and 22 swallows from subjects 1–6, respectively) and 123 post-lesion swallows (14, 19, 26, 14, 25, and 25 swallows from subjects 1–6, respectively).
Postmortem Dissection
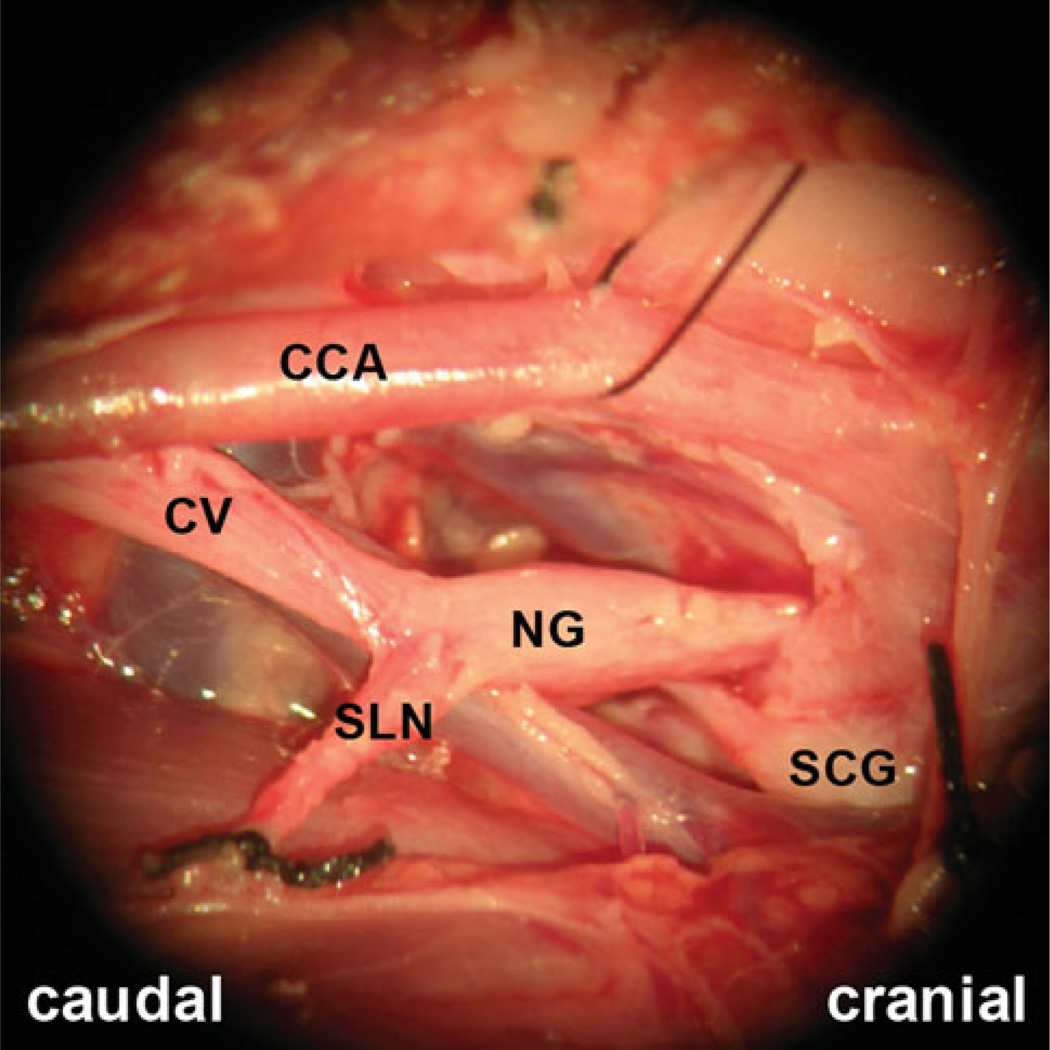
Three days after the nerve lesion procedure and recording, the animal was euthanized with an intracardiac injection of sodium pentobarbital (>200 mg/kg) under a deep plane of isoflurane anesthesia. During the subsequent dissection, we identified both cut ends of the right SLN by the two silk suture knots. We traced one end to the CNX and the other to the thyrohyoid membrane where the internal branch of the SLN pierces the larynx. The postmortem evaluation confirmed that the nerve lesioned was the right-side SLN and the markers and electrodes were implanted in the correct positions in every case (Fig. 1). This confirming postmortem dissection was performed by a laboratory staff member not involved in the original surgery. In addition, we dissected 15 fresh pig cadavers to confirm the innervation of the cricothyroid muscle by the external branch of the SLN. Macroscopically, we found that all external SLNs, except one, provided branches to the cricothyroid muscle and to the inferior pharyngeal constrictor muscle [27].
Fig. 1.
Right-side carotid sheath of a pig post dissection showed the cut SLN and its surrounding structures. One end is knotted with black silk suture, in the lower-left corner of the photo, originated from the nodose ganglion of the cervical vagus nerve. The other end, in the lower-right corner of the photo, pierced through the thyrohyoid membrane into the larynx CCA common carotid artery, CV cervical vagus nerve, IJV internal jugular vein, NG nodose ganglion, SCG superior cervical sympathetic ganglion, SLN superior laryngeal nerve
Data Analysis
The blinded examiner examined the videos of each swallow, frame by frame, to determine the incidence of aspiration or penetration, defined below. We also digitized, frame by frame, the Cartesian coordinates (x, y) of the epiglottis marker using the software ImageJ (National Institutes of Health, Bethesda, MD, USA) and the software MaxTRAQ 2D (Innovision Systems, Inc., Columbiaville, MI, USA). We calibrated radiologic magnification using the digitized images of the 12-mm inner diameter of the nipple on the feeding bottle when the teat was in a relaxed state, i.e., prior to any compression. The response or dependent variables in this study were aspiration incidence, duration of the suck and suck–swallow cycles, latency of suck to pharyngeal swallow, and suck–swallow ratio. They are defined as follows:
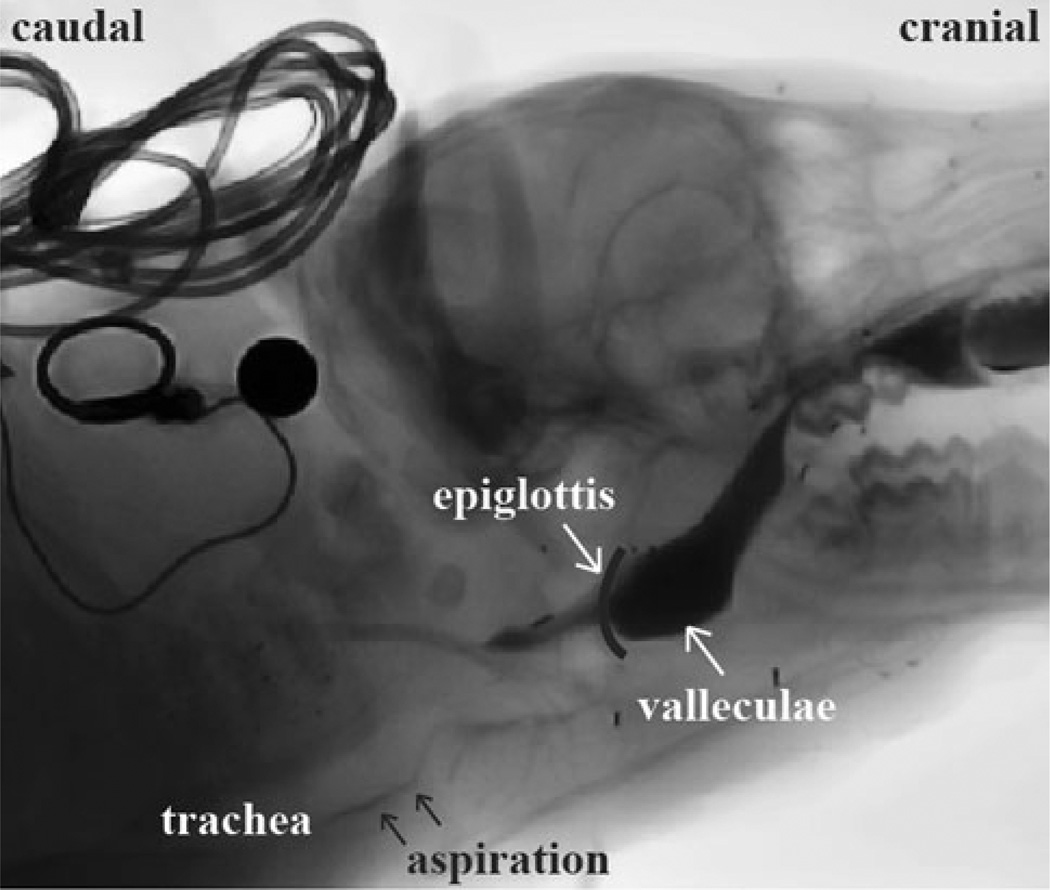
Aspiration incidence aspiration was scaled into one of three categories: no penetration or aspiration (“no P/A”), laryngeal penetration (P), and tracheal aspiration (A). The location of the aspirated bolus, i.e., above or below the vocal cords, was the basis for determining whether it was laryngeal penetration or tracheal aspiration (Fig. 2). If the bolus enters the supraglottic space and remains above the vocal folds after the epiglottis returns back to its resting position during a swallow, it is defined as penetration. If the bolus enters the supraglottic space and then passes below the vocal folds, it is defined as aspiration. Based on the time of the occurrence of aspiration during the pharyngeal swallow, aspiration was classified as “before swallow aspiration,” “during swallow aspiration,” or “after swallow aspiration.”
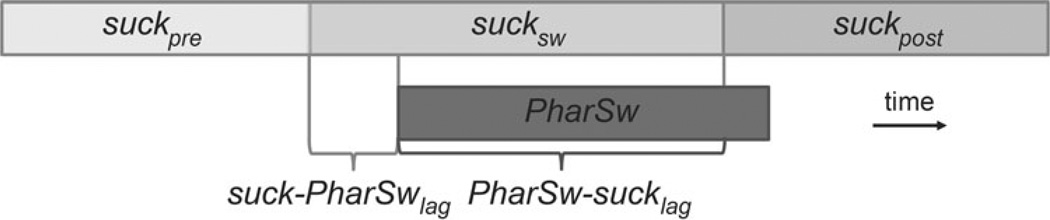
Pharyngeal swallow the onset of a pharyngeal swallow (PharSwon) was defined as the time of the start of epiglottis flipping, when the epiglottis just starts to move caudally. The end of a pharyngeal swallow (PharSwoff) was defined as the time of the frame following the abrupt return of the epiglottis from its caudal to its cranial position. The duration of a pharyngeal swallow (PharSwDur) is equal to PharSwoff − PharSwon (Table 1; Fig. 3).
Three types of sucks in this study we divided sucks into a pattern similar to that of Thexton et al. [12], but one using radiographic criteria: preswallow suck (Suckpre), suck–swallow cycle (Sucksw), and post-swallow suck (Suckpost) based on the temporal relationships between suck cycles and the suck–swallow cycles. The suck in which Swon occurred was designated Sucksw. The suck immediately prior to this was Suckpre, and the one following was Suckpost. Temporally, the three types of suck cycles are contiguous with each other (Table 1 and Fig. 3). Using videofluoroscopy, the suck onset was defined by the time at which milk started to issue from the nipple and enter the mouth, and the termination of that suck cycle was defined by the start of next suck cycle.
Lag of suck to swallow the Sucksw and the Suckpre overlapped with the pharyngeal swallow defined by epiglottal movement. Based on the temporal relationship between the onset of the Sucksw, the Suckpre, and the onset of the pharyngeal swallow, we defined two latencies: Suck − PharSwlag and PharSw − Sucklag (Table 1; Fig. 3). These are similar to the phase divisions defined by Thexton et al. [12].
Suck–swallow ratio the suck–swallow ratio was the ratio of the suck cycles to the pharyngeal swallows. It was equivalent to how many suck cycles followed by one pharyngeal swallow.
Fig. 2.
Aspiration during feeding in an infant pig. The milk containing barium was visible in the larynx and trachea, as indicated by the arrows
Table 1.
Definition of suck, swallow, duration, and latency
| Terms | Definitions |
|---|---|
| Suckpre | The suck prior to the pharyngeal swallow occurs |
| Sucksw | The suck containing the beginning of the pharyngeal swallow |
| Suckpost | The suck following the Sucksw |
| PharSwon | The start of epiglottis flipping when the epiglottis just starts to move caudally. The frame time with the biggest caudal-cranial value before flipping caudally was set to the PharSwon |
| PharSwoff | The frame time after an abrupt return from caudal to cranial position, whose X-axis amplitude distance from the next frame position was less than 1.5 mm |
| PharSwDur | The duration of a pharyngeal swallow: PharSwDur = PharSwoff − PharSwon |
| suckon | The start of milk going into the oral cavity from the nipple |
| suckoff | The start of a new suck |
| suckDur | The duration of a suck: suckDur = suckoff − suckon |
| suck − PharSwlag | The lag of the Sucksw onset and the pharyngeal swallow onset: suck − PharSwlag = PharSwon − suckon* |
| PharSw − sucklag | The lag of the pharyngeal swallow onset and the Suckpost onset: PharSw − sucklag = suckon** − PharSwon |
All temporal terms are italic
suckon refers to the onset of Sucksw
suckon refers to the onset of Suckpost
Fig. 3.
Three types of suck cycles relative to the pharyngeal swallow. The durations are indicated by the bars. The suck − PharSwlag is the latency between the onset of the Sucksw and the onset of pharyngeal swallow. PharSw − sucklag is the latency between the onset of pharyngeal swallow and the onset of the post-swallow suck cycle
Statistics
We used a paired t-test to compare the duration and latency data between the pre-lesion and the post-lesion SLN swallows (SYSTAT 2010, Systat Software, San Jose, CA, USA). A χ2 test was used to compare the incidence of aspiration before and after lesion. Multinomial logistic regression was used to predict the aspiration value from the suck–swallow ratio, durations, and latencies.
Results
Aspiration Incidence
Penetration occurred in 27.8 % (40/144) of the pre-lesion swallows. However, in 27.1 % (39/144) of all pre-lesion swallows there was no aspiration, even though the bolus penetrated to the supraglottic area. Only one pig had a single incident of aspiration prior to lesion for an overall rate of 0.7 % (1/144 swallows).
In contrast to the control pigs, all SLN-lesioned pigs experienced at least one instance of penetration or aspiration. The overall incidence of penetration was 75.6 % (93/123) of all swallows. Among all swallows, 41.5 % (51/123) involved aspiration and 34.1 % (42/123) did not. The incidence of aspiration/penetration post-lesion was significantly greater than in pre-lesion (χ2 = 87.946; p < 0.001). In the SLN-lesioned swallows, 90.3 % (84/93) of swallows with penetration or aspiration occurred during swallowing and 9.7 % (9/93) occurred after swallowing. All swallows were continuous and natural, and no pigs coughed in the middle of a swallow, even when severe aspiration occurred.
Duration
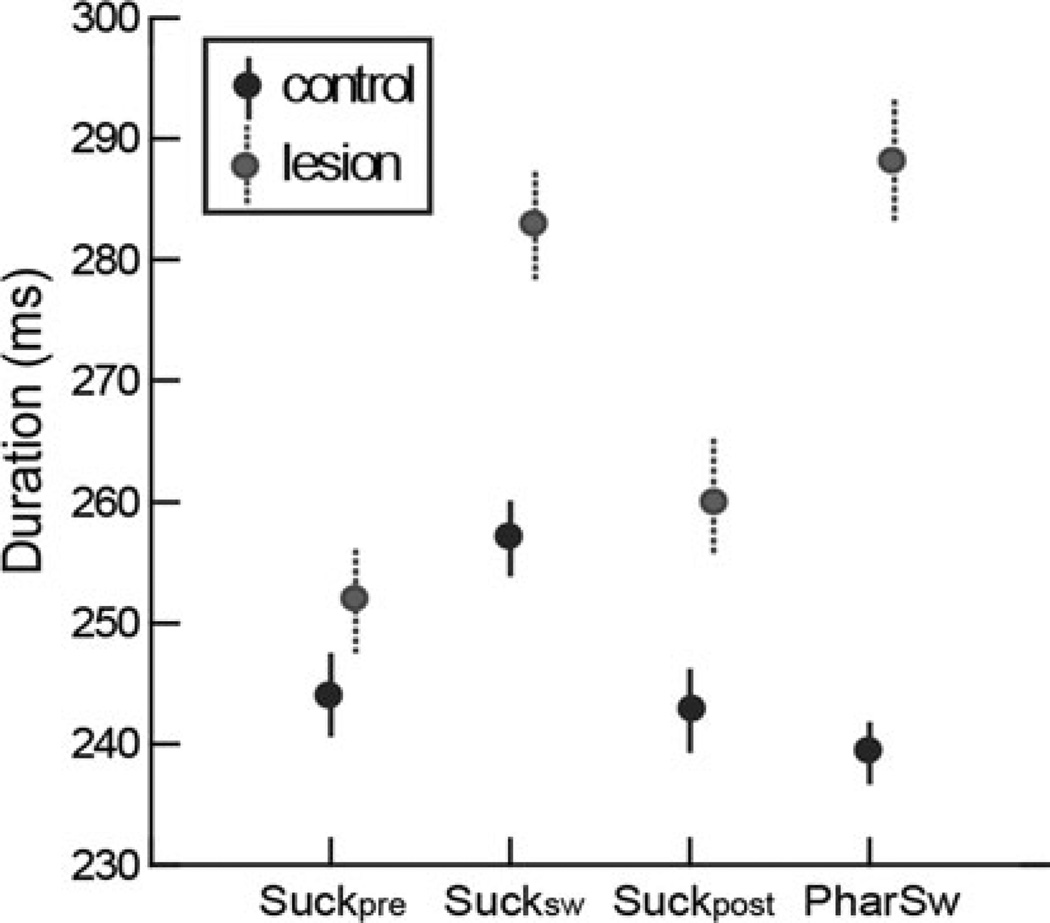
There was no difference in duration of the Suckpre between the control and lesioned swallows [244 (mean) ± 36 (SD) vs. 252 ± 45 ms; p = 0.068]. However, the durations of the Sucksw (257 ± 35 vs. 283 ± 47 ms; p < 0.001) and the Suckpost (243 ± 37 vs. 259 ± 49 ms; p < 0.001) were shorter in the control swallows than in the lesioned swallows. The duration of the pharyngeal swallow was also different (239 ± 28 vs. 288 ± 53 ms; p < 0.001). These differences are shown in Fig. 4.
Fig. 4.
Comparison of duration between the control and SLN-lesioned swallows in six subjects. There was no difference in the duration of the Suckpre (p = 0.068). The durations of the Sucksw (p < 0.001), the Suckpost (p < 0.001), and the pharyngeal swallow (p < 0.001) were significantly different between the two sets of swallows
Latency
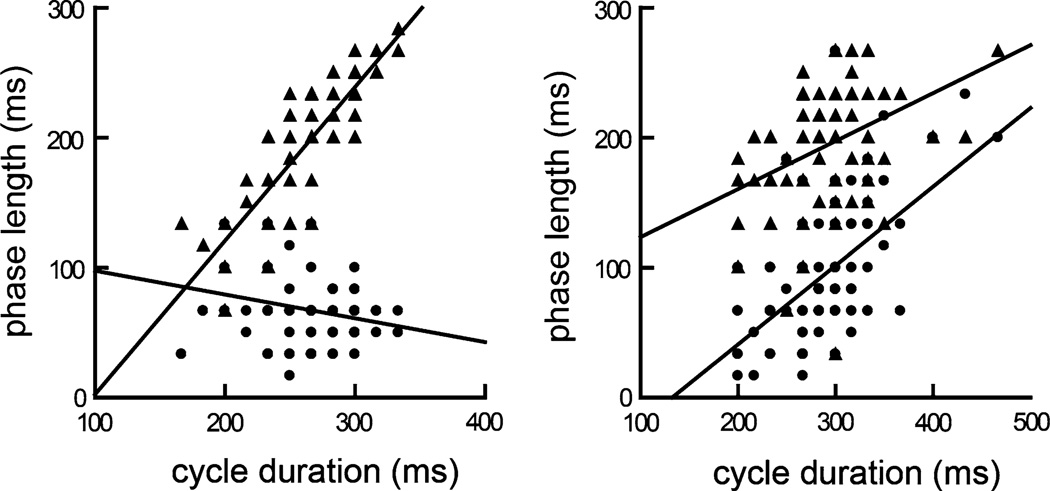
The first lag, Suck − PharSwlag, was longer in the control swallows than in the lesioned swallows (69 ± 29 vs. 91 ± 49 ms; p < 0.001). For the second lag, PharSw − Sucklag, there was no significant difference between the control and lesioned swallows (188 ± 50 vs. 191 ± 42 ms; p = 0.299). In the control data, there was a marginal relationship between Suck − PharSwlag, the first phase, and swallow duration PharSwDur (p = 0.06) when all animals were pooled. An interesting finding is that this relationship appears to be slightly inverse, i.e., as the cycle duration increased, the duration of the first phase decreased. In the PharSw − Suckla, the second phase, the linear relationship was statistically significant (p < 0.001). In the lesioned data, both lags (phases) had significant positive relationships to the PharSwdur (p < 0.001) (Fig. 5).
Fig. 5.
The suck − PharSwlag (squares) and the PharSw − sucklag (triangles) against total pharyngeal swallow cycle duration in six subjects. a In the control group. b In the SLN-lesioned group
Suck–Swallow Ratio
The average number of suck cycles between epiglottal flips in the control swallows was 2.64 (1.44), and for the lesioned animals it was 3.59 (1.48). The two-sample t-test using pooled variance showed the lesioned swallows had significantly more sucks between swallows than did the control swallows (t = 5.189; p < 0.00).
Relationship Between the Occurrence of Aspiration and Suck–Swallow Ratio, Durations, and Latencies
In the lesioned swallows, some variables were useful for predicting the occurrence of aspiration or penetration. A higher suck–swallow ratio (penetration 3 ± 1.7 vs. no P/A 4 ± 1.1; p = 0.011) and a longer duration of Suckpre (penetration 260 ± 42 ms vs. no P/A 235 ± 47 ms; p = 0.021) and PharSwdur (penetration 298 ± 60 ms vs. no P/A 251 ± 24 ms; p < 0.001) in the lesioned swallows than in the “no P/A” subset of swallows predicted the occurrence of penetration. A shorter PharSw − Sucklag (aspiration 176 ± 44 ms vs. penetration 200 ± 47 ms; p = 0.011) was the only variable to predict aspiration as distinct from penetration in the lesioned swallows. In the pre-lesion swallows, we analyzed the regression relation between “no P/A” and the penetration subset of swallows. We did not consider the aspiration subset of swallows because there was only one swallow with aspiration in the control swallows. The results showed that PharSwDur (penetration 256 ± 22 ms vs. no P/A 232 ± 28 ms; p < 0.001) and the duration of the two lags Suck − PharSwlag (penetration 84 ± 37 ms vs. no P/A 64 ± 23 ms; p < 0.001) and PharSw-Sucklag (penetration 175 ± 70 ms vs. no P/A 192 ± 40 ms; p < 0.001) were predictors of the incidence of penetration, whereas the suck durations and suck–swallow ratios had no significant relationship to penetration. In the penetration subset of swallows, the durations of PharSwDur and Suck − PharSwlag were longer and the duration of PharSw − Sucklag was shorter compared to the “no P/A” subset of swallows.
Discussion
Unilateral SLN lesions had a clear impact on the timing of a number of aspects of sucking, particularly the relationships between suck cycles and swallowing. The following measures were all increased after the SLN had been lesioned: the suck–swallow ratio, the average duration of sucks, the duration of the pharyngeal swallows, and the time interval between the suck preceding a swallow and the pharyngeal swallow itself. These time-related changes were, in turn, related to a significantly increased incidence of both laryngeal penetration and tracheal aspiration of liquid boluses. After unilateral SLN lesions, the increase in the incidence of aspiration supported our original hypothesis that a unilateral SLN lesion would increase the incidence of aspiration in this animal model.
These results also provided some insight into the potential mechanisms for producing aspiration. In intact pigs, the pharyngeal swallow occurs with a relatively fixed time lag following the start of the Sucksw relative to a total cycle length [12, 13]. In our study, the equivalent time interval (Suck − PharSwlag) was also constant relative to cycle length. However, after SLN lesion, this relationship changed (Fig. 5b) and varied as a function of cycle length. Thus, the longer the cycle length, the longer milk remained in the vallecula and piriform sinuses prior to the caudal flexion of the epiglottis. In general, the duration of the PharSw in the lesioned swallows was longer, implying either a slower speed of epiglottal flipping or a delay inserted in the movement. In either case, this may also have increased the risk of milk spilling into the larynx. Furthermore, the vast majority of penetration or aspiration incidences occurred during the swallow, implying that inadequate laryngeal closure may be attributed to the delay in the pharyngeal swallow initiation.
The lesion of the eSLN may be another possible reason for aspiration. When the SLN was detached from its origin on the vagus, both internal and external branches were denervated. The iSLN is a sensory branch that innervates the laryngopharynx and loss of this sensory input was initially considered to be the prime reason for the changes leading to aspiration. However, the eSLN, which is a motor branch that traditionally innervates only the ipsilateral cricothyroid muscle, has also been found in man to innervate the ipsilateral thyroarytenoid muscle (46 %) and subglottic mucosa (67 %) or connect with the recurrent laryngeal nerve (25 %) in humans [16] and to innervate the inferior pharyngeal constrictor muscle [17]. We dissected 15 fresh pig cadavers and found that all the eSLNs, except one, innervated the cricothyroid muscle and the inferior pharyngeal constrictor muscle. It is therefore reasonable to suggest that a significant component of the dysfunction following a lesion of the SLN results from loss of the motor activity that is normally provided via the external branch of the SLN.
A constant temporal relationship between the onset of the Sucksw and the pharyngeal swallow (Suck − PharSwlag), meant that, in control swallows, a stable interval existed between the time of entry of the milk bolus into the mouth and the time the epiglottis started flipping to cover the larynx inlet. It would seem reasonable to suggest that the duration of this interval, which corresponded to the period of intraoral transport and temporary containment of liquid in the vallecula and the piriform sinuses, could be important in protecting the airway. The post-lesion increase in the duration of this interval was associated with an increased incidence of penetration and aspiration in this study, which could be explained by a post-lesion failure to increase the swallowrelated cross-sectional area of the oropharynx and piriform fossae and by a lack of glottal closing force that would normally be produced by activity in an intact external branch of the SLN [9, 10]. Similarly, in stroke victims, increased pharyngeal transit time is associated with an increased severity of aspiration [18].
In the control sucking pigs, laryngeal penetration was not uncommon during normal swallowing. We considered that this was a benign, normal occurrence, likely due to immaturity of the swallowing mechanism, as described in human infants [19]. Even in normal human adults under the age of 50, penetration occurs in 7.4 % of swallows, while in people age 50 and over, penetration occurred in 16.8 % of swallows [20]. Another human study reported an incidence of penetration on a liquid bolus of 9.3 % in elderly individuals aged >65 years and an incidence of 14.3 % in adults aged <65 years [21].
A mechanism that could possibly explain penetration in the control animals of our study was that the pharyngeal swallow was initiated late in the Sucksw. The Suck − PharSwlag in the “penetration” subset of swallows was also 20 ms longer than in the “no P/A” subset of swallows. In such cases, a delayed onset and prolonged duration of the pharyngeal phase could extend toward or into the following cycle, the Suckpost cycle. This variation in temporal coordination between sucking and pharyngeal swallowing would appear to have the potential to affect airway protection.
It should be noted that none of our animals coughed during penetration or aspiration. While in adult humans silent penetration or aspiration may be due to sensory loss of the supraglottic area innervated by the iSLN, the situation is different in neonates and infants. In a human study, Newman et al. [22] reported that almost all infants who showed penetration and aspiration had no cough. Following laryngeal penetration by fluids, reflex coughing is weak in both neonatal and infant humans and animals. Conversely, reflex apnea is correspondingly more prolonged [23], which suggests that there is no sensory deficit; this pattern of no cough but respiratory apnea does, however, reverse with maturation.
The rhythm of sucking is generated in a central pattern generator (CPG) within the brainstem, involving the nucleus ambiguus, tractus solitarius, nucleus trigeminalis, and hypoglossus. In the infant pig, sucking and swallowing movements can be generated by these central nervous system structures in the absence of higher centers [11]. However, during the normal postnatal period of brainstem and suprabulbar maturation [24, 25], infants increase their ability to vary and control their feeding. An increase in the oral sensory input has distributed beneficial effects on an improved nutritive sucking and swallow–respiration coordination [26].
In our study, the data of bottle-fed infant pigs demonstrated that loss of hypopharyngeal sensory input and loss of motor output via the eSLN resulted in a significant increase in duration of both the Sucksw and the Suckpost as well as the duration of epiglottal movement, and produced a bigger suck–swallow ratio. The eSLN, however, supplies only the cricothyroid muscle. This increase in the duration of suck cycles suggests that the loss of afferent activity from the iSLN with or without the combined effect of the eSLN due to the anatomic variations [27] not only affects swallowing itself but also affects the generator for trigeminal rhythmic oral activity. Although this could be an indirect effect, Daun et al. [28] have proved that reduction or withdrawal of excitatory afferent activity from CPGs results in slower rhythm generation.
The pharyngeal swallow has traditionally been considered a purely reflexive pattern [29], although it probably reflects the operation of a brainstem pattern generator [30]. In either case, maturation of descending pathways from the cerebral hemispheres may influence the pattern of EMG activity in the pharyngeal swallow [13]. The bolus characteristics can also change the timing of a normal oropharyngeal swallow [31]. In our study, the increased duration of the pharyngeal swallow after unilateral SLN lesioning indicated that the unilateral loss of sensory input could still affect the overall pattern of the pharyngeal swallow as well as suck cycles. Although it is unproven that the increased duration of the Sucksw and of the pharyngeal swallow is due to decreased drive to a central pattern generator for the swallow, the alternative view that it results from a decreased “reflex” action is problematical, as one would expect that to generate a shorter-duration pharyngeal swallow, not a longer one, although its latency might increase.
This study described a useful animal model for the study of the effects of peripheral nerve lesions on swallowing function. After unilateral SLN lesion, all animals exhibited penetration and an increased incidence of aspiration compared to controls. In humans, a comparable study design would be limited by many factors that do not apply in animal experiments. While topical anesthesia or local nerve block to imitate SLN lesions is ethically possible in humans, such models are not wholly reliable in totally eliminating only specific components of the normal sensory input. A more significant consideration in human studies is the duration of radiation exposure; the accepted limit for a human subject is only 3–5 min under VFSS. Furthermore, in human studies, it is not easy to insert radiopaque markers in the soft tissues of the pharynx and larynx to allow accurate assessment of movement [32]. In our animal model, we can identify the nerve accurately, lesion it precisely with microsurgery, and finally confirm it in a postmortem dissection. Without a time limitation on VFSS, we were able to record data with a significant sample size, both before and after lesioning. One limitation of our study is that we analyzed only the kinematic data without the muscular function results. In a future study we will combine the kinematic data with our EMG data to better understand the mechanism of the SLN-related swallowing disorder.
Clinically it is still a challenging task to diagnose and treat unilateral SLN injury due to the absence of objective and generally accepted diagnostic criteria and the heterogeneity of its clinicopathological presentations [4]. This study indicated that clinicians should be more aware of the complication of aspiration that can follow a unilateral SLN injury.
Conclusion
Using an established animal model of swallowing, we have shown that a unilateral SLN lesion increased the incidence of aspiration and changed the temporal relationships among sucking and swallowing activities. The longer transit time and the temporal coordinative dysfunction between suck and swallow cycles may contribute to the aspiration. The pig is a validated model of aspiration and could be developed for further dysphagia studies.
Acknowledgments
We appreciate Laurie Pipitone for her help with the radiologic test. We also thank Judy Cook, Melanie Albano, and Kristy Koenig for their assistance in the animal surgery. This study was funded by the National Institutes of Health (DC9980 to RZG).
Footnotes
Conflict of interest The authors have no conflicts of interest to disclose.
Contributor Information
Peng Ding, Email: pding3@jhmi.edu, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, 98 N. Broadway, Suite 409, Baltimore, MD 21231, USA.
Regina Campbell-Malone, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, 98 N. Broadway, Suite 409, Baltimore, MD 21231, USA.
Shaina D. Holman, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, 98 N. Broadway, Suite 409, Baltimore, MD 21231, USA; Department of Pain and Neural Sciences, University of Maryland School of Dentistry, 650 West Baltimore St, Baltimore, MD 21201, USA.
Stacey L. Lukasik, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, 98 N. Broadway, Suite 409, Baltimore, MD 21231, USA.
Takako Fukuhara, Division of Dysphagia Rehabilitation, Niigata University Graduate School of Medical and Dental Sciences, Niigata 951-8514, Japan.
Estela M. Gierbolini-Norat, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, 98 N. Broadway, Suite 409, Baltimore, MD 21231, USA.
Allan J. Thexton, Division of Physiology, King’s College, London WC2R 2LS, UK.
Rebecca Z. German, Department of Physical Medicine and Rehabilitation, Johns Hopkins University School of Medicine, 98 N. Broadway, Suite 409, Baltimore, MD 21231, USA; Department of Pain and Neural Sciences, University of Maryland School of Dentistry, 650 West Baltimore St, Baltimore, MD 21201, USA.
References
- 1.Steele CM, Miller AJ. Sensory input pathways and mechanisms in swallowing: a review. Dysphagia. 2010;25(4):323–333. doi: 10.1007/s00455-010-9301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ertekin C, et al. Effect of mucosal anaesthesia on oropharyngeal swallowing. Neurogastroenterol Motil. 2000;12(6):567–572. doi: 10.1046/j.1365-2982.2000.00232.x. [DOI] [PubMed] [Google Scholar]
- 3.Teismann IK, et al. Functional oropharyngeal sensory disruption interferes with the cortical control of swallowing. BMC Neurosci. 2007;8:62. doi: 10.1186/1471-2202-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sulica L. The superior laryngeal nerve: function and dysfunction. Otolaryngol Clin North Am. 2004;37(1):183–201. doi: 10.1016/S0030-6665(03)00175-0. [DOI] [PubMed] [Google Scholar]
- 5.Sulica L, Hembree A, Blitzer A. Swallowing and sensation: evaluation of deglutition in the anesthetized larynx. Ann Otol Rhinol Laryngol. 2002;111(4):291–294. doi: 10.1177/000348940211100402. [DOI] [PubMed] [Google Scholar]
- 6.Jafari S, et al. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol. 2003;550(Pt 1):287–304. doi: 10.1113/jphysiol.2003.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aviv JE, et al. Supraglottic and pharyngeal sensory abnormalities in stroke patients with dysphagia. Ann Otol Rhinol Laryngol. 1996;105(2):92–97. doi: 10.1177/000348949610500202. [DOI] [PubMed] [Google Scholar]
- 8.Sasaki CT, Hundal JS, Kim YH. Protective glottic closure: biomechanical effects of selective laryngeal denervation. Ann Otol Rhinol Laryngol. 2005;114(4):271–275. doi: 10.1177/000348940511400404. [DOI] [PubMed] [Google Scholar]
- 9.Amis TC, et al. Pharyngeal dilation associated with cricothyroid muscle contraction in dogs. J Appl Physiol. 1992;73(2):762–766. doi: 10.1152/jappl.1992.73.2.762. [DOI] [PubMed] [Google Scholar]
- 10.Amis TC, et al. Effects of cricothyroid muscle contraction on upper airway flow dynamics in dogs. J Appl Physiol. 1992;72(6):2329–2335. doi: 10.1152/jappl.1992.72.6.2329. [DOI] [PubMed] [Google Scholar]
- 11.Thexton AJ, et al. Impact of rhythmic oral activity on the timing of muscle activation in the swallow of the decerebrate pig. J Neurophysiol. 2009;101(3):1386–1393. doi: 10.1152/jn.90847.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thexton AJ, Crompton AW, German RZ. EMG activity in hyoid muscles during pig suckling. J Appl Physiol. 2012;112(9):1512–1519. doi: 10.1152/japplphysiol.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol. 2009;102(2):1017–1025. doi: 10.1152/jn.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.German RZ, et al. Volume and rate of milk delivery as determinants of swallowing in an infant model animal (Sus scrofia) Dysphagia. 2004;19(3):147–154. doi: 10.1007/s00455-004-0001-x. [DOI] [PubMed] [Google Scholar]
- 15.German RZ, Crompton AW, Thexton AJ. The role of animal models in understanding feeding behavior in infants. Int J Orofacial Myol. 2004;30:20–30. [PubMed] [Google Scholar]
- 16.Mu L, Sanders I. The human cricothyroid muscle: three muscle bellies and their innervation patterns. J Voice. 2009;23(1):21–28. doi: 10.1016/j.jvoice.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Kochilas X, et al. Surgical anatomy of the external branch of the superior laryngeal nerve and its clinical significance in head and neck surgery. Clin Anat. 2008;21(2):99–105. doi: 10.1002/ca.20604. [DOI] [PubMed] [Google Scholar]
- 18.Power ML, et al. Deglutitive laryngeal closure in stroke patients. J Neurol Neurosurg Psychiatry. 2007;78(2):141–146. doi: 10.1136/jnnp.2006.101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delzell PB, et al. Laryngeal penetration: a predictor of aspiration in infants? Pediatr Radiol. 1999;29(10):762–765. doi: 10.1007/s002470050690. [DOI] [PubMed] [Google Scholar]
- 20.Daggett A, et al. Laryngeal penetration during deglutition in normal subjects of various ages. Dysphagia. 2006;21(4):270–274. doi: 10.1007/s00455-006-9051-6. [DOI] [PubMed] [Google Scholar]
- 21.Allen JE, et al. Prevalence of penetration and aspiration on videofluoroscopy in normal individuals without dysphagia. Otolaryngol Head Neck Surg. 2010;142(2):208–213. doi: 10.1016/j.otohns.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Newman LA, et al. Swallowing function and medical diagnoses in infants suspected of Dysphagia. Pediatrics. 2001;108(6):E106. doi: 10.1542/peds.108.6.e106. [DOI] [PubMed] [Google Scholar]
- 23.Thach BT. Maturation of cough and other reflexes that protect the fetal and neonatal airway. Pulm Pharmacol Ther. 2007;20(4):365–370. doi: 10.1016/j.pupt.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qureshi MA, et al. Changes in rhythmic suckle feeding patterns in term infants in the first month of life. Dev Med Child Neurol. 2002;44(1):34–39. doi: 10.1017/s0012162201001621. [DOI] [PubMed] [Google Scholar]
- 25.Bosma JF. Postnatal ontogeny of performances of the pharynx, larynx, and mouth. Am Rev Respir Dis. 1985;131(5):S10–S15. doi: 10.1164/arrd.1985.131.S5.S10. [DOI] [PubMed] [Google Scholar]
- 26.Fucile S, et al. Oral and nonoral sensorimotor interventions facilitate suck-swallow-respiration functions and their coordination in preterm infants. Early Hum Dev. 2012;88(6):345–350. doi: 10.1016/j.earlhumdev.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding P, Tufano RP, German RZ. Anatomical anomalies of the laryngeal branches of the vagus nerve in pigs (Sus scrofa) Lab Anim. 2012;46(4):338–340. doi: 10.1258/la.2012.012091. [DOI] [PubMed] [Google Scholar]
- 28.Daun S, Rubin JE, Rybak IA. Control of oscillation periods and phase durations in half-center central pattern generators: a comparative mechanistic analysis. J Comput Neurosci. 2009;27(1):3–36. doi: 10.1007/s10827-008-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doty RW, Bosma JF. An electromyographic analysis of reflex deglutition. J Neurophysiol. 1956;19(1):44–60. doi: 10.1152/jn.1956.19.1.44. [DOI] [PubMed] [Google Scholar]
- 30.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev. 2001;81(2):929–969. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 31.Logemann JA. Swallowing disorders. Best Pract Res Clin Gastroenterol. 2007;21(4):563–573. doi: 10.1016/j.bpg.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Matsuo K, Palmer JB. Kinematic linkage of the tongue, jaw, and hyoid during eating and speech. Arch Oral Biol. 2010;55(4):325–331. doi: 10.1016/j.archoralbio.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]