Abstract
The nucleoprotein (N) of Borna disease virus (BDV) is the major target of the disease-inducing antiviral CD8 T-cell response in the central nervous system of mice. We established two transgenic mouse lines which express BDV-N in either neurons (Neuro-N) or astrocytes (Astro-N). Despite strong transgene expression, neurological disease or gross behavioral abnormalities were not observed in these animals. When Neuro-N mice were infected as adults, replication of BDV was severely impaired and was restricted to brain areas with a low density of transgene-expressing cells. Notably, the virus failed to replicate in the transgene-expressing granular and pyramidal neurons of the hippocampus (which are usually the preferred host cells of BDV). When Neuro-N mice were infected within the first 5 days of life, replication of BDV was not suppressed in most neurons, presumably because the onset of transgene expression in the brain occurred after these cells became infected with BDV. Astro-N mice remained susceptible to BDV infection, but they were resistant to BDV-induced neurological disorder. Unlike their nontransgenic littermates, Neuro-N mice with persistent BDV infection did not develop neurological disease after immunization with a vaccinia virus vector expressing BDV-N. In contrast to the situation in wild-type mice, this treatment also failed to induce N-specific CD8 T cells in the spleens of both transgenic mouse lines. Thus, while resistance to BDV infection in N-expressing neurons appeared to result from untimely expression of a viral nucleocapsid component, the resistance to BDV-induced neuropathology probably resulted from immunological tolerance.
Borna disease virus (BDV) is a noncytolytic virus that can infect a broad range of warm-blooded animal species and possibly also humans (36, 42). It has a strong tropism for neurons of the central nervous system (CNS) (18). BDV is the causative agent of a severe meningoencephalitis in horses and sheep, resulting in behavioral abnormalities and, often, fatal neurologic disease (36) which is mediated by CNS-infiltrating virus-specific immune cells (44). Mouse-adapted BDV variants cause meningoencephalitis and lead to fatal neurological disease with high frequency in MRL mice (22). In contrast, B10.BR or C57BL/6 mice show low susceptibility to spontaneous neurological disease after experimental infection with BDV, although the virus replicates well in the brain of these animals (22, 23). Disease in susceptible H-2k mice and Lewis rats is mediated by CD8 T cells recognizing immunodominant epitopes derived from the viral nucleoprotein (N) (BDV-N) (33, 39). Our group recently demonstrated that cell lines persistently infected with BDV are resistant to subsequent superinfection with the same virus (15), a phenomenon termed superinfection exclusion or homologous interference (1, 28). It was further shown that this effect can be mimicked by expression of single BDV nucleocapsid components (17).
Transgenic models of infectious diseases represent valuable tools for studying host-pathogen interactions and for elucidating the mechanisms of viral replication under in vivo conditions. Well-known examples are transgenic mice expressing the human immunodeficiency virus (HIV) protein Tat (48, 51) or Nef (31) or the full-length hepatitis B virus genome (20). CNS-specific expression of HIV-gp120 was employed to investigate the pathogenesis of cerebral HIV infection (46). Nucleo- and glycoprotein of lymphocytic choriomeningitis virus (LCMV) were expressed in oligodendrocytes of transgenic mice to study the development of CD8 T-cell-dependent CNS autoimmune disease (13).
CD8 T cells are major mediators of CNS inflammation during various viral infections (14, 29, 32, 45). Transgenic mice artificially overexpressing the major histocompatibility complex (MHC) class I Db molecule in neurons can clear infection with LCMV more rapidly than nontransgenic mice and develop more severe neurological symptoms (35). Studies using murine models of viral infections that mainly target neurons yielded controversial results with respect to antigen presentation by MHC class I molecules on this cell type. Whereas direct recognition of neurons by CD8 T cells was postulated in BDV-induced neuropathology in the rat brain (2), indirect effects of antiviral CD8 T cells were suggested by a study in which MHC class I expression in Sindbis virus-infected C57BL/6 mice was analyzed (29).
We now established a transgenic model for BDV-induced immunopathology by expressing BDV-N in either neurons or astrocytes. The study presented here was conducted to answer two major questions. First, we wanted to analyze whether transgenic expression of the viral N in the major host cell type of BDV would interfere with replication of BDV and whether a possible interference with viral replication would obey the same rules as has been found in studies of cell cultures. Second, we wanted to determine whether and under which conditions an autoimmune type of disease would be inducible by peripheral immunization with a viral vector expressing the transgene and whether this disease would resemble spontaneous neurological disease after BDV infection in either or both types of transgenic mice. By establishing such transgenic mice, we attempted to create a neuroimmunological model with reduced complexity compared to that of the infection situation. This model should enable us to dissect crucial factors and mechanisms involved in CD8 T-cell-mediated disease in the CNS. We found that expression of BDV-N in neurons but not astrocytes impaired the spread of BDV in the CNS and blocked infection of neurons in the hippocampus. Our transgenic mice showed no signs of gross disturbances in behavior, general health, or the cytoarchitecture of the CNS. Peripheral immunization with BDV-N did not result in CNS inflammation and immune-mediated neurological disease due to impaired BDV-N-specific CD8 T-cell responses, indicating that expression of neo-self-antigens under the control of the Thy-1.2 or glial fibrillary acidic protein (GFAP) promoter is not compatible with induction of CNS immunopathology by simple immunization with a vector expressing the transgene. Induction of immune-mediated CNS disorder in this model will require more sophisticated methods such as adoptive transfer of short-term N-specific T-cell lines.
MATERIALS AND METHODS
Virus infections.
A rat-adapted strain of BDV was adapted to the mouse by four consecutive passages through brains of newborn BALB/c mice and two further passages through brains of adult MRL mice. The seed virus which was originally assumed to be derived from strain He/80 (22) has recently been identified as strain rat BDV (also designated RW98) (15). BDV strain H215 was isolated as described previously (17) and adapted to the mouse by two consecutive passages through brains of newborn-infected MRL mice, selecting for high pathogenicity. Mice were infected intracerebrally into the thalamic region of the left brain hemisphere at the indicated time points by using a Hamilton syringe to inject 10-μl samples of a 10% brain homogenate containing 103 focus-forming units of strain RW98 or 50 focus-forming units of strain H215. For induction of disease in mice persistently infected with BDV or the analysis of N-specific CD8 T-cell responses, animals were intravenously infected with 5 × 106 PFU of a recombinant vaccinia virus (VV) expressing BDV-N (VV-N) (23). For induction of N-specific CD8 T cells in Astro-N-25 mice, animals were primed by intramuscular injection of 107 PFU of recombinant parapoxvirus ovis strain D1701-VrVp40 expressing BDV-N (kindly provided by H.-J. Rziha, Tübingen, Germany) followed by an intravenous booster infection with 5 × 106 PFU of VV-N 7 days later.
Transgenic mice.
Transgenic mice expressing BDV-N in neurons or astrocytes were generated by pronuclear DNA microinjection of fertilized B6D2F2 mouse oocytes. The construct used for Neuro-N mice carried the complete coding sequence for the N of BDV strain He/80FR under the control of a Thy-1.2 promoter fragment that permits neuron-specific expression in mice (10, 34). The construct used for Astro-N mice carried the complete coding sequence for the BDV HE/80FR N under the control of a modified promoter of the murine GFAP (26). Transgenic offspring were identified by PCR analysis of DNA from tail biopsies with primers N-303-F (GGTATAGGGCATGAGAAGGA) and N-304-R (AGAGACAACACAAAGGAGCC). Founder animals were back-crossed to B10.BR inbred mice to establish transgenic lines. Lines Neuro-N-44 and Astro-N-25 used in this study strongly expressed the transgene in neurons and astrocytes, respectively. They were maintained as hemizygous lines in a conventional animal house facility.
Histology and immunohistochemical analysis.
Brains from sacrificed animals were divided along the midline upon removal, and the left hemispheres were immersed in Zamboni's fixative (4% paraformaldehyde and 15% picric acid in 0.25 M sodium phosphate, pH 7.5) for at least 24 h. The right hemispheres were snap frozen in liquid nitrogen for preparation of tissue lysates in Laemmli's sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer. Fixed brain hemispheres were embedded in paraffin. Immunostaining of brain sections was performed overnight at 4°C with a monoclonal mouse antibody against BDV-N (Bo18; kindly provided by J. Richt, Giessen, Germany) or monoclonal antibodies 21E7 or 30H8 directed against BDV-P, the viral phosphoprotein (P) (kind gifts of L. Stitz, Tübingen, Germany). Blocking and antibody dilutions were done in phosphate-buffered saline containing 5% normal goat serum. After extensive washing, bound antibody was detected using a peroxidase-based Vectastain Elite ABC kit (Vector Laboratories). Diaminobenzidine was used as the substrate according to the manufacturer's instructions. Counterstaining was done with hematoxylin.
Immunofluorescence analysis on microsections.
Brain microsections were prepared as described above and incubated overnight with antibodies against BDV-N (Bo18 [1:100 dilution]), BDV-P (30H8 [1:100] and polyclonal rabbit antiserum [1:1,000]), and GFAP (Dako) ([1:1,000]). Secondary antibodies coupled to the fluorochrome Cy2 or Cy3 were used to visualize bound antibodies. Distribution of labeled virus antigen-positive cells and GFAP-positive astrocytes was evaluated by laser confocal microscopy.
Immunoblot analysis.
For Western blot analyses, crude lysates from the indicated organs were prepared by homogenization in SDS-PAGE loading buffer. A total of 8 μg of protein per lane was subjected to SDS-PAGE (12 to 15% gels) and transferred to nitrocellulose membranes. Following treatment with 10% nonfat dry milk or blocking buffer (Sigma-Genosys), the membranes were developed using an electrochemoluminescent detection system (Amersham) and the indicated BDV-specific antibodies and appropriate secondary reagents.
In vitro cytotoxicity assay.
Spleens from immunized animals were harvested 7 to 14 days after VV infection, and single-cell suspensions were prepared. For in vitro restimulation, naive splenocytes were treated with mitomycin C and pulsed with TELEISSI peptide (39) at a concentration of 10−6 M for 60 min, washed, and grown in cultures with splenocytes from immunized animals at an effector/stimulator ratio of 10:1 for 9 to 14 days in Iscove's modified Dulbecco's medium supplemented with 10% fetal calf serum and 50 μM β-mercaptoethanol. Cultures were used as effector cells in 51chromium release assays in threefold serial dilutions (as indicated). Target cells were prepared by labeling 5 × 106 L929 (H-2k) cells in suspension with 200 μCi of Na2 51CrO4 (ICN) for 2 h at 37°C and 10−4 M TELEISSI peptide or the H-2Kk-restricted CTL epitope FEANGNLI derived from the hemagglutinin of influenza virus strain A/PR/8/34 (H1N1) (19). Target cells were then diluted to a final concentration of 4 × 104 cells per ml in Iscove's modified Dulbecco's medium, dispensed into 96-well round-bottom microtiter plates at 4 × 103 cells per well, and incubated with different numbers of effector cells in a total volume of 200 μl for 6 h at 37°C. The percentage of specific 51Cr release was calculated according to the following formula: 100 × [(test release − spontaneous release)/(total release − spontaneous release)].
RESULTS
Characterization of Neuro-N transgenic mice.
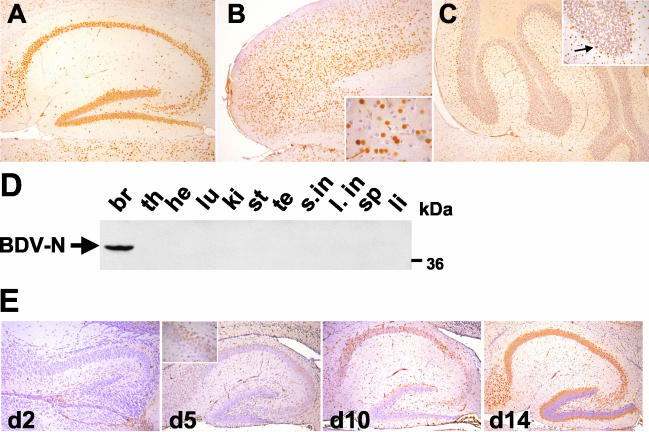
Transgenic animals expressing the N of BDV under the control of the murine neuron-specific promoter elements of the Thy-1.2 gene were generated on a (C57BL/6 × DBA/2)F2 genetic background. Of 48 animals analyzed, 4 founder mice were obtained that all transmitted the transgene to their offspring. Expression of the transgene was analyzed by immunohistochemistry on paraffin-embedded brain tissue with the monoclonal antibody (MAb) Bo18, which specifically recognizes BDV-N (21). Animals from all four transgenic lines expressed N in high amounts and with similar expression patterns in the brain (data not shown). Prominent N expression in brains from Neuro-N-44 mice included that of neurons from the hippocampus (Fig. 1A), cortex (Fig. 1B), thalamic region (data not shown), and cerebellum (Fig. 1C). The transgene product mainly localized to the nuclei of neurons in line 44 animals (Fig. 1B and C, inserts). In two other Neuro-N transgenic lines, N could additionally be detected in higher amounts in the neuronal cytoplasm and in the cellular processes (data not shown). Whereas in most CNS regions (such as the thalamus and cortex) a subpopulation of neurons without detectable expression could be observed (Fig. 1B, insert), interestingly, virtually all neurons in the dentate gyrus and CA1 and CA3 regions of line Neuro-N-44 brains expressed N (Fig. 1A). In the cerebellum, N was found in most Purkinje cells, molecular layer interneurons, and granule cells (Fig. 1C). As determined on the basis of morphological criteria, expression of N in Neuro-N animals only occurred in neurons (consistent with previous findings regarding the cell type specificity of the modified Thy-1.2 promoter) (10). Because BDV preferentially targets hippocampal CA3 pyramidal neurons and dentate gyrus granular neurons, we chose line 44 for further analyses.
FIG. 1.
Cell- and tissue-specific expression pattern of BDV-N in Neuro-N-44 transgenic mice. (A to C) Sagittal sections (8 μm in thickness) of paraffin-embedded brain hemispheres from 8- to 12-week-old Neuro-N-44 mice were stained with anti-N MAb Bo18. Sections were counterstained with hematoxylin. (A) Hippocampus; (B) frontal cortex. The insert shows a fourfold-higher magnification of the frontal cortex. Note the transgene-expressing and nonexpressing neurons in the immediate vicinity. (C) Cerebellum. The insert shows a 2.5-fold-higher magnification of one cerebellar lobe. The arrow indicates an N-expressing Purkinje cell. (D) Tissue-specific expression pattern of transgenic N. Organs were taken from a 7-week-old Neuro-N-44 male, homogenized, and subjected to SDS-PAGE. Immunoblotting (using MAb Bo18) for detection of N was performed. Samples are from brain (br), thymus (th), heart (he), lung (lu), kidney (ki), stomach (st), testis (te), small intestine (s.in), large intestine (l. in), spleen (sp), and liver (li). The arrow indicates the BDV-N-specific band at about 40 kDa. (E) Kinetics of modified Thy-1.2 promoter-controlled transgenic N expression in the CNS. Transgene expression in the hippocampus of Neuro-N-44 animals of the indicated ages was analyzed on sagittal brain sections by staining with MAb Bo18. The insert shows the CA3 region at a higher magnification.
To determine the tissue-specific expression pattern of the BDV-N transgene, whole-protein lysates from all major organs of an animal from the Neuro-N-44 line were prepared and analyzed by immunoblotting using MAb Bo18. An N-specific band migrating at about 40 kDa was detected in the brain lysate (Fig. 1D). No N-specific signals were detected in lysates of any other organs (Fig. 1D) even after long exposure (data not shown).
In agreement with the known properties of the Thy-1.2 promoter (10), the earliest time point of detection of transgenic N expression by immunohistochemistry was at postnatal day 5 in pyramidal neurons of the hippocampal CA3 region (Fig. 1E). At day 14, most of the CA3 and CA1 pyramidal neurons expressed N whereas transgene expression in the dentate gyrus granule cells was still confined to the outmost layer of cells at this time point (Fig. 1E).
Characterization of Astro-N transgenic mice.
Transgenic mice expressing BDV-N in astrocytes under the control of the GFAP promoter were generated on a mixed (C57BL/6 × DBA/2)F2 background, and transmission of the transgene to progeny was analyzed. All seven founders transmitted the transgene. Initial analysis by immunohistochemistry of N expression in the CNS of 55 transgenic animals revealed that only a low percentage of mice descending from three founders expressed detectable levels of N in the CNS. The vast majority of these transgenic mice showed no transgene expression detectable by immunohistochemistry. After crossing Astro-N animals of line 25 once with B10.BR mice, the proportion of transgenic animals expressing N in high amounts in CNS cells increased to 70% whereas the remaining 30% showed no transgene expression. After a second backcross with B10.BR mice, the number of animals expressing the transgene at high levels reached 100% and remained at this level for all further backcross generations. Thus, transgene expression controlled by the GFAP promoter was strongly dependent on the genetic background. The B10.BR genetic background seemed to favor high-level expression from the GFAP promoter used in this study.
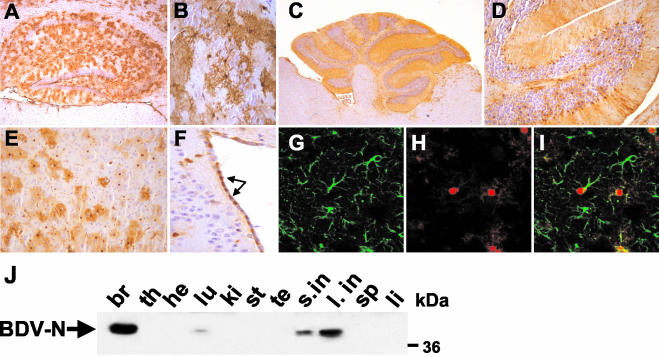
In the CNS, N-expressing astrocytes were mainly found in the hippocampus (Fig. 2A and B), cerebellum (Fig. 2C and D), and the cortex (Fig. 2E). In the hippocampus, most N-expressing astrocytes were located in the hippocampal white matter. Some BDV-N-positive astrocytes were interspersed between the neurons of the dentate gyrus and the CA3 and CA1 regions (Fig. 2B). In the cerebellum, N was expressed by astrocytes located in the granular layer and by Bergmann glial cells (Fig. 2C and D). N was present not only in the nuclei and cell bodies of astrocytes but also in their processes. Processes of Bergmann glial cells which are located at the boundary between granular and molecular layer (Fig. 2D) span the whole molecular layer in the cerebellum, leading to intense transgene-specific staining of the complete molecular layer. Apart from the expression by astrocytes, N was also expressed by ependymal cells lining the ventricle walls (Fig. 2F) and endothelial cells of the choroid plexus (data not shown). Double immunofluorescence labeling using MAb Bo18 and an antibody directed against the astrocytic marker protein GFAP revealed that the cells of the major cell type expressing the transgene were indeed astrocytes (Fig. 2G to I), but only a subset of astrocytes in a given region expressed the transgene (Fig. 2I).
FIG. 2.
Cell- and tissue-specific expression pattern of BDV-N in Astro-N-25 transgenic mice. (A to I) Sagittal sections (8 μm in thickness) of paraffin-embedded brain hemispheres from 8- to 12-week-old Astro-N-25 mice were stained with anti-N MAb Bo18 (A to F) or a mixture of Bo18 and rabbit anti-GFAP (G to I). Bound antibodies were visualized using a biotinylated secondary antibody (A to F) or a mixture of Cy2-labeled anti-rabbit antibody and Cy3-labeled anti-mouse antibody (G to I). Sections in panels A to F were counterstained with hematoxylin. (A) Hippocampus; (B) CA3 region of hippocampus; (C) cerebellum; (D) 2.5-fold-higher magnification image of cerebellum; (E) frontal cortex; (F) ventricle wall (arrows point to N-expressing ependymal cells). (G to I) Confocal laser-scanning microscopy analysis of double-labeled cortical section. (G) anti-GFAP staining; (H) anti-N staining; (I) overlaid images from panels G and H. (J) Tissue-specific expression pattern of transgenic N. Organs were taken from a 6-week-old Astro-N-25 male and homogenized, 80-μg samples were subjected to SDS-PAGE, and immunoblotting using MAb Bo18 for detection of N was performed. Samples are from brain (br), thymus (th), heart (he), lung (lu), kidney (ki), stomach (st), testis (te), small intestine (s.in), large intestine (l. in), spleen (sp), and liver (li). The arrow indicates the BDV-N-specific band at about 40 kDa.
The tissue-specific expression pattern of Astro-N-25 mice was determined by immunoblot analysis of organ homogenates. The highest amount of transgenic N antigen was detected in the brain (Fig. 2J). Significant amounts of N were also present in the small and large intestines (Fig. 2J). The cells of the cell type expressing GFAP promoter-controlled genes in these organs are enteric glial cells of the mucosa (6). A weak N signal was also observed in extracts from lung tissue, which could have been due to the presence of chondrocytes within the bronchial system of the respiratory tract that have been shown to express GFAP (49). Expression of BDV-N in astrocytes of the cerebellum, hippocampus, and cortex started between day 2 and day 5 after birth and reached high levels at postnatal day 10 (data not shown).
Neuro-N and Astro-N mice are healthy.
Neuro-N as well as Astro-N mice up to 1 year of age were examined for neurological symptoms and behavioral changes. No obvious clinical abnormalities could be detected. The transgenic mice showed normal fertility and nursing of pups. We never observed abnormal limb flexion (which is an early sign of neurological disease in BDV-infected MRL mice) (22) in Neuro-N or Astro-N mice or in transgenic mice expressing Tau in neurons under the control of the modified Thy-1.2 promoter (34). Histologically, no alterations in the cytoarchitecture of the CNS were observed (Fig. 1 and 2), indicating that high-level expression of N was not toxic to neurons and astrocytes.
Transgenic expression of N interferes with BDV replication in neurons.
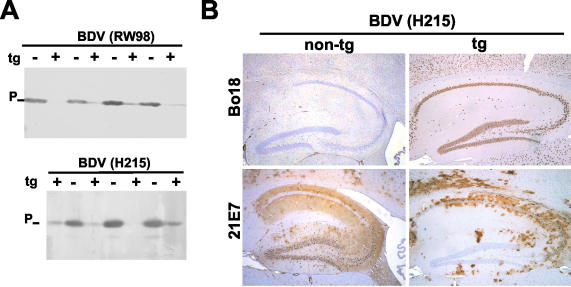
To determine whether susceptibility toward BDV was altered in N-expressing transgenic animals, Neuro-N-44 and nontransgenic littermates were challenged with BDV strain RW98 or H215 at postnatal weeks 3 to 5 (when transgene expression was maximal) and sacrificed 9 weeks (RW98) or 6 weeks (H215) later. None of the infected animals showed signs of neurological disorder. This outcome was expected, because the transgene was crossed into the B10.BR genetic background (which confers resistance to BDV-induced disease) (23). The use of rabbit antiserum to BDV-P for Western blot analysis of brain homogenates revealed differing but greatly reduced levels of viral antigen in transgenic mice (Fig. 3A), indicating impaired replication of both virus strains in the CNS of these mice. Immunohistochemical analysis of brain sections was performed either with MAb 21E7 (which recognizes BDV-P) or with Bo18 (which recognizes transgenically expressed N but fails to recognize the N of strain H215) (17). The H215 challenge virus had disseminated through most parts of the brain in nontransgenic mice. Dentate gyrus and CA3 neurons contained particularly high levels of virus-encoded antigen (Fig. 3B, lower left panel). In contrast, only scattered BDV-infected cells were observed in the hippocampus of transgenic mice (Fig. 3B, lower right panel). Importantly, the dentate gyrus and the CA3 region (both of which harbored a particularly high proportion of transgene-expressing neurons in Neuro-N-44 mice) were spared BDV infection. These hippocampal structures are usually highly susceptible to BDV, which is true for both B10.BR (Fig. 3B, lower left panel) and MRL (38) mice as well as for other experimentally and naturally infected mammalian species such as rats, horses, and sheep (8, 18). BDV replication in transgenic mice was mainly restricted to brain areas with lower numbers of transgene-expressing neurons (data not shown). Thus, BDV replication was apparently blocked in neurons in which the transgene product was present.
FIG. 3.
Neurons of transgenic mice expressing BDV-N are resistant to BDV infection. (A) Transgenic (t) (+) and nontransgenic (−) Neuro-N-44 animals 3 to 5 weeks of age were infected by the intracerebral route with mouse-adapted variants of BDV strain RW98 or strain H215. At 6 weeks (H215) or 9 weeks (RW98) postinfection, mice were sacrificed and samples of brain homogenates were analyzed by SDS-PAGE and immunoblotting using a rabbit antiserum against BDV-P. (B) Immunohistochemical analysis of transgene expression and virus replication 5 weeks postinfection in the hippocampus of selected transgenic (tg+) and nontransgenic (tg−) Neuro-N-44 mice infected with H215 at the age of 4 weeks. Sagittal brain sections were stained with either MAb Bo18 (which selectively detects the transgene product) or MAb 21E7 (which detects viral P antigen in infected cells). The lower left panel shows the typical distribution of BDV-infected cells in the hippocampus of nontransgenic mice. Note that the majority of hippocampal neurons in brains of transgenic mice did not contain viral P antigen (lower right panel), indicating a lack of infection.
Effect of transgenic N expression on an established BDV infection.
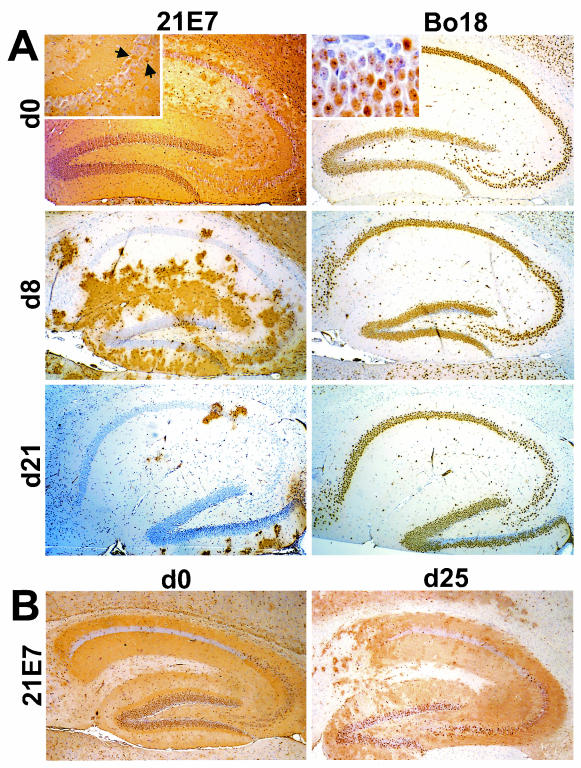
The late onset of transgenic N expression in Neuro-N-44 mice enabled us to design experiments in which BDV infection of dentate gyrus neurons is established before the transgene is expressed. Neuro-N-44 mice were infected with BDV either as newborns or at postnatal days 5, 8, and 21 and analyzed by immunohistochemistry for virus spread in the hippocampus at 5 weeks postinfection. As shown in Fig. 4A (upper left panel), almost all dentate gyrus neurons of Neuro-N-44 mice were positive for virus antigen when the animals were infected as newborns. Pyramidal neurons of the CA3 region were frequently virus antigen negative (Fig. 4A, top left panel and insert). The transgene-derived N was clearly present in infected dentate gyrus neurons (Fig. 4A, top right panel), indicating that transgenically expressed N and BDV can coexist in neurons with established viral infection. Interestingly, a spot-like distribution of transgenically expressed N was observed in a number of persistently infected dentate gyrus granule cells (Fig. 4A, top right panel insert). A similar staining pattern never occurred in uninfected granule cells expressing transgenic N, suggesting a redistribution of transgenically expressed N in persistently infected neurons. When animals were inoculated at postnatal day 8, a significant number of interneurons but only a minor portion of dentate gyrus neurons were virus infected. After inoculation at day 21, dentate gyrus granule cells and pyramidal neurons of the CA3 region as well as most interneurons eventually failed to replicate BDV (Fig. 4A). In contrast, a high proportion of dentate gyrus neurons of nontransgenic littermates harbored BDV when infected at the age of 25 days, although the number of infected dentate gyrus neurons was slightly higher when nontransgenic animals were infected as newborns (Fig. 4B). Independently of the time of inoculation, almost all CA3 region neurons in nontransgenic mice were infected (Fig. 4B). Analysis of nontransgenic animals thus demonstrated that the age of the animals at the time of infection had no significant influence on susceptibility of the hippocampus to BDV infection within the first 3 to 4 weeks of life and cannot explain the observed resistance of hippocampi of 8- to 21-day-old Neuro-N-44 mice to BDV infection. On the basis of these findings, we conclude that transgenically expressed N can prevent de novo infection but is not able to terminate an established BDV infection in neurons.
FIG. 4.
Age-dependent BDV resistance of dentate gyrus neurons in Neuro-N-44 mice. (A) Transgenic Neuro-N-44 animals were infected with mouse-adapted BDV strain H215 at the indicated ages (d0, day 0; d8, day 8; d21, day 21). Animals were sacrificed 5 weeks postinfection. Brain hemispheres were paraffin embedded, and 8-μm-thick sections were stained with anti-P MAb 21E7 (left panels) to visualize virus-infected cells or with MAb Bo18 (right panels) to visualize transgenically expressed N. Note the high number of infected neurons in the dentate gyrus of Neuro-N animals infected as newborns. The insert in the upper left panel shows a higher-magnification image of the CA3 region; arrows point to rare infected pyramidal CA3 neurons. The insert in the upper right panel shows a higher-magnification image of BDV-infected dentate gyrus neurons expressing transgenic N. Note the punctate staining pattern in most granular neurons, indicating a redistribution of transgenic N in infected cells. (B) Nontransgenic littermates were infected as newborns (left panel) or at the age of 25 days (right panel) and sacrificed 5 weeks postinfection. Brains were processed as described for panel A, and 8-μm-thick paraffin sections were stained with anti-P MAb 21E7 to visualize infected cells.
BDV susceptibility of Astro-N-25 mice.
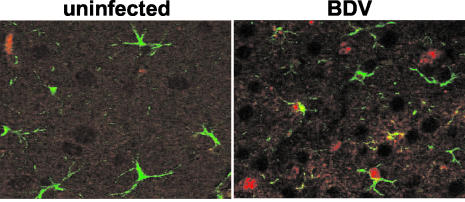
BDV infection of astrocytes was described for experimentally infected rats (8, 9) and naturally infected horses (18) but had previously not been demonstrated well with mice. We therefore used antibodies against the viral N and GFAP to perform double-labeling experiments on sections of wild-type B10.BR mouse brains infected with BDV for 5 weeks. Indirect immunofluorescence clearly revealed the presence of virus antigen-positive astrocytes (Fig. 5). The overall frequency of infected astrocytes was rather low in the cerebrum. In the cerebellum, however, the majority of infected cells appeared to be Bergmann glia cells, a specialized type of cerebellar astrocytes. Viral antigen was present in cell bodies as well as in the processes of Bergmann glia cells that span the molecular layer of the cerebellum (data not shown). After prolonged infection times (generally more than 6 weeks), increasing numbers of infected Purkinje neurons were also observed. Purkinje cells were only rarely infected at earlier times even when high numbers of infected Bergmann glia cells were present. This pattern of BDV spread through the cerebellum was observed in MRL, B10.BR, and C57BL/6 mice and was thus independent of the strain of mice (data not shown).
FIG. 5.
Mouse astrocytes can be infected with BDV. Newborn wild-type B10.BR mice were infected with BDV strain H215. At 5 weeks of age, the animals were sacrificed and sagittal sections (8 μm in thickness) of paraffin-embedded brain hemispheres were stained with a mixture of anti-P MAb 30H8 and rabbit anti-GFAP. Bound antibodies were visualized using a mixture of Cy2-labeled anti-rabbit antibody and Cy3-labeled anti-mouse antibody. An uninfected animal served as the control.
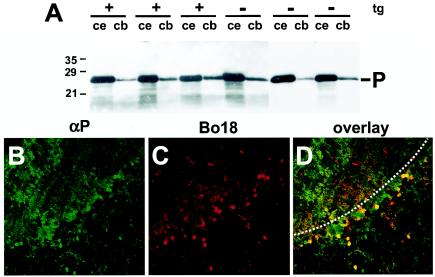
Since most cells that were initially infected in the cerebellum appeared to be Bergmann glia cells, we hypothesized that the spread of BDV infection to the cerebellum might be diminished or delayed in Astro-N transgenic mice. We therefore infected Astro-N-25 mice 3 to 5 weeks of age with BDV and analyzed viral spread to the cerebrum and cerebellum 4 to 6 weeks later. Immunohistochemical analysis indicated that BDV was still able to spread to the cerebellum of Astro-N-25 transgenic animals with efficiency comparable to that seen with nontransgenic littermates. Immunoblot analysis of brain lysates for viral P antigen revealed no significant alteration in the amount of viral antigen in the cerebrum or cerebellum of Astro-N-25 mice compared to the results seen with nontransgenic littermates (Fig. 6A). Analysis of infected cells in the cerebellum by double immunofluorescence labeling revealed that a significant proportion of infected cells expressed the transgene (Fig. 6B to D). Since these double-positive cells were mainly located at the boundary between molecular and granular layers, they likely represented Bergmann glial cells. It remains unclear whether this finding indicates that N-mediated resistance to BDV is not operative in astrocytes or whether other factors (such as the level and/or the kinetics of transgene expression) can explain the presence of BDV in transgene-expressing Bergmann glia cells of Astro-N-25 mice.
FIG. 6.
Transgenic N expression in astrocytes does not impair the efficacy of BDV infection. Astro-N-25 transgenic mice and nontransgenic littermates were infected with BDV at the age of 3 weeks, and brain hemispheres were taken at 9 weeks postinfection for immunoblot analysis (A) and immunohistological analysis (B to D) of brain homogenates. (A) Cerebella (cb) and the remaining parts of the brain hemispheres (ce) of three transgenic (tg) (+) and three nontransgenic (−) animals were separately homogenized, and samples were analyzed by immunoblotting using a rabbit antiserum against BDV-P (-P). Sagittal sections (8 μm in thickness) of paraffin-embedded brain hemispheres (used as described for panel A) from selected animals were double stained with a rabbit polyclonal serum against P (αP) to detect virus infection and with MAb Bo18 detecting transgenically expressed N. Bound antibodies were visualized using a mixture of Cy2-labeled anti-rabbit antibody and Cy3-labeled anti-mouse antibody. Confocal laser-scanning microscopy showed virus-infected, P-antigen-positive cells (B) and BDV-N transgene-expressing cells (C); panel D shows overlaid images from panels B and C. Double-positive cells appear in yellow, and uninfected transgene-expressing cells appear in red. The border between the molecular layer (top) and the granular layer (bottom) is indicated by a dotted line.
The BDV-N-specific CD8 T-cell response is impaired in Neuro-N-44 mice.
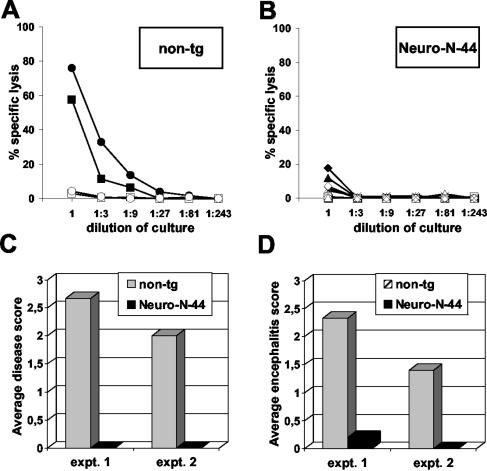
Neuro-N-44 mice were refractory to induction of an autoimmune-like meningoencephalitis or neurological disorder by vaccination directed against the neo-self-antigen (data not shown). To analyze the N-specific T-cell response, Neuro-N-44 mice and nontransgenic littermates were intravenously infected with a recombinant VV-N. At 7 days later, the animals were sacrificed and the spleens were removed. After in vitro restimulation with TELEISSI-loaded antigen-presenting cells, the N-specific cytotoxic activity of splenic CD8 T cells was determined. Splenocytes from nontransgenic mice efficiently and specifically lysed L929 target cells coated with the immunodominant, BDV-N-specific peptide TELEISSI (Fig. 7A). In contrast, only very low levels of lytic activity were detected in splenocyte cultures derived from transgenic animals (Fig. 7B). Thus, the CD8 T-cell response of Neuro-N-44 transgenic animals directed against BDV-N appeared to be impaired.
FIG. 7.
N-specific CD8+ T-cell response is impaired in Neuro-N-44 mice. (A and B) Spleens from two VV-N-immunized nontransgenic (non-tg) (A) and five Neuro-N-44 (B) mice were recovered 7 days post-VV infection, and single cell suspensions were restimulated for 9 days with TELEISSI peptide. Cytolytic activity of restimulated cultures was determined in a 51chromium release assay using L929 target cells coated with TELEISSI (closed symbols) or the irrelevant control peptide FEANGNLI (open symbols). Results are representative of two independent experiments. (C and D) Severity of disease (C) and degree of meningoencephalitis (D) in persistently BDV-infected Neuro-N-mice and nontransgenic littermates after infection with VV-N are shown as mean values for the following groups of mice: two Neuro-N-44 mice and five nontransgenic littermates infected with BDV strain H215 at the age of 5 days (expt. 1) and five Neuro-N-44 mice and three nontransgenic littermates infected as newborns (expt. 2). VV-N infection was performed at day 65 (expt. 1) or 64 (expt. 2). Animals were observed for clinical symptoms until day 13 post-VV infection (C). Severity of disease was scored in a range from 0 to 3 (0, no symptoms; 1, low degree of ataxia and increased anxiety; 2, clear ataxia, torticollis, uncontrolled movements of extremities when the animal was held up by the tail, rough fur or hunched posture, and characteristic position of hind limbs when animal was lifted by the tail; 3, pronounced weight loss, severe ataxia and torticollis, paraparesis, apathy, and morbidity). (D) Meningoencephalitis was scored on an arbitrary scale from 0 to 3 (0, no infiltrates; 1, up to two perivascular infiltrates per brain section, with one or two layers of cells and some mononuclear cells in meninges; 2, three to five perivascular infiltrates per brain section (mostly with multilayer appearance), incidental spread into parenchyma, and intermediate meningitis; 3, more than five perivascular infiltrates per brain section (with multiple layers of cells), strong infiltration of parenchyma at multiple sites, and strong meningitis.
This observation prompted us to determine whether Neuro-N-44 mice exhibited reduced susceptibility to BDV-induced immunopathology and neurological disease. The B10.BR genetic background favors symptomless persistent infection, but Borna disease-like neurological disease can be induced in such mice by peripheral infection with VV-N, which triggers an N-specific CD8 T-cell response (23). Therefore, Neuro-N-44 mice and nontransgenic littermates were infected with BDV at the age of 1 to 5 days (which largely circumvents the inhibitory effect of transgenic N on BDV spread) (Fig. 4). At 9 weeks later, the animals were immunized by infection with VV-N. At the time of the VV challenge, all mice were healthy. Until day 10 postimmunization, however, all eight non-transgenic animals showed moderate to severe neurological disease. By contrast, none of the seven VV-N-challenged transgenic littermates displayed signs of neurological disease (Fig. 7C). Histological analysis showed that meningoencephalitis was prominent in the brains of all the nontransgenic animals (Fig. 7D). By contrast, mononuclear cell infiltrates were not detected in the brains of immunized transgenic animals (with the exception of a single animal, which showed low-level meningoencephalitis) (Fig. 7D). Thus, BDV-infected Neuro-N-44 transgenic animals were largely resistant to vaccination-induced neurological disease. RNA extracted from thymi of transgenic and nontransgenic mice, as well as RNA from brains of transgenic animals, was analyzed by reverse transcriptase PCR (RT-PCR) for presence of N gene-derived transcripts. We did not attempt to use nested RT-PCR, because this method is prone to contamination artifacts. N-specific RNA was detectable by nonnested RT-PCR in brain RNA samples but not in thymic RNA samples (data not shown). Using a single-tube RT-PCR assay, N-specific RNA was detectable in 50% of the samples (data not shown). This indicates that the N gene is expressed in the thymus of Neuro-N-44 mice but probably at very low levels which are difficult to detect by nonnested RT-PCR. We conclude that the observed lack of a BDV-N-specific CD8+ T-cell response in Neuro-N animals is most likely due to central immunological tolerance towards the transgene product.
Impaired BDV-N-specific CD8 T-cell response in Astro-N-25 mice.
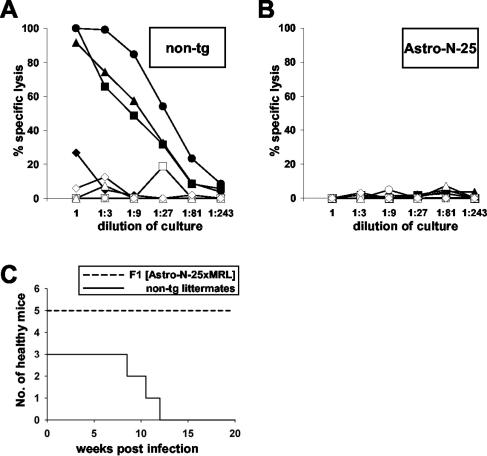
As seen with Neuro-N mice, N-specific immunization of Astro-N-25 mice did not result in autoimmune-like meningoencephalitis or neurological disease. To assess the ability of Astro-N-25 mice to mount an N-specific CD8 T-cell response, we immunized 5- to 6-week-old transgenic mice and nontransgenic littermates through the use of a prime-boost protocol. Animals were primed by intramuscular injection of a recombinant parapoxvirus ovis vector expressing N followed by a booster infection with VV-N. High N-specific cytolytic activity was detected in restimulated splenocyte cultures from three out of four nontransgenic mice (Fig. 8A), whereas splenocyte cultures from all four Astro-N-25 mice showed no N-specific cytolytic activity (Fig. 8B).
FIG. 8.
Impaired BDV-N-specific CD8 T-cell response in Astro-N-25 mice. (A and B) Spleen cells from immunized nontransgenic (A) and Astro-N-25 (B) mice were restimulated with TELEISSI peptide-loaded antigen-presenting cells. Cytolytic activity levels of restimulated cultures were determined in a 51chromium release assay using L929 target cells coated with TELEISSI (closed symbols) or the irrelevant control peptide FEANGNLI (open symbols). (C) Five (Astro-N-25 × MRL)F1 mice (solid line) and three nontransgenic littermates (dashed line) were infected with BDV strain H215 at the age of 16 days. They were observed for clinical symptoms until 20 weeks postinfection.
Since B10.BR mice show a high degree of resistance to BDV-induced disease (22), we crossed Astro-N-25 with MRL mice to produce F1 offspring with intermediate resistance. The resulting transgenic and nontransgenic mice were infected with BDV and observed for neurological symptoms. The nontransgenic animals started to show severe neurological symptoms at 2 to 3 months postinfection. In contrast, none of the Astro-N-25 animals showed any disease symptoms until the experiment was terminated at 20 weeks postinfection (Fig. 8C). Histological analysis demonstrated that BDV had spread at similar levels in the brains of nontransgenic and transgenic mice (data not shown). Inflammation was observed in brains of nontransgenic animals, whereas the brains of transgenic Astro-N-25 mice showed no mononuclear cell infiltration (data not shown), indicating that disease was caused by N-specific CD8 T cells, as seen with MRL mice (22). It is highly unlikely that the spontaneous disease observed after long-term infection of nontransgenic B10.BR mice was due to CD8 T cells with specificities for other viral proteins and that activation of such CD8 T cells was also impaired in Astro-N-25 mice by an unknown mechanism. We have previously shown that the disease-inducing CD8 T cells are directed against the viral N (23). Moreover, only peripheral immunization with N, but not with other viral proteins, induced neurological disease in symptomless persistently infected B10.BR mice. Thus, an impaired N-specific CD8 T-cell response appeared to be responsible for the resistance of Astro-N-25 mice to BDV-induced CD8 T-cell-mediated disease.
DISCUSSION
We report the establishment of two transgenic mouse lines that express the N of BDV in neurons or astrocytes at high levels. We found that neurons were resistant to BDV when the transgene was strongly expressed before virus infection. Resistance to BDV infection was particularly evident in the hippocampus, a preferred site of BDV replication in the CNS. We noted that virtually all hippocampus neurons of adult Neuro-N-44 mice expressed the transgene very well. Interestingly, BDV was able to replicate in hippocampal neurons when mice were infected early in life before transgenic N expression reached maximal levels, emphasizing the need for preformed BDV-N for efficient viral interference. With both Neuro-N-44 and Astro-N-25 mice, autoimmune-like meningoencephalitis and neurological disease could not be induced by peripheral immunization of the animals with a recombinant VV-N. Similarly, these diseases could not be induced when Neuro-N mice with persistent BDV infections were infected with VV-N. In contrast, meningoencephalitis and neurological disease was readily induced under these conditions in nontransgenic littermates. These observations can be explained by our finding that the N-specific CD8 T-cell response was severely impaired in both types of transgenic mice. Our transgenic mice appear to exhibit immunological tolerance toward the transgene product.
Although the expression levels of the N transgene in Neuro-N-44 and Astro-N-25 mice were surprisingly high, no obvious functional deficits or disturbances of the CNS cytoarchitecture were observed. This indicated that expression of BDV-N alone in either neurons or astrocytes is nontoxic. Neurotoxic effects have been reported after transgenic expression of HIV gp120 (12, 46) and human foamy virus proteins (4, 47) in the CNS. The low-level pathogenic potential of singly expressed BDV-N was not unexpected, because BDV is noncytolytic in cell culture. It is further well tolerated in brains of mice devoid of antiviral CD8 T cells (22). Negative effects of BDV replication in the CNS of mice lacking a fully competent immune system were only detectable in very sensitive learning tests (38). Very recently, a study analyzing transgenic mice expressing the P of BDV under the control of a GFAP promoter also did not detect any gross neuroanatomical alterations such as neuronal loss or astrogliosis (27). Interestingly, however, detailed analysis of brains from BDV-P-transgenic mice revealed some neurobiological abnormalities, including reduced synaptic density and lower expression of brain-derived neurotrophic factor and serotonin receptor (27). At present, we cannot rule out the possibility that subtle changes in brain cytoarchitecture or behavior might also have occurred in our N-transgenic animals.
It has recently been demonstrated that cells grown in cultures and rat brains infected with BDV resist infection by a second strain of BDV (15, 17), a phenomenon termed superinfection exclusion or homologous interference (1, 28). Resistance to BDV infection was virus specific and mediated by unbalanced expression of the BDV nucleocapsid components N, P, and X in stably expressing cell lines (17). We now present evidence that unbalanced expression of nucleocapsid components can also mediate resistance to BDV infection in vivo. Brains of adult transgenic mice expressing N in neurons showed drastically reduced susceptibility to BDV infection. Areas with a high density of transgene-expressing neurons (such as the dentate gyrus and the CA3 and CA1 regions of the hippocampus) were fully resistant to BDV infection (Fig. 3). However, the CNS of Neuro-N-44 mice could still be infected with BDV. Since BDV spreads intraaxonally along neuronal connections (18), a preceding BDV infection might block the pathways required for dissemination of a second incoming virus more efficiently than transgenic expression of a single BDV component. In most brain regions of Neuro-N-44 mice, transgene-expressing neurons were interspersed with neurons that do not express the transgene (Fig. 1). This pattern of transgene expression in neurons most probably does not reflect the existing neuronal connections. The stochastic distribution of N-expressing and nonexpressing neurons along synaptic connections might explain the residual spread of BDV.
The mechanism by which transgenically expressed BDV-N can block the establishment of a persistent BDV infection remains unknown. Experiments with cell cultures indicated that inhibition is not due to interfering RNA (17). Since preexisting BDV-P and -X proteins were also inhibitory, it is also unlikely that downregulation of the viral entry receptor can account for the resistance phenomenon. These experiments favored the view that unbalanced expression of nucleocapsid components is detrimental for replication initiation of incoming BDV (17). Using the recently established system for reconstitution of a functionally active BDV polymerase complex in cell culture, it could be shown that efficient reconstitution of active BDV polymerase can only be achieved when the molar ratio of N to P was within a narrowly defined range from 30:1 to 10:1 (40). This result clearly supports the notion that the relative amounts of nucleocapsid components are very important for the formation of an active BDV polymerase complex and that disturbance of the optimal ratio can abolish viral replication. In contrast, BDV infection was not abolished when overexpression of N started subsequent to establishment of persistent infection of the cell culture (17). Similarly, BDV could spread in transgenic mice infected as newborns almost as efficiently as in wild-type animals, most probably because efficient expression of the transgene does not occur before 2 weeks of age, when the virus has already reached most neurons.
These observations demonstrate that once an infection is established and the BDV polymerase complex is active within an infected cell, polymerase activity is less sensitive to the increased amounts of N which alter the optimal N/P ratio. Thus, creating an imbalance in the amounts of N and P most severely impairs the activity of the BDV polymerase complex during initiation of the infection. In contrast, no detectable impairment occurs when the infection has already been established. Interestingly, these mechanisms of BDV inhibition by overexpressed N in cells grown in cultures and in CNS neurons of the living host are probably identical. The specificity of the inhibition has been demonstrated in cell cultures by two independent approaches. First, stable cell lines expressing the N of the unrelated Thogoto virus were as susceptible to BDV infection as the parental UTA-6 cell line (17). Second, cell lines expressing BDV-N were still susceptible to infection with both vesicular stomatitis virus and influenza A virus (17), which replicate in the cytoplasm and the nucleus, respectively.
At first glance, the unhindered spread of BDV in the CNS of Asto-N-25 mice was rather surprising. However, because only a limited number of astrocytes expressed the transgene at detectable levels in the cerebrum (Fig. 2G to I) and because BDV mainly replicates in neurons, no strong inhibitory effect would be expected even if the transgene product were active in this cell type. In the cerebellum, astrocytes serve more frequently as host cells for BDV. Virus inhibition in transgenic mice might therefore be most pronounced in this brain region. For unclear reasons, we also found no inhibitory effect of astrocyte-specific expression of BDV-N in the cerebellum. Since variation in transgene expression controlled by a GFAP promoter has been observed in transgenic mouse lines expressing β-galactosidase (5, 16, 26) (for a review, see reference 6), it is possible that the critical threshold concentration of N was not reached in the Bergmann glia cells. The possibility remains that the levels of transgenic N expression were not constant over time. Circumstantial evidence indeed suggests that expression of transgenes under the control of the GFAP promoter is episodic (37). Levels of transgenic N might therefore intermittently fall below the putative inhibitory threshold and thus allow virus spread. This might lead to progressive infection of most Bergmann glia cells in the presence of a constant number of transgene-expressing cells. A more trivial explanation (that infection occurred before the onset of transgenic N expression) seems unlikely, because BDV usually does not reach the cerebellum before 2 to 3 weeks postinoculation into the thalamic region and because transgenic N expression was already detectable in the cerebellum at postnatal day 5. An alternative explanation is that the N-mediated block of BDV replication might not be operative in astrocytes. Unfortunately, this cannot easily be evaluated by in vitro experiments using explant cultures of astrocytes (because mouse cells grown in cultures usually do not support BDV infection) (43).
The importance of direct recognition of neurons by antigen-specific CD8 T cells in the development and maintenance of CNS inflammation is still unclear. Viral infections that mainly target neurons, such as LCMV in newborn mice (14), neurovirulent Sindbis virus (25), and BDV (18, 22), are valuable models for studying such interactions in the CNS. The upregulation of MHC class I molecules on astrocytes and neurons in vivo upon infection or other proinflammatory stimuli remains controversial (2, 24, 30). Important issues that can now be addressed with our transgenic model of BDV-induced disease include the question of whether and under which circumstances BDV-N-specific CD8 T cells can recognize N-expressing neurons in the CNS. Astro-N-25 mice should represent a positive control for such experiments. It has been shown that by immunization with purified β-galactosidase, CD4 and CD8 T-cell-mediated inflammation can be induced in animals expressing β-galactosidase in astrocytes (3). Furthermore, intravenous application of CD8 T cells specific for a peptide derived from influenza virus A/PR8/34 hemagglutinin induced monophasic brain inflammation in mice expressing this protein transgenically in astrocytes (7), indicating that CD8 T cells can recognize MHC class I-restricted antigens in the resting CNS.
Since the N-specific CD8 T-cell response is impaired in our transgenic mice, future experiments aimed at inducing a BDV-N-dependent inflammation in the brain of these animals will require the transfer of sufficient amounts of N-specific CD8 T cells. The impaired N-specific CD8 T-cell response is probably a consequence of a central tolerance towards the neo-self-antigen due to low-level thymic expression of N. The Thy-1.2 expression cassette used to construct Neuro-N-44 transgenic mice lacks the entire open reading frame of Thy-1 and has been reported to eliminate elements required for transgene expression in the thymus (50). However, we are not aware of any studies investigating the immune responses against transgene products expressed under control of this promoter. The assumption that low-level expression of the transgene in the thymus is responsible for the impaired N-specific CD8 T-cell response is supported by our finding that a highly sensitive RT-PCR analysis of thymic RNA samples from Neuro-N-44 mice detects N-specific RNA. Since our study was not aimed at the investigation of possible mechanisms of tolerance to overexpressed transgene products, we did not extend such RT-PCR studies to Astro-N mice. However, since low-level thymic expression has been demonstrated for a number of CNS-specific antigens (11), it may be assumed that GFAP-promoter driven genes are also expressed to low levels in the thymus. Alternatively, expression of N in the intestinal tissue of Astro-N-25 mice might have induced peripheral tolerance. Such extrathymic tolerance induction has been demonstrated in mice overexpressing an H-2Kb transgene under the control of the GFAP promoter (41). It should be noted that in studies of GFAP-β-galactosidase mice, no transgene-specific tolerance which might be related to the characteristics of the various types of GFAP promoters used was reported (3). Future studies using our transgenic mice for investigations of the interaction of CD8 T cells with N-expressing neurons and astrocytes will need to be performed by adoptive transfer of N-specific CD8 T cells induced by immunization of non-transgenic mice.
Acknowledgments
We thank Rosita Frank for excellent technical assistance, Lothar Stitz and Jürgen Richt for providing antibodies, and Hanns-Joachim Rziha for providing recombinant parapoxvirus ovis D1701-VrVp40. We further thank Karen Baur and Otto Haller for critical comments on the manuscript.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) to J.H. and grants from the Swiss National Science Foundation and Olga Mayenfisch Foundation to J.G.
REFERENCES
- 1.Adams, R. H., and D. T. Brown. 1985. BHK cells expressing Sindbis virus-induced homologous interference allow the translation of nonstructural genes of superinfecting virus. J. Virol. 54:351-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilzer, T., and L. Stitz. 1994. Immune-mediated brain atrophy. CD8+ T cells contribute to tissue destruction during borna disease. J. Immunol. 153:818-823. [PubMed] [Google Scholar]
- 3.Borrow, P., J. L. Cornell, M. D. Ruppe, and L. Mucke. 1995. Immunization-induced inflammatory infiltration of the central nervous system in transgenic mice expressing a microbial antigen in astrocytes. J. Neuroimmunol. 61:133-149. [DOI] [PubMed] [Google Scholar]
- 4.Bothe, K., A. Aguzzi, H. Lassmann, A. Rethwilm, and I. Horak. 1991. Progressive encephalopathy and myopathy in transgenic mice expressing human foamy virus genes. Science 253:555-557. [DOI] [PubMed] [Google Scholar]
- 5.Brenner, M., W. C. Kisseberth, Y. Su, F. Besnard, and A. Messing. 1994. GFAP promoter directs astrocyte-specific expression in transgenic mice. J. Neurosci. 14:1030-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner, M., and A. Messing. 1996. GFAP transgenic mice. Methods 10:351-364. [DOI] [PubMed] [Google Scholar]
- 7.Cabarrocas, J., J. Bauer, E. Piaggio, R. Liblau, and H. Lassmann. 2003. Effective and selective immune surveillance of the brain by MHC class I-restricted cytotoxic T lymphocytes. Eur. J. Immunol. 33:1174-1182. [DOI] [PubMed] [Google Scholar]
- 8.Carbone, K. M., T. R. Moench, and W. I. Lipkin. 1991. Borna disease virus replicates in astrocytes, Schwann cells and ependymal cells in persistently infected rats: location of viral genomic and messenger RNAs by in situ hybridization. J. Neuropathol. Exp. Neurol. 50:205-214. [DOI] [PubMed] [Google Scholar]
- 9.Carbone, K. M., B. D. Trapp, J. W. Griffin, C. S. Duchala, and O. Narayan. 1989. Astrocytes and Schwann cells are virus-host cells in the nervous system of rats with Borna disease. J. Neuropathol. Exp. Neurol. 48:631-644. [DOI] [PubMed] [Google Scholar]
- 10.Caroni, P. 1997. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J. Neurosci. Methods 71:3-9. [DOI] [PubMed] [Google Scholar]
- 11.Derbinski, J., A. Schulte, B. Kyewski, and L. Klein. 2001. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2:1032-1039. [DOI] [PubMed] [Google Scholar]
- 12.D'Hooge, R., F. Franck, L. Mucke, and P. P. De Deyn. 1999. Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur. J. Neurosci. 11:4398-4402. [DOI] [PubMed] [Google Scholar]
- 13.Evans, C. F., M. S. Horwitz, M. V. Hobbs, and M. B. Oldstone. 1996. Viral infection of transgenic mice expressing a viral protein in oligodendrocytes leads to chronic central nervous system autoimmune disease. J. Exp. Med. 184:2371-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans, C. F., J. M. Redwine, C. E. Patterson, S. Askovic, and G. F. Rall. 2002. LCMV and the central nervous system: uncovering basic principles of CNS physiology and virus-induced disease. Curr. Top. Microbiol. Immunol. 263:177-195. [DOI] [PubMed] [Google Scholar]
- 15.Formella, S., C. Jehle, C. Sauder, P. Staeheli, and M. Schwemmle. 2000. Sequence variability of Borna disease virus: resistance to superinfection may contribute to high genome stability in persistently infected cells. J. Virol. 74:7878-7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galou, M., S. Pournin, D. Ensergueix, J. L. Ridet, J. L. Tchelingerian, L. Lossouarn, A. Privat, C. Babinet, and P. Dupouey. 1994. Normal and pathological expression of GFAP promoter elements in transgenic mice. Glia 12:281-293. [DOI] [PubMed] [Google Scholar]
- 17.Geib, T., C. Sauder, S. Venturelli, C. Hassler, P. Staeheli, and M. Schwemmle. 2003. Selective virus resistance conferred by expression of Borna disease virus nucleocapsid components. J. Virol. 77:4283-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gosztonyi, G., and H. Ludwig. 1995. Borna disease—neuropathology and pathogenesis. Curr. Top. Microbiol. Immunol. 190:39-73. [PubMed] [Google Scholar]
- 19.Gould, K. G., H. Scotney, and G. G. Brownlee. 1991. Characterization of two distinct major histocompatibility complex class I Kk-restricted T-cell epitopes within the influenza A/PR/8/34 virus hemagglutinin. J. Virol. 65:5401-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guidotti, L. G., B. Matzke, H. Schaller, and F. V. Chisari. 1995. High-level hepatitis B virus replication in transgenic mice. J. Virol. 69:6158-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haas, B., H. Becht, and R. Rott. 1986. Purification and properties of an intranuclear virus-specific antigen from tissue infected with Borna disease virus. J. Gen. Virol. 67:235-241. [DOI] [PubMed] [Google Scholar]
- 22.Hallensleben, W., M. Schwemmle, J. Hausmann, L. Stitz, B. Volk, A. Pagenstecher, and P. Staeheli. 1998. Borna disease virus-induced neurological disorder in mice: infection of neonates results in immunopathology. J. Virol. 72:4379-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hausmann, J., W. Hallensleben, J. C. De la Torre, A. Pagenstecher, C. Zimmermann, H. Pircher, and P. Staeheli. 1999. T cell ignorance in mice to Borna disease virus can be overcome by peripheral expression of the viral nucleoprotein. Proc. Natl. Acad. Sci. USA 96:9769-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horwitz, M. S., C. F. Evans, F. G. Klier, and M. B. Oldstone. 1999. Detailed in vivo analysis of interferon-gamma induced major histocompatibility complex expression in the central nervous system: astrocytes fail to express major histocompatibility complex class I and II molecules. Lab. Investig. 79:235-242. [PubMed] [Google Scholar]
- 25.Jackson, A. C., T. R. Moench, D. E. Griffin, and R. T. Johnson. 1987. The pathogenesis of spinal cord involvement in the encephalomyelitis of mice caused by neuroadapted Sindbis virus infection. Lab. Investig. 56:418-423. [PubMed] [Google Scholar]
- 26.Johnson, W. B., M. D. Ruppe, E. M. Rockenstein, J. Price, V. P. Sarthy, L. C. Verderber, and L. Mucke. 1995. Indicator expression directed by regulatory sequences of the glial fibrillary acidic protein (GFAP) gene: in vivo comparison of distinct GFAP-lacZ transgenes. Glia 13:174-184. [DOI] [PubMed] [Google Scholar]
- 27.Kamitani, W., E. Ono, S. Yoshino, T. Kobayashi, S. Taharaguchi, B. J. Lee, M. Yamashita, M. Okamoto, H. Taniyama, K. Tomonaga, and K. Ikuta. 2003. Glial expression of Borna disease virus phosphoprotein induces behavioral and neurological abnormalities in transgenic mice. Proc. Natl. Acad. Sci. USA 100:8969-8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karpf, A. R., E. Lenches, E. G. Strauss, J. H. Strauss, and D. T. Brown. 1997. Superinfection exclusion of alphaviruses in three mosquito cell lines persistently infected with Sindbis virus. J. Virol. 71:7119-7123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kimura, T., and D. E. Griffin. 2000. The role of CD8+ T cells and major histocompatibility complex class I expression in the central nervous system of mice infected with neurovirulent Sindbis virus. J. Virol. 74:6117-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Linda, H., H. Hammarberg, S. Cullheim, A. Levinovitz, M. Khademi, and T. Olsson. 1998. Expression of MHC class I and β2-microglobulin in rat spinal motoneurons: regulatory influences by IFN-gamma and axotomy. Exp. Neurol. 150:282-295. [DOI] [PubMed] [Google Scholar]
- 31.Lindemann, D., R. Wilhelm, P. Renard, A. Althage, R. Zinkernagel, and J. Mous. 1994. Severe immunodeficiency associated with a human immunodeficiency virus 1 NEF/3′-long terminal repeat transgene. J. Exp. Med. 179:797-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray, P. D., K. D. Pavelko, J. Leibowitz, X. Lin, and M. Rodriguez. 1998. CD4+ and CD8+ T cells make discrete contributions to demyelination and neurologic disease in a viral model of multiple sclerosis. J. Virol. 72:7320-7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Planz, O., and L. Stitz. 1999. Borna disease virus nucleoprotein (p40) is a major target for CD8+-T-cell-mediated immune response. J. Virol. 73:1715-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Probst, A., J. Gotz, K. H. Wiederhold, M. Tolnay, C. Mistl, A. L. Jaton, M. Hong, T. Ishihara, V. M. Lee, J. Q. Trojanowski, R. Jakes, R. A. Crowther, M. G. Spillantini, K. Burki, and M. Goedert. 2000. Axonopathy and amyotrophy in mice transgenic for human four-repeat tau protein. Acta Neuropathol. (Berlin) 99:469-481. [DOI] [PubMed] [Google Scholar]
- 35.Rall, G. F., L. Mucke, and M. B. Oldstone. 1995. Consequences of cytotoxic T lymphocyte interaction with major histocompatibility complex class I-expressing neurons in vivo. J. Exp. Med. 182:1201-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rott, R., and H. Becht. 1995. Natural and experimental Borna disease in animals. Curr. Top. Microbiol. Immunol. 190:17-30. [DOI] [PubMed] [Google Scholar]
- 37.Sarthy, V., and H. Egal. 1995. Transient induction of the glial intermediate filament protein gene in Muller cells in the mouse retina. DNA Cell Biol. 14:313-320. [DOI] [PubMed] [Google Scholar]
- 38.Sauder, C., D. P. Wolfer, H. Lipp, P. Staeheli, and J. Hausmann. 2001. Learning deficits in mice with persistent Borna disease virus infection of the CNS associated with elevated chemokine expression. Behav. Brain Res. 120:189-201. [DOI] [PubMed] [Google Scholar]
- 39.Schamel, K., P. Staeheli, and J. Hausmann. 2001. Identification of the immunodominant H-2Kk-restricted cytotoxic T-cell epitope in the Borna disease virus nucleoprotein. J. Virol. 75:8579-8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schneider, U., M. Naegele, P. Staeheli, and M. Schwemmle. 2003. Active Borna disease virus polymerase complex requires a distinct nucleoprotein-to-phosphoprotein ratio but no viral X protein. J. Virol. 77:11781-11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schonrich, G., U. Kalinke, F. Momburg, M. Malissen, A. M. Schmitt-Verhulst, B. Malissen, G. J. Hammerling, and B. Arnold. 1991. Down-regulation of T cell receptors on self-reactive T cells as a novel mechanism for extrathymic tolerance induction. Cell 65:293-304. [DOI] [PubMed] [Google Scholar]
- 42.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of borna disease virus. J. Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 43.Staeheli, P., M. Sentandreu, A. Pagenstecher, and J. Hausmann. 2001. Alpha/beta interferon promotes transcription and inhibits replication of borna disease virus in persistently infected cells. J. Virol. 75:8216-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stitz, L., T. Bilzer, and O. Planz. 2002. The immunopathogenesis of Borna disease virus infection. Front. Biosci. 7:D541-D555. [DOI] [PubMed] [Google Scholar]
- 45.Stitz, L., B. Dietzschold, and K. M. Carbone. 1995. Immunopathogenesis of Borna disease. Curr. Top. Microbiol. Immunol. 190:75-92. [DOI] [PubMed] [Google Scholar]
- 46.Toggas, S. M., E. Masliah, E. M. Rockenstein, G. F. Rall, C. R. Abraham, and L. Mucke. 1994. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 367:188-193. [DOI] [PubMed] [Google Scholar]
- 47.Tschopp, R. R., S. Brandner, S. Marino, K. Bothe, I. Horak, A. Rethwilm, and A. Aguzzi. 1996. Analysis of the determinants of neurotropism and neurotoxicity of HFV in transgenic mice. Virology 216:338-346. [DOI] [PubMed] [Google Scholar]
- 48.Vellutini, C., N. Horschowski, V. Philippon, D. Gambarelli, K. A. Nave, and P. Filippi. 1995. Development of lymphoid hyperplasia in transgenic mice expressing the HIV tat gene. AIDS Res. Hum. Retrovir. 11:21-29. [DOI] [PubMed] [Google Scholar]
- 49.Viale, G., C. Doglioni, P. Dell'Orto, G. Zanetti, P. Iuzzolino, L. Bontempini, and G. Coggi. 1988. Glial fibrillary acidic protein immunoreactivity in human respiratory tract cartilages and pulmonary chondromatous hamartomas. Am. J. Pathol. 133:363-373. [PMC free article] [PubMed] [Google Scholar]
- 50.Vidal, M., R. Morris, F. Grosveld, and E. Spanopoulou. 1990. Tissue-specific control elements of the Thy-1 gene. EMBO J. 9:833-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vogel, J., S. H. Hinrichs, R. K. Reynolds, P. A. Luciw, and G. Jay. 1988. The HIV tat gene induces dermal lesions resembling Kaposi's sarcoma in transgenic mice. Nature 335:606-611. [DOI] [PubMed] [Google Scholar]