Abstract
Binding of avian sarcoma and leukosis virus (ASLV) to its cognate receptor on the cell surface causes conformational changes in its envelope protein (Env). It is currently debated whether low pH is required for ASLV infection. To elucidate the role of low pH, we studied the association between ASLV subgroup B (ASLV-B) and liposomes and fusion between effector cells expressing Env from ASLV-A and ASLV-B and target cells expressing cognate receptors. Neither EnvA nor EnvB promoted cell-cell fusion at neutral pH, but lowering the pH resulted in quick and extensive fusion. As expected for a low-pH-triggered reaction, fusion was a steep function of pH. Steps that required low pH were identified. Binding a soluble form of the receptor caused ASLV-B to hydrophobically associate with liposome membranes at neutral pH, indicating that low pH is not required for insertion of Env's fusion peptides into membranes. But both cell-cell hemifusion and fusion pore formation were pH dependent. It is proposed that fusion peptide insertion stabilizes the conformation of ASLV Env into a form that can be acted upon by low pH. At this point, but not before, low pH can induce fusion and is in fact required for fusion to occur. However, low pH is no longer necessary after formation of the initial fusion pore: pore enlargement does not require low pH.
After an enveloped virus binds to receptors on plasma membranes, one of two triggers directly causes the membrane fusion that allows the virus to deposit its genome into the cell. In the first case, association of the fusion protein with receptors at the plasma membrane is the trigger for the conformational changes required for fusion at neutral pH. In the second case, the virus is internalized and trafficked to an endosome, where the low-pH environment triggers these changes in the viral glycoprotein. For most viruses that fuse within endosomes at low pH, receptor binding does no more than anchor the virus to the cell membrane and all of the fusogenic conformational changes are induced solely by low pH. Thus, it had been thought that the trigger for viral fusion was either receptor binding or low pH but never both. This view was recently challenged by evidence that strongly indicated that avian sarcoma and leukosis virus (ASLV) entry is not triggered through only one of these known patterns but instead utilizes both in combination (35).
ASLV entry into a host cell is strictly receptor dependent. ASLVs are classified into ten subgroups (A through J) according to their receptor specificities (22). Expressing the TVA and TVB receptors on cells deficient in the receptor renders those cells susceptible to infection by ASLV subgroup A (ASLV-A) or ASLV-B, respectively (2, 4, 8). Moreover, binding soluble forms of the ectodomains of the receptors (soluble TVA [sTVA] or sTVB) to their respective viral counterparts allow infection of receptor-deficient cells (6, 12, 25, 40-43).
There is strong evidence to indicate that both receptor interaction and low pH are required for ASLV entry. When reverse transcription was used as an early marker of ASLV infection, it was observed that viral entry was blocked by the addition of lysosomotropic agents that act to raise the pH of acidic organelles and inhibit entry of pH-dependent viruses (35, 37). Unlike the results seen with classical pH-dependent viruses, ASLV particles seem to reside stably in the lumen of neutralized intracellular organelles for many hours in the presence of lysosomotropic reagents and infection rapidly resumes following inhibitor removal (14, 35, 37). Additional evidence that ASLV entry involves trafficking to a low-pH endosomal compartment was obtained when it was shown that ASLV infectivity is reduced by expressing a dominant-negative form of dynamin to inhibit clathrin- and caveola-mediated endocytosis (35) (see also reference 14).
Importantly, the lysosomotropic reagents affect the activity of the ASLV envelope protein (Env) rather than that of the viral core; this was determined by using mixed murine leukemia virus (MLV)-ASLV-pseudotyped virions. In contrast to ASLV, MLV uses a pH-independent viral entry mechanism (35). Pseudotyped viruses containing ASLV Env with either ASLV or MLV cores were strictly pH dependent, whereas those containing MLV Env infected cells by a pH-independent mechanism (35). Moreover, ASLV Env imposes a strict pH dependence on the entry of mixed virions that contain the core of human immunodeficiency virus type 1 (HIV-1) (14) (R. J. O. Barnard, S. Narayan, M. Miller, and J. A. T. Young, unpublished data), which is widely accepted to be a pH-independent virus (30, 39, 44). These data are compatible with a model in which low pH is required to trigger the complete fusion reaction between virions containing ASLV Env and cell membranes that leads to nucleocapsid release into the cytoplasm. However, these data do not support the model put forward by others, according to which low pH might not be required for formation of a fusion pore but instead might be required for a step downstream of fusion (15). We believe that that model is extremely unlikely, because it requires that ASLV Env impose (for a step downstream of fusion) a strict pH dependence on either its own core or on heterologous cores that show absolutely no pH dependence when associated with their own viral Env proteins.
Fusion between cells expressing fusion proteins and cells expressing receptors is a standard model system for isolating the functions of fusion proteins from the multitude of processes that occur during viral entry. It is agreed that low pH promotes ASLV Env-induced cell-cell fusion. However, some groups have found that after many hours some fusion occurs at neutral pH (15, 17, 21) whereas others have found that cell-cell fusion only occurs at low pH (35). The present study was initiated to rigorously investigate whether ASLV Env-induced cell-cell fusion had the characteristics expected of a bona fide pH-dependent fusion protein and to determine which steps of the fusion process do require low pH. By fusing cells expressing ASLV Env to cells expressing the cognate receptor of Env, we found that fusion does not take place between cells at neutral pH. Instead, we show that fusion is steeply pH dependent, occurring soon after the pH is lowered. But for low pH to induce fusion, the receptor must have already bound Env, causing interaction with the target membrane. Furthermore, we demonstrate that low pH is required for hemifusion and pore formation but not for pore growth.
MATERIALS AND METHODS
Reagents.
The fluorescent dyes CMAC (7-amino-4-chloromethylcoumarin), calcein AM, and DiI-C18 (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) were purchased from Molecular Probes Inc. (Eugene, Oreg.). Chlorpromazine (CPZ) and bovine serum albumin were obtained from Sigma Chemical Co. (St. Louis, Mo.). Dioleoylphosphatidylethanolamine (DOPE), dioleoylphosphatidylcholine (DOPC), lauroyl-lysoPC (LPC), and cholesterol were purchased from Avanti Polar Lipids (Alabaster, Ala.). Cell culture media and solutions were purchased from Gibco BRL (Gaithersburg, Md.). Anti-p27 antibody was obtained from SPAFAS, Inc. (North Franklin, Conn.).
Virus preparation.
ASLV-B virions were produced using a protocol described previously for ASLV-A virus (40). Briefly, cells of the chicken fibroblast cell line DF-1 were transfected with the subgroup B vector RCASBP(B)-eGFP and propagated until all the cells were infected with virus, as determined using fluorescence microscopy to ascertain levels of enhanced green fluorescent protein (eGFP) expression. The chronically infected cells were plated in 150-mm-diameter dishes, and (upon reaching >90% confluency) virus was harvested in the cell supernatant every 12 h over a 96-h period. The harvested cell supernatants containing ASLV-B virus were pooled, centrifuged at 1,000 × g for 15 min at 4°C, filtered through a 0.45-μm-pore-size sterile filter, and frozen in 1-ml aliquots. The viral titer of ASLV-B (determined on 293 cells expressing death domain-deleted TVB (TVBS1-5ΔDD) (3) was 106 eGFP-transducing units/ml. The envelope glycoprotein in the RCASBP(B)-eGFP virus consists of the signal peptide and receptor binding determinants of the subgroup B virus (RAV-2), with all other domains derived from the subgroup A virus (Schmidt-Ruppin A) Env. This chimeric construct has been used widely to study subgroup B ASLV-receptor interactions. For experiments that utilize virus rather than cells, we employ this chimeric Env and refer to the virus as ASLV-B.
Production of liposomes.
A mixture of 3.6 μmol of DOPC, 1.2 μmol of DOPE, and 1.2 μmol of cholesterol (mol/mol/mol ratio, 3:1:1) in chloroform was dried down to a thin film in glass tubes under a constant stream of nitrogen at room temperature for 30 min. The lipid film was then further dried under a vacuum for 30 min at room temperature. Lipids were hydrated in 1 ml of 1× HB (150 mM NaCl, 20 mM HEPES, pH 7.4) and resuspended by extensive vortexing. Liposomes (∼100 nm in diameter) were produced following five rapid freeze-thaw cycles in liquid nitrogen and a subsequent extrusion through a 0.1-μm-pore-size polycarbonate filter (Avanti Polar Lipids). Liposomes were purified on a Sephadex-G100 column, and their sizes were confirmed by quasi-dynamic light scattering in a Coulter N4 Plus Dynamic Light Scattering instrument (Beckmann Coulter, Fullerton, Calif.).
Virus liposome binding.
A total of 5,000 eGFP-transducing units of ASLV-B (preincubated with or without sTVB for 1 h at 4°C) was incubated with 125 μl of liposomes (3 to 6 mM) at the indicated temperature for 30 min. In hydrophobic association experiments, liposome-virus preparations were mixed 1:1 with 2 M NaCl-HB, 20 mM Na2CO3 (pH 11.0)-HB, 8 M urea-HB, or 0.2% Triton X-100-HB for 10 min at room temperature. An equal volume of ice-cold 80% sucrose-HB was added to the preparation, and 200 μl of this solution was overlaid with 550 μl of 30% sucrose-HB and 50 μl of 5% sucrose-HB. The samples were ultracentrifuged at 150,000 × g at 4°C for 2 h. Four 200-μl aliquots were taken from the air-liquid interphase. The top and bottom 200 μl of each sample were mixed with 25 μl of 10× SDS reducing sample buffer, boiled, and run on sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Virions were detected with the anti-ASLV p27 antibody raised against the capsid protein of ASLV, and sTVB was detected using SUBrIgG, as described previously (1).
Construction of ASLV Env expression plasmids and cell lines.
Nucleotides corresponding to amino acids 6 to 613 of the Schmidt-Ruppin B Env gene were PCR amplified from the Schmidt-Ruppin B proviral DNA construct pLD6 (26) and cloned in frame with nucleotides encoding the first 10 amino acids of the Schmidt-Ruppin A Env signal peptide in a packageable MLV-based internal ribosome entry site (IRES)-eGFP-containing vector (pCMMP-IRES-GFP, a kind gift from Bill Sugden, University of Wisconsin), generating pRB011. The ASLV-A Env protein was PCR amplified from the EnvA expression construct pAB7 (6) and was also cloned into the MLV-based pCMMP-IRES-GFP expression vector, generating the plasmid pRB013. To produce stable cell lines expressing ASLV EnvA, EnvB, or TVBS1-5ΔDD, pseudotyped MLV particles were generated that contained the vesicular stomatitis virus spike protein with a pRB011-, pRB013-, or pAB-1 (3)-derived genome, respectively. These viral particles were produced from 293 cells following a tripartite transfection with pMD.old.gagpol (MLVGag and Gag-pol proteins), pMD.G (encoding the vesicular stomatitis virus spike protein) (6), and pRB013, pRB011, or pAB1. The viral supernatants were collected 48 and 72 h posttransfection, centrifuged at 1,000 × g for 15 min at 4°C, filter sterilized through a 0.45-μm-pore-size filter, and stored at −80°C. These viral stocks were then used to infect either human 293 or mouse 3T3 BALB/c cells. Cells were sorted for high-level expression of EnvA, EnvB, or TVBs1-5ΔDD by flow cytometry with eGFP for the EnvA construct, TVBS3rIgG (made by PCR amplification of residues 1 to 148 of TVBS3 and cloned in frame into the rabbit immunoadhesin expression plasmid pSK100, generating plasmid pSK101) for the EnvB construct, or SUBrIgG for TVBS1-5ΔDD, as described previously (2). The SK100 plasmid was generated by PCR cloning residues 175 to 402 of the rabbit immunoglobulin heavy chain into the mammalian expression vector pCI-NEO (Promega, Madison, Wis.). 293 and 3T3 BALB/c cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 10% bovine calf serum, respectively. Production of 293(TVA950) and 293(TVA800) cell lines was described previously (37). For EnvA cell-cell fusion experiments, EnvA-expressing (3T3/EnvA) cells were used as effector cells to fuse to target TVA-expressing cells [either 293(TVA800) or 293(TVA950)] (37). For EnvB cell-cell fusion experiments, 3T3/EnvB cells were used as effector cells to fuse to target 293(TVB) cells.
Preparation of sTVB.
sTVB was prepared as outlined previously (1). Briefly, 293 cells were grown to 40% confluency and transfected (using the calcium phosphate precipitation method) with a plasmid encoding the soluble ectodomain of sTVB (pAB22) (1). At 48 and 72 h later, the supernatant (which contained the sTVB) from these cells was centrifuged at 1,000 × g for 15 min, filtered through a 0.22-μm-pore-size filter, aliquoted into 1-ml aliquots, and stored at −80°C. The concentration of sTVB in the cell supernatant was estimated by quantitative immunoblotting and compared to known concentrations of affinity-purified His6-tagged sTVB through the use of SUBrIgG (8) and an 35S-labeled anti-rabbit antibody (catalog number SJ424-50; Amersham) as binding probes.
Cell-cell fusion.
Fusion was monitored by a three-color fluorescence assay (33). Briefly, the effector cells were loaded with calcein AM (green emission at 515 nm) and the target cells were loaded with CMAC (blue emission at 466 nm) according to the manufacturer's instructions, with modifications described elsewhere (33). To monitor lipid mixing, we colabeled the target cells with the membrane dye DiI (orange emission at 565 nm) (33). To determine the extent of fusion, effector and target cells were mixed and coincubated in eight-well slides (Lab-Tek, Naperville, Ill.) at the temperature and pH indicated. Unless otherwise specified, fusion was triggered by exposing cells to pH 5.4 followed by additional incubation at neutral pH for 15 min at 37°C. The extent of fusion was quantified by fluorescence microscopy, determining the fraction of cells for which dyes mixed out of the total number of effector and target cells in contact. Care was taken to exclude false positives that resulted from colocalization of dye of overlaid effector and target cells rather than from actual mixing. At least four independent measurements were made for each datum point. For each measurement, ∼100 effector and target cell pairs within each well were identified and the fraction that fused was determined by the fraction of cell pairs that exhibited aqueous dye mixing. All data points were obtained from at least four such measurements. The error bars on all fusion graphs represent standard errors of the means.
To measure the kinetics of fusion, a coverslip containing cells was placed in a Peltier (20/20 Technology, Wilmington, N.C.)-cooled chamber (total volume, 1.5 ml) and dye transfer was recorded with a digital charge-coupled device camera (Retiga EX; Q-Imaging, Vancouver, British Columbia, Canada. Images were saved to disk and subsequently analyzed off line with commercial software (Northern Eclipse; Empix Imaging Inc., Mississauga, Ontario, Canada). To change temperature rapidly after reaching an intermediate state of fusion, the chamber was maintained at 4°C and the temperature was rapidly increased by illuminating the field of view with an infrared laser diode (28, 33). By subsequent reduction of the laser illumination, the temperature could be lowered to desired values at any time. In addition, we implemented a means to vary the pH in the vicinity of a cell pair. A low-pH solution (2 to 3 μl) was ejected from a micropipette that was positioned adjacent to a selected cell pair. Termination of ejection was used to reneutralize the solution surrounding the cell pair.
In experiments monitoring pore growth, effector cells (expressing GFP) were loaded with calcein and fused to target cells that were not labeled. Pore permeability was obtained as has been described in detail previously (28). Briefly, the changes in fluorescence of the effector and target cells were obtained from images that were captured at the rate of ∼3/s. Because the fluorescence of calcein was not quenched, fluorescence was proportional to dye concentration. The ratio of the rate of change in fluorescence (the flux) and the difference in dye concentrations between effector and target cells (the driving force for dye movement) yielded the permeability of the fusion pore as a function of time. To account for dye mobility as a function of temperature, we assumed that the diffusion coefficient increased 20% for every 10°C increase in temperature. To test whether any dye was compartmentalized or otherwise immobilized within effector cells and thus unable to move through the fusion pore, we added saponin (100 μg/ml) to permeabilize the cells and monitored the decrease in cell fluorescence.
RESULTS
Fusion between Env- and receptor-expressing cells requires low pH.
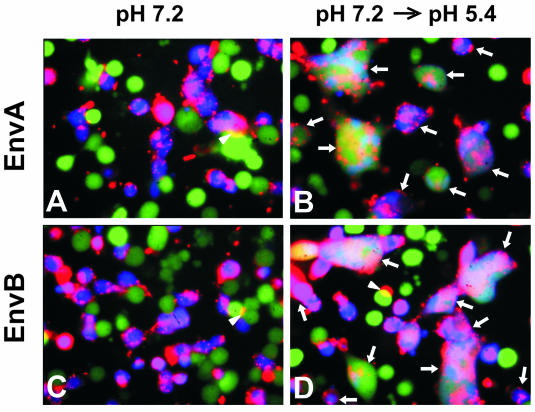
ASLV Env-mediated cell-cell fusion was monitored by lipid and aqueous dye redistribution with fluorescence microscopy (33, 36). Cells expressing either EnvA or EnvB (effector cells) were mixed with TVA or TVB receptor-expressing cells (target cells), respectively, and incubated for 2 h at 37°C in a pH 7.2 medium. Neither lipid transfer nor content transfer was detected (Fig. 1A and C). But when the pH was briefly lowered, almost all of the effector cells and target cells that were in contact fused (Fig. 1B and D). Control experiments showed that EnvA- and EnvB-expressing cells fused only to target cells containing their own cognate receptors (Fig. 2).
FIG. 1.
ASLV Env-mediated fusion monitored by a three-color fluorescence assay. 3T3/EnvA (A and B) or 3T3/EnvB cells (C and D) were loaded with calcein (green) and cocultured with 293(TVA950) or 293(TVB) target cells, respectively, colabeled with CMAC (blue) and DiI (red). Cells were coincubated for 2 h at 37°C (pH 7.2) followed by exposure to neutral pH (A and C) or to a pH 5.4 solution (B and D) for 15 min. Fusion occurred only when pH was lowered (B and D). Fused cells were positive for all three dyes and are marked by arrows. Regions of cells that partially overlap (marked by arrowheads) are readily distinguished from those of fused cells.
FIG. 2.
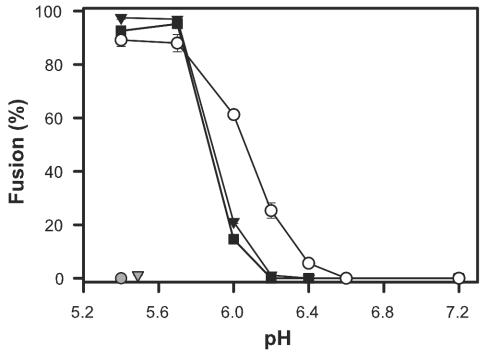
The pH dependence of cell-cell fusion. 3T3/EnvA cells were preincubated with either 293(TVA-800) (filled squares) or 293(TVA-950) cells (filled triangles), and 3T3/EnvB cells (open circles) were preincubated with 293(TVB) cells. In all cases, preincubation was for 1 h at 37°C, after which cells were exposed to solutions of different acidity levels for 10 min. Fusion was quantified by the transfer of aqueous dye 30 min after reneutralizing the external solution at 37°C. EnvA did not promote fusion when paired with target cells expressing TVB (shaded triangle); EnvB did not support fusion when paired with TVA-950-expressing cells (shaded circle).
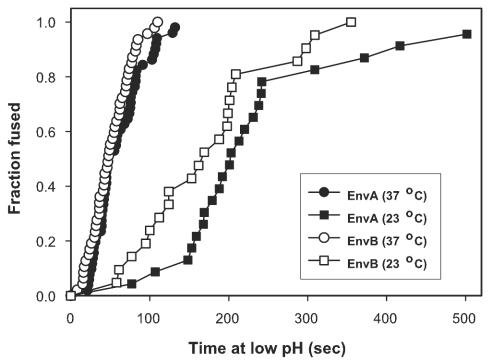
Env-induced fusion varied steeply with pH, as expected for a low-pH-triggered process (Fig. 2). EnvA can utilize either glycosylphosphatidylinositol-linked TVA-800 or membrane-spanning TVA-950 as a receptor (37) (Fig. 2). The same pH dependence of fusion resulted independently of which of these receptors was expressed on the target cells. The slope representing pH dependence of EnvB-induced fusion to cells expressing TVB was somewhat less steep than that for EnvA, and the threshold (∼pH 6.4) was somewhat higher than that for EnvA (∼6.0). These thresholds are significantly higher than those for most strains of influenza hemagglutinin (7, 32, 46) but are similar to those observed for Semliki Forest virus and vesicular stomatitis virus fusion proteins (5, 24, 45). Fusion occurred quickly for both EnvA and EnvB after acidification—completed within 2 min upon lowering the pH at 37°C to 5.4 (Fig. 3) and within 5 min at 23°C—providing further evidence that low pH is a true trigger for the ASLV fusion.
FIG. 3.
Kinetics of low pH-induced fusion. 3T3/EnvA (filled symbols) and 3T3/EnvB (open symbols) cells were coincubated with 293(TVA-950) and 293(TVB) cells, respectively, for 1 h at neutral pH and 37°C before a pH 5.4 solution was introduced at either 23°C (squares) or at 37°C (circles). The waiting times from application of the low-pH solution to the onset of fluorescent dye redistribution were measured, ranked, and plotted as cumulative distributions.
While some ASLV-A Env-mediated cell-cell fusion has been reported to proceed at neutral pH (15, 17), in our system no fusion occurred until the pH was lowered. In control experiments, effector and target cells were maintained at neutral pH for 20 h to better match the protocol of Earp et al. (15); the spread of aqueous dyes had occurred in less than 3% of the cells. When these cells were exposed to pH 5.4 for 15 min and the solution was then reneutralized for 30 min, in contrast, the dyes had spread for ∼90% of the cell pairs (data not shown).
At neutral pH, receptor-binding causes ASLV-B to become hydrophobically associated with liposomes.
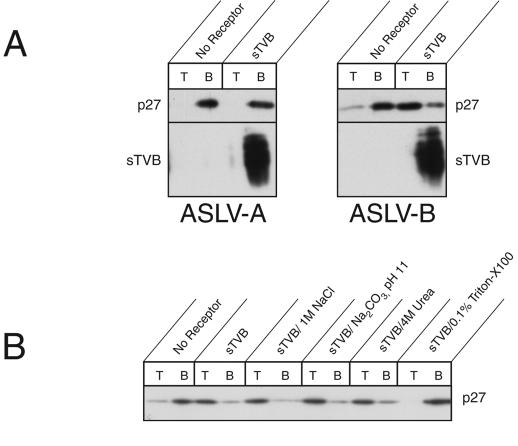
It had been shown (using a liposome coflotation assay) that solubilized ectodomain of ASLV-A Env (13, 20) and ASLV-A virions (15) bind to liposomes at neutral pH in the presence of sTVA. The binding was judged to be hydrophobic and mediated, at least in part, by Env's fusion peptide (which is internal to the transmembrane [TM] subunit). Using the coflotation assay, we have also found that ASLV-B virions associated with liposomes upon binding sTVB: the heavy virus remained in the bottom fractions of sucrose gradients but migrated to the top fraction when associated with the lighter liposomes (Fig. 4A). The ASLV particles migrated to the top only when incubated with sTVB in the presence of liposomes (Fig. 4A). When liposomes were not present, virions remained in the bottom fractions even when sTVB was added (data not shown). In contrast to the results seen with ASLV-B, ASLV-A particles were always located in the bottom fraction regardless of whether sTVB and/or liposomes were present (Fig. 4A), providing a control for specificity of binding between sTVB and EnvB. The ASLV receptor did not colocalize with virions at the top of the gradient; instead, sTVB was always found in the bottom fraction (Fig. 4A). The virions bound to liposomes through hydrophobic interactions: receptor-activated ASLV-B remained bound to liposomes in the presence of 1 M sodium chloride, 10 mM sodium carbonate (pH 11.0), or 4 M urea, all affirming a hydrophobic interaction between virions and liposomal membranes (Fig. 4B). As has been found for ASLV-A binding (13), however, the presence of 6 M urea did cause ASLV-B to dissociate from the target membrane (data not shown). It is possible that high concentrations of urea alter a higher-order structure of Env. The data indicate that binding of sTVB to ASLV-B virions causes exposure of the internal fusion peptide of EnvB, which is then inserted into liposomes.
FIG. 4.
Receptor-activated ASLV-B associates with liposomes through a hydrophobic interaction. Association between ASLV and liposomes was monitored as levels of comigration in sucrose gradients. (A) ASLV-A and ASLV-B particles were incubated with or without sTVB and mixed with liposomes at 37°C. After ultracentrifugation on a sucrose step gradient, the top (lanes T) and bottom (lanes B) fractions were subjected to SDS-polyacrylamide gel electrophoresis and the ASLV capsid protein and sTVB were detected in immunoblot analysis using an anti-ASLV capsid antibody and SUBrIgG, respectively. (B) The stability of the receptor-activated ASLV-B particle-liposome interaction was assessed (following liposome association) by incubation in the presence of 1 M NaCl, 10 mM Na2CO3, 4 M urea, or 0.5% Triton X-100.
In the case of sTVA, the soluble receptor remains bound for long times to soluble EnvA but readily dissociates when EnvA binds hydrophobically to liposomes (13, 20). Thus, insertion of the fusion peptide into lipid bilayers could cause conformational changes in the SU subunit of Env, which releases the receptor. In the case of ASLV-B particles, infectivity is retained for several hours after binding sTVB (6), suggesting that the receptor remains bound to Env when fusion peptides are not inserted into membranes. However, as sTVB did not comigrate with membrane-associated ASLV-B (Fig. 4A), receptor might be released upon hydrophobic association of EnvB with liposomes, as is also the case for EnvA (13, 20).
For fusion to occur, receptor-activated Env must interact with target cells before pH is reduced.
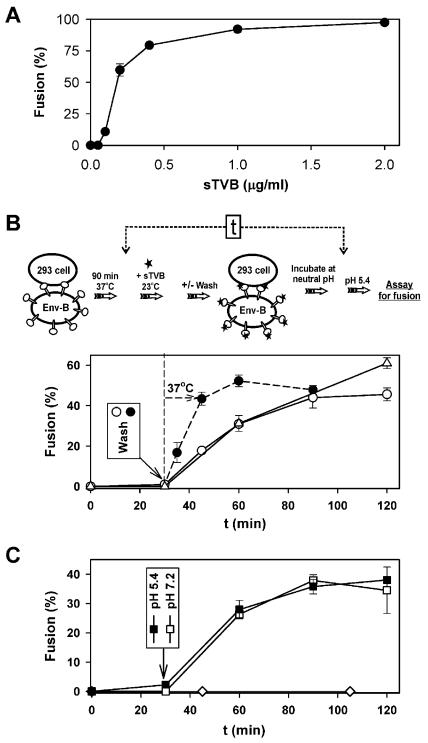
Binding soluble receptor to ASLV-B allows the virus to infect receptor-deficient cells (6, 25, 43). We utilized sTVB to address this question: where in the fusion process is low pH effective? “Receptor-activated” designates the state of Env that results from receptor binding without fusion peptide insertion into target membranes. This state occurs after binding soluble receptor in the absence of target membranes or after binding receptor on target membranes but prior to the insertion of fusion peptides. First, we verified that for cell-cell fusion, sTVB binding to EnvB is adequate to induce fusion to receptor-deficient cells: sTVB (2 μg/ml) was bound to effector cells that had been coincubated at neutral pH with human 293T cells (that lack TVB). Efficient fusion occurred upon lowering the pH to 5.4 (Fig. 5A). Thus, in agreement with earlier findings (13, 25) the cognate receptor of ASLV Env does not have to be anchored to a target membrane to support fusion. We adjusted the concentration of sTVB and used the same experimental protocol and found that the extent of fusion increased superlinearly with low concentrations of sTVB, indicating that multiple copies of EnvB cooperatively participate in the fusion process. A similar finding and interpretation have been presented for EnvA (13).
FIG. 5.
(A) Fusion between cells expressing EnvB and receptor-deficient 293T cells in the presence of various concentrations of sTVB. Effector and target cells were coincubated for 1 h at 37°C with the indicated concentrations of sTVB. Fusion was triggered by exposure to pH 5.4 for 10 min, and dye spread was monitored after an additional incubation of 15 min at neutral pH (37°C). (B) Time dependence for acquisition of the ability to fuse at low pH for soluble receptor-activated effector cells that are in contact with receptor-deficient target cells. 3T3/EnvB and 293T cells were preincubated at 37°C for 1.5 h, exposed to 1.5 μg of sTVB/ml for 30 min at 23°C, washed, and incubated for various times at 23°C (open circles) or 37°C (filled circles). Alternatively, sTVB was never removed during the 23°C incubation (open triangles). Fusion was subsequently induced by lowering the pH to 5.4 for 2 min at 37°C and was quantified after an additional incubation at 37°C at neutral pH for 15 min. The indicated time (t) values refer to the times of acidification after addition of sTVB (time = 0), as shown in the panel illustrating the experimental protocol. (C) Effector-target cell pairs were incubated with sTVB for 30 min, and then sTVB was removed by washing. Cells were acidified to pH 5.4 for 2 min and reneutralized, and (at the indicated times) the pH was again lowered for 2 min (closed squares). Alternatively, cells were maintained at neutral pH and then the pH was lowered at the indicated time for 2 min followed by reneutralization (open squares). Fusion was not observed when 3T3/EnvB cells were preincubated with sTVB, washed, and only then incubated with target 293T cells for 1 h (open diamonds).
Effector cells were then allowed to bind to receptor-deficient cells for 1.5 h and were treated with sTVB (1.5 μg/ml) for different time periods at 23°C before the pH was lowered to 5.4. When the pH was lowered 30 min after adding sTVB, fusion did not occur. But an appreciable extent of fusion was observed when the pH was instead lowered 60 min after sTVB was added (Fig. 5B). Clearly, soluble-receptor-activated Env increasingly interacted with the receptor-deficient target membrane over time, most likely due to insertion of fusion peptides at neutral pH (Fig. 4) (13, 15, 20). To explore postbinding interactions between Env and the target cell prior to the lowering of the pH, sTVB was bound for 30 min at 23°C and then unbound sTVB was removed (see schematic protocol in Fig. 5B). The cells were then incubated at either 23 or 37°C for various times at neutral pH before the pH was lowered to 5.4 (Fig. 5B). As expected, virtually no fusion occurred when the pH was lowered immediately after unbound sTVB was removed (i.e., time = 30 min). However, the extent of fusion increased as the time between the removal of sTVB and the lowering of pH was increased. The time course of this increase was the same as that seen when sTVB was never removed. Thus, receptor binding was complete by the time sTVB was washed out but interaction between EnvB and the target membrane continued after washout. The increase in fusion with increased incubation time indicates that if fusion is to occur, Env must associate with a target membrane by the time the pH is lowered. The time course of the interaction at neutral pH was temperature dependent: at 23°C, more than 40 min had to elapse after the addition of sTVB for low pH to induce fusion (Fig. 5B), but at 37°C, it took only ∼15 min (data not shown). When sTVB was removed following a 30-min incubation at 23°C, fusion was slower when 23°C was maintained than when the temperature was raised to 37°C.
The time courses for acquiring fusion competence were the same regardless of whether a conditioning acidification (pH 5.4 for 2 min) was or was not applied (immediately after removal of sTVB) prior to the incubation at neutral pH (Fig. 5C). Clearly, the conditioning acidification did not inactivate receptor-activated Env or promote the reconfigurations necessary to induce fusion. These experiments emphasize that for low pH to affect Env, Env must already be associated with target membranes. This conclusion is further underscored by experiments in which effector cells were pretreated with sTVB and bound to receptor-deficient target cells (after removing unbound sTVB). Here, fusion did not occur even after more than 1 h of cell coincubation (Fig. 5C). It is possible that activated Env enters the endocytic pathway unless it is anchored to the target membrane. The simplest explanation, however, is that the release of fusion peptides (caused by binding of sTVB) can be reversed in the absence of a target membrane; but when the peptides insert in the lipid bilayer of a target membrane they become trapped, and it is this membrane-bound conformation of Env that responds to low pH to induce fusion.
Hemifusion requires low pH.
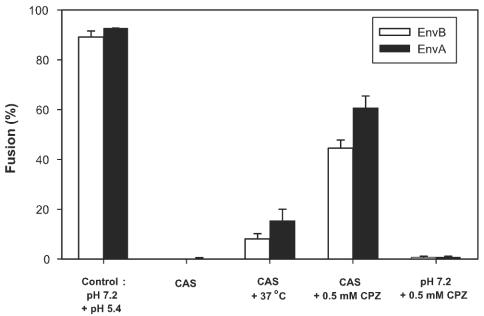
Effector cells expressing either EnvA or EnvB and target cells were preincubated at 37°C at neutral pH (Fig. 6). They were transiently exposed to pH 5.4 at either 4 or 12°C (temperatures that do not support fusion) and then returned to neutral pH without a further change in temperature. Neither lipid (data not shown) nor content mixing (Fig. 6) was detected for as long as 30 min after the reneutralization step. We refer to the stage reached by lowering pH at low temperature as the cold-arrested stage (CAS). It is well known that a lack of observable lipid dye transfer between cells does not necessarily mean that membrane continuity (hemifusion) has not been established. When a state of hemifusion has been reached that can proceed to full fusion, lipid dye has generally not yet spread, possibly because the high density of fusion proteins at the local site of hemifusion impedes lipid movement (9, 10, 34). This state can be revealed by treating cells with CPZ, an agent that destabilizes the hemifusion diaphragm and promotes aqueous dye spread (9, 31, 34). When CPZ was added at CAS, fusion was reasonably efficient, indicating that the CAS is a state in which local hemifusion has been achieved. In contrast, when CPZ was applied to effector and target cells that had been incubated at neutral pH (but not exposed to low pH) neither lipid nor content mixing was detected, indicating that local hemifusion does not occur at neutral pH even though Env has definitely engaged its receptor and has perhaps inserted its fusion peptide into the target membrane. When the temperature was raised to 37°C after achieving the CAS, there was some fusion but it was not extensive. In separate experiments, CAS was generated at 12°C (a temperature too low for fusion) rather than at 4°C, as shown in Fig. 6. The same phenomena were qualitatively observed, but (quantitatively) a somewhat greater extent of fusion was observed after the temperature was raised to 37°C for 12°-CAS (data not shown). Therefore, for both EnvA and EnvB low pH is not only necessary for the local hemifusion at CAS but also must be present for subsequent steps that lead to pore formation.
FIG. 6.
Cold-arrested intermediate of ASLV Env-induced fusion. 3T3/EnvA and 3T3/EnvB cells were preincubated with 293(TVA950) cells and 293(TVB) cells, respectively, for 1 h at neutral pH and 37°C. Bound effector and target cells were treated with pH 5.4 for 15 min at 4°C and then reneutralized at the same temperature. Neither lipid (DiI [data not shown]) nor aqueous (calcein [bars]) dye had mixed at this point (CAS). Raising the temperature to 37°C after the CAS was reached led to some fusion (CAS + 37°C), but the level of fusion fell far short of the full extent (Control: pH 7.2 + pH 5.4). The precise extent of fusion upon raising temperature from that of the CAS depended on the density of Env that was expressed on the effector cells. The addition of 0.5 mM CPZ at the CAS for 1 min at 23°C (CAS + 0.5 mM CPZ) led to significant aqueous dye mixing. In control experiments, dye did not spread when CPZ was added to bound cells that had not been exposed to low pH (pH 7.2 + 0.5 mM CPZ).
Pore growth is independent of pH.
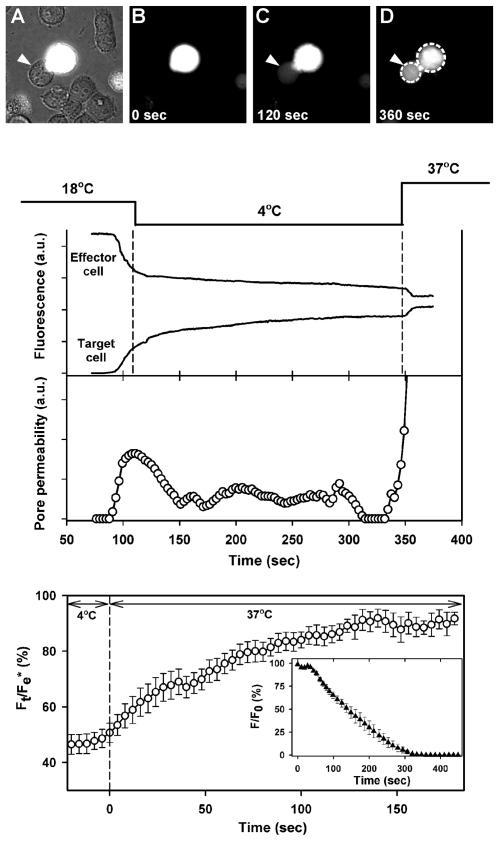
It has been suggested that ASLV Env-mediated fusion occurs at neutral pH and that any need for low pH is restricted to pore enlargement (15). Having established that low pH is, in fact, required for pore formation, we determined whether pore enlargement proceeds efficiently at neutral pH. To monitor pore growth, Env-expressing cells (also expressing GFP; see Materials and Methods) were loaded with calcein and bound to TVB-expressing cells on a coverslip for 1 h at 37°C and then transferred to a chamber maintained at 4°C (Fig. 7A and B). The temperature of a cell pair was locally raised to 18°C by laser illumination, and the pH of the surrounding solution was transiently lowered to 5.4 via ejection from a pipette for 1 min (see Materials and Methods). Shortly after the perfusion was stopped to reneutralize the surrounding solution, calcein was observed to begin transferring from the target to the effector cell, showing that a fusion pore had formed (Fig. 7C). (Several low-pH viral fusion proteins, such as those from influenza virus [27] and Semliki Forest virus [38], can induce fusion at 18°C.) As soon as dye transfer was detected, the laser was shut off, lowering temperature to 4°C. (In control experiments, the entire solution in the chamber was replaced by a fresh neutral pH solution to make certain that subsequent pore growth occurred at neutral pH.) The dye continued to transfer but at a slower rate (upper graph), showing that the pore remained open. However, not only was pore growth arrested but the pore itself would shrink when the temperature was lowered to 4°C. We showed that the fluorescence was not quenched (data not shown and see reference 28); hence, fluorescence intensity was proportional to dye concentration. Pore permeability was calculated from the rate of change of dye concentration and the difference in concentrations of dye in the two cells (28). The cessation of pore growth and pore shrinkage are readily seen from the calculated pore permeabilities (middle graph).
FIG. 7.
Pore growth proceeds at neutral pH. Effector (containing calcein and GFP) and target cells were bound together at 37°C and then placed in a 4°C chamber (image A, phase contrast overlaid with fluorescence; image B, fluorescence). Cell pairs were locally exposed to pH 5.4 at 18°C by ejection of solution from a micropipette; ejection was stopped after 1 min. Immediately after aqueous dye was observed to spread (image C), the temperature was lowered to 4°C. Middle panel: the rate of dye spread (fluorescence [upper graph]) and the calculated pore permeabilities (open circles [lower graph]) decreased. Raising temperature to 37°C at neutral pH led to rapid dye transfer due to growth of the pore. Dye concentration quickly reached a steady state (image D and upper graph). The temperature protocol is shown above the graphs. In this particular experiment, the fluorescence of the effector cell remained somewhat greater than that of the target cell. After saponin was added, the majority of the fluorescence of the effector cell quickly (within seconds) decayed but a steady-state level remained above background. Therefore, a small fraction of dye within the effector cell was immobile; the pore was not the cause of the fluorescence inequality. In general, the morphology of the cells changed after pore formation. In the illustrated experiment, the cell boundaries did not appreciably change after fusion, aiding visual clarity, although there was a small immobile fraction of dye. Bottom panel: the ratio of the fluorescence of the target cell (Ft) to the final florescence of the effector cell after all dye movement ceased (Fe*) as a function of time (averaged for nine fusion experiments). Individual fluorescence traces were aligned at the time of the temperature jump to 37°C (defined as time = 0). Inset: the time course for release of dye from isolated effector cells after adding saponin at 4°C (averaged forseven cells). F/F0 represents the ratio of the cell fluorescence (F) at times after adding saponin normalized to the initial fluorescence level (F0). Error bars represent standard errors of the means.
When the temperature was subsequently raised to 37°C, dye movement was immediately augmented, showing that the pore had not closed at 4°C; it rapidly enlarged at neutral pH. After the temperature was raised, the fluorescence of the effector and target cells quickly reached steady-state levels. This is the same pattern of pore growth previously observed for fusion pores induced by HIV Env (28). Pore enlargement was always observed at neutral pH when the temperature was raised, as can be seen from the time course for the fluorescence of the target cell normalized to the final fluorescence of the fused effector cell (Fig. 7, bottom graph). Raising the temperature from 4 to 37°C consistently led to faster dye movement. As can be seen from the averaged time courses, about 50% of the dye that would transfer into the target cell had yet to do so at the time temperature was raised from 4°C (bottom graph). Thus, we were able to conduct our quantitative analysis of pore growth on the basis of a large change in fluorescence. For the batches of cells used for these averaging experiments, the fluorescence levels of the fused effector and target cells became equal, showing that both calcein and the larger GFP moved freely through the fusion pore. For these batches, the fluorescence of effector cells fully decayed to the background level when saponin was added at 4°C (bottom graph, inset). Because all dyes within these effector cells were mobile (rather than compartmentalized), even at low temperature the increased dye transfer upon raising temperature must have been due to enlargement of the fusion pore. In short, it is clear that ASLV Env-induced pores reliably and rapidly enlarge at neutral pH. Low pH (required for hemifusion and then for fusion to occur) is no longer needed once the pore has formed.
DISCUSSION
Our results confirm that ASLV Env-dependent fusion has a strict requirement for receptor binding followed by low pH (35). Incubating a soluble form of TVB with ASLV-B virions leads to hydrophobic association of virions with liposomes, showing that receptor binding induces a conformational change in Env at neutral pH. The hydrophobic association indicates that it is most likely that receptor binding is the trigger for fusion peptide insertion into the target membrane. Fusion of EnvA- and EnvB-expressing cells with target cells expressing their cognate receptors was absolutely dependent on low pH and occurred rapidly following acidification—the process was complete within a couple of minutes at 37°C. Fusion varied steeply with pH, as expected if low pH is required to trigger the fusion process. Our results show that conformational changes of Env that induce hemifusion and fusion require low pH. Subsequent pore enlargement does not require low pH.
The sequential steps of ASLV Env fusion require a series of triggers.
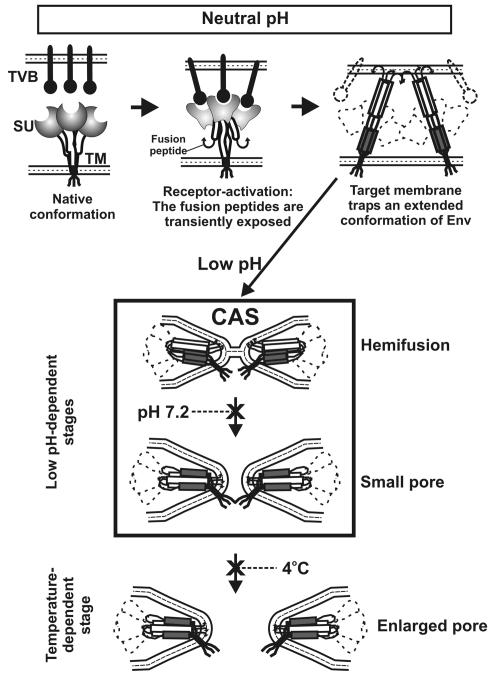
We suggest that the fusion process for ASLV Env follows these steps (Fig. 8): Env binds receptor at neutral pH and then releases its fusion peptides, which insert into the target membrane (top panel). At this point, the trimeric coiled coil has formed (top panel, right). Env is now in a state that permits (and, in fact, requires) low pH to induce the additional conformational changes that lead to hemifusion and fusion (as shown within the box). Pore enlargement utilizes pH-independent but temperature-dependent steps (bottom panel).
FIG. 8.
A model for ASLV Env-induced fusion. (Top panel) Upon binding receptors (situated within the target membrane), the SU subunits of Env (shaded circles) undergo conformational changes (denoted by altered shaded shapes) and the internal fusion peptides (curved arrows of the TM subunit) are released. After insertion of the fusion peptides into the target membrane, the N-terminal heptad repeats (open boxes) and the C-terminal helices (gray boxes) are exposed, creating an extended conformation of Env. Receptor has dissociated from Env. (Center panel) After insertion of the fusion peptides into the target membrane, low pH-induced conformational changes lead to hemifusion. Hemifusion can occur at 4°C. Subsequent conformation changes that lead to pore formation also require acidic pH, but a higher temperature is needed than that for hemifusion. Because peptides corresponding to the C-terminal repeats of the TM subunit of ASLV Env inhibit ASLV infectivity and cell-cell fusion (15), it is almost certain that ASLV Env forms a six-helix bundle. A particular sequence by which Env may fold into a six-helix bundle is illustrated, but the actual sequence is not yet known. (Bottom panel) Pore enlargement can proceed at neutral pH. The processes of hemifusion, pore formation, and pore enlargement proceed as Env undergoes conformational changes.
Engagement of the target membrane by Env is a dynamic process. Binding to receptor is the first attachment between Env and the target membrane. Liposome binding experiments indicate that the conformational changes in Env that allow the fusion peptides to insert into the target membrane might also cause the receptor to dissociate from Env. Thus, Env's engagement with the plasma membrane is switched from receptor binding to fusion peptide insertion. This could have implications for the internalization of ASLV. It has recently been shown that the uptake and intracellular trafficking pathways of ASLV-A differ depending on whether TVA is localized to lipid rafts (37). If ASLV Env does dissociate from its cognate receptor, therefore, the receptor should not directly dictate the internalization pathway of the virus; instead, the lipid environment (such as a lipid raft) into which the fusion peptide has inserted might be the determinant. Our finding with the cell-cell fusion system (that ASLV Env must have already interacted with the target membrane for pH to cause fusion) probably also holds for viral infection. The block of viral infection created by using bafilomycin A1 to prevent endosomal acidification could be overcome by transiently lowering pH of the external solution, but pH could not be lowered immediately—the virus had to be incubated with the cells for 15 min at neutral pH and 37°C for infection to occur (35). It is probable that virus increasingly interacted with receptor-containing membranes in that study.
The freedom that Env gains in detaching from its receptor could facilitate the subsequent low-pH conformational changes that induce the lipid rearrangements of hemifusion and pore formation. The potential importance of this freedom for the fusion process has previously been emphasized (13).
The results of this study show that the sequential steps in ASLV fusion are tightly regulated: several steps require a trigger that can be effected only after the preceding steps have been completed. The insertion of fusion peptides into target membranes requires that Env bind to receptor. This renders Env sensitive to low pH. In our model, low pH cannot promote fusion until the fusion peptides of Env are membrane inserted (i.e., until after conformational changes in Env driven by interaction with receptor and the target membrane). For viruses in general, the target membrane plays a passive role in the fusion process. (There are, however, notable exceptions, such as Semliki Forest virus, in which particular lipids are required in the target membrane) (11, 24). It is acted upon by the insertion of the fusion peptides, and fusion proceeds without further triggers. We hypothesize that for ASLV, the presence of the low pH trigger subsequent to receptor activation leads to a subtle but important difference in the role of the target membrane: as the fusion peptides “grasp” the target membrane, they lock Env into a configuration that then allows low pH to induce the further conformational changes required for fusion.
Prior conclusions that ASLV fuses at neutral pH were made on the basis of assays that failed to detect the low pH-dependent step.
Reports arguing that ASLV infects cells at neutral pH have been based on infection protocols that necessitated washing out the lysosomotropic agents; typically the cells were maintained for days after removing the agent (15, 17). Because removal of the reversible lysosomotropic agent NH4Cl for even 1 min allows ASLV entry to proceed (37), all fusion that led to infection had probably occurred at low pH. In fact, reverse transcription-PCR assays that can be performed in the presence of lysosomotropic agents have shown that virally encoded reverse transcription products occur only when the neutralizing agents are removed (37). That is, experiments that allow continuous control of endosomal pH show that low pH is essential for ASLV infection.
Infection by viruses that have been shown to definitely require low pH to initiate fusion, such as influenza and Semliki Forest virus, was inhibited even after the lysosomotropic agents were removed (15, 18, 19, 29). It has therefore been argued that the ability of ASLV to induce fusion despite the addition of lysosomotropic agents demonstrates that ASLV fuses at neutral pH. But the difference may be that the classical low-pH viruses within a neutralized endosome are readily degraded (19) whereas ASLV is stable within a neutralized endosome for many hours (35, 37). In other words, the observed differences in behavior do not necessarily stem from low-pH requirements but rather from viral stability, perhaps due to differences in their endosomal trafficking pathways.
A small amount of EnvA-induced cell-cell fusion has been observed at neutral pH (15). In these experiments, bound cells were incubated for about 16 h at neutral pH; exposure to low pH for just 5 min greatly increased the extent of fusion. For our cells expressing either EnvA or EnvB, aqueous dye transfer did not occur after 2 to 4 h at neutral pH and only a negligible amount occurred after ∼18 h; fusion was extensive and was completed within minutes of lowering pH. It may be that the quantitative difference between these systems at neutral pH is due to high-protein-expression levels from butyrate induction and from the use of an avian vaccinia virus expression system as employed by Earp et al. (15). The entire process of viral infection takes a few hours, with ASLV DNA synthesis readily detectable within that time frame; any fusion between cells observed only after long times is unlikely to be biologically relevant to the activity of ASLV Env during infection. In our system and at relevant time scales, low pH is an absolute requirement for ASLV Env-induced cell-cell fusion. The inability of CPZ to promote aqueous dye spread between bound cells that were maintained at neutral pH provides strong evidence that not even hemifusion occurs without lowering of pH. To try to reconcile disparate claims for the need for low pH, it has been suggested that low pH does not promote opening of pores but rather promotes their growth (15). This diverges from our finding that after the pore forms at low pH, its growth is efficient and complete at neutral pH.
Lipid mixing has been observed between pyrene-labeled virions and plasma membranes of target cells that express receptor (15). This indicates that ASLV Env-driven fusion reaches the lipid-mixing stage at neutral pH. However, because of the high particle/infectivity ratio of ASLV and the high-level multiplicity of infection that was used in these lipid mixing studies, it is possible that the viral particles that disperse lipid dye at neutral pH are not those that cause infection but those that merely terminate nonproductively at a state of hemifusion (9, 10). The finding of and significance of lipid mixing from virus to cells at neutral pH clearly need to be further explored. Fusion between cells induced by viral proteins generally occurs through the same fundamental mechanism as fusion of the virus itself to cells. It is of course possible that ASLV Env is anomalous in this respect, as suggested previously (15). But we have now demonstrated the hallmarks of a bona fide low-pH fusion mechanism for ASLV Env induced cell-cell fusion, suggesting that it behaves in the same manner as that for other low pH viral proteins: fusion occurs quickly after lowering pH and exhibits steep pH dependence.
At present, the requirement of low pH is specific to a small subset of retroviruses, including mouse mammary tumor virus and ASLV. While members of many classes of viruses fuse only at low pH, fusion of retroviruses generally is not pH dependent. For example, fusion between effector cells expressing HIV-1 Env and target cells expressing CD4 and coreceptor was not augmented by low pH (our unpublished results). Also, the envelope glycoprotein of MLV is activated in a pH-independent manner (35). The amino acid sequence of the TM subunit of ASLV Env is highly homologous to that of the GP2 subunit of the fusion protein of Ebola virus (16), and their glycosylation characteristics are similar (23). As the Ebola virus fusion protein fuses at low pH (47), it seems logical that ASLV would do so as well. It remains to be determined, however, whether it is necessary for receptor binding to activate Ebola virus GP before low pH can induce fusion.
Acknowledgments
We thank Sofya Brener for excellent technical assistance and Angeline Babel for help in production of the 3T3 BALB/c TVB and 293 EnvB cells. We also thank K. Schell and members of the flow cytometry facility at the University of Wisconsin Comprehensive Cancer Center for performing the cell sorting.
This work was supported by NIH grants GM27367 (F.S.C.), GM54787 (G.B.M.), and CA70810 (J.A.T.Y.) and by a Rush Medical College grant-in-aid (R.M.M.).
REFERENCES
- 1.Adkins, H. B., S. C. Blacklow, and J. A. Young. 2001. Two functionally distinct forms of a retroviral receptor explain the nonreciprocal receptor interference among subgroups B, D, and E avian leukosis viruses. J. Virol. 75:3520-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins, H. B., J. Brojatsch, J. Naughton, M. M. Rolls, J. M. Pesola, and J. A. T. Young. 1997. Identification of a cellular receptor for subgroup E avian leukosis virus. Proc. Natl. Acad. Sci. USA 94:11617-11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adkins, H. B., J. Brojatsch, and J. A. Young. 2000. Identification and characterization of a shared TNFR-related receptor for subgroup B, D, and E avian leukosis viruses reveal cysteine residues required specifically for subgroup E viral entry. J. Virol. 74:3572-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates, P., J. A. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74:1043-1051. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal, R., A. Bali-Puri, A. Walter, D. Covell, and O. Eidelman. 1987. pH-dependent fusion of vesicular stomatitis virus with Vero cells. Measurement by dequenching of octadecyl rhodamine fluorescence. J. Biol. Chem. 262:13614-13619. [PubMed] [Google Scholar]
- 6.Boerger, A. L., S. Snitkovsky, and J. A. Young. 1999. Retroviral vectors preloaded with a viral receptor-ligand bridge protein are targeted to specific cell types. Proc. Natl. Acad. Sci. USA 96:9867-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulay, F., R. W. Doms, R. G. Webster, and A. Helenius. 1988. Posttranslational oligomerization and cooperative acid activation of mixed influenza hemagglutinin trimers. J. Cell Biol. 106:629-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brojatsch, J., J. Naughton, M. M. Rolls, K. Zingler, and J. A. Young. 1996. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell 87:845-855. [DOI] [PubMed] [Google Scholar]
- 9.Chernomordik, L. V., V. A. Frolov, E. Leikina, P. Bronk, and J. Zimmerberg. 1998. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell Biol. 140:1369-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen, F. S., R. M. Markosyan, and G. B. Melikyan. 2002. The process of membrane fusion: nipples, hemifusion, pores, and pore growth. Curr. Top. Membr. 52:501-529. [Google Scholar]
- 11.Corver, J., L. Moesby, R. K. Erukulla, K. C. Reddy, R. Bittman, and J. Wilschut. 1995. Sphingolipid-dependent fusion of Semliki Forest virus with cholesterol-containing liposomes requires both the 3-hydroxyl group and the double bond of the sphingolipid backbone. J. Virol. 69:3220-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damico, R., and P. Bates. 2000. Soluble receptor-induced retroviral infection of receptor-deficient cells. J. Virol. 74:6469-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damico, R. L., J. Crane, and P. Bates. 1998. Receptor-triggered membrane association of a model retroviral glycoprotein. Proc. Natl. Acad. Sci. USA 95:2580-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz-Griffero, F., S. A. Hoschander, and J. Brojatsch. 2002. Endocytosis is a critical step in entry of subgroup B avian leukosis viruses. J. Virol. 76:12866-12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Earp, L. J., S. E. Delos, R. C. Netter, P. Bates, and J. M. White. 2003. The avian retrovirus avian sarcoma/leukosis virus subtype A reaches the lipid mixing stage of fusion at neutral pH. J. Virol. 77:3058-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallaher, W. R. 1996. Similar structural models of the transmembrane proteins of Ebola and avian sarcoma viruses. Cell 85:477-478. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert, J. M., D. Mason, and J. M. White. 1990. Fusion of Rous sarcoma virus with host cells does not require exposure to low pH. J. Virol. 64:5106-5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helenius, A., J. Kartenbeck, K. Simons, and E. Fries. 1980. On the entry of Semliki forest virus into BHK-21 cells. J. Cell Biol. 84:404-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helenius, A., M. Marsh, and J. White. 1982. Inhibition of Semliki forest virus penetration by lysosomotropic weak bases. J. Gen. Virol. 58:47-61. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez, L. D., R. J. Peters, S. E. Delos, J. A. Young, D. A. Agard, and J. M. White. 1997. Activation of a retroviral membrane fusion protein: soluble receptor-induced liposome binding of the ALSV envelope glycoprotein. J. Cell Biol. 139:1455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez, L. D., and J. M. White. 1998. Mutational analysis of the candidate internal fusion peptide of the avian leukosis and sarcoma virus subgroup A envelope glycoprotein. J. Virol. 72:3259-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter, E. 1997. Viral entry and receptors, p. 71-119. In J. Coffin, S. M. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 23.Jeffers, S. A., D. A. Sanders, and A. Sanchez. 2002. Covalent modifications of the Ebola virus glycoprotein. J. Virol. 76:12463-12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kielian, M. 1995. Membrane fusion and the alphavirus life cycle. Adv. Virus Res. 45:113-151. [DOI] [PubMed] [Google Scholar]
- 25.Knauss, D. J., and J. A. Young. 2002. A fifteen-amino-acid TVB peptide serves as a minimal soluble receptor for subgroup B avian leukosis and sarcoma viruses. J. Virol. 76:5404-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kopchick, J. J., and D. W. Stacey. 1984. Differences in intracellular DNA ligation after microinjection and transfection. Mol. Cell. Biol. 4:240-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markosyan, R., G. B. Melikyan, and F. S. Cohen. 2001. Evolution of intermediates of influenza virus hemagglutinin-mediated fusion revealed by kinetic measurements of pore formation. Biophys. J. 80:812-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markosyan, R. M., F. S. Cohen, and G. B. Melikyan. 2003. HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation. Mol. Biol. Cell 14:926-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matlin, K., H. Reggio, A. Helenius, and K. Simons. 1981. Infectious entry pathway of influenza virus in a canine kidney cell line. J. Cell Biol. 91:601-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McClure, M. O., M. Marsh, and R. A. Weiss. 1988. Human immunodeficiency virus infection of CD4-bearing cells occurs by a pH-independent mechanism. EMBO J. 7:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melikyan, G. B., S. A. Brener, D. C. Ok, and F. S. Cohen. 1997. Inner but not outer membrane leaflets control the transition from glycosylphosphatidylinositol-anchored influenza hemagglutinin-induced hemifusion to full fusion. J. Cell Biol. 136:995-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melikyan, G. B., S. Lin, M. G. Roth, and F. S. Cohen. 1999. Amino acid sequence requirements of the transmembrane and cytoplasmic domains of influenza virus hemagglutinin for viable membrane fusion. Mol. Biol. Cell 10:1821-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melikyan, G. B., R. M. Markosyan, H. Hemmati, M. K. Delmedico, D. M. Lambert, and F. S. Cohen. 2000. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J. Cell Biol. 151:413-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melikyan, G. B., R. M. Markosyan, M. G. Roth, and F. S. Cohen. 2000. A point mutation in the transmembrane domain of the hemagglutinin of influenza virus stabilizes a hemifusion intermediate that can transit to fusion. Mol. Biol. Cell 11:3765-3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 36.Munoz-Barroso, I., S. Durell, K. Sakaguchi, E. Appella, and R. Blumenthal. 1998. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J. Cell Biol. 140:315-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Narayan, S., R. J. Barnard, and J. A. Young. 2003. Two retroviral entry pathways distinguished by lipid raft association of the viral receptor and differences in viral infectivity. J. Virol. 77:1977-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samsonov, A. V., P. K. Chatterjee, V. I. Razinkov, C. H. Eng, M. Kielian, and F. S. Cohen. 2002. Effects of membrane potential and sphingolipid structures on fusion of Semliki Forest virus. J. Virol. 76:12691-12702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinangil, F., A. Loyter, and D. J. Volsky. 1988. Quantitative measurement of fusion between human immunodeficiency virus and cultured cells using membrane fluorescence dequenching. FEBS Lett. 239:88-92. [DOI] [PubMed] [Google Scholar]
- 40.Snitkovsky, S., T. M. Niederman, B. S. Carter, R. C. Mulligan, and J. A. Young. 2000. A TVA-single-chain antibody fusion protein mediates specific targeting of a subgroup A avian leukosis virus vector to cells expressing a tumor-specific form of epidermal growth factor receptor. J. Virol. 74:9540-9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Snitkovsky, S., T. M. Niederman, R. C. Mulligan, and J. A. Young. 2001. Targeting avian leukosis virus subgroup A vectors by using a TVA-VEGF bridge protein. J. Virol. 75:1571-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Snitkovsky, S., and J. A. Young. 1998. Cell-specific viral targeting mediated by a soluble retroviral receptor-ligand fusion protein. Proc. Natl. Acad. Sci. USA 95:7063-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Snitkovsky, S., and J. A. Young. 2002. Targeting retroviral vector infection to cells that express heregulin receptors using a TVA-heregulin bridge protein. Virology 292:150-155. [DOI] [PubMed] [Google Scholar]
- 44.Stein, B. S., S. D. Gowda, J. D. Lifson, R. C. Penhallow, K. G. Bensch, and E. G. Engleman. 1987. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell 49:659-668. [DOI] [PubMed] [Google Scholar]
- 45.White, J., K. Matlin, and A. Helenius. 1981. Cell fusion by Semliki Forest, influenza, and vesicular stomatitis viruses. J. Cell Biol. 89:674-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White, J. M., and I. A. Wilson. 1987. Anti-peptide antibodies detect steps in a protein conformational change: low-pH activation of the influenza virus hemagglutinin. J. Cell Biol. 105:2887-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wool-Lewis, R. J., and P. Bates. 1998. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 72:3155-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]