Abstract
Objective
Electrical stimulation of the vagus nerve at relatively high voltages (e.g., >10V) can induce bronchoconstriction. However, low voltage (≤2V) vagus nerve stimulation (VNS) can attenuate histamine-invoked bronchoconstriction. Here, we identify the mechanism for this inhibition.
Methods
In urethanea-nesthetized guinea pigs, bipolar electrodes were attached to both vagus nerves and changes in pulmonary inflation pressure were recorded in response to i.v. histamine and during VNS. The attenuation of the histamine response by low-voltage VNS was then examined in the presence of pharmacologic inhibitors or nerve ligation.
Results
Low-voltage VNS attenuated histamine-induced bronchoconstriction (4.4 ± 0.3 vs. 3.2 ± 0.2 cm H2O, p < 0.01) and remained effective following administration of a nitric oxide synthase inhibitor, NG-nitro-L-arginine methyl ester, and after sympathetic nerve depletion with guanethidine, but not after the β-adrenoceptor antagonist propranolol. Nerve ligation caudal to the electrodes did not block the inhibition but cephalic nerve ligation did. Low-voltage VNS increased circulating epinephrine and norepinephrine without but not with cephalic nerve ligation.
Conclusion
These results indicate that low-voltage VNS attenuates histamine-induced bronchoconstriction via activation of afferent nerves, resulting in a systemic increase in catecholamines likely arising from the adrenal medulla.
Keywords: Asthma, bronchoconstriction, catecholamine, guinea pig, vagus nerve stimulation
INTRODUCTION
Asthma is a complex disorder characterized by chronic inflammation, edema, and airway smooth muscle hyperresponsiveness that can be triggered by both allergic and nonallergic processes. However, despite improvements in treatments and in the understanding of its etiology, asthma continues to be a major health burden, affecting 8.5% of the population in the USA (1). Recently, vagus nerve stimulation (VNS) using a percutaneous device (Resolve RPS-1000 Stimulator and Proximity Electrode, ElectroCore LLC, Morris Plains, NJ, USA) was tested in patients with acute asthma exacerbations (ClinicalTrials.gov Identifier: NCT00762931) under an FDA Investigational Device Exemption. Although this phase 1 study was limited in size and scope, the use of percutaneous VNS was associated with respiratory improvements as measured by forced expiration volume in 1 sec and perceived dyspnea (2,3). Following the completion of this study, a new transcutaneous VNS device was developed for the treatment of bronchoconstriction that is approved for use outside the United States.
The rationale for these devices was based on our previous study that found low-voltage VNS reduced histamine-induced broncho-constrictive responses in guinea pigs and swine (4). Here, we elucidate the mechanism for this VNS-mediated reduction of histamine-induced bronchoconstriction in guinea pigs in vivo.
MATERIALS AND METHODS
Animals
Male Hartley guinea pigs (400–500 g) were acclimated to our facility for >2 weeks prior to use. They were housed in pairs at 22°C on a 12-hour light/dark cycle and provided food and water ad libitum. All animal experiments were performed under protocols approved by the Animal Care and Use Committee of Columbia University (New York, NY) and in accordance with standards established by the U.S. Animal Welfare Acts set forth in the National Institutes of Health guidelines. Animals were randomly selected to receive different treatments as defined below.
Procedure
This study utilized a well-characterized guinea pig model of bron-choconstriction and procedures previously described (4–6). Briefly, animals were anesthetized with intraperitoneal injections of ure-thane (1.5 ± 0.2 g/kg) which has a long duration (>8 hours) and is without respiratory nerve interference (7). Presurgical depth of anesthesia was monitored via toe pinch reflex and respiration rate, with additional anesthesia dosed (0.2 g/kg) until the animal was no longer responsive to stimulation. Body temperature was maintained at 37°C through the use of a homeothermic heating pad. A tracheostomy was performed using a 14G angiocatheter connected to a microventilator (Model 683, Harvard Apparatus Co., South Natick, MA, USA) to provide positive pressure, constant volume ventilation (62 stokes/min, tidal volume 2.5 cc). A side tap between the ventilator and the tracheal cannula enabled airway pressure recording using a pressure transducer and data acquisition system (TSD160C with model MP100A, Biopac Systems Inc., Goleta, CA, USA). Bilateral external jugular vein catheters, using PE-50 tubing, provided independent access for continuous infusion of a paralyzing agent, succinylcholine, and for histamine, acetylcholine, or pre-treatment dosing of inhibitors. Succinylcholine (1.25 mg/kg bolus then 5 mg/kg/hour) removed any confounding effects of chest wall and diaphragm muscle contractions on airway pressure measurements. Following muscle paralysis, hemodynamic changes (hypertension and tachycardia) were monitored to assure that the depth of anesthesia was sufficient. The left carotid artery was cannulated with PE-50 tubing for monitoring blood pressure and heart rate (HR) (Model TSD104A pressure transducer, Biopac Systems). Both cervical vagus nerves were isolated and vertically elevated above the surrounding tissue by applying slight upward traction to custom bipolar silver electrodes. This insured consistent electrical contact with the nerves while avoiding undesired electrical stimulation of surrounding structures. In some studies, both nerves were tightly ligated with 4-0 silk sutures but not transected, which ensured consistent contact pressure with the electrodes. Saline-soaked cotton was placed alongside the nerves to prevent drying. The electrodes were then connected to a Grass S88 stimulator (Grass Technologies, Quincy, MA, USA). Airway and blood pressure measurements were captured and analyzed using Acknowledge software, version 3.90 (Biopac Systems, Inc.). Following preparation, animals were allowed to stabilize for >20 min before testing commenced.
Treatments
Intravenous bolus histamine (4–16µg/kg) and acetylcholine (4–10µg/kg) were dosed using a syringe pump (Model 11 Plus, Harvard Apparatus Co., South Natick, MA, USA) and titrated to elicit a ~2–6 cm H2O increase in pulmonary inflation pressure (Ppi). At these doses, the bronchoconstriction responses were rapid and spontaneously resolved in less than 1 min. If airway pressure failed to completely return to baseline, the expiratory port of the ventilator was occluded for 3–4 breaths to re-expand atelactatic lungs and subsequent challenges delayed to guard against deep inspiration effects (8).
VNS
To evaluate the effect of low-voltage VNS on histamine-induced increases in Ppi, VNS (25 Hz, 0.2 msec pulse duration and 0.5–2 V) was applied starting 20 sec prior to the histamine challenge and continued for 10 sec past the peak response. In each animal a voltage was chosen that minimally influenced blood pressure but reduced histamine-induced increases in Ppi. In one group of animals, the duration of low-voltage VNS effectiveness was tested where the VNS stimulation was applied for 30 sec then discontinued for 10 sec prior to the histamine challenge. The ability of low-voltage VNS to attenuate histamine-induced increases in Ppi was also tested, with the vagal nerves ligated by silk sutures either cephalic or caudal to the electrodes. In some animals, electrical stimulation of both vagal nerves with a relatively high voltage (10 V) and brief pulse trains (25 Hz, 0.2 msec pulse duration for 7 sec) was used to confirm the classic parasympathetic responses including broncho-constriction and bradycardia.
To investigate the mechanism of action for low-voltage VNS effects on intravenous histamine-induced bronchoconstriction, atropine (1 mg/kg i.v.), NG-nitro-L-arginine methyl ester (L-NAME) (50 mg/kg i.v.), guanethidine (10 mg/kg i.v.), and propranolol (1 mg/kg i.v.) were administered as indicated. To examine the mechanism for the increase in blood pressure by low-voltage VNS, phentolamine (3 mg/kg i.v.) was administered as indicated.
Animals served as their own controls, with bronchoconstriction to histamine or acetylcholine compared with and without low-voltage VNS stimulation. The extent of pulmonary and hemodynamic changes following histamine challenge was determined using AcqKnowledge Software (Biopac Systems Inc.). To account for secretion-induced spike artifacts found on some tracings, all airway pressure waveforms were first filtered by replotting as maximum peak to peak waveforms followed by the application of a 1-sec smoothing algorithm. The Ppi response was then calculated as the difference between the baseline peak value over a 20-sec period preceding vs. the peak value during the histamine or acetylcholine response. To guard against adaptation, all VNS + histamine or VNS + acetylcholine challenges were preceded by and followed by control (no nerve stimulation) histamine or acetylcholine challenges. If these controls varied by >20%, the comparison was rejected. Multiple bracketed measurements were obtained per animal and averaged to yield a single value for that animal. This averaged single value from each animal was then compared between animals.
The blood pressure data were analyzed to generate a mean arterial blood pressure (MAP) waveform and HR waveform with each, including a 5-sec smoothing algorithm. The reported values represent the percent change from a 20-sec pre-dose baseline.
Catecholamine Levels
Blood samples were obtained from the carotid artery catheter at baseline and during low-voltage VNS, pre-histamine dosing, and immediately placed on ice. All samples were collected into 1 mM ethylenediaminetetraacetic acid, centrifuged for 15 min, 2000 × g at 4°C, and plasma was stored at −20°C until analyzed by a dual adrenaline/noradrenaline ELISA kit (Labor Diagnostika Nord GmbH & Co. KG, Nordhorn, Germany) according to the manufacturer’s instructions. In each animal, all pre-stimulation (baseline) sample results were averaged and all samples collected during stimulation were averaged. The mean value from each condition for each animal was then compared.
Chemicals
Urethane, histamine, acetylcholine, atropine, L-NAME, guanethi-dine, phentolamine, and propranolol were obtained from Sigma-Aldrich (St. Louis, MO, USA) and made up in 0.9% saline. Succinylcholine (Hospira, Lake Forest, IL, USA) was diluted to 1 mg/mL with 0.9% saline and continuously infused via a Medfusion 2010 microinfuser pump (Medex, Kensington, MD, USA).
Statistics
Results are reported as means ± standard errors and as percent change from baseline (pre histamine challenge). For significance, pairwise comparisons were performed using a two-tailed Student’s t distribution with 95% confidence intervals.
RESULTS
Model Characteristics
The application of low-voltage bilateral stimulation (≤2V, 25 Hz, 0.2 msec pulse width) to the vagus nerves did not increase resting pulmonary airway pressure. When the voltage was increased to 10 volts (25 Hz, 0.2 msec pulse width for 7 sec), stimulation induced the expected parasympathetic responses, including bronchoconstriction (airway pressure: 8.8 ± 0.2 vs. 15.8 ± 1.7 cm H2O, p < 0.01, N = 21, before VNS vs. during high-voltage VNS, respectively), hypotension (MAP: 47.6 ± 2.8 vs. 33.0 ± 1.7 mmHg, p < 0.01), and bradycardia (HR: 310 ± 6 vs. 138 ± 10 bpm, p < 0.01).
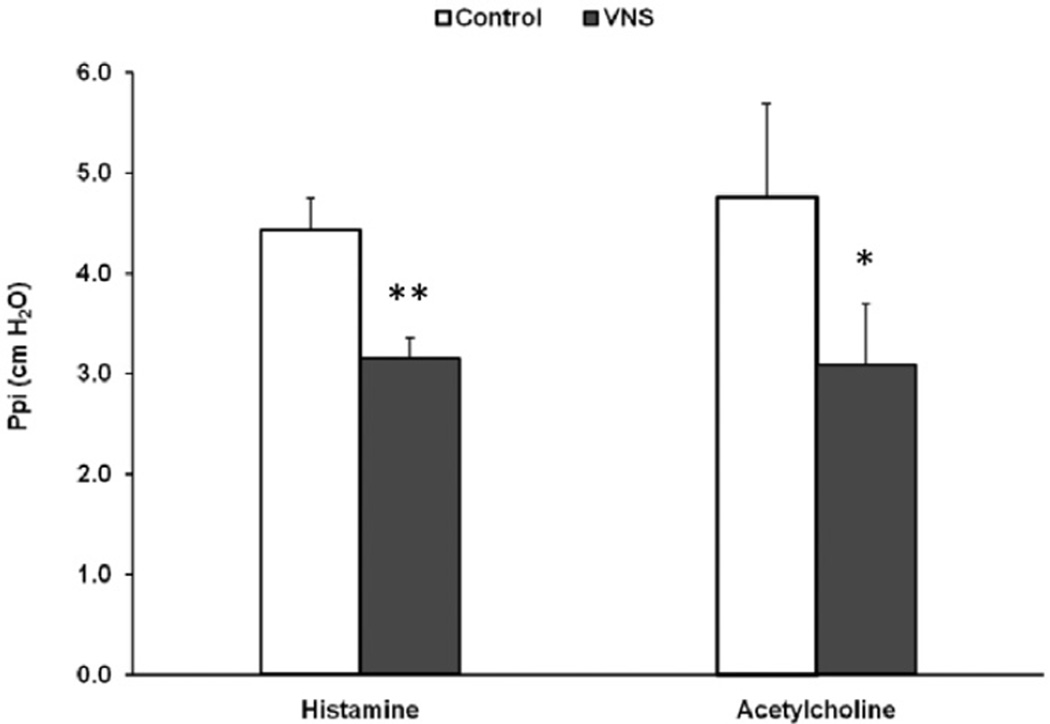
The low-voltage stimulation did not accentuate responses to histamine or acetylcholine. In contrast, when bronchoconstriction was induced using histamine, low-voltage VNS significantly reduced the Ppi increase (4.4 ± 0.3 vs. 3.2 ± 0.2 cm H2O, N = 26, p < 0.01) (Fig. 1). In a group of animals that were challenged with i.v. acetylcholine, low-voltage VNS also reduced the bronchoconstrictive response (4.8 ± 0.9 vs. 3.1 ± 0.6 cm H2O, N = 6, p < 0.05) (Fig. 1).
Figure 1.
Pulmonary inflation pressure (Ppi) was monitored during histamine (N = 26) or acetylcholine (N = 6) administration (control) and compared with the response when low-voltage vagus nerve stimulation (VNS) treatment was applied 20 sec before and during the administration of histamine or acetylcho-line (VNS). *p < 0.05 and **p < 0.01 compared with respective controls.
To determine the degree of contraction attributable to histamine inducing parasympathetic nerve acetylcholine release vs. histamine directly activating histamine receptors on airway smooth muscle, the muscarinic acetylcholine receptor antagonist, atropine, was administered at the conclusion of selected experiments. Atropine significantly reduced the bronchoconstriction response to histamine (4.5 ± 1.1 vs. 0.9 ± 0.2 cm H2O, N = 8, p < 0.01), indicating that the predominant mechanism of bronchoconstriction following i.v. histamine was via activation of parasympathetic nerves inducing the release of acetylcholine.
Pharmacologic Inhibitors
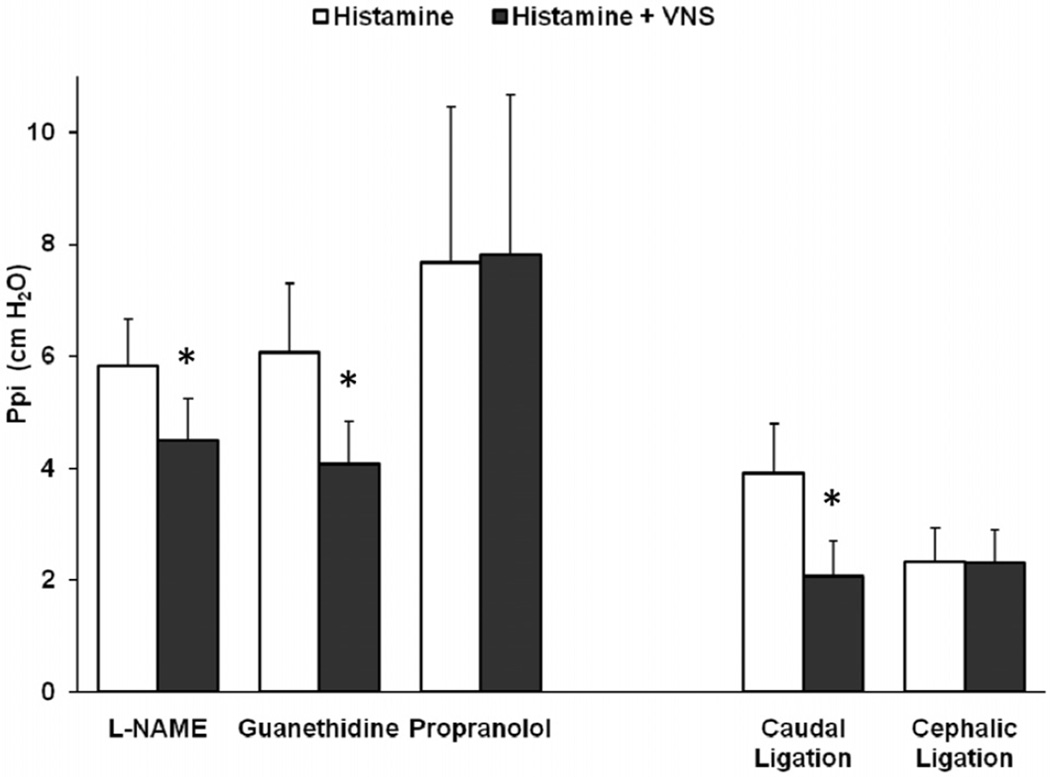
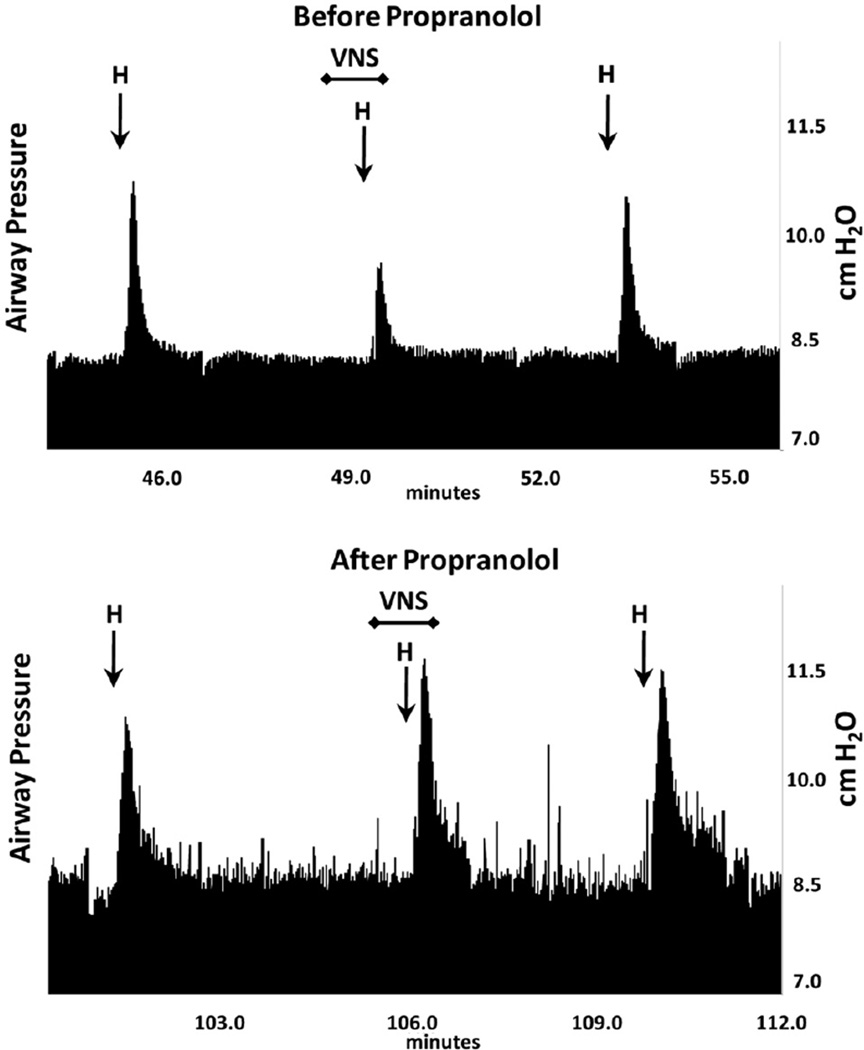
The nitric oxide synthase inhibitor, L-NAME, was administered in seven animals to deplete the inhibitory non-adrenergic non-cholinergic iNANC nerves of nitric oxide to determine their role in the VNS reduction of the histamine response. In these studies, L-NAME significantly increased baseline blood pressure (49 ± 3 vs. 101 ± 9 mmHg, p < 0.01) and the airway responsiveness to histamine (3.3 ± 0.4 vs. 5.8 ± 0.8 cm H2O, p < 0.01), as similarly reported by others (9,10). However, L-NAME did not block the ability of low-voltage VNS to attenuate bronchoconstriction (5.8 ± 0.8 vs. 4.5 ± 0.7 cm H2O, p < 0.05) (Fig. 2). To verify that the low-voltage VNS response was not through sympathetic nerves, guanethidine was used to inhibit norepinephrine release from presynaptic terminals and adequate dosing confirmed through observed sustained dramatic decreases in blood pressure. Guanethidine pretreatment did not prevent VNS from attenuating histamine-induced bronchoconstriction (6.1 ± 1.2 vs. 4.1 ± 0.8 cm H2O, N = 6, p < 0.05) (Fig. 2). Guanethidine induced a dramatic and sustained decrease in blood pressure, indicating effective blockade of sympathetic nerves. The contribution of β-adrenoceptors on airway smooth muscle to the VNS attenuation of histamine-induced bronchoconstriction was examined using the nonselective β antagonist, propranolol. Propranolol pretreatment increased the Ppi response to histamine (4.2 ±1.3 vs. 10.1 ± 2.4 cm H2O, N = 6, p < 0.05) as others have also reported (11). Subsequent i.v. histamine doses were reduced to compensate for this elevated response of Ppi to histamine before testing the effect of low-voltage VNS in the presence of propranolol. After propranolol treatment, low-voltage VNS was no longer effective in attenuating the histamine bronchoconstriction (7.7 ± 2.8 vs. 7.8 ± 2.9 cm H2O, N = 6, not significant [NS]) (Figs. 2 and 3).
Figure 2.
Following treatment with chemical inhibitors or ligation of the vagus nerve, the pulmonary inflation pressure (Ppi) response to histamine was compared without and during low-voltage vagus nerve stimulation (VNS). NG-nitro-L-arginine methyl ester (L-NAME), N = 7, Guanethidine, N = 6, Propranolol, N = 6, Caudal Ligation, N = 7, Cephalic Ligation, N = 3. *p < 0.05.
Figure 3.
Representative traces of the airway pressure responses to i.v. histamine alone (H) and during low-voltage vagus nerve stimulation (VNS). Upper trace demonstrates responses before propranolol was administered. Bottom trace shows the responses in the presence of propranolol.
Low-voltage VNS can increase blood pressure (3), and for most studies we titrated VNS to a level that reduced histamine bronchoconstriction while minimizing blood pressure elevation. However, to examine pressor effects more closely, in selected studies we used voltages that increased MAP and then compared the response of blood pressure with low-voltage VNS in the presence of phentola-mine. With phentolamine pretreatment, the low-voltage VNS-induced increase in MAP was eliminated (20.8 ± 2.1% [VNS] vs. 1.8 ± 0.5% [VNS after phentolamine pretreatment], N = 3, p < 0.01).
Vagus Nerve Ligation
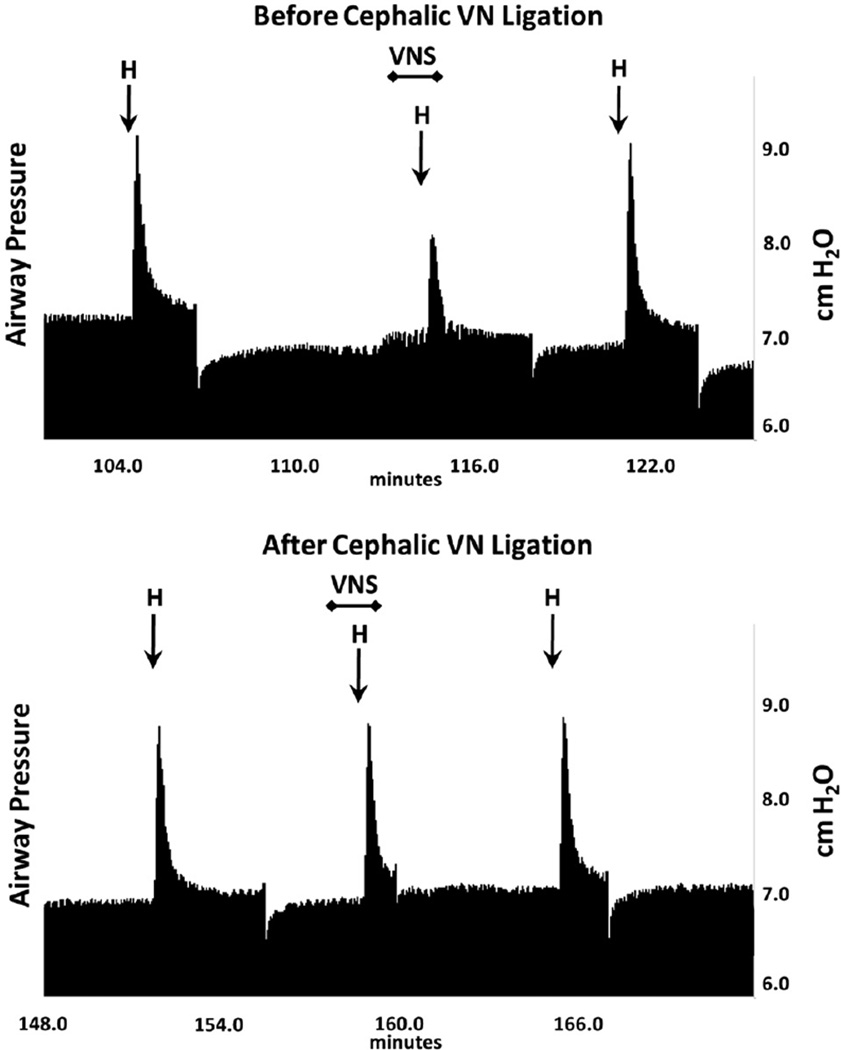
Bilateral vagus nerve ligation was used to identify the neural pathways governing the VNS response. When nerve ligation was caudal to the electrodes, low-voltage VNS reduced histamine-induced airway bronchoconstriction (3.9 ± 0.9 vs. 2.1 ± 0.6 cm H2O, N = 7, p <0.05) (Fig. 2). In contrast, when nerve ligation was cephalic to the electrode, low-voltage VNS did not reduce the histamine response (2.3 ± 0.6 vs. 2.3 ± 0.6 cm H2O, N = 3, NS) (Figs. 2 and 4), indicating that an afferent nerve signal traveling toward the central nervous system was mediating the low-voltage VNS attenuation of histamine-induced bronchoconstriction.
Figure 4.
Representative traces of the airway pressure responses to i.v. histamine (H) alone and during vagus nerve stimulation (VNS). Upper trace shows responses prior to placement of a ligature around the vagus nerve. Bottom trace shows i.v. histamine responses without and during low voltage VNS after the vagus nerve was ligated cephalic to the electrodes.
Low-Voltage VNS Bronchoprotection after Discontinuation of VNS
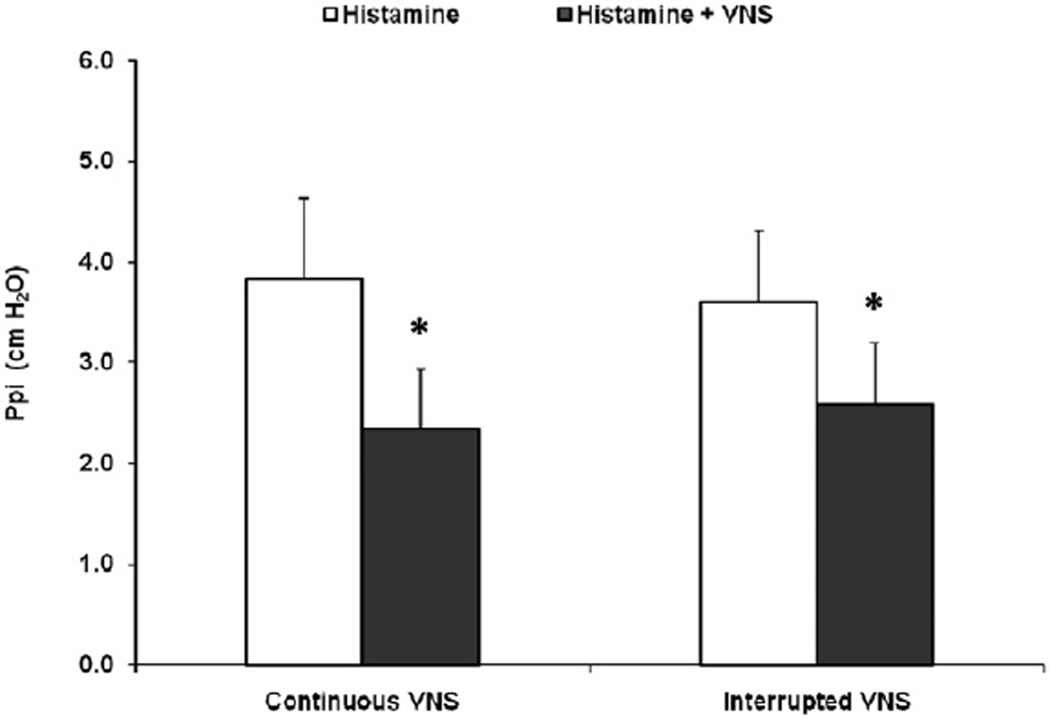
In four animals, low-voltage VNS was applied for 30 sec then discontinued for 10 sec prior to the injection of histamine and was compared with leaving the VNS on for 20 sec before and through the histamine injection. Low-voltage VNS remained effective at inhibiting bronchoconstriction when the VNS signal was interrupted 10 sec prior to i.v. histamine (3.6 ± 0.7 vs. 2.6 ± 0.6 cm H2O, p < 0.05), and was similar to applying VNS continuously during i.v. histamine (3.8 ± 0.8 vs. 2.3 ± 0.6 cm H2O, p < 0.05) (histamine vs. histamine with VNS, respectively) (Fig. 5).
Figure 5.
Pulmonary inflation pressure (Ppi) was monitored in five animals and the responses to i.v. histamine and to i.v. histamine plus a low-voltage vagus nerve stimulation (VNS) treatment compared. In the comparisons on the left, VNS was applied for 20 sec before and continued through the administration of i.v. histamine (Continuous VNS). In the comparisons on the right, the low-voltage VNS treatment was applied for 30 sec and then discontinued for 10 sec prior to administration of i.v. histamine (Interrupted VNS). *p < 0.05.
Catecholamine Analysis
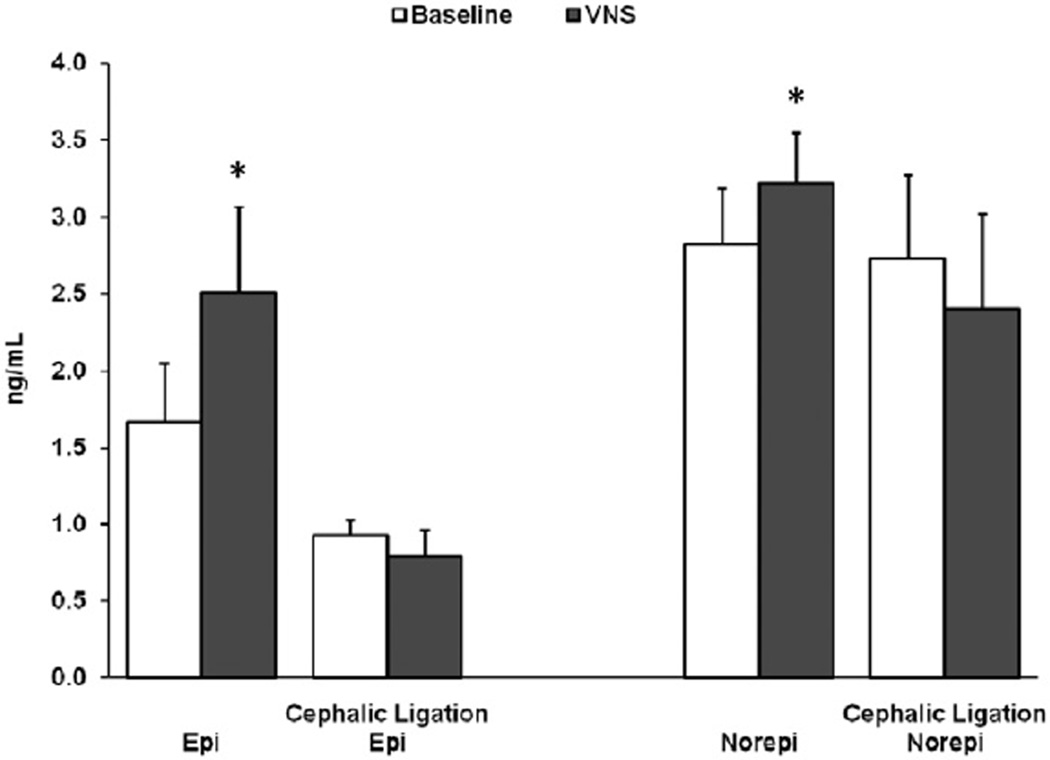
Low-voltage VNS increased plasma epinephrine levels above baseline (1.67 ± 0.38 vs. 2.51 ± 0.56 ng/mL, N = 4, p < 0.05) and norepinephrine levels above baseline (2.82 ± 0.37 vs. 3.22 ± 0.33 ng/mL, N = 4, p < 0.05). In three animals following cephalic nerve ligation, low-voltage VNS no longer increased catecholamine levels (epinephrine: 0.93 ± 0.10 vs. 0.79 ± 0.17 ng/mL, NS, and norepinephrine: 2.73 ± 0.55 vs. 2.40 ± 0.62 ng/mL, NS) (Fig. 6).
Figure 6.
Arterial epinephrine (Epi) and norepinephrine (Noepi) levels were measured at baseline and during vagus nerve stimulation (VNS), N = 4. Three of these animals underwent ligation of the vagus nerve cephalic to the electrodes. Following ligation, epinephrine (Cephalic Ligation Epi) and norepinephrine (Cephalic Ligation Norepi) levels were remeasured at baseline and during VNS. *p < 0.05.
DISCUSSION
The primary findings of the current study are that low-voltage nerve stimulation of guinea pig cervical vagus nerves attenuates histamine-induced increases in Ppi and that this attenuation required an intact afferent (but not efferent) vagus nerve. Secondly, the low-voltage VNS attenuation of the histamine-induced bronchoconstriction could be blocked by systemic pretreatment with a β-adrenoceptor antagonist. In contrast, pretreatment with a nitric oxide synthase inhibitor or depletion of sympathetic nerve terminals did not interfere with low-voltage VNS attenuation of histamine-induced increases in Ppi. Additionally, low-voltage VNS was able to attenuate acetylcholine-induced increases in Ppi. These findings confirm our previous report and identify a likely mechanism to account for this protective effect of low-voltage VNS on bronchoconstriction in our animal studies and in early human clinical trials.
Classically, stimulation of the vagus nerve with relatively high voltages (10–25 V) results in direct bronchoconstriction due to the release of acetylcholine from efferent parasympathetic nerves traveling in the cervical vagus nerve to the lung (5,6,12,13). Additionally, this high-voltage stimulation induces bradycardia (5,14). However, we have demonstrated that lower voltages attenuated histamine-induced bronchoconstriction without reaching the threshold for stimulating efferent parasympathetic lung and heart responses.
We tested whether the attenuation of histamine-induced increases in Ppi was mediated by efferent stimulation of sympathetic nerve fibers traveling within the cervical vagus nerve or by indirect stimulation of iNANC nerves in the guinea pig lung. Our results rule out both of these possibilities since ligation of the vagal nerve caudal to the electrodes did not eliminate the bronchopro-tection afforded by low-voltage VNS. Moreover, pretreatment with guanethidine or L-NAME to block norepinephrine or nitric oxide release from sympathetic or iNANC nerves, respectively, did not block the bronchoprotection afforded by low-voltage VNS.
Intravenously administered histamine binds to H1 receptors on the vagus nerve, inducing the release of acetylcholine, which binds to muscarinic receptors on airway smooth muscle causing constriction (15). Additionally, histamine binds directly to hista-mine receptors on airway smooth muscle cells, resulting in constriction. In the present study, atropine pretreatment blocked the vagal nerve contribution to the bronchoconstriction (by antagonizing the effect of acetylcholine at the airway smooth muscle), revealing that the direct airway smooth muscle response to hista-mine comprised ~20% of the contractile response. Direct bron-choconstriction following activation of airway smooth muscle muscarinic receptors with i.v. acetylcholine was also attenuated by low-voltage VNS. This suggests that the mechanism of bronchop-rotection afforded by low-voltage VNS did not involve efferent parasympathetic fibers in the vagus nerve but was by an alternative neural pathway.
The histamine-induced bronchoconstriction in this study had a rapid onset and recovery such that each airway and blood pressure response was completed in less than 1 min. In preliminary tests, we observed that low-voltageVNS was more effective if it preceded the histamine challenge than if administered simultaneously; therefore, we used a 20-sec pretreatment in our mechanistic investigations. In addition, we found in selected studies that low-voltage VNS remained effective even if discontinued 10 sec prior to the hista-mine challenge. This prolonged effectiveness is contrary to normal fast neural responses exhibited by sympathetic and parasympa-thetic nerves. However, it is consistent with the characteristics of iNANC nerves, which have a slow response with long duration (11,16–18).
iNANC nerves release nitric oxide and are capable of modulating both tracheal (19–21) and bronchial (17,22) smooth muscle tone. The synthesis of nitric oxide and its modulation of airway tone can be inhibited by L-NAME (9,21,22). However, in our study, VNS remained capable of attenuating bronchoconstriction in the presence of L-NAME. Since we had concerns with the ability of reagents to fully block other iNANC neurotransmitters such as vasoactive intestinal peptide, we performed vagal nerve ligation caudal to the electrodes to interrupt all efferent iNANC control of airway responses. Despite ligation, low-voltage VNS remained effective in attenuating bronchoconstriction.
In order to demonstrate iNANC nerve function, it is necessary to first isolate the response using cholinergic inhibitors, adrenergic inhibitors, and, frequently, beta antagonists prior to eliciting iNANC responses (16,23–25). These inhibitors were not necessary to demonstrate the inhibition of the histamine response by VNS in this study. Indeed, it was the failure of propranolol to attenuate VNS-induced relaxation of guinea pig tracheal pouch pressure that led Chesrown et al. (26) to conclude that their mechanism was iNANC nerves. In our study, propranolol did attenuate the low-voltage VNS reduction of bronchoconstriction. Thus, while the contribution of iNANC nerves might not have been completely eliminated, they were not the primary pathway responsible for the low-voltage VNS inhibition.
Another possible mechanism for the reduction of the histamine response during low-voltage VNS could be the release of norepi-nephrine from the sympathetically innervated pulmonary vascula-ture (27–29), with subsequent norepinephrine binding to β2-adrenoreceptors on airway smooth muscle (30). This norepineph-rine outflow can also bind to α1 adrenergic receptors on vascular smooth muscle to increase blood pressure. When we administered the α1 antagonist, phentolamine, the blood pressure response from low-voltage VNS was eliminated. However, when we administered guanethidine to deplete sympathetic nerves but not interfere with catecholamine release from the adrenal glands (31–33), low-voltage VNS remained effective at attenuating histamine-induced increases in Ppi. In addition, vagus nerve ligation between the electrode and the pulmonary vasculature would interrupt this nerve pathway and norepinephrine’s release yet the VNS bronchoprotection remained. Thus, it is unlikely that this efferent sympathetic pathway was responsible for the low-voltage VNS effect.
In contrast to efferent vagus nerve ligation, ligation cephalic to the electrode blocked afferent nerve transmission and eliminated the ability of low-voltage VNS to attenuate histamine-induced increases in Ppi. Furthermore, we noticed that cephalic but not caudal ligation interfered with the ability of low-voltage VNS to increase blood pressure. The adrenal medulla is a source for both circulating norepinephrine and epinephrine and is innervated by preganglionic sympathetic splanchnic nerves. Afferent VNS has been shown to reach the hypothalamus and activate the hypothalamic-pituitary-adrenal axis (34), and direct electrical stimulation of the hypothalamus can induce the adrenal medulla to release epinephrine (35). Thus, in the present study, afferent low-voltage VNS stimulation may have led to efferent stimulation of the adrenal medulla and the release of circulating catecholamines, which could explain the effects on airway and blood pressure responses.
The peak measured serum concentration of epinephrine in the present study was 2.35 ng/mL, equating to a concentration of approximately 18 nM. This concentration is within the reported Kd of epinephrine for the β2-adrenoceptor in some studies (23.9 nM) (36) but falls well below the reported Kd (200 nM) for epinephrine binding at the β-adrenoceptor in other studies (37). However, significant bronchodilation has been reported in humans at a serum concentration of epinephrine similar to that reported in the present study (38). Additionally, pharmacologic receptor-effector studies have suggested the presence of “spare β-adrenoceptors receptors” (39) and the concept that with excess receptors present, ligand occupancy of only a small fraction of the receptor pool is necessary for full activation of the downstream cellular signaling pathway. Although spare β-adrenoceptors have been reported in airway smooth muscle (40,41), overexpression studies suggest the opposite (42): that the quantity of β-adrenoceptors present in airway smooth muscle is the rate-limiting step in activation of the β-adrenoceptor-Gs-adenylyl cyclase signaling pathway. Additional molecular explanations for this apparent discrepancy is a concept of a rapid off rate in the kinetics of epinephrine binding such that a single molecule of ligand can rapidly and sequentially activate neighboring receptors, amplifying the signaling event, or that a single activated receptor can activate multiple molecules of the Gs protein, again leading to amplification of the signaling event (43).
Parasympathetic pulmonary nerves are normally tonically active and trigger smooth muscle contractions in response to ongoing afferent nerve activity (20,44), while circulating catecholamines have been reported to provide tonal balance (38,45–47). Using adrenalectomy techniques, several investigators (30,48,49) linked these circulating catecholamines to adrenal release and binding to β2-adrenoceptors to reverse bronchoconstriction. Other studies have indicated a possible impaired sympathoadrenal response to exercise in asthmatics (50,51). Both epinephrine and norepineph-rine bind to β2-adrenergic receptors, and blocking these receptors with propranolol can induce bronchoconstriction in asthmatics. We found that the bronchoconstriction response to histamine was augmented by propranolol, as did others (11,30,52), while Matsu-moto (53) reported a similar finding for acetylcholine. The finding that the VNS bronchoprotection was eliminated by propranolol but not guanethidine suggests circulating catecholamines as the common modality in this and previous studies. This would also explain the temporal nature of the low-voltage VNS response that required VNS treatment to commence several seconds prior to the histamine challenge but which remained effective at reducing the histamine response even after terminating stimulation.
In an isolated lung perfusion model, it was found that during sympathetic electric field stimulation, norepinephrine levels were increased while epinephrine levels remained below detection (29). This is in agreement with the general view that the adrenal medulla is the primary source of circulating epinephrine. Since we measured an increase in plasma epinephrine levels with stimulation, it suggests that we were indeed triggering an efferent sympathetic stimulation of adrenal chromaffin cells. Furthermore, the adrenal gland secretes proportionally more epinephrine than norepinephrine. In this study, we found epinephrine made up 68.0% of the increase in catecholamines.
There are several limitations to our study. The increases in cat-echolamine concentrations we reported were modest, but this was in keeping with the study design. The guinea pig model of bron-choconstriction used here utilized urethane anesthesia, which is known to accentuate blood pressure elevations from stimulation (54). In addition, increases in VNS voltage could negatively affect the HR and blood pressure. We therefore adjusted stimulation to minimize these effects. Although the caudal ligation technique would minimize the neuronal chronotropic and inotropic cardiac contribution, it would not interfere with the afferent mediated central nervous system responses leading to adrenal medulla stimulation. It is possible that higher voltages could increase the secretion of circulating catecholamines and have greater utility for treating bron-choconstriction without cardiovascular implications.
In a study of conscious individuals with an implanted vagus nerve stimulator for treating epilepsy, a test with increasing levels of stimulation was not associated with increases in blood pressure and HR due to the natural ability of the body to compensate (55). In two studies using low-dose infusions of epinephrine (38,45), the infusions produced dose-related bronchodilation in asthmatic patients prior to any changes in blood pressure or HR. However, norepinephrine was not effective (45). Therefore, the increase in epinephrine levels from low-voltage VNS that we found may have utility for treating asthma without concurrent changes in blood pressure or HR.
CONCLUSION
This study has confirmed that low-voltage vagal nerve stimulation can attenuate histamine-induced bronchoconstriction in guinea pigs. The primary mechanism for this effect is not through blockade of efferent vagally induced bronchoconstriction, nor iNANC nerves, nor norepinephrine outflow from sympathetic innervated pulmonary vasculature. Rather, this study suggests that the response is through an afferent nerve pathway leading to efferent stimulation of chromaffin cells in the adrenal medulla and the release of cat-echolamines into the systemic circulation. These catecholamines, especially epinephrine, bind to β2-adrenergic receptors to relax airway smooth muscle. The induced release of low levels of epi-nephrine by VNS would be in a manner that is tolerable and without local complications associated with epinephrine infusions. This mechanism may explain the improvements in FEV1 and the perception of dyspnea that were associated with percutaneous VNS in asthma patients that had been reported (3), but this must be confirmed in future studies.
Acknowledgments
Source(s) of financial support: This study was funded by ElectroCore LLC.
Footnotes
Conflicts of Interest: Thomas Hoffmann and Bruce Simon are employees of ElectroCore LLC. Charles Emala’s laboratory received grant support.
Disclaimers: None.
Authorship Statements
All authors contributed to the design, conduction, and interpretation of this study. Each has reviewed and approved the version to be published.
REFERENCES
- 1.Trends in asthma morbidity and mortality. New York, NY: American Lung Association; 2011. 4-1-2011. http://lungusa.org/finding-cures/our-research/trend-reports/asthma-trend-report.pdf. [Google Scholar]
- 2.Sepulveda P, Bohill G, Hoffmann T. Treatment of asthmatic bronchoconstriction by percutaneous low voltage vagal nerve stimulation: case report. Internet J Asthma Allergy Immunol. 2009;7:2. [Google Scholar]
- 3.Miner J, Lewis L, Mosnaim G, Varon J, Theodoro D, Hoffmann T. Percutaneous vagal nerve stimulation for the treatment of acute asthma exacerbations. Acad Emerg Med. 2012;19:421–429. doi: 10.1111/j.1553-2712.2012.01329.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann TJ, Mendez S, Staats P, Emala CW, Guo P. Inhibition of histamine-induced bronchoconstriction in guinea pig and swine by pulsed electrical vagus nerve stimulation. Neuromodulation. 2009;12:261–269. doi: 10.1111/j.1525-1403.2009.00234.x. [DOI] [PubMed] [Google Scholar]
- 5.Jooste E, Zhang Y, Emala CW. Neuromuscular blocking agents’ differential bron-choconstrictive potential in guinea pig airways. Anesthesiology. 2007;106:763–772. doi: 10.1097/01.anes.0000264763.48920.c9. [DOI] [PubMed] [Google Scholar]
- 6.Gleason NR, Gallos G, Zhang Y, Emala CW. The GABAA agonist muscimol attenuates induced airway constriction in guinea pigs in vivo. J Appl Physiol. 2009;106:1257–1263. doi: 10.1152/japplphysiol.91314.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg R, Antonaccio MJ, Steinbacher T. Thromboxane A2 mediated broncho-constriction in the anesthetized guinea pig. Eur J Pharmacol. 1982;80:19. doi: 10.1016/0014-2999(82)90173-x. [DOI] [PubMed] [Google Scholar]
- 8.Gunst SJ, Shen X, Ramchandani R, Tepper RS. Bronchoprotective and bronchodila-tory effects of deep inspiration in rabbits subjected to bronchial challenge. J Appl Physiol. 2001;91:2511–2516. doi: 10.1152/jappl.2001.91.6.2511. [DOI] [PubMed] [Google Scholar]
- 9.Lei Y-H, Barnes PJ, Rogers D. Regulation of NANC neural bronchoconstriction in vivo in the guinea-pig: involvement of nitric oxide, vasoactive intestinal peptide and soluble guanylyl cyclase. Br J Pharmacol. 1993;108:228–235. doi: 10.1111/j.1476-5381.1993.tb13467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto S, Takeda M, Saiki C, Takahashi T, Ojima K. Effects of tachykinins on rapidly adapting pulmonary stretch receptors and total lung resistance in anesthetized, artificially ventilated rabbits. J Pharmacol Exp Ther. 1997;283:1026–1031. [PubMed] [Google Scholar]
- 11.Canning BJ, Reynolds SM, Mazzone SB. Multiple mechanisms of reflex broncho-spasm in guinea pigs. J Appl Physiol. 2001;91:2642–2653. doi: 10.1152/jappl.2001.91.6.2642. [DOI] [PubMed] [Google Scholar]
- 12.Blaber LC, Fryer AD, Maclagan J. Neuronal muscarinic receptors attenuate vagally-induced contraction of feline bronchial smooth muscle. Br J Pharmacol. 1985;86:723–728. doi: 10.1111/j.1476-5381.1985.tb08951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichinose M, Inoue H, Miura M, Yafuso N, Nogami H, Takishima T. Possible sensory receptor of nonadrenergic inhibitory nervous system. J Appl Physiol. 1987;63:923–929. doi: 10.1152/jappl.1987.63.3.923. [DOI] [PubMed] [Google Scholar]
- 14.Verbout NG, Jacoby DB, Gleich GJ, Fryer AD. Atropine-enhanced, antigen challenge-induced airway hyperreactivity in guinea pigs is mediated by eosinophils and nerve growth factor. Am J Physiol Lung Cell Mol Physiol. 2009;297:L228–L237. doi: 10.1152/ajplung.90540.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aizawa H, Inoue H, Miyazaki N, Hara N. Histamine-induced increase in isometric tension of smooth muscle is mediated by local vagus nerve in human bronchus. Respiration. 2000;67:652–656. doi: 10.1159/000056295. [DOI] [PubMed] [Google Scholar]
- 16.Diamond L, O’Donnell M. A nonadrenergic vagal inhibitory pathway to feline airways. Science. 1980;208:185–188. doi: 10.1126/science.7361114. [DOI] [PubMed] [Google Scholar]
- 17.Aizawa H, Tanaka H, Sakai J, Takata S, Hara N, Ito Y. L-NAME-sensitive and -insensitive nonadrenergic noncholinergic relaxation of cat airway in vivo and in vitro. Eur Respir J. 1997;10:314–321. doi: 10.1183/09031936.97.10020314. [DOI] [PubMed] [Google Scholar]
- 18.Krishnakumar S, Holmes EP, Moore RM, Kappel L, Venugopal CS. Non-adrenergic non-cholinergic excitatory innervation in the airways: role of neurokinin-2 receptors. Auton Autacoid Pharmacol. 2002;22:215–224. doi: 10.1046/j.1474-8673.2002.00262.x. [DOI] [PubMed] [Google Scholar]
- 19.Fischer A, Mayer B, Kummer W. Nitric oxide synthase in vagal sensory and sympathetic neurons innervating the guinea-pig trachea. J Auton Nerv Syst. 1996;56:157–160. doi: 10.1016/0165-1838(95)00085-2. [DOI] [PubMed] [Google Scholar]
- 20.Kesler BS, Mazzone SB, Canning BJ. Nitric oxide-dependent modulation of smooth-muscle tone by airway parasympathetic nerves. Am J Respir Crit Care Med. 2002;165:481–488. doi: 10.1164/ajrccm.165.4.2004005. [DOI] [PubMed] [Google Scholar]
- 21.Li CG, Rand MJ. Evidence that part of the NANC relaxant response of guinea-pig trachea to electrical field stimulation is mediated by nitric oxide. Br J Pharmacol. 1991;102:91–94. doi: 10.1111/j.1476-5381.1991.tb12137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis JL, Undem BJ. Inhibition by L-N6-nitro-l-arginine of nonadrenergic-noncholinergic-mediated relaxations of human isolated central and peripheral airways. Am Rev Respir Dis. 1992;146:1543–1547. doi: 10.1164/ajrccm/146.6.1543. [DOI] [PubMed] [Google Scholar]
- 23.Coleman RA, Levy GP. A nonadrenergic inhibitory nervous pathway in guinea pig trachea. Br J Pharmacol. 1974;52:167. doi: 10.1111/j.1476-5381.1974.tb09697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson J, Beland J. Nonadrenergic inhibitory nervous system in human airways. J Appl Physiol. 1976;41:764. doi: 10.1152/jappl.1976.41.5.764. [DOI] [PubMed] [Google Scholar]
- 25.Richardson JB, Bouchard T. Demonstration of a nonadrenergic inhibitory nervous system in the trachea of the guinea pig. J Allergy Clin Immunol. 1975;56:473. doi: 10.1016/0091-6749(75)90065-2. [DOI] [PubMed] [Google Scholar]
- 26.Chesrown SE, Venugopalan CS, Gold WM, Drazen JM. In vivo demonstration of nonadrenergic inhibitory innervation of the guinea pig trachea. J Clin Invest. 1980;65:314–320. doi: 10.1172/JCI109674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ainsworth GA, Garland LG, Payne AN. Modulation of bronchoconstrictor responses to histamine in pithed guinea pigs by sympathetic nerve stimulation. Br J Pharmacol. 1982;77:249. doi: 10.1111/j.1476-5381.1982.tb09293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Racke K, Bahring A, Brunn G, Elsner M, Wessler I. Characterization of endogenous noradrenaline release from intact and epithelium-denuded rat isolated trachea. Br J Pharmacol. 1991;103:1213–1217. doi: 10.1111/j.1476-5381.1991.tb12326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamane K, Kawata M. Catecholamine release from isolated guinea pig lungs during sympathetic stimulation with varied ventilation and perfusion. Exp Anim. 1999;48:65–72. doi: 10.1538/expanim.48.65. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi Y, Ohno H, Misawa M. Beta 2- but not beta 1-adrenoceptors mediate the adrenergic component of reflex tracheal dilatation during bronchoconstriction in guinea pigs in vivo. Res Commun Mol Pathol Pharmacol. 1996;93:301–318. [PubMed] [Google Scholar]
- 31.Wakade AR, Malhotra RK, Wakade TD, Dixon WR. Simultaneous secretion of cat-echolamines from the adrenal medulla and of [3H]norepinephrine from sympathetic nerves from a single test preparation: different effects of agents on the secretion. Neuroscience. 1986;18:877–888. doi: 10.1016/0306-4522(86)90106-5. [DOI] [PubMed] [Google Scholar]
- 32.Grewal RS, Kaul CL. Mechanism of the antagonism of the hypotensive action of guanethidine by propranolol. Br J Pharmacol. 1970;38:771–775. doi: 10.1111/j.1476-5381.1970.tb09886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKechnie K, Dean HG, Furman BL, Parratt JR. Plasma catecholamines during endo-toxin infusion in conscious unrestrained rats: effects of adrenal demedullation and/or guanethidine treatment. Circ Shock. 1985;17:85–94. [PubMed] [Google Scholar]
- 34.Hosoi T, Okuma Y, Nomura Y. Electrical stimulation of afferent vagus nerve induces IL-1beta expression in the brain and activates HPA axis. Am J Physiol Regul Integr Comp Physiol. 2000;279:R141–R147. doi: 10.1152/ajpregu.2000.279.1.R141. [DOI] [PubMed] [Google Scholar]
- 35.Tsuchimochi H, Nakamoto T, Matsukawa K. Centrally evoked increase in adrenal sympathetic outflow elicits immediate secretion of adrenaline in anaesthetized rats. Exp Physiol. 2010;95:93–106. doi: 10.1113/expphysiol.2009.048553. [DOI] [PubMed] [Google Scholar]
- 36.U’Prichard DC, Bylund DB, Snyder SH. (+/−)-[3H]Epinephrine and (−)[3H]dihydroalprenolol binding to beta1- and beta2-noradrenergic receptors in brain, heart, and lung membranes. J Biol Chem. 1978;253:5090–5102. [PubMed] [Google Scholar]
- 37.Maguire ME, Wiklund RA, Anderson HJ, Gilman AG. Binding of (125I)iodohydroxy-benzylpindolol to putative beta-adrenergic receptors of rat glioma cells and other cell clones. J Biol Chem. 1976;251:1221–1231. [PubMed] [Google Scholar]
- 38.Knox AJ, Campos-Gongora H, Wisniewski A, MacDonald IA, Tattersfield AE. Modification of bronchial reactivity by physiological concentrations of plasma epine-phrine. J Appl Physiol. 1992;73:1004–1007. doi: 10.1152/jappl.1992.73.3.1004. [DOI] [PubMed] [Google Scholar]
- 39.Stephenson RP. A modification of receptor theory. Br J Pharmacol Chemother. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avner BP, Wilson S. Possible existence of “spare” beta-receptors in rat tracheal smooth muscle. Proc West Pharmacol Soc. 1979;22:177–181. [PubMed] [Google Scholar]
- 41.Lemoine H, Overlack C. Highly potent beta-2 sympathomimetics convert to less potent partial agonists as relaxants of guinea pig tracheae maximally contracted by carbachol. Comparison of relaxation with receptor binding and adenylate cyclase stimulation. J Pharmacol Exp Ther. 1992;261:258–270. [PubMed] [Google Scholar]
- 42.McGraw DW, Forbes SL, Kramer LA, et al. Transgenic overexpression of beta(2)-adrenergic receptors in airway smooth muscle alters myocyte function and ablates bronchial hyperreactivity. J Biol Chem. 1999;274:32241–32247. doi: 10.1074/jbc.274.45.32241. [DOI] [PubMed] [Google Scholar]
- 43.Stickle D, Barber R. Evidence for the role of epinephrine binding frequency in activation of adenylate cyclase. Mol Pharmacol. 1989;36:437–445. [PubMed] [Google Scholar]
- 44.Jammes Y, Mei N. Assessment of the pulmonary origin of bronchoconstrictor vagal tone. J Physiol. 1979;291:305–316. doi: 10.1113/jphysiol.1979.sp012814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berkin KE, Inglis GC, Ball SG, Thomson NC. Effect of low dose adrenaline and nora-drenaline infusions on airway calibre in asthmatic patients. Clin Sci. 1986;70:347–352. doi: 10.1042/cs0700347. [DOI] [PubMed] [Google Scholar]
- 46.Ind PW, Causon RC, Brown MJ, Barnes PJ. Circulating catecholamines in acute asthma. Br Med J (Clin Res Ed) 1985;290:267–269. doi: 10.1136/bmj.290.6464.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warren JB, Dalton N, Turner C, Clark TJ. Protective effect of circulating epinephrine within the physiologic range on the airway response to inhaled histamine in non-asthmatic subjects. J Allergy Clin Immunol. 1984;74:683–686. doi: 10.1016/0091-6749(84)90230-6. [DOI] [PubMed] [Google Scholar]
- 48.Underwood DC, Matthews JK, Osborn RR, Novak LB, Bochnowicz S, Meunier LD. Catecholamine and beta-adrenoceptor influences on airway reactivity to antigen in guinea pigs. Int Arch Allergy Immunol. 1996;109:286–294. doi: 10.1159/000237251. [DOI] [PubMed] [Google Scholar]
- 49.Ikezono K, Kamata M, Mori T. Adrenal influences on the inhibitory effects of pro-caterol, a selective beta-two-adrenoceptor agonist, on antigen-induced airway microvascular leakage and bronchoconstriction in guinea pigs. Pharmacology. 2005;73:209–215. doi: 10.1159/000083299. [DOI] [PubMed] [Google Scholar]
- 50.Tsuda H, Tsuda A, Ito M, Nambu M, Mayumi M, Mikawa H. Roles of eosinophils and catecholamines in pathophysiology of exercise-induced asthma. Pediatr Allergy Immunol. 1993;4:221–225. doi: 10.1111/j.1399-3038.1993.tb00096.x. [DOI] [PubMed] [Google Scholar]
- 51.Barnes PJ, Brown MJ, Silverman M, Dollery CT. Circulating catecholamines in exercise and hyperventilation induced asthma. Thorax. 1981;36:435–440. doi: 10.1136/thx.36.6.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ney UM. Propranolol-induced airway hyperreactivity in guinea-pigs. Br J Pharmacol. 1983;79:1003–1009. doi: 10.1111/j.1476-5381.1983.tb10548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsumoto S. Functional evidence of excitatory M1 receptors in the rabbit airway. J Pharmacol Exp Ther. 1997;281:531–539. [PubMed] [Google Scholar]
- 54.mase-Michel C, Tran MA, Montastruc JL, Montastruc P. Effect of losartan on afferent nerve stimulation. Eur J Pharmacol. 1998;355:125–132. doi: 10.1016/s0014-2999(98)00488-9. [DOI] [PubMed] [Google Scholar]
- 55.Binks AP, Paydarfar D, Schachter SC, Guz A, Banzett RB. High strength stimulation of the vagus nerve in awake humans: a lack of cardiorespiratory effects. Physiol. 2001;127:125–133. doi: 10.1016/s0034-5687(01)00252-3. [DOI] [PubMed] [Google Scholar]