Abstract
Studies over the last decade using FM dyes to label vesicles at many terminals, including the calyx-type nerve terminal, led to a well accepted “principle” that only a small fraction of vesicles (∼5–20%) participate in recycling under physiological conditions. This principle imposes a large challenge in maintaining synaptic transmission during repetitive firing, because the small recycling pool may limit the number of available vesicles for release and nerve terminals would have to distinguish the recycling pool from the reserve pool and keep reserve pool vesicles from being used. By recording the presynaptic capacitance changes and the postsynaptic EPSC at rat calyx of Held synapses in the absence or presence of transmitter glutamate in nerve terminals, we developed a new method to count functional recycling vesicles. We found that essentially all vesicles in calyces participated in recycling, challenging the small-recycling-pool principle established by FM dye labeling. Nerve terminals may use all available vesicles to maximize their ability in maintaining synaptic transmission during repetitive firing.
Introduction
Maintaining synaptic transmission relies on vesicle recycling. FM dye labeling in the last decade suggests that only ∼5–20% vesicles participate in recycling during firing at ≤ 30 Hz at many synapses, including neuromuscular junctions, hippocampal synapses, and calyx-type synapses (Harata et al., 2001b; de Lange et al., 2003; Richards et al., 2003; Denker et al., 2011). This well accepted small-recycling-pool principle (Rizzoli and Betz, 2005) imposes large challenges for neurons to maintain synaptic transmission during repetitive firing. It raises the question as to why most vesicles do not participate in recycling and how recycling vesicles are distinguished from non-recycling vesicles.
Here we developed a technique to count recycling vesicles at the calyx of Held nerve terminal, where FM dye labeling showed morphologically a small recycling pool (∼5%) (de Lange et al., 2003). By preventing glutamate uptake into recycled vesicles, we found an activity-dependent reduction of the EPSC reflecting recycling of vesicles containing no glutamate. The sum of the EPSC, which reflected the functional recycling pool size, was as large as the entire vesicle pool, challenging the small-recycling-pool “principle.”
Materials and Methods
Preparation of brainstem slices containing calyces of Held from Wistar rats of either sex (7–10 d old), whole-cell measurements of presynaptic calcium currents and capacitance (EPC-10 amplifier), and the EPSCs and mEPSCs (Axopatch 200B amplifier) at room temperature (22–24°C) have been described previously (Wu et al., 2009). The holding potential for presynaptic and postsynaptic recordings was −80 mV. Data were expressed as the mean ± SEM. The statistical test used was the t test.
The presynaptic pipette (2.5–4.5 MΩ) solution contained the following (in mm): 125 Cs-gluconate, 20 CsCl, 4 MgATP, 10 Na2-phosphocreatine, 0.3 GTP, 10 HEPES, and 0.05 BAPTA, pH 7.2 adjusted with CsOH. When glutamate (potassium l-glutamate, 10 mm) was added, Cs-gluconate was reduced to maintain the same osmolarity (310–325 mOsm). The bath solution contained the following (in mm): 105 NaCl, 20 TEA-Cl, 2.5 KCl, 1 MgCl2, 2 CaCl2, 25 NaHCO3, 1.25 NaH2PO4, 25 dextrose, 0.4 ascorbic acid, 3 myo-inositol, 2 sodium pyruvate, 0.001 tetrodotoxin, 0.1 3,4-diaminopyridine, 0.05 d-APV, pH 7.4 when bubbled with 95% O2 and 5% CO2. The postsynaptic pipette (2–3 MΩ) solution contained the following (in mm): 125 K-gluconate, 20 KCl, 4 MgATP, 10 Na2-phosphocreatine, 0.3 GTP, 10 HEPES, and 0.5 EGTA, pH 7.2, adjusted with KOH. The series resistance (<12 MΩ) was compensated by 90% (lag 10 μs).
Fura-2 fluorescence imaging was performed on an upright epifluorescence microscope (Olympus BX51WI, Achroplan 40×, numerical aperture 0.75, Zeiss) equipped with a monochromator (TILL Photonics), a dichroic mirror (400 nm), an emission filter (510 nm long pass), and a CCD camera (EM CCD 9100, Hamamatsu Photonics). Images were analyzed using the software Openlab 5.5 (Improvision).
Results
Activity-dependent EPSC decrease in the absence of glutamate
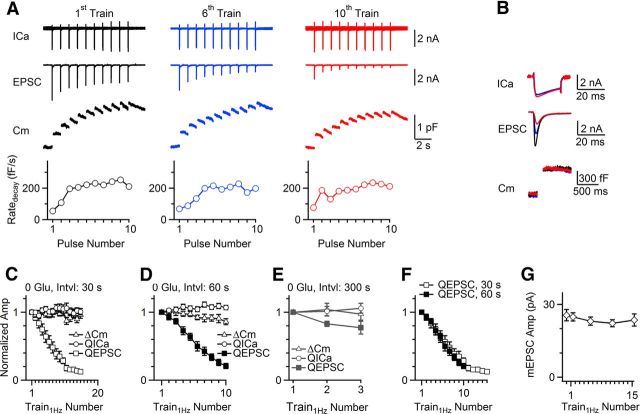
The calyx and the postsynaptic neuron were voltage-clamped. Kynurenic acid (1 mm, bath) was included to relieve AMPA receptor saturation (Sun and Wu, 2001; Scheuss et al., 2002; Wong et al., 2003). At 1–2 min after whole-cell break-in, the calyx was stimulated with 10 pulses of 20 ms depolarization (from −80 to +10 mV) at 1 Hz (Train1Hz). Train1Hz induced a presynaptic calcium current charge (QICa) of 366 ± 42 pC, a membrane capacitance jump (ΔCm) of 2898 ± 276 fF (the sum of ΔCm induced by each 20 ms depolarization, not the net increase) and an EPSC charge (QEPSC) of 94 ± 11 pC (n = 8, Fig. 1A,B).
Figure 1.
Activity-dependent EPSC decrease in the absence of glutamate. A, Sampled presynaptic calcium currents (ICa), EPSCs, and presynaptic Cm induced by the first, sixth, and 10th Train1Hz applied every 30 s. The rate of Cm decay (Ratedecay) in the interval between each 20 ms depolarization during Train1Hz is also plotted. The presynaptic pipette contained 0 mm glutamate (Glu applies to A–G). B, ICa, EPSC, and Cm induced by the first 20 ms depolarization during first (black), sixth (blue), and 10th (red) Train1Hz shown in A are superimposed. C–E, The QICa, ΔCm, and QEPSC induced by each Train1Hz are plotted versus the number of Train1Hz applied every 30 s (C, n = 8), 60 s (D, n = 8), or 300 s (E, n = 5). Data were normalized to the amplitude (Amp) induced by the first Train1Hz, and expressed as the mean ± SEM (applied to all figures). F, QEPSC in C and D are plotted versus the Train1Hz number. G, The mean mEPSC amplitude recorded between Train1Hz applied every 30 s for 15 times (n = 4 synapses, no kynurenic acid in bath).
As Train1Hz was repeated every 30 s (n = 8, Fig. 1C), 60 s (n = 8, Fig. 1D), or 300 s (n = 5, Fig. 1E), ΔCm and QICa induced by Train1Hz did not decrease significantly, whereas QEPSC decreased to near 0 at ≥10th Train1Hz. QEPSC decrease was fastest at 30 s interval, slower at 60 s interval, and very slowly at 300 s interval (Fig. 1C–E). Plotting QEPSC versus the number of Train1Hz applied every 30 or 60 s showed similar decreases at the same Train1Hz number (Fig. 1F). Thus, the QEPSC decrease is activity dependent. The mEPSC amplitude did not change as Train1Hz was repeated every 30 s 15 times in the absence of glutamate and kynurenic acid (Fig. 1G; n = 4 synapses).
The activity-dependent EPSC decrease (Fig. 1) and the persistence of endocytosis in ∼10 min recording (Xu et al., 2008) (Fig. 1A) suggests that mixing of recycled vesicles lacking glutamate causes the EPSC decrease. The lack of mEPSC amplitude changes (Fig. 1G) suggests that recycled vesicles contained no glutamate, not a fraction of a normal amount.
Activity-dependent EPSC decrease is due to lack of glutamate refilling
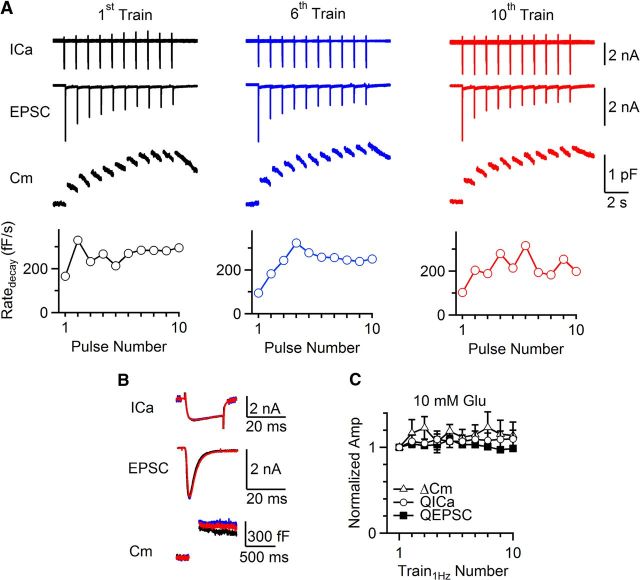
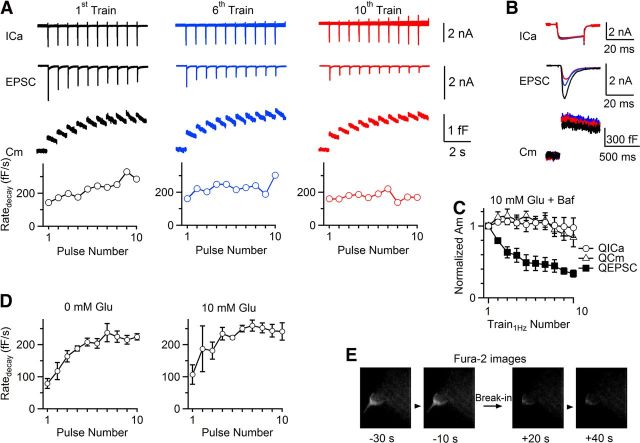
To confirm that vesicle recycling without glutamate causes the EPSC decrease, we included 10 mm glutamate in the pipette, at which the mEPSC amplitude does not change (Ishikawa et al., 2002). As Train1Hz was repeated every 60 s for 10 times, QEPSC, ΔCm and QICa did not decrease significantly (Fig. 2A–C; n = 3). However, in the presence of bafilomycin A1 (6 μm in the bath, 20–30 min) that blocks the V-type ATPase-dependent vesicle reacidification and transmitter refilling (Ikeda and Bekkers, 2009), QEPSC, but not ΔCm or QICa decreased to 34 ± 5% of control (n = 4; Fig. 3A–C; p < 0.01). The QEPSC (34 ± 5%) was slightly larger than that without glutamate (21 ± 4%, n = 8), perhaps due to incomplete block of glutamate refilling. These results strengthen our suggestion that the activity-dependent EPSC decrease is due to recycling of vesicles containing no glutamate.
Figure 2.
The EPSC does not decrease in the presence of glutamate. A–C, Same arrangements as in Figure 1A,B,D, respectively, except that the presynaptic pipette contained 10 mm glutamate (n = 3 synapses; Train1Hz interval: 60 s).
Figure 3.
Block of glutamate uptake causes activity-dependent EPSC decrease. A–C, Same arrangements as in Fig. 2A–C (10 mm glutamate), respectively, except that the bath contained bafilomycin A1 (Baf, 6 μm, 20–30 min, n = 4 synapses). D, Capacitance rate of Cm decay (Ratedecay) after each 20 ms depolarization (from ∼300 to 950 ms after depolarization) during the first Train1Hz with 0 mm (left, n = 8, data analyzed from Fig. 1) or 10 mm (right, n = 3, data from Fig. 2) glutamate in the pipette. E, Fura-2 images of a calyx before (−30, −10 s) and after (+20, +40 s) whole-cell break-in with a pipette containing no fura-2. Before taking these images, the calyx was preloaded with fura-2 using another whole-cell pipette containing 150 μm fura-2, which was withdrawn ∼1 min later and the calyx resealed.
Negligible glutamate for vesicle uptake
The following calculations/experiments further support our conclusion that recycled vesicles uptake negligible glutamate. At calyces, glutamate release is complete, because even kiss-and-run fusion takes ∼200 ms with a pore that releases all glutamate within ∼5 ms (He et al., 2006). Since the EPSC decayed to near baseline at ∼10–20 ms during depolarization (Figs. 1B, 2B), and release at the end of 20 ms depolarization was minimal (Wölfel et al., 2007), the cleft glutamate concentration was negligible after a 20 ms depolarization. The only time endocytosis might uptake glutamate is the first 10 ms of a 20 ms depolarization (Figs. 1B, 2B), during which endocytosis was negligible because endocytosis is much longer than 10 ms (Wu et al., 2007). More quantitatively, the capacitance decay rate at the interval between 20 ms depolarization during Train1Hz reached a similar plateau level (<260 fF/s) in the absence (Fig. 3D, left; n = 8) or presence of glutamate (Fig. 3D, right; n = 3, p = 0.11). Applying this rate (<260 fF/s) to the first 10 ms of depolarization for 10 times, we estimated that <26 fF (260 fF/s × 0.01 s × 10) or <1% (26/2898 fF) of ΔCm (2898 ± 276 fF, n = 8) induced by Train1Hz was retrieved. Thus, glutamate uptake from synaptic clefts was negligible.
Since the pipette solution (∼5 μl) was much more than the calyx cytosol, cytosolic glutamate should be washed out soon after whole-cell break-in. Indeed, when we loaded calyces with fura-2 by whole-cell break-in with a pipette containing fura-2 (150 μm) for 1 min, withdrawal of the pipette, and break-in with another pipette containing no fura-2 decreased fura-2 fluorescence to a near background level within ∼20 s after break-in (Fig. 3E; n = 4). Residual fura-2 fluorescence remained similar afterward (Fig. 3E), likely due to fura-2 binding to immobile molecules. Since fura-2's molecular weight was larger than glutamate's, glutamate washout should be faster and should occur before Train1Hz application. Partial refilling from locally synthesized glutamate is highly unlikely, because synthesized glutamate should be quickly diluted and the mEPSC amplitude did not decrease (Fig. 1G).
Large recycling pool
Since the activity-dependent EPSC decrease is due to vesicle recycling without glutamate, the accumulated QEPSC as Train1Hz was repeated every 30 s without glutamate (ΣQEPSC; Fig. 4A) reflected glutamate-containing vesicles undergoing exocytosis for the first time. The ΣQEPSC increased exponentially as Train1Hz was repeated and approached a plateau level (Fig. 4A), reflecting depletion of glutamate-containing releasable vesicles, i.e., the recycling vesicle pool. The plateau level obtained from monoexponential fit (Fig. 4A) was 46 times the QEPSC induced by the first 20 ms depolarization during the first Train1Hz, or 46 times the readily releasable pool (RRP) size because a 20 ms depolarization depletes the RRP (Wu and Borst, 1999; Xu and Wu, 2005). Since the vesicle number estimated from the EPSC or the ΔCm induced by a 20 ms depolarization matched approximately (Wölfel et al., 2007), we used ΔCm (464 ± 28 fF, n = 8; Fig. 1A,B) induced by the first 20 ms depolarization during the first Train1Hz to estimate the RRP size. The RRP contained 6356 (464/0.073) vesicles, considering that a vesicle's membrane capacitance is ∼0.073 fF (He et al., 2006). Accordingly, the recycling pool contained ∼292,000 (46 × 6356) vesicles, in the same order as an electron microscopic estimate (∼188,000 vesicles) of the vesicle number in the calyx (de Lange et al., 2003). Our value is larger, perhaps because we selected large calyces for patch-clamping.
Figure 4.
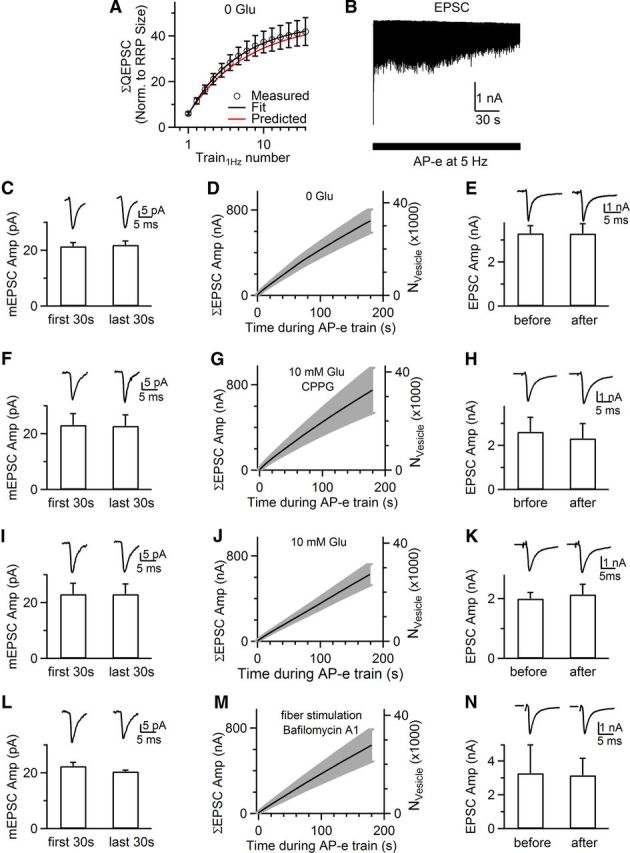
Large recycling pool. A, The accumulated QEPSC (ΣQEPSC, circles) induced by Train1Hz applied every 30 s is plotted versus the Train1Hz number in the absence of glutamate (analyzed from Fig. 1C). ΣQEPSC is normalized to the QEPSC induced by the first 20 ms depolarization of the first Train1Hz, i.e., the RRP size. Black curve is a monoexponential fit with a plateau value of 46 times the RRP size. The red curve is the predicted change assuming random mixture of recycling vesicles (see Results). B, Sampled EPSCs induced by a presynaptic AP-e train at 5 Hz for 180 s (bar) with no glutamate in the pipette (applies to B–E). C, The mEPSC amplitude (n = 8 synapses) in the first and the last 30 s during the AP-e train at 5 Hz for 180 s. Sampled mean mEPSCs at the same time period from a synapse shown in B are also plotted above the bar graph (left: averaged of 97 mEPSCs; right: 93 mEPSCs). D, The cumulative EPSC amplitude (ΣEPSC Amp) and the number of vesicles (Nvesicle) released by an AP-e train at 5 Hz (n = 8 synapses). Data are shown as the mean (solid curve) ± SEM (gray). E, The amplitude of EPSCs (bottom) and sampled EPSCs (top, from one synapse) induced by an AP-e at ∼30 s before (left) and after (right) an AP-e train at 5 Hz for 180 s (n = 8 synapses). F–H, Same arrangements as in C–E, respectively, except that the pipette contained 10 mm glutamate and the bath contained 300 μm CPPG (n = 5 synapses). mEPSC traces in F: left, averaged of 47 mEPSCs; right, 56 mEPSCs. I–K, Same arrangements as in C–E, respectively, except that the pipette contained 10 mm glutamate (no CPPG in bath, n = 5 synapses). mEPSC traces in I: left, averaged of 136 mEPSCs; right, 58 mEPSCs. L–N, Same arrangements as in C–E, respectively, except that whole-cell AP-e was replaced with an extracellular stimulation that induced axonal action potentials (see Results), and the bath contained 6 μm bafilomycin A1 (n = 4, synapses). mEPSC traces in M: left, 44 mEPSCs; right, 51 mEPSCs.
Assuming random mixing of recycled vesicles, we predicted the normalized QEPSC based on the measured recycling pool size (46 times the RRP size) and ΔCm induced by the first Train1Hz (6.0 times the RRP size; Fig. 4A). The predicted QEPSC (normalized to the RRP size) was 6.0, 5.2 [(1 − 6.0/46) × 6.0], etc… as Train1Hz was repeated 1, 2, etc… times, which matched well with the measured QEPSC (Fig. 4A), confirming that the recycling pool is ∼46 times the RRP size.
Large recycling pool during action potential-equivalent stimuli
Our estimate of the recycling pool (∼292,000 vesicles) was much larger than that labeled with FM dye (∼20,000 vesicles) during action potential stimulation at 5 Hz (de Lange et al., 2003). To determine whether this discrepancy is due to a different stimulation protocol, we mimicked an action potential by a 1 ms depolarization to +7 mV (AP-e) (Sun et al., 2002; Xu and Wu, 2005) and delivered the AP-e at 5 Hz for 180 s without glutamate (in pipette) or kynurenic acid (in bath; Fig. 4B). Since the mEPSC amplitude in the first and last 30 s of stimulation did not change significantly (Fig. 4C), we divided the accumulated EPSC by the mean mEPSC, yielding a total release of 33,000 ± 5000 vesicles at the end of stimulation (n = 8; Fig. 4D). If there were only 20,000 vesicles in the recycling pool as FM dye labeling estimated (de Lange et al., 2003), the AP-e train should release them all and thus reduce the EPSC to 0 in the absence of glutamate. However, the EPSC evoked by an AP-e at 30–60 s before (3.30 ± 0.35 nA, n = 8) and after (3.29 ± 0.44 nA, n = 8) the AP-e train was similar (p > 0.9; Fig. 4E), suggesting that 33,000 vesicles represent only a small fraction of the large recycling pool. Consistently, a recycling pool containing 292,000 vesicles, as estimated during repetitive Train1Hz (Fig. 4A), predicted only a small (∼11%) EPSC reduction when 33,000 vesicles are recycled without glutamate. In two synapses where we applied a 300 s AP-e train at 5 Hz, we observed an ∼20–25% reduction of the EPSC at 30–60 s after the train (data not shown), further supporting a large recycling pool.
The large recycling pool was obtained when cytosolic glutamate was washed out. Would glutamate washout influence group III metabotropic glutamate receptor (mGluR) at calyces (Billups et al., 2005), and thus the recycling pool size? Two sets of evidence exclude this possibility. First, we repeated experiments in Figure 4B–E, but with 10 mm glutamate in the pipette and 300 or 0 μm (R,S)-cyclopropyl-4-phosphonophenylglycine (CPPG), the group III mGluR antagonist, in the bath. In the presence (Fig. 4F–H; n = 5) or absence (Fig. 4I–K; n = 5) of CPPG, neither the mEPSC nor the EPSC was decreased after an AP-e train at 5 Hz for 180 s, similar to those obtained without glutamate in the pipette (Fig. 4C–E). Second, the activity-dependent EPSC decrease was observed in the absence of glutamate or in the presence of glutamate and bafilomycin A1 (Figs. 1, 3A–C).
The large recycling pool was obtained at the whole-cell configuration. To determine whether presynaptic whole-cell dialysis influences our conclusion, we replaced presynaptic whole-cell stimulation with a bipolar extracellular electrode at the midline of the trapezoid body (∼5–15 V, 0.1 ms, horizontal brainstem slice) to induce action potentials. In the presence (n = 4; Fig. 4L–N) or absence (n = 4) of bafilomycin A1 (6 μm, bath), the mEPSC and the EPSC measured 30–60 s before and after a 5 Hz train for 180 s were similar (p > 0.6), suggesting that the recycling pool is too large to be depleted substantially. In three of these recorded synapses in the presence of bafilomycin A1, we resumed stimulation at 5 Hz for another 5 min. The EPSC at 30–60 s after the train was reduced by <30%, consistent with the large recycling pool. These results were similar to those obtained without glutamate in the presynaptic pipette (Fig. 4C–E). Thus, whole-cell presynaptic dialysis does not affect our finding of a large recycling pool.
Discussion
We found that at single calyces, the recycling pool contained ∼292,000 vesicles or ∼46 times the vesicles in the RRP during repetitive Train1Hz or AP-e train at 5 Hz. This number was similar to the total vesicle number previously estimated by electron microscopy in the calyx (de Lange et al., 2003). Thus, the calyx maximizes its capacity in maintaining synaptic transmission by using essentially all vesicles for recycling.
The recycling pool we measured with EPSC recordings was much larger than the recycling pool (∼5–20% of total vesicles) morphologically labeled with FM dye at many synapses, including the calyx-type synapse (Harata et al., 2001b; Richards et al., 2003; de Lange et al., 2003; Rizzoli and Betz, 2004, 2005; Denker et al., 2011). The reason for this discrepancy is unclear. It has not been demonstrated that FM dye can label every endocytosed vesicle. For example, in a classical study, FM1–43 shows up in 86% of cisternae thought to reflect bulk endocytosis, whereas FM2–10 shows up in only 41% of cisternae (Richards et al., 2000). Based on the observation that prolonged higher-frequency or high potassium stimulation induced more FM dye-labeled vesicles, labeling of a small fraction of vesicles at low-frequency firing is suggested to reflect a small recycling pool. However, the possibility that FM dye uptake is different in different stimulation conditions has not been excluded. Different stimulation conditions induced different forms of endocytosis, including rapid, slow, bulk endocytosis and endocytosis overshoot (Richards et al., 2000; Clayton et al., 2007; Wu et al., 2009), which might take up FM dye differentially (Richards et al., 2000; Aravanis et al., 2003). A narrow fusion pore that closes rapidly, which has been suggested to take place in chromaffin cells (Chan and Smith, 2001; Chan and Smith, 2003), hippocampal synapses (Zhang et al., 2009), and calyces (Wu et al., 2005) during mild stimulation conditions, might prevent FM dye uptake into endocytosed vesicles. While this provides a potential explanation for a small recycling pool labeled with FM dye during low-frequency stimulation, whether this explanation holds remains to be tested. The present work developed a technique to count functional recycling vesicles at calyces, where only ∼5% vesicles are labeled by FM dye during firing at 5 Hz (de Lange et al., 2003). Our technique relies on washout of cytosolic glutamate while counting all released vesicles containing glutamate. We found that nearly all vesicles participate in recycling, which challenges the generally accepted small-recycling-pool principle.
Many studies of functional vesicle pools are in line with our findings. For example, when endocytosis is blocked, release of >20,000 vesicles (measured as ∼1.5 pF in the ΔCm) did not affect subsequent release (Wu et al., 2009). If the recycling pool contains only 20,000 vesicles (de Lange et al., 2003), subsequent release should be abolished. At calyces dialyzed with GTP-γS (and 0 mm glutamate) to block endocytosis, repetitive AP-e stimuli at 200 Hz releases approximately eight times the RRP, or ∼40,000–50,000 vesicles, but only an ∼20% reduction in the EPSC measured 80 s later (Xu et al., 2008), consistent with a recycling pool of much >50,000 vesicles. A gradual block of release was observed when endocytosis was abolished by inhibition of dynamin at hippocampal synapses and Drosophila neuromuscular junctions (Koenig and Ikeda, 1989, 1996; Delgado et al., 2000; Newton et al., 2006), which seems consistent with a large recycling pool. At physiological temperature, the recycling pool labeled by FM dye is approximately two times larger than at room temperature at hippocampal synapses (Micheva and Smith, 2005). A gradual block of EPSCs in the presence of bafilomycin A1 is observed at hippocampal synapses. By indirectly estimating the number of boutons involved in release, it is estimated that the recycling pool contained ∼80–130 vesicles per bouton (Ikeda and Bekkers, 2009), which is higher than ∼31–64 vesicles estimated with imaging methods (Harata et al., 2001a; Balaji and Ryan, 2007). While the difference could be potentially small (could be 80 vs 64 in the extreme), it might be due to several factors difficult to control at hippocampal synapses, such as the indirect estimate of the bouton number, and the lack of endocytosis measurements and an independent estimate of exocytosis other than the EPSC measurement. By controlling all these factors at calyces, including the calyx number, endocytosis, and measurements of exocytosis (ΔCm) other than the EPSC, we found that the functional recycling pool (∼292,000 vesicles) is >10-fold larger than the morphological estimate (∼20,000) (de Lange et al., 2003). Together, it is likely that many synapses use all available vesicles for recycling to maximize their ability in maintaining synaptic transmission.
Footnotes
This work was supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program.
References
- Aravanis AM, Pyle JL, Tsien RW. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–647. doi: 10.1038/nature01686. [DOI] [PubMed] [Google Scholar]
- Balaji J, Ryan TA. Single-vesicle imaging reveals that synaptic vesicle exocytosis and endocytosis are coupled by a single stochastic mode. Proc Natl Acad Sci U S A. 2007;104:20576–20581. doi: 10.1073/pnas.0707574105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups B, Graham BP, Wong AY, Forsythe ID. Unmasking group III metabotropic glutamate autoreceptor function at excitatory synapses in the rat CNS. J Physiol. 2005;565:885–896. doi: 10.1113/jphysiol.2005.086736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SA, Smith C. Physiological stimuli evoke two forms of endocytosis in bovine chromaffin cells. J Physiol. 2001;537:871–885. doi: 10.1113/jphysiol.2001.012838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SA, Smith C. Low frequency stimulation of mouse adrenal slices reveals a clathrin-independent, protein kinase C-mediated endocytic mechanism. J Physiol. 2003;553:707–717. doi: 10.1113/jphysiol.2003.053918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EL, Evans GJ, Cousin MA. Activity-dependent control of bulk endocytosis by protein dephosphorylation in central nerve terminals. J Physiol. 2007;585:687–691. doi: 10.1113/jphysiol.2007.137539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange RP, de Roos AD, Borst JG. Two modes of vesicle recycling in the rat calyx of Held. J Neurosci. 2003;23:10164–10173. doi: 10.1523/JNEUROSCI.23-31-10164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado R, Maureira C, Oliva C, Kidokoro Y, Labarca P. Size of vesicle pools, rates of mobilization, and recycling at neuromuscular synapses of a Drosophila mutant, shibire. Neuron. 2000;28:941–953. doi: 10.1016/S0896-6273(00)00165-3. [DOI] [PubMed] [Google Scholar]
- Denker A, Bethani I, Kröhnert K, Körber C, Horstmann H, Wilhelm BG, Barysch SV, Kuner T, Neher E, Rizzoli SO. A small pool of vesicles maintains synaptic activity in vivo. Proc Natl Acad Sci U S A. 2011;108:17177–17182. doi: 10.1073/pnas.1112688108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harata N, Pyle JL, Aravanis AM, Mozhayeva M, Kavalali ET, Tsien RW. Limited numbers of recycling vesicles in small CNS nerve terminals: implications for neural signaling and vesicular cycling. Trends Neurosci. 2001a;24:637–643. doi: 10.1016/S0166-2236(00)02030-0. [DOI] [PubMed] [Google Scholar]
- Harata N, Ryan TA, Smith SJ, Buchanan J, Tsien RW. Visualizing recycling synaptic vesicles in hippocampal neurons by FM 1–43 photoconversion. Proc Natl Acad Sci U S A. 2001b;98:12748–12753. doi: 10.1073/pnas.171442798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Wu XS, Mohan R, Wu LG. Two modes of fusion pore opening revealed by cell-attached recordings at a synapse. Nature. 2006;444:102–105. doi: 10.1038/nature05250. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Bekkers JM. Counting the number of releasable synaptic vesicles in a presynaptic terminal. Proc Natl Acad Sci U S A. 2009;106:2945–2950. doi: 10.1073/pnas.0811017106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Sahara Y, Takahashi T. A single packet of transmitter does not saturate postsynaptic glutamate receptors. Neuron. 2002;34:613–621. doi: 10.1016/S0896-6273(02)00692-X. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Disappearance and reformation of synaptic vesicle membrane upon transmitter release observed under reversible blockage of membrane retrieval. J Neurosci. 1989;9:3844–3860. doi: 10.1523/JNEUROSCI.09-11-03844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Smith SJ. Strong effects of subphysiological temperature on the function and plasticity of mammalian presynaptic terminals. J Neurosci. 2005;25:7481–7488. doi: 10.1523/JNEUROSCI.1801-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AJ, Kirchhausen T, Murthy VN. Inhibition of dynamin completely blocks compensatory synaptic vesicle endocytosis. Proc Natl Acad Sci U S A. 2006;103:17955–17960. doi: 10.1073/pnas.0606212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Betz WJ. Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron. 2000;27:551–559. doi: 10.1016/S0896-6273(00)00065-9. [DOI] [PubMed] [Google Scholar]
- Richards DA, Guatimosim C, Rizzoli SO, Betz WJ. Synaptic vesicle pools at the frog neuromuscular junction. Neuron. 2003;39:529–541. doi: 10.1016/S0896-6273(03)00405-7. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. The structural organization of the readily releasable pool of synaptic vesicles. Science. 2004;303:2037–2039. doi: 10.1126/science.1094682. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Scheuss V, Schneggenburger R, Neher E. Separation of presynaptic and postsynaptic contributions to depression by covariance analysis of successive EPSCs at the calyx of Held synapse. J Neurosci. 2002;22:728–739. doi: 10.1523/JNEUROSCI.22-03-00728.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JY, Wu LG. Fast kinetics of exocytosis revealed by simultaneous measurements of presynaptic capacitance and postsynatpic currents at a central synapse. Neuron. 2001;30:171–182. doi: 10.1016/S0896-6273(01)00271-9. [DOI] [PubMed] [Google Scholar]
- Sun JY, Wu XS, Wu LG. Single and multiple vesicle fusion induce different rates of endocytosis at a central synapse. Nature. 2002;417:555–559. doi: 10.1038/417555a. [DOI] [PubMed] [Google Scholar]
- Wölfel M, Lou X, Schneggenburger R. A mechanism intrinsic to the vesicle fusion machinery determines fast and slow transmitter release at a large CNS synapse. J Neurosci. 2007;27:3198–3210. doi: 10.1523/JNEUROSCI.4471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AY, Graham BP, Billups B, Forsythe ID. Distinguishing between presynaptic and postsynaptic mechanisms of short-term depression during action potential trains. J Neurosci. 2003;23:4868–4877. doi: 10.1523/JNEUROSCI.23-12-04868.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LG, Borst JG. The reduced release probability of releasable vesicles during recovery from short-term synaptic depression. Neuron. 1999;23:821–832. doi: 10.1016/S0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- Wu LG, Ryan TA, Lagnado L. Modes of vesicle retrieval at ribbon synapses, calyx-type synapses, and small central synapses. J Neurosci. 2007;27:11793–11802. doi: 10.1523/JNEUROSCI.3471-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Xu J, Wu XS, Wu LG. Activity-dependent acceleration of endocytosis at a central synapse. J Neurosci. 2005;25:11676–11683. doi: 10.1523/JNEUROSCI.2972-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XS, McNeil BD, Xu J, Fan J, Xue L, Melicoff E, Adachi R, Bai L, Wu LG. Ca(2+) and calmodulin initiate all forms of endocytosis during depolarization at a nerve terminal. Nat Neurosci. 2009;12:1003–1010. doi: 10.1038/nn.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46:633–645. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Xu J, McNeil B, Wu W, Nees D, Bai L, Wu LG. GTP-independent rapid and slow endocytosis at a central synapse. Nat Neurosci. 2008;11:45–53. doi: 10.1038/nn2021. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Li Y, Tsien RW. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science. 2009;323:1448–1453. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]