Abstract
Cutaneous exposure to chronic solar UVA-radiation is a causative factor in photocarcinogenesis and photoaging. Recently, we have identified the thiol-dependent cysteine-protease cathepsin B as a novel UVA-target undergoing photo-oxidative inactivation upstream of autophagic-lysosomal dysfunction in fibroblasts. In this study, we examined UVA effects on a wider range of cathepsins and explored the occurrence of UVA-induced cathepsin inactivation in other cultured skin cell types. In dermal fibroblasts, chronic exposure to non-cytotoxic doses of UVA caused pronounced inactivation of the lysosomal cysteine-proteases cathepsin B and L, effects not observed in primary keratinocytes and occurring only to a minor extent in primary melanocytes. In order to determine if UVA-induced lysosomal impairment requires single or dual inactivation of cathepsin B and/or L, we used a genetic approach (siRNA) to selectively downregulate enzymatic activity of these target cathepsins. Monitoring an established set of protein markers (including LAMP1, LC3-II, and p62) and cell ultrastructural changes detected by electron microscopy, we observed that only dual genetic antagonism (targeting both CTSB and CTSL expression) could mimic UVA-induced autophagic-lysosomal alterations, whereas single knockdown (targeting CTSB or CTSL only) did not display ‘UVA-mimetic’ effects failing to reproduce the UVA-induced phenotype. Taken together, our data demonstrate that chronic UVA inhibits both cathepsin B and L enzymatic activity and that dual inactivation of both enzymes is a causative factor underlying UVA-induced impairment of lysosomal function in dermal fibroblasts.
Keywords: UVA, skin photodamage, cathepsin B, cathepsin L, fibroblast
1. Introduction
Most (>95%) of the solar UV energy incident on human skin is in the UVA range (320–400 nm), and cutaneous exposure to chronic UVA-radiation is an established causative factor in photocarcinogenesis and photoaging [1–9]. UVA effects on human skin involve induction of photooxidative stress mediated by reactive oxygen species (ROS), causing the structural alteration and functional modulation of various molecular targets including lipids, nucleic acids, and proteins [10–14]. Indeed, recent research has identified a wide range of target proteins thought to be modulated by UVA-induced photooxidative stress including structural proteins (e.g. actin, collagen, elastin, keratin), enzymes (e.g. GAPDH, IRP-1, p38 MAPK, PTP1B, calcineurin, MMPs, proteasome), and transcription factors (e.g. AP-1, AP-2, NFκB) [14–27].
Recently, employing an unbiased proteomic approach we have identified the thiol-dependent cysteine-protease cathepsin B as a novel UVA target undergoing photo-oxidative inactivation and altered posttranslational maturation in cultured dermal fibroblasts [28,29]. In response to chronic exposure to noncytotoxic doses of UVA, photooxidative impairment of cathepsin B enzymatic activity occurred with accumulation of autofluorescent aggregates colocalizing with lysosomes, an effect also observed by electron microscopy that revealed massive accumulation of lipofuscin-containing lysosomal vesicles. Consistent with UVA-induced lysosomal expansion and impaired autophagic clearance, upregulated cellular protein levels of the lysosomal marker glycoprotein Lamp-1, the lipidated autophagosomal membrane constituent LC3-II, and the autophagy substrates p62 (sequestosome 1) and α-synuclein were detected in UVA-exposed fibroblasts. Strikingly, pharmacological inhibition of cathepsin B mimicked UVA-induced cellular changes including lysosomal expansion and accumulation of lipofuscin and autophagy substrates, and the ‘UVA-mimetic’ effects of pharmacological cathepsin inhibition on unirradiated fibroblasts led us to conclude that cathepsin inactivation is a causative factor in UVA-photodamage upstream of autophagic-lysosomal dysregulation [28,29].
In this follow-up study, we aimed at further elucidating the identity of UVA-sensitive cathepsins beyond cathepsin B in dermal fibroblasts and other skin cell types including primary keratinocytes and melanocytes. Furthermore, we employed siRNA-based genetic target modulation in order to identify the key cathepsins involved in ‘UVA-mimetic’ lysosomal dysfunction in cultured human fibroblasts. Here we demonstrate for the first time that chronic UVA inhibits both cathepsin B and L enzymatic activities and that UVA-inhibition of cathepsin B and L predominantly occurs in cultured fibroblasts with only minor effects observed in primary cutaneous keratinocytes and melanocytes. Moreover, we report that dual siRNA-based genetic antagonism [suppressing both cathepsin B (siCTSB) and L (siCTSL) expression in unirradiated cells] mimics UVA-induced autophagic-lysosomal alterations in dermal fibroblasts, whereas single antagonism (siCTSB or siCTSL only) does not reproduce the UVA-induced phenotype, providing mechanistic evidence that dual inactivation of both enzymes is the crucial molecular event underlying impairment of lysosomal function in UVA-exposed dermal fibroblasts.
2. Materials and methods
2.1. Chemicals
[L-3-trans-(propylcarbamoyl)oxirane-2-carbonyl]-L-isoleucyl-L-proline methyl ester (CA074Me) was purchased from Enzo Life Sciences (Plymouth Meeting, PA), and [L-3-trans-(propylcarbamoyl)oxirane-2-carbonyl]-L-isoleucyl-L-proline (CA074) was from EMD chemicals (Billerica, MA). All other chemicals were purchased from Sigma (St. Louis, MO).
2.2. Cell Culture
Dermal neonatal foreskin Hs27 fibroblasts from ATCC (Manassas, VA) were cultured in DMEM containing 10% bovine calf serum (BCS). Primary human epidermal keratinocytes (HEK; neonatal HEKn-APF, from Cascade Biologics, Portland, OR) were cultured using Epilife medium supplemented with EDGS growth supplement (Cascade Biologics). Primary human epidermal melanocytes (HEMa, from Cascade Biologics) were cultured using M254 supplemented with HMGS-2. Cells were maintained at 37°C in 5% CO2, 95% air in a humidified chamber. HEK and HEMa were passaged using recombinant trypsin/EDTA and defined trypsin inhibitor.
2.3. Irradiation with solar UVA
A KW large area light source solar simulator, model 91293, from Oriel Corporation (Stratford, CT) was used, equipped with a 1000 W Xenon arc lamp power supply, model 68920, and a VIS-IR bandpass blocking filter plus UVB and C blocking filter (output 320–400 nm plus residual 650–800 nm, for UVA) [28,29]. The output was quantified using a dosimeter from International Light Inc. (Newburyport, MA), model IL1700, with a SED033 detector for UVA (range 315–390 nm, peak 365 nm), at a distance of 365 mm from the source, which was used for all experiments. Using UVB/C blocking filter, the dose at 365 mm from the source was 5.39 mJ cm−2 sec−1 UVA radiation with a residual UVB dose of 3.16 µJ cm−2 sec−1. For chronic UVA treatment (‘1 week’ UVA regimen), an exposure regimen was selected that delivered a physiologically relevant dose of UVA without causing compromised cell viability or altered proliferative rate after reseeding. Cells were seeded at 5×105 cells/ 100 mm dish and incubated overnight prior to UV exposure. Cells were exposed to 9.9 J/cm2 UVA for four consecutive days (39.6 J/cm2 total UVA dose). Before each irradiation, cells were first washed with PBS and then irradiated under PBS. After irradiation, PBS was removed and fresh culture medium was added. For mock UV treatments, cells were washed with PBS, placed in 10 ml PBS and then incubated at room temperature in the dark for 25 min. For analysis, cells were harvested one hour after final UV exposure.
2.4. Flow cytometric analysis of cell viability
Cell viability was determined using flow cytometric analysis of annexinV (AV)-propidium iodide (PI) stained cells using an apoptosis detection kit (APO-AF, Sigma, St. Louis, MO) according to the manufacturer’s specifications as published before [28,30].
2.5. Measurement of cathepsin B enzymatic activity
Cathepsin B activity was measured using the fluorimetric cathepsin B assay kit from BioVision, Inc. (Milpitas, CA) according to the manufacturer’s instructions [28,29]. Cells (5×105) were lysed in 0.5 ml of chilled lysis buffer. After 10 min incubation on ice, lysates were centrifuged at 10,000 g at 4 °C for 5 min and supernatant was retained for analysis. 50 µL of cell lysate was incubated with 50 µL of reaction buffer and cathepsin B substrate (Ac-Arg-Arg-AFC; 200 µM final concentration; 1 h at 37 °C). As a negative control, analysis was performed in the presence of the cathepsin B/L inhibitor Z-Phe-Phe-FMK (200 µM final concentration). The release of free amino-4-trifluoromethylcoumarin (AFC) was measured using a fluorescence plate reader (λex 400 nm, λem 505 nm; SpectraMax Gemini, Molecular Devices, Sunnyvale, CA). Additionally, protein concentration of cell lysates was determined using the BCA assay and cathepsin B activity was normalized to protein concentration per sample.
2.6. Measurement of Cathepsin L enzymatic activity
Cathepsin L activity was measured using the fluorimetric cathepsin L activity assay kit from BioVision, Inc. according to the manufacturer’s instructions. Sample processing and protocol were identical to the cathepsin B activity assay with the following modifications: After lysis, 50 µL lysate were incubated with 50 µL of reaction buffer containing Ca074 (1 µM, 15 min) to irreversibly inhibit cathepsin B, thereby eliminating interference from cathepsin B-dependent cleavage of the substrate [31,32]. Cathepsin L substrate (Ac-Phe-Arg-AFC; 200 µM final concentration) was then added and mixture was incubated for 1 h at 37 °C followed by AFC detection (λex 400 nm, λem 505 nm). As a negative control, analysis was performed in the presence of the cathepsin B/L inhibitor Z-Phe-Phe-FMK (200 µM final concentration).
2.7. Measurement of cathepsin D activity
Cathepsin D activity was measured using the fluorimetric cathepsin D activity assay kit from BioVision, Inc. according to the manufacturer’s instructions. Sample processing and protocol were identical to the cathepsin B activity assay with the following modifications: Using cathepsin D substrate [MCA-GKPILFFRLK(Dnp)-DR-NH2; 200 µM final concentration] the release of free 7-methoxycoumarin-4-yl acetyl (MCA) was measured using a fluorescence plate reader (λex 328 nm, λem 460 nm).
2.8. Proteasome activity assay
The Proteasome-GloTM Cell-Based Assay kit (Promega, Madison, WI), a homogeneous, bioluminescent assay for all three proteasome activities, was used according to manufacturer’s specifications [33]. After final UVA exposure cells were harvested and placed on an opaque 96-well plate (5000 cells/well). After 1 h, an equal volume of reagent containing the appropriate substrate (luminogenic peptide-conjugated aminoluciferin; Suc-LLVY-aminoluciferin, Z-LRR-aminoluciferin, Z-nLPnLD-aminoluciferin for chymotrypsin-like, trypsin-like, and caspase-like proteasome activities, respectively) was added to each well (with inclusion of two proprietary inhibitors blocking nonspecific proteases for determination of trypsin-like activity). Plates were incubated at room temperature (10 min) before measuring luminescence signal using a Synergy 2 microplate reader. Data are expressed as % activity relative to untreated control cells [means ± SD (n = 3)].
2.9. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) activity assay
UVA-induced alteration of GAPDH specific enzymatic activity was assessed in cytosolic cell extracts prepared from Hs27 fibroblasts (5 × 106 per sample) according to a published standard procedure measuring increase in absorbance at 340 nm (formation of NADH) in a reaction mixture containing 0.4 mM NAD+, 50 mM sodium arsenate, 0.1 mM DTPA, 0.9 mM D,L-glyceraldehyde-3-phosphate, and 50 mM Tris HCl, pH 8.8. [22]. Reaction was started by the addition of cytosolic extract normalized for protein content using the BCA protein assay (detailed in 2.10). One unit of enzyme was defined as the amount forming 1 µmol/min NADH at 25° C.
2.10. Gene expression array analysis
Total cellular RNA (5 × 106 cells) was prepared using the RNeasy kit from Qiagen (Valencia, California). Reverse transcription was performed using the RT2 First Strand kit (SA Biosciences, Frederick, MD) and 1 µg total RNA. Expression array analysis using the Human Oxidative Stress and Antioxidant Defense RT2 Profiler™ (PAHS-065) and Human Stress and Toxicity RT2 Profiler™ PCR Expression Array (PAHS-003; SA Biosciences) was performed as published recently [28,29,34]. All reactions were run using the following PCR conditions: 95 °C for 10 min, followed by 40 cycles of 95°C for 15 s alternating with 60 °C for 1 min (Applied Biosystems 7000 SDS, Foster City, CA). Gene-specific product was normalized to GAPDH and quantified using the comparative (ΔΔCt) Ct method as described in the ABI Prism 7000 sequence detection system user guide.
2.11. Gene expression analysis by real time RT-PCR
One hour after last UVA exposure, total cellular RNA (5×106 cells) was prepared using the RNEasy kit from Qiagen (Valencia, CA). Reverse transcription was performed using TaqMan Reverse Transcription Reagents (Roche Molecular Systems, Branchburg, NJ) and 200 ng of total RNA in a 50 µl reaction. Reverse transcription was primed with random hexamers and incubated at 25°C for 10 min followed by 48°C for 30 min, 95°C for 5 min, and a chill at 4°C. Each PCR reaction consisted of 3.75 µl of cDNA added to 12.5 µl of TaqMan Universal PCR Master Mix (Roche Molecular Systems), 1.25 µl of gene-specific primer/probe mix [Assays-by-Design; Applied Biosystems: CTSB (assay ID Hs00947433_m1), CTSL (assay ID HS00964650_m1), SQSTM1 (assay ID Hs00177654_m1), LAMP1 (assay ID Hs00174766_m1), or GAPDH (assay ID Hs99999905_m1)] and 7.5 µl of PCR water. PCR conditions were: 95°C for 10 min, followed by 40 cycles of 95°C for 15 s alternating with 60°C for 1 min (Applied Biosystems 7000 SDSGene-specific product was normalized to GAPDH and quantified using the comparative (ΔΔCt) Ct method described in the ABI Prism 7000 sequence detection system user guide. Expression values were averaged across three independent experiments (mean ± SD).
2.12. Transmission Electron Microscopy
Cells were trypsinized, reseeded and cultured for 4h. Cells were fixed in situ with 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH7.4), postfixed in 1% osmium tetroxide in cacodylate buffer, washed, scraped and pelleted. Cells were then stained in 2% aqueous uranyl acetate, dehydrated through a graded series (50,70, 90 and 100%) of ethanol and infiltrated with Spurr's resin, then allowed to polymerize overnight at 60 °C. Sections (50 nm) were cut, mounted onto uncoated 150 mesh copper grids, and stained with 2% lead citrate. Sections were examined in a CM12 Transmission Electron Microscope (FEI, Hillsboro, OR) operated at 80 kV with digital image collection (AMT, Danvers, MA).
2.13. Immunoblot detection
One hour after last irradiation, cells were washed with PBS, lysed in 1× SDS-PAGE sample buffer (0.375 M Tris HCl pH 6.8, 50% glycerol, 10% SDS, 5% β-mercaptoethanol, 0.25% bromophenol blue) and heated for 3 min at 95°C. Samples were separated by 12% SDS-PAGE followed by transfer to nitrocellulose membranes (Optitran, Whatman, Piscataway, NJ). Membranes were incubated with primary antibody in 5% milk-TBST overnight at 4°C. HRP-conjugated goat anti-rabbit or goat anti-mouse secondary antibody (Jackson Immunological Research, West Grove, PA) was used at 1:20,000 in 5% milk-TBST followed by visualization using enhanced chemiluminescence detection reagents. Equal protein loading was examined by β-actin-detection. The following primary antibodies were used: rabbit anti-cathepsin B polyclonal antibody, 1:200 (BioVision, Inc.); mouse anti-cathepsin L (BD Biosciences, San Jose, CA); rabbit anti-HO-1 polyclonal antibody, 1:5,000 (Stressgen Bioreagents, Ann Arbor, MI); rabbit anti-Hsp70 polyclonal antibody, 1:1,500 (Stressgen Bioreagents); rabbit anti-Lamp-1 monoclonal antibody, 1:1,000 (Cell Signaling Technology, Danvers, MA); mouse anti-sequestosome 1 (p62) monoclonal antibody, 1:200 (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-Nrf2 polyclonal antibody, 1:4000 (Santa Cruz Biotechnology); rabbit anti-LC3 polyclonal antibody, 1:500 (Novus Biologics, Littleton, CO); rabbit anti-eIF2α, 1:1000 (Cell Signaling Technology), rabbit anti-phospho-eIF2α, 1:1000 (Ser51; Cell Signaling Technology); mouse anti-actin monoclonal antibody, 1:1,500 (Sigma).
2.14. Detection of 4-HNE adducted cathepsin B
Cells (1×107) were lysed in radioimmunoprecipitation (RIPA) buffer containing 50 mM Tris-Cl, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, and a cocktail of protease inhibitors (Roche, Indianapolis, IN). After pre-clearing cell lysate with Protein G Sepharose™ beads (GE Healthcare, Piscataway, NJ) to remove proteins that nonspecifically bind to the beads, protein concentrations were quantified (BCA). Cell lysate (500 µg) was incubated overnight with 5 µL anti–cathepsin B antibody (200 µg/mL; BioVision, Inc.). Protein G Sepharose™ beads (50 µL) were added and incubated for an additional 4 hr. After 4 washes with RIPA buffer, the immunoprecipitates were boiled in 1× SDS-PAGE sample buffer 5 min. Samples were separated by 12% SDS-PAGE followed by transfer to nitrocellulose membranes (Optitran, Whatman). Membranes were incubated with anti-4-HNE primary antibody (polyclonal, rabbit) in 5% milk-TBST overnight at 4°C. HRP-conjugated goat anti-rabbit secondary antibody was used at 1:20,000 in 5% milk-PBST followed by visualization using enhanced chemiluminescence detection reagents.
2.15. Small interfering RNA Cathepsin B/Cathepsin L transfection
Hs27 fibroblasts were transiently transfected with a 50 nmol pool of four small interfering RNA (siRNA) oligonucleotides (oligos) targeting CTSB and/or CTSL or a 50 nmol pool of four nontargeting siRNA oligos using the DharmaFECT 1 transfection reagent (Dharmacon RNA Technologies, Lafayette, CO). The sequences of siGENOME CTSB SMARTpool (CTSB siRNA; GenBank: NM_147783) were GGCACAACUUCUACAACGU, GGAUGAGCUGGUCAACUAU GGAACUUCUGGACAAGAAA, and GGAUCACUGUGGAAUCGAA. The sequences of siGENOME CTSL SMARTpool (CTSL siRNA; GenBank: NM_001912) were CAGCUACUCUAACAUUUGA, UCCAGUAUGUUCAGGAUAA, GGAGAAACCAUUGUGGAAU, and CAGAUUAUACGGCAUGAAU. The oligos were resuspended in Dharmacon siRNA buffer and incubated in serum free media for 5 min. DharmaFECT 1 was also incubated in serum free media for 5 min before the addition of the siRNA oligos. The oligos were incubated with the transfection reagent for 20 min before cellular treatment. Complete media was added to the siRNA oligo mixture and the cells were incubated with the siRNAs in appropriate cell culture conditions for 48 h. Cells were then re-transfected with another 50 nmol pool of four siRNA oligonucleotides targeting CTSB and/or CTSL or a 50 nmol pool of four nontargeting siRNA oligonucleotides and incubated for an additional 48 h prior to analysis.
2.16. Statistical analysis
The results are presented as means (± SD) of at least three independent experiments. Where indicated, results are presented as means (±SEM). Data were analyzed employing the two-sided Student’s t-test using the Prism 4.0 software. Differences were considered significant at p < 0.05 (*p < 0.05; **p < 0.01; ***p < 0.001).
3. Results
3.1. UVA-induced inhibition of cathepsin B and L enzymatic activity differs between skin cell types, occurring primarily in dermal fibroblasts with minor effects in keratinocytes and melanocytes
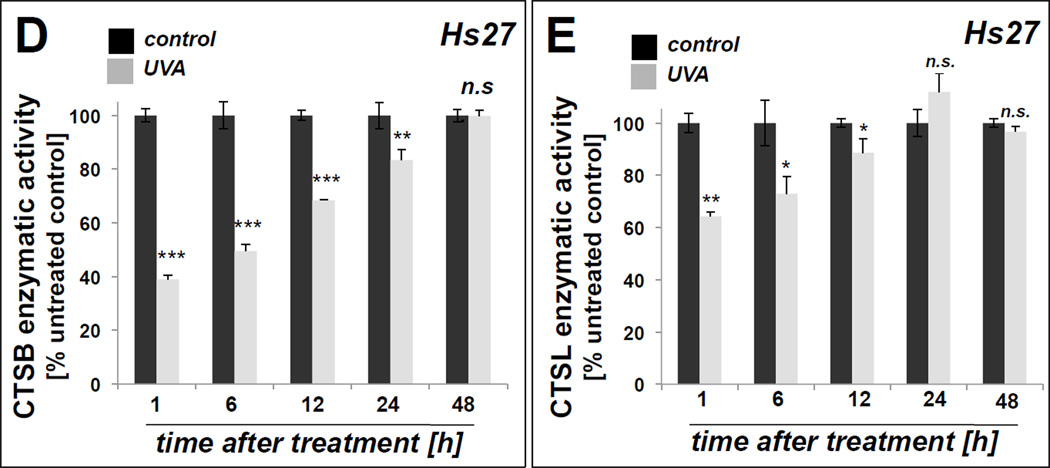
Our earlier experiments have demonstrated that chronic exposure to solar simulated UVA delivered at noncytotoxic doses causes photo-oxidative inactivation of cathepsin B leading to a dramatic loss in enzymatic activity in cultured human dermal fibroblasts [28,29]. In an attempt to further investigate the potential cutaneous relevance of UVA-induced cathepsin inactivation we examined UVA effects on a wider range of cathepsins beyond the cysteine-dependent cathepsin B focusing on other prominent cutaneous cathepsins, including the cysteine-dependent cathepsin L, and the aspartate-dependent cathepsin D (Fig. 1). Moreover, we examined the occurrence of UVA-induced cathepsin inactivation in other cultured skin cell types. To this end, a UVA exposure regimen (termed ‘1 week’ regimen; 9.9 J/cm2 UVA, q.d., 4 consecutive days) was employed that did not reduce viability in either Hs27 dermal fibroblasts (Fig. 1A), primary epidermal keratinocytes (HEK; Fig, 1B), or primary epidermal melanocytes (HEMa; Fig. 1C). However, it was observed that viability of unirradiated populations of HEK and HEMa cells was slightly reduced as compared to Hs27 fibroblasts (percentage viable cells of total population as indicated in lower left quadrant). As observed earlier, in Hs27 fibroblasts exposed to UVA (‘1 week’ regimen) the cysteine cathepsins B displayed pronounced photo-inactivation [60.7 % ± 13.0 residual protease activity]. Equally pronounced UVA-inhibition occurred with cathepsin L [60.7 % ± 13.0 residual protease activity], whereas only moderate inhibition of the aspartate-dependent protease cathepsin D was observed in response to chronic UVA [79.2 % ± 8.6 residual protease activity (n=3; mean ± SD; p < 0.05)] (Fig. 1A).
Figure 1. UVA modulation of cathepsin B and cathepsin L specific enzymatic activity in cultured human skin cell types.
Skin cells were exposed to UVA (‘1 week’ regimen). After completion of the UVA regimen, viability was determined by flow cytometric analysis of annexin V-propidium iodide stained cells (left panels; numbers indicate % viable of total gated cells; n=3, mean ± SD). (A) UVA-modulation of cathepsin B, cathepsin L, cathepsin D, and GAPDH enzymatic activities in human dermal fibroblasts. (B) UVA-modulation of cathepsin B and cathepsin L enzymatic activities in human primary keratinocytes (HEK). (C) UVA-modulation of cathepsin B and cathepsin L enzymatic activities in human primary melanocytes (HEMa). (D) Recovery kinetics (assessed at 1–48 h after last UVA exposure) of cathepsin B activity in human dermal fibroblasts. (E) Recovery kinetics (assessed at 1–48 h after last UVA exposure) of cathepsin L activity in human dermal fibroblasts. All bar graphs represent data as means ± SD (n=3).
In stark contrast to UVA effects observed in fibroblasts, UVA-inhibition of cathepsin B and L activity occurred to a minor extent in primary melanocytes and did not even reach the level of statistical significance in primary keratinocytes (Fig 1B–C).
We also examined the possibility that other cysteine-dependent enzymes might be subject to photo-oxidative stress in fibroblasts. Since GAPDH, an active site cysteine-containing glycolytic enzyme, has been shown earlier to be a target of cytotoxic oxidative insult including Rose Bengal-dependent photosensitization and UVA-inactivation [20,35], we examined if UVA-induced changes in activity would occur (Fig. 1A). However, the specific enzymatic activity of GAPDH remained unaffected by UVA exposure. In Hs27 dermal fibroblasts, we also examined recovery of enzymatic activity as a function of time after irradiation. After UVA, recovery of enzymatic activity occurred slowly over the course of 24–48 h for both cathepsin B and cathepsin L. Cathepsin B activity recovered to 80% of untreated cells within 24 h after UVA and returned to control levels within 48 h (Fig. 1D). Similarly, enzymatic activity of cathepsin L returned to that of untreated controls within 24 h (Fig. 1E).
3.2. UVA-induced cathepsin inactivation is accompanied by oxidative and proteotoxic stress response gene expression and occurs without impairment of proteasomal activity
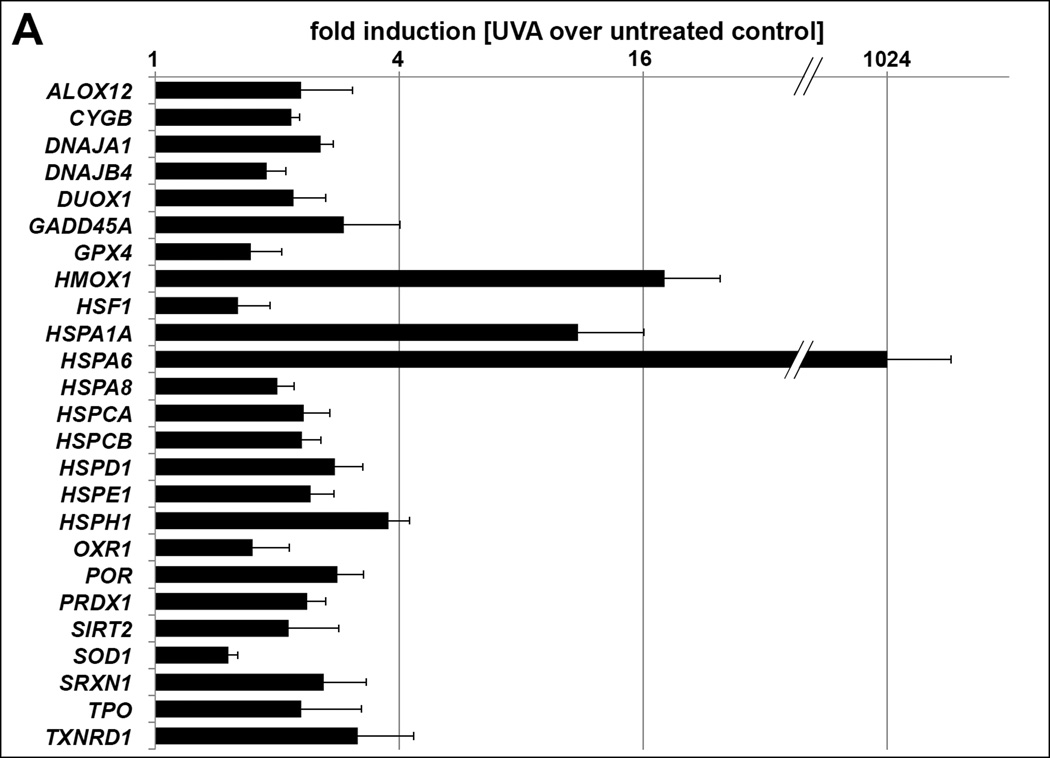
Given the pronounced UVA-effects on cathepsin function observed in dermal fibroblasts, we focused our follow-up experiments on this cell type. Gene expression array analysis revealed that cathepsin inhibition and proteasome activation observed in dermal fibroblasts upon repetitive exposure to nonlethal doses of UVA occur in the context of significant oxidative and proteotoxic stress response upregulation (Fig 2A). Consistent with our prior observations on pronounced induction of photo-oxidative stress in UVA-exposed fibroblasts significant upregulation was observed for a wide range of oxidative (e.g. ALOX12, HMOX1, PRDX1, SOD1, SRXN1, TXNRD1) and proteotoxic/heat shock (e.g. DNAJA1, HSPA1A, HSPA6, HSPD1, HSPH1) stress response genes, alterations that occur without changes affecting mRNA levels of cathepsin B (CTSB) or L (CTSL) [28,29]. Immunoblot analysis confirmed upregulation of Hsp70 (encoded by HSPA1A/HSPA6) and demonstrated inhibitory phosphorylation of the translation initiation factor eIF2α, indicative of UVA-induced proteotoxic stress response and ER stress signaling (Fig. 2B). Moreover, UVA-activation of the cellular antioxidant response was substantiated by immunodetection of increased protein levels of the transcription factor Nrf2 and its downstream target heme oxygenase-1 (HO-1; encoded by HMOX1) [36–38]. Supporting the mechanistic involvement of photo-oxidative stress in UVA inactivation of both cysteine-dependent cathepsins B and L, loss of cathepsin B and L enzymatic activities was antagonized significantly if irradiation occurred in the presence of the thiol-antioxidant N-acetyl-L-cysteine (NAC, 10 mM), a protective effect not observed with the aspartate-dependent cathepsin D (Fig. 2C). Among other antioxidants tested in addition to NAC, significant suppression of UVA-induced cathepsin B and L inactivation was achieved using the phenolic antioxidant tiron (4,5-dihydroxy-1,3-benzenedisulfonic acid disodium salt; 250 µM) and the thiol-based cytoprotectant L-ergothioneine (2-mercapto-L-histidine-trimethylbetaine; 1 mM), but not the reducing antioxidant L-ascorbate (1 mM) (data not shown), an observation consistent with the oxidative nature of cathepsin B/L inactivation potentially reflecting the differential efficacy of structurally diverse antioxidants to reach and protect lysosomal enzymes.
Figure 2. UVA-induced upregulation of oxidative/proteotoxic stress responses and proteasomal activity in dermal fibroblasts.
(A) UVA-modulation of stress response gene expression as assessed by quantitative RT-PCR using the Stress and Toxicity Pathway Finder™ array technology. The bar graph depicts statistically significant differences (n=3; p < 0.05) between UVA-exposed and untreated groups. (B) Immunoblot analysis of cellular stress response protein levels; β-actin: loading control. (C) Antioxidant modulation of cathepsin (CTSB, CTSL, CTSD) enzymatic activities in human dermal fibroblasts exposed to UVA (‘1 week’ regimen) in the presence or absence of NAC (10 mM). (D) 4-HNE adduct formation targeting cathepsin B in fibroblasts exposed to chronic UVA. Human dermal fibroblasts were lysed following chronic UVA or mock treatment as specified in Materials and methods. Cathepsin B was immunoprecipitated (IP) and immunoblot (IB) analysis of 4-HNE adduction was performed (top panel). As a loading control, IB analysis of cathepsin B [double chain (DC) and single chain (SC) form; bottom pabel] was performed from equal portions of cell lysates as used for immunoprecipitation. (E) Analysis of proteasome enzymatic activities [chymotrypsin-like, trypsin-like, and caspase (PGPH)-like] one hour after single (9.9 J/cm2) or cumulative UVA exposure (‘1 week’ regimen). Treatment with MG132 (10 µM, 6 h) served as a positive control. All bar graphs represent data as means ± SD (n=3).
Gene symbols and names: ALOX12: Arachidonate 12-lipoxygenase; CYGB: Cytoglobin; CYP2E1: Cytochrome P450, family 2, subfamily E, polypeptide 1; DNAJA1: DnaJ (Hsp40) homolog, subfamily A, member 1; DNAJB4: DnaJ (Hsp40) homolog, subfamily B, member 4; DUOX1: Dual oxidase 1; GADD45A: Growth arrest and DNA-damage-inducible, alpha; GDF15: Growth differentiation factor 15; GPX4: Glutathione peroxidase 4 (phospholipid hydroperoxidase); HMOX1: Heme oxygenase (decycling) 1; HSF1: Heat shock transcription factor 1; HSPA1A: Heat shock 70kDa protein 1A; HSPA6: Heat shock 70kDa protein 6 (HSP70B'); HSPA8: Heat shock 70kDa protein 8; HSPCA: Heat shock protein 90kDa alpha (cytosolic), class A member 2; HSPCB: Heat shock protein 90kDa alpha (cytosolic), class B member 1; HSPD1: Heat shock 60kDa protein 1 (chaperonin); HSPE1: Heat shock 10kDa protein 1 (chaperonin 10); HSPH1: Heat shock 105kDa/110kDa protein 1; OXR1: Oxidation resistance 1; POR: P450 (cytochrome) oxidoreductase; PRDX1: Peroxiredoxin 1; SIRT2: Sirtuin 2; SOD1: Superoxide dismutase 1, soluble; SRXN1: Sulfiredoxin 1; TPO: Thyroid peroxidase; TXNRD1: Thioredoxin reductase 1.
Prior research indicates that covalent modification of cathepsin B by electrophilic species such as ROS, lipid peroxidation products including 4-hydroynonenal (4-HNE), and drug metabolites can lead to loss of cathepsin B enzymatic activity [39–42]. We therefore tested for the UVA-induced occurrence of cathepsin B covalent adduction by 4-HNE) (Fig. 2D). To this end, cathepsin B was immunoprecipitated from cell lysates of untreated or UVA-exposed Hs27 fibroblasts followed by subsequent immunoblot detection of 4-HNE protein-epitopes. For the purpose of calculating the relative amount of modified protein, cathepsin B levels in total cell lysates were also determined by immunodetection. As reported earlier, UVA exposure caused the accumulation of the single chain form (SC; Mw: 29 kDa) with concomitant depletion of the fully mature double chain form (DC; large fragment, Mw: 24 kDa) of cathepsin B, changes in molecular size attributed to loss of cathepsin B enzymatic activity impairing the proteolytic autoprocessing from SC to DC form [28,29,43]. Pronounced immunostaining for 4-HNE adduction of cathepsin B [SC and DC form] was detected in Hs27 cells exposed to chronic UVA, a change confirmed by densitometric quantification of 4-HNE-modification [SC: 1.5 ± 0.3 fold increase; DC: 4.2 ± 0.7 fold increase; UVA-treated versus untreated control fibroblasts; mean ± SD] (Fig. 2D).
Next, we examined the possibility that UVA-induced inactivation might impair other major proteolytic systems beyond lysosomal cathepsins, specifically the proteasome, known to be modulated by cellular oxidative and proteotoxic stress. To this end, we assessed proteasome activity in cells exposed to UVA, employing a homogeneous luminescence-based assay that monitors all three proteasomal activities [chymotrypsin-, trypsin-, and caspase-like/PGPH (peptidylglutamyl-peptide hydrolyzing); (Fig. 2E)] [33]. As a positive control, the established proteasome inhibitor MG132 was used (10 µM, 6 h exposure time). In contrast to UVA-inhibition of cathepsin-dependent proteolytic activity, proteasome-dependent proteolytic activity (chymotrypsin-, trypsin- and caspase-like/PGPH) increased in fibroblasts exposed to UVA. Remarkably, even in response to one single UVA exposure (9.9 J/cm2), induction of caspase-like/PGPH activity was observable (Fig. 2E).
Taken together, these data indicate that (i) repetitive UVA exposure at doses that do not compromise viability of dermal fibroblasts upregulates photooxidative and proteotoxic stress response gene expression, that (ii) UVA-induced cathepsin B and L inactivation can be antagonized by antioxidant intervention, and that (iii) UVA effects on cathepsin B and L are not associated with impairment of proteasomal function.
3.3. Only dual siRNA-based genetic antagonism targeting both CTSB and CTSL mimics UVA-induced lysosomal dysfunction
Consistent with our prior research we observed that together with cathepsin B inactivation, chronic UVA exposure causes dramatic autophagic-lysosomal alterations in dermal fibroblasts substantiated by EM-based ultrastructural changes and an established set of protein markers (including LAMP1, LC3-II, and p62) (Fig. 3A and B) [28,29]. We also had reported earlier that pharmacological cathepsin inhibition using CA074Me induces ‘UVA-mimetic’ phenotypic changes in fibroblasts, as observable at the ultrastructural (Fig. 3A, right panel) and protein level (Fig. 3C) [28,29].
Figure 3. UVA-induced autophagic-lysosomal dysfunction in dermal fibroblasts is mimicked using CTSB-/CTSL-directed dual pharmacological inhibition.
(A) After exposure to UVA (‘1 week’ regimen), CA074Me (1µM, q.d., 4 consecutive days), or mock treatment (control) lysosomal alterations were visualized by transmission electron microscopy (TEM): L (Osmiophilic vesicles indicative of lysosomal lipofuscin accumulation; M (mitochondrion); N (nucleus). (B and C) Immunoblot detection of autophagic-lysosomal marker proteins (Lamp-1, LC3-I, LC3-II, p62; β-actin: loading control) in the treatment groups (panel B: control, UVA; panel C: control, CA074Me) as observed earlier [29]. (D) Inhibitory profile of CA074Me on cathepsin B, cathepsin L, and cathepsin D enzymatic activities as assessed in fibroblasts exposed to CA074Me (1µM, q.d., 4 consecutive days). All bar graphs represent data as means ± SD (n=3).
Since the inhibitory specificity of CA074Me for cathepsin B versus L in human dermal fibroblasts remained undefined in our earlier studies, we decided to examine the inhibitory profile of CA074Me directed against CTSB, CTSL, and CTSD (Fig. 3D). Enzymatic analysis revealed that CA074Me displays potent dual inhibitory activity against cathepsin B and cathepsin L without affecting cathepsin D in fibroblasts, a finding consistent with earlier research performed in murine fibroblasts [44]. Next, in order to test the hypothesis that lysosomal alterations may occur as a consequence of cathepsin inactivation and in order to examine if these changes result from single or dual inactivation of cathepsin B and/or L, we used a genetic target modulation approach (siRNA) (Fig. 4). First, we confirmed that siRNA-based intervention enabled selective downregulation of cathepsin B and/or L activity, with 10% residual cathepsin B activity (siCTSB) versus transfection control (siControl) and 25% residual cathepsin L activity (siCTSL) versus transfection control (siControl), respectively (Fig. 4A). Efficacy of knockdown was also confirmed at the mRNA and protein levels (Fig. 4B–C).
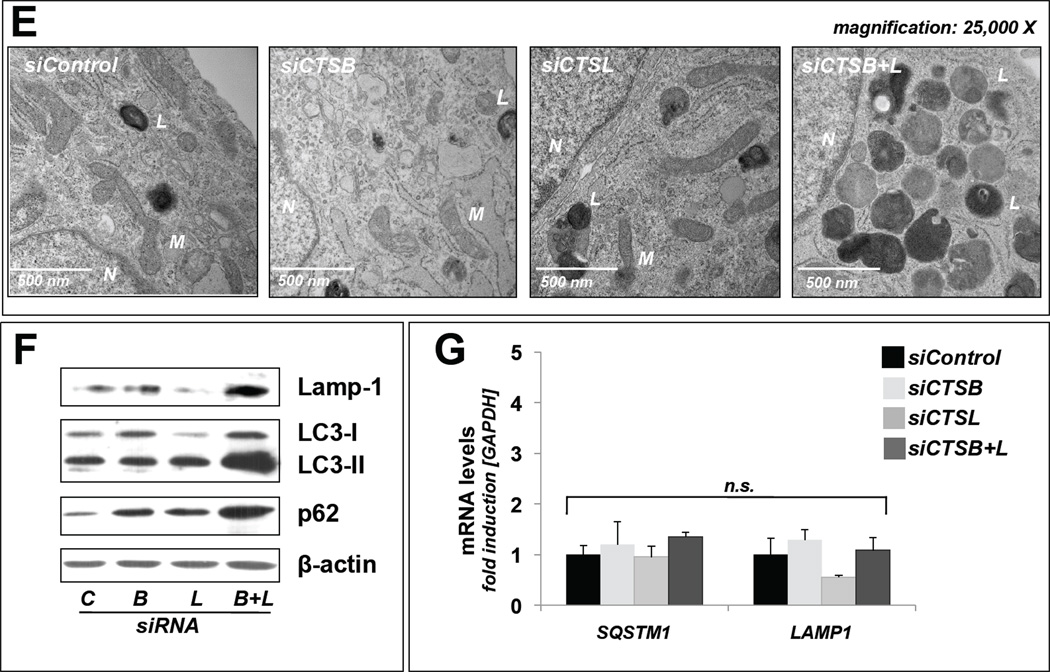
Figure 4. UVA-induced lysosomal dysfunction is mimicked only by dual but not single siRNA-based genetic antagonism targeting CTSB and CTSL in dermal fibroblasts.
(A–C) Confirmation of cathepsin specific expression knockdown: (A) Cathepsin B and cathepsin L enzymatic activities as assessed in fibroblasts after transfection with (i) siControl, (ii) siCTSB, (iii) siCTSL, or (iv) siCTSB ± siCTSL. (B) mRNA transcript levels detected by quantitative RT-PCR of CTSB and CTSL; groups as in (A). (C) Immunoblot detection of cellular cathepsin B and cathepsin L protein levels [double chain (DC) and single chain (SC) form]; groups as in (A); β-actin: loading control. (D–E) Lysosomal alterations in treatment groups versus untreated control as visualized by transmission electron microscopy (magnification, D: 10,000 ×; E: 25,000 ×). L (Osmiophilic vesicles indicative of lysosomal lipofuscin accumulation; M (mitochondrion); N (nucleus). (F). Immunoblot detection of autophagic-lysosomal marker proteins (Lamp-1, LC3-I, LC3-II, p62); groups as in (A); β-actin: loading control. (G) mRNA transcript levels of genes encoding autophagic-lysosomal markers (LAMP1, SQSTM1) detected by quantitative RTP-CR; groups as in (A). All bar graphs represent data as means ± SD (n=3).
Strikingly, only dual genetic antagonism (targeting both CTSB and CTSL expression) induced dramatic autophagic-lysosomal alterations (Fig. 4D–F) that mimicked those caused by UVA exposure (Fig. 3A–B and Fig. 4 D–E), an observation substantiated by EM and immunoblot analysis as employed before [28,29]. In contrast, single knockdown (targeting CTSB or CTSL only) did not reproduce the UVA-induced phenotype (Fig. 4D–F). Specifically, immunoblot analysis revealed similar accumulation of lysosomal-associated membrane protein (Lamp-1), an established lysosomal marker, in siCTSB/L- (Fig. 4F) or UVA-treated cells (Fig. 3B), a finding further confirmed by EM that showed increased levels of lysosomal vesicles containing lipofuscin-like osmiophilic material (Figs. 3A and 4D–E). Similarly, consistent with autophagic-lysosomal impairment downstream of cathepsin inactivation, increased cellular levels of the autophagic cargo receptor and substrate p62, and the autophagosome component LC3-II were detected in siCTSB/L- (Fig. 4F) or UVA-treated cells (Fig. 3B), changes that occurred exclusively at the protein but not mRNA level (Fig. 4G).
Taken together, these data indicate that only dual genetic antagonism (suppressing both CTSB and CTSL expression in unirradiated cells) reproduces UVA-induced autophagic-lysosomal alterations. The fact that single knockdown (targeting CTSB or CTSL only) did not reproduce the UVA-induced phenotype suggests a compensatory relationship between cathepsin B and L in dermal fibroblasts, a finding consistent with observations in other cellular systems, where either cathepsin can maintain lysosomal function in the absence of the other, and functional deficits become apparent if both cathepsin B and L are compromised [45,46].
4. Discussion
Recent research has substantiated a causative role of cathepsins, a family of lysosomal proteases, in UV-induced cutaneous alterations and photodamage [47,48] [49–52]. Recently, we have identified the lysosomal cysteine protease cathepsin B as a novel target of UVA-induced photooxidative stress in cultured human Hs27 skin fibroblasts, a finding consistent with other research suggesting a mechanistic involvement of UVA-induced cathepsin B impairment in skin photoaging [28,29,53,54].
The data presented here demonstrate for the first time that repetitive exposure to solar simulated UVA at noncytotoxic doses inhibits not only cathepsin B but also causes similar impairment of cathepsin L enzymatic activity in dermal fibroblasts, while only minor UVA-effects were observed in primary keratinocytes and melanocytes (Fig. 1). The molecular mechanism underlying differential UVA sensitivity of dermal fibroblasts, primary keratinocytes, and primary melanocytes to UVA-induced cathepsin B and L inactivation remains unknown and awaits further mechanistic examination, even though it may be speculated that differences in constitutive antioxidant defense capacity may be involved. In our follow up pilot experiments performed in mouse embryonal fibroblasts (MEFs) isolated from Nrf2 wildtype and KO mice, we observed that the absence of Nrf2 caused hypersensitivity to UVA-induced inhibition of cathepsin activity, consistent with the role of the Nrf2-orchestrated cellular antioxidant defense in antagonizing UVA-induced cathepsin inactivation in fibroblasts (data not shown). Moreover, it has been demonstrated earlier that human skin keratinocytes are less susceptible to UVA-induced oxidative stress and lipid peroxidation than dermal fibroblasts [55,56], and it has been documented that cathepsin B in melanocytes is localized in part in melanosomes potentially providing melanin-based protection from UVA-induced inactivation [57].
Earlier research has demonstrated that cathepsin deficiency may cause impairment of autophagic-lysosomal function: Genetic suppression of cathepsin B [macrophages from CTSB-deficient (CTSB−/−) mice] is sufficient to cause delay of autophagic flux, and cathepsin L deficiency impairs turnover of autophagolysosomes observable in CTSL−/− mice. Our earlier research has provided evidence that pharmacological cathepsin inactivation mimics UVA-induced autophagic-lysosomal dysfunction in human dermal fibroblasts [28,29]. In order to further elucidate the relative mechanistic involvement of the UVA-target cathepsins B and L in dermal fibroblasts (Fig. 1), we therefore examined effects of single and combinatorial genetic antagonism on lysosomal morphology and function (Fig. 4). Remarkably, only dual genetic antagonism (suppressing both CTSB and CTSL expression in unirradiated cells) mimics UVA-induced autophagic-lysosomal alterations (Fig. 3A–B and 4D–F), whereas single genetic antagonism (siCTSB or siCTSL only) does not reproduce the UVA-induced phenotype. Consistent with these findings, we were able to demonstrate that the pharmacological inhibitor CA074Me, shown earlier to cause ‘UVA-mimetic’ effects on autophagic-lysosomal function, impairs both cathepsin B and L activity in fibroblasts (Fig. 3D). Taken together, these data provide mechanistic evidence that dual inactivation of both enzymes, cathepsin B and L, is the necessary and sufficient causative event underlying impairment of lysosomal function in UVA-exposed dermal fibroblasts, a molecular scenario consistent with earlier findings that document functional redundancy between cathepsin B and L. For example, CTSB/CTSL KO mice display severe neurodegenerative dysfunction that can be rescued by expression of cathepsin L [45]. Interestingly, in mice lacking both cathepsin B and L neurodegeneration is preceded by an accumulation of lipofuscin and lysosomal bodies in large cortical neurons [58], a finding reminiscent of the ultrastructural changes observed by us in dermal fibroblasts exposed to either UVA-, combined CTSB/CTSL-directed genetic knockdown, or CA074Me-based dual pharmacological inhibition (Figs. 3–4) [28,29].
Our data suggest that UVA-induced changes affecting cathepsin B and L enzymatic activity may occur as a result of specific molecular mechanisms that do not cause the indiscriminate inactivation of other cysteine-dependent enzymes such as GAPDH (Fig. 1A). Inactivation of cysteine-cathepsins in response to electrophilic stressors (including dye sensitization/singlet oxygen, nitroxyl, and reactive carbonyl species such as 4-HNE, methylglyoxal, and glyoxal) has been documented earlier, and it has been shown that protein hydroperoxides impair enzymatic activity of cathepsins B and L (but not D) in macrophages [35,39–42,59]. Our own immunoblot analysis performed on immunoprecipitated cathepsin B from UVA-exposed fibroblasts versus control cells is consistent with the UVA-induced covalent adduction of cathepsin B by the lipid peroxidation product 4-HNE (Fig. 2D), but no experiments were performed that would identify the specific site of aduction. If operative at active site cysteine (or histidine) residues, 4-HNE-adduction may indeed represent the mechanistic determinant of cathepsin B photooxidative inactivation by UVA, a hypothesis consistent with other reports describing cathepsin adduction and inactivation by lipid peroxidation products including 4-HNE observed in non-cutaneous cell types [39,41]. A more stringent proteomic determination of chemical nature and site of cathepsin B (and potentially L) adduction in UVA-exposed fibroblasts is currently being pursued in our laboratory. It should be mentioned that in addition to direct photooxidative target alteration it is also possible that other molecular mechanisms contribute to UVA-induced functional alterations affecting cathepsins in dermal fibroblasts. For example, recent research indicates that UVA exposure can cause alternate trafficking of cathepsin L in dermal fibroblasts leading to extracellular release [51]. However, using ELISA-based detection in cell culture medium, we were unable to observe UVA-induced cathepsin B/L secretion from fibroblasts exposed to our treatment regimen (data not shown).
It remains to be examined if other redox-sensitive cysteine-dependent cathepsins that are important for cutaneous function (including cathepsins K, S, C, and V) are subject to UVA-induced inactivation. Indeed, our own unpublished data indicate that cathepsin K, another cysteine-dependent protease involved in cutaneous elastin processing [49], displays analogous UVA-sensitivity, losing enzymatic activity in dermal fibroblasts exposed to UVA dose regimens employed in this study (data not shown). Interestingly, even non-cysteine-dependent cathepsins have been shown to be inactivated in response to oxidative stress, an effect thought to originate from reactive oxygen dependent modification of non-catalytic redox-sensitive amino acids. For example, in neutrophils cathepsin G, a serine-dependent cathepsin, activity is lost with conversion of methionine to methionine-sulfoxide [60]. It is therefore possible that the moderate loss of activity observed by us with the aspartate-cathepsin cathepsin D in response to UVA may result from similar redox modifications. Similarly, general lysosomal dysfunction downstream of cathepsin B and L inactivation may impair proteolytic processing affecting non-cysteine-dependent cathepsins and possibly other lysosomal enzymes.
Guided by the emerging inhibitory effects of UVA on lysosomal proteolysis and function mediated through cathepsin inactivation, we also examined the possibility that repetitive delivery of low doses of UVA may impair another major intracellular proteolytic system, the proteasome (Fig. 2E). Indeed, it has been reported earlier that acute exposure to UVA delivered at a dose range up to 120 J/cm2 impairs proteasomal chymotrypsin-like activity in cultured fibroblasts and human skin [21]. Enzyme inhibition is thought to occur as an indirect effect attributed to UVA-induced accumulation of oxidatively damaged carbonylated and crosslinked protein aggregates known to impair proteasome-dependent proteolysis [21,61,62]. Our experimentation indicates that repetitive exposure to non-lethal UVA doses causes upregulation of all three proteasomal activities (i.e. chymotrypsin-, trypsin- and caspase (PGPH)-like; Fig. 2E) suggesting that UVA-induced autophagic-lysosomal alterations (e.g. accumulation of LAMP1, LC3-II, and p62) are not paralleled by proteasomal impairment. Upregulation of proteasome activity is an important adaptation to increased oxidative stress, and recent research indicates that the proteasome system is under Nrf2-dependent transcriptional control [63]. It is therefore tempting to speculate that upregulation of proteasomal enzymatic activity as observed in our experiments may occur in the context of UVA-induced Nrf2 activation [36,37], observed by us at the protein level and substantiated further by upregulation of Nrf2-regulated genes encoding antioxidant enzymes (SRXN1, TXNRD1, PRDX1, HMOX; Fig. 2A–B). In addition to stress-induced upregulation of proteasomal gene expression, direct redox activation of the 20S proteasome may occur through posttranslational glutathionylation under conditions of non-lethal oxidative stress, an emerging regulatory mechanism potentially underlying the efficient removal of oxidized or unfolded proteins that might also be involved in UVA-induced proteasomal activation [64]. However, the molecular mechanism underlying upregulated proteasome activity and its potential function as a compensatory mechanism balancing oxidative UVA challenge and lysosomal proteolytic imapirment remains to be explored.
Earlier research has suggested a role of UVA in skin photodamage based on photo-oxidative rupture of lysosomal membranes followed by cytoplasmic release of proteases including cathepsin B involved in apoptosis and degradation of ferritin with mobilization of redox-active iron, phenomena observed upon single exposure to UVA (or combined UVA/B) at high cytotoxic fluences [47,48,65]. Based on our data we hypothesize that repetitive exposure to solar UVA delivered at doses that do not compromise viability contributes to photodamage in dermal fibroblasts through a ‘double-hit’ mechanism: UVA not only inflicts primary photo-oxidative insult on cellular components but also causes dual inactivation of cathepsin B and L upstream of impaired autophagic-lysosomal proteolytic clearance of damaged molecules and organelles, a novel molecular pathway as envisioned in Fig. 5. Given the complex role of cathepsin-dependent lysosomal proteolysis in cellular protein homeostasis, maintenance of autophagic flux, and endosomal trafficking, the impact of UVA-induced impairment of cathepsin B and L on dermal fibroblast function and its mechanistic implications for photocarcinogenesis and photoaging remain to be substantiated by future experimentation in cell culture and live human skin.
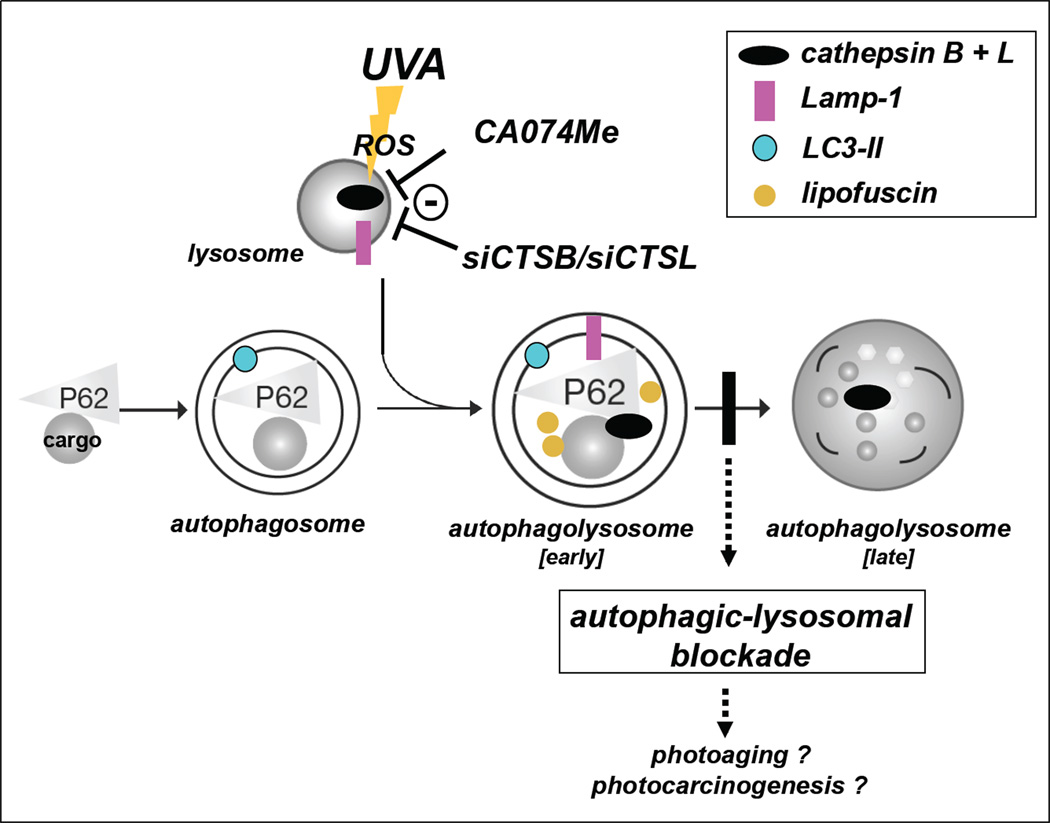
Figure 5. Cathepsin B and L as critical molecular targets of UVA-induced cutaneous photooxidative stress impairing autophagic-lysosomal function.
Our data suggest that inactivation of cysteine-dependent, redox-sensitive lysosomal proteases (cathepsin B and L) may occur as a consequence of (i) UVA-induced photooxidative stress mediated through reactive oxygen species (ROS) or (ii) pharmacological (CA074Me) or (iii) genetic (siCTSB/siCTSL) antagonism, causing autophagic-lysosomal blockade in human dermal fibroblasts. In the early autophagolysosome, formed upon fusion between lysosome and autophagosome, loss of cathepsin B and L enzymatic activity causes lysosomal expansion and induces accumulation of Lamp-1, LC3-II, and autophagic cargo proteins (e.g. p62). The vertical black bar indicates the resulting autophagic-lysosomal blockade suppressing the formation of late autophagolysosomes. Dysfunctional and peroxidized cargo that remains undigested now forms autofluorescent lipofuscin pigments that further compromise lysosomal function. The potential role of cathepsin B/L inhibition induced by chronic UVA exposure in cutaneous photoaging and photocarcinogenesis remains to be established.
UVA selectively inactivates lysosomal cysteine-proteases (cathepsin B and L)
Chronic UVA causes lysosomal dysfunction in skin fibroblasts
Dual genetic antagonism (siCTSB/CTSL) mimics UVA-induced lysosomal alterations
Single knockdown (siCTSB or siCTSL only) does not display ‘UVA-mimetic’ effects
Cathepsin B/L dual inactivation is a causative factor in lysosomal impairment by UVA
Acknowledgements
Supported in part by grants from the National Institutes of Health [R01CA122484, R03CA167580, ES007091, ES006694, Arizona Cancer Center Support Grant CA023074].
Abbreviations
- AV
annexin V
- CTSB
cathepsin B
- CTSL
cathepsin L
- CTSD
cathepsin D
- DC
double chain
- DMEM
Dulbecco’s modified Eagle’s medium
- EM
electron microscopy
- FITC
fluorescein isothiocyanate
- HEK
primary human epidermal keratinocyte
- HEMa
primary human epidermal melanocyte
- 4-HNE
4-hydroxynonenal
- HO-1
heme oxygenase 1
- Lamp-1
lysosomal-associated membrane protein
- NAC
N-acetyl-L-cysteine
- PGPH
peptidylglutamylpeptide hydrolyzing activity
- PI
propidium iodide
- ROS
reactive oxygen species
- SC
single chain
- UVA
ultraviolet A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Preliminary data from this research were part of an oral presentation at the 36th Meeting of the American Society for Photobiology, June 23–28, 2012, in Montreal, Canada. Flow cytometric analysis was performed at the Arizona Cancer Center flow cytometry core facility.
References
- 1.de Laat A, van der Leun JC, de Gruijl FR. Carcinogenesis induced by UVA (365-nm) radiation: the dose-time dependence of tumor formation in hairless mice. Carcinogenesis. 1997;18:1013–1020. doi: 10.1093/carcin/18.5.1013. [DOI] [PubMed] [Google Scholar]
- 2.Wlaschek M, Tantcheva-Poor I, Naderi L, Ma W, Schneider LA, Razi-Wolf Z, Schuller J, Scharffetter-Kochanek K. Solar UV irradiation and dermal photoaging. J Photochem Photobiol B. 2001;63:41–51. doi: 10.1016/s1011-1344(01)00201-9. [DOI] [PubMed] [Google Scholar]
- 3.Runger TM. How different wavelengths of the ultraviolet spectrum contribute to skin carcinogenesis: the role of cellular damage responses. J Invest Dermatol. 2007;127:2103–2105. doi: 10.1038/sj.jid.5700988. [DOI] [PubMed] [Google Scholar]
- 4.Cadet J, Douki T, Ravanat JL, Di Mascio P. Sensitized formation of oxidatively generated damage to cellular DNA by UVA radiation. Photochem Photobiol Sci. 2009;8:903–911. doi: 10.1039/b905343n. [DOI] [PubMed] [Google Scholar]
- 5.Zastrow L, Groth N, Klein F, Kockott D, Lademann J, Renneberg R, Ferrero L. The missing link--light-induced (280-1,600 nm) free radical formation in human skin. Skin Pharmacol Physiol. 2009;22:31–44. doi: 10.1159/000188083. [DOI] [PubMed] [Google Scholar]
- 6.Runger TM, Farahvash B, Hatvani Z, Rees A. Comparison of DNA damage responses following equimutagenic doses of UVA and UVB: a less effective cell cycle arrest with UVA may render UVA-induced pyrimidine dimers more mutagenic than UVB-induced ones. Photochem Photobiol Sci. 2012;11:207–215. doi: 10.1039/c1pp05232b. [DOI] [PubMed] [Google Scholar]
- 7.Marrot L, Meunier JR. Skin DNA photodamage and its biological consequences. J Am Acad Dermatol. 2008;58:S139–S148. doi: 10.1016/j.jaad.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Kostyuk V, Potapovich A, Stancato A, De Luca C, Lulli D, Pastore S, Korkina L. Photo-oxidation products of skin surface squalene mediate metabolic and inflammatory responses to solar UV in human keratinocytes. PLoS One. 2012;7:e44472. doi: 10.1371/journal.pone.0044472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheller M, Jones AM. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: A role for UVA in human skin carcinogenesis. PNAS. 2004;101:4954–4959. doi: 10.1073/pnas.0401141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girotti AW, Kriska T. Role of lipid hydroperoxides in photo-oxidative stress signaling. Antioxid Redox Signal. 2004;6:301–310. doi: 10.1089/152308604322899369. [DOI] [PubMed] [Google Scholar]
- 11.Scharffetter-Kochanek K, Wlaschek M, Brenneisen P, Schauen M, Blaudschun R, Wenk J. UV-induced reactive oxygen species in photocarcinogenesis and photoaging. Biol Chem. 1997;378:1247–1257. [PubMed] [Google Scholar]
- 12.Wondrak GT, Jacobson MK, Jacobson EL. Endogenous UVA-photosensitizers: mediators of skin photodamage and novel targets for skin photoprotection. Photochem Photobiol Sci. 2006;5:215–237. doi: 10.1039/b504573h. [DOI] [PubMed] [Google Scholar]
- 13.Cadet J, Mouret S, Ravanat JL, Douki T. Photoinduced damage to cellular DNA: direct and photosensitized reactions. Photochem Photobiol. 2012;88:1048–1065. doi: 10.1111/j.1751-1097.2012.01200.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Bowden GT. Activation of p38 MAP kinase and JNK pathways by UVA irradiation. Photochem Photobiol Sci. 2012;11:54–61. doi: 10.1039/c1pp05133d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djavaheri-Mergny M, Pieraggi MT, Maziere C, Santus R, Lageron A, Salvayre R, Dubertret L, Maziere JC. Early alterations of actin in cultured human keratinocytes and fibroblasts exposed to long-wavelength radiations. Possible involvement in the UVA-induced perturbations of endocytotic processes. Photochem Photobiol. 1994;59:48–52. doi: 10.1111/j.1751-1097.1994.tb05000.x. [DOI] [PubMed] [Google Scholar]
- 16.Thiele JJ, Hsieh SN, Briviba K, Sies H. Protein oxidation in human stratum corneum: susceptibility of keratins to oxidation in vitro and presence of a keratin oxidation gradient in vivo. J Invest Dermatol. 1999;113:335–339. doi: 10.1046/j.1523-1747.1999.00693.x. [DOI] [PubMed] [Google Scholar]
- 17.Klotz LO, Kroncke KD, Sies H. Singlet oxygen-induced signaling effects in mammalian cells. Photochem Photobiol Sci. 2003;2:88–94. doi: 10.1039/b210750c. [DOI] [PubMed] [Google Scholar]
- 18.Wondrak GT, Roberts MJ, Cervantes-Laurean D, Jacobson MK, Jacobson EL. Proteins of the Extracellular Matrix Are Sensitizers of Photo-oxidative Stress in Human Skin Cells. J Invest Dermatol. 2003;121:578–586. doi: 10.1046/j.1523-1747.2003.12414.x. [DOI] [PubMed] [Google Scholar]
- 19.Gulati P, Markova B, Gottlicher M, Bohmer FD, Herrlich PA. UVA inactivates protein tyrosine phosphatases by calpain-mediated degradation. EMBO Rep. 2004;5:812–817. doi: 10.1038/sj.embor.7400190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voss P, Hajimiragha H, Engels M, Ruhwiedel C, Calles C, Schroeder P, Grune T. Irradiation of GAPDH: a model for environmentally induced protein damage. Biol Chem. 2007;388:583–592. doi: 10.1515/BC.2007.068. [DOI] [PubMed] [Google Scholar]
- 21.Catalgol B, Ziaja I, Breusing N, Jung T, Hohn A, Alpertunga B, Schroeder P, Chondrogianni N, Gonos ES, Petropoulos I, Friguet B, Klotz LO, Krutmann J, Grune T. The proteasome is an integral part of solar ultraviolet a radiation-induced gene expression. J Biol Chem. 2009;284:30076–30086. doi: 10.1074/jbc.M109.044503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linetsky M, Chemoganskiy VG, Hu F, Ortwerth BJ. Effect of UVA light on the activity of several aged human lens enzymes. Invest Ophthalmol Vis Sci. 2003;44:264–274. doi: 10.1167/iovs.02-0597. [DOI] [PubMed] [Google Scholar]
- 23.He YY, Council SE, Feng L, Chignell CF. UVA-induced cell cycle progression is mediated by a disintegrin and metalloprotease/epidermal growth factor receptor/AKT/Cyclin D1 pathways in keratinocytes. Cancer Res. 2008;68:3752–3758. doi: 10.1158/0008-5472.CAN-07-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giordani A, Martin ME, Beaumont C, Santus R, Morliere P. Inactivation of iron responsive element-binding capacity and aconitase function of iron regulatory protein-1 of skin cells by ultraviolet A. Photochem Photobiol. 2000;72:746–752. doi: 10.1562/0031-8655(2000)072<0746:ioireb>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Smit N, Musson R, Romijn F, van Rossum H, van Pelt J. Effects of ultraviolet A-1 radiation on calcineurin activity and cytokine production in (skin) cell cultures. Photochem Photobiol. 2010;86:360–366. doi: 10.1111/j.1751-1097.2009.00650.x. [DOI] [PubMed] [Google Scholar]
- 26.Musson RE, Hensbergen PJ, Westphal AH, Temmink WP, Deelder AM, van Pelt J, Mullenders LH, Smit NP. UVA1 radiation inhibits calcineurin through oxidative damage mediated by photosensitization. Free Radic Biol Med. 2011;50:1392–1399. doi: 10.1016/j.freeradbiomed.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Pattison DI, Rahmanto AS, Davies MJ. Photo-oxidation of proteins. Photochem Photobiol Sci. 2012;11:38–53. doi: 10.1039/c1pp05164d. [DOI] [PubMed] [Google Scholar]
- 28.Lamore SD, Qiao S, Horn D, Wondrak GT. Proteomic identification of cathepsin B and nucleophosmin as novel UVA-targets in human skin fibroblasts. Photochem Photobiol. 2010;86:1307–1317. doi: 10.1111/j.1751-1097.2010.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamore SD, Wondrak GT. Autophagic-lysosomal dysregulation downstream of cathepsin B inactivation in human skin fibroblasts exposed to UVA. Photochem Photobiol Sci. 2012;11:163–172. doi: 10.1039/c1pp05131h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamore SD, Cabello CM, Wondrak GT. The topical antimicrobial zinc pyrithione is a heat shock response inducer that causes DNA damage and PARP-dependent energy crisis in human skin cells. Cell Stress Chaperones. 2010;15:309–322. doi: 10.1007/s12192-009-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werle B, Ebert W, Klein W, Spiess E. Assessment of cathepsin L activity by use of the inhibitor CA-074 compared to cathepsin B activity in human lung tumor tissue. Biol Chem Hoppe Seyler. 1995;376:157–164. doi: 10.1515/bchm3.1995.376.3.157. [DOI] [PubMed] [Google Scholar]
- 32.Levicar N, Dewey RA, Daley E, Bates TE, Davies D, Kos J, Pilkington GJ, Lah TT. Selective suppression of cathepsin L by antisense cDNA impairs human brain tumor cell invasion in vitro and promotes apoptosis. Cancer Gene Ther. 2003;10:141–151. doi: 10.1038/sj.cgt.7700546. [DOI] [PubMed] [Google Scholar]
- 33.Moravec RA, O'Brien MA, Daily WJ, Scurria MA, Bernad L, Riss TL. Cell-based bioluminescent assays for all three proteasome activities in a homogeneous format. Anal Biochem. 2009;387:294–302. doi: 10.1016/j.ab.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Cabello CM, Bair WB, 3rd, Lamore SD, Ley S, Bause AS, Azimian S, Wondrak GT. The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radic Biol Med. 2009;46:220–231. doi: 10.1016/j.freeradbiomed.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahmanto AS, Morgan PE, Hawkins CL, Davies MJ. Cellular effects of photo-generated oxidants and long-lived, reactive, hydroperoxide photoproducts. Free Radic Biol Med. 2010 doi: 10.1016/j.freeradbiomed.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 36.Hirota A, Kawachi Y, Itoh K, Nakamura Y, Xu X, Banno T, Takahashi T, Yamamoto M, Otsuka F. Ultraviolet A irradiation induces NF-E2-related factor 2 activation in dermal fibroblasts: protective role in UVA-induced apoptosis. J Invest Dermatol. 2005;124:825–832. doi: 10.1111/j.0022-202X.2005.23670.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhong JL, Edwards GP, Raval C, Li H, Tyrrell RM. The role of Nrf2 in ultraviolet A mediated heme oxygenase 1 induction in human skin fibroblasts. Photochem Photobiol Sci. 2010;9:18–24. doi: 10.1039/b9pp00068b. [DOI] [PubMed] [Google Scholar]
- 38.Komori R, Taniguchi M, Ichikawa Y, Uemura A, Oku M, Wakabayashi S, Higuchi K, Yoshida H. Ultraviolet a induces endoplasmic reticulum stress response in human dermal fibroblasts. Cell Struct Funct. 2012;37:49–53. doi: 10.1247/csf.11041. [DOI] [PubMed] [Google Scholar]
- 39.Crabb JW, O'Neil J, Miyagi M, West K, Hoff HF. Hydroxynonenal inactivates cathepsin B by forming Michael adducts with active site residues. Protein Sci. 2002;11:831–840. doi: 10.1110/ps.4400102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng J, Dunlop RA, Rodgers KJ, Davies MJ. Evidence for inactivation of cysteine proteases by reactive carbonyls via glycation of active site thiols. Biochem J. 2006;398:197–206. doi: 10.1042/BJ20060019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krohne TU, Kaemmerer E, Holz FG, Kopitz J. Lipid peroxidation products reduce lysosomal protease activities in human retinal pigment epithelial cells via two different mechanisms of action. Exp Eye Res. 2010;90:261–266. doi: 10.1016/j.exer.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Mirkovic B, Sosic I, Gobec S, Kos J. Redox-based inactivation of cysteine cathepsins by compounds containing the 4-aminophenol moiety. PLoS One. 2011;6:e27197. doi: 10.1371/journal.pone.0027197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mach L, Schwihla H, Stuwe K, Rowan AD, Mort JS, Glossl J. Activation of procathepsin B in human hepatoma cells: the conversion into the mature enzyme relies on the action of cathepsin B itself. Biochem J. 1993;293(Pt 2):437–442. doi: 10.1042/bj2930437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montaser M, Lalmanach G, Mach L. CA-074, but not its methyl ester CA-074Me, is a selective inhibitor of cathepsin B within living cells. Biol Chem. 2002;383:1305–1308. doi: 10.1515/BC.2002.147. [DOI] [PubMed] [Google Scholar]
- 45.Sevenich L, Pennacchio LA, Peters C, Reinheckel T. Human cathepsin L rescues the neurodegeneration and lethality in cathepsin B/L double-deficient mice. Biol Chem. 2006;387:885–891. doi: 10.1515/BC.2006.112. [DOI] [PubMed] [Google Scholar]
- 46.Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120:3421–3431. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pourzand C, Watkin RD, Brown JE, Tyrrell RM. Ultraviolet A radiation induces immediate release of iron in human primary skin fibroblasts: the role of ferritin. Proc Natl Acad Sci U S A. 1999;96:6751–6756. doi: 10.1073/pnas.96.12.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basu-Modak S, Ali D, Gordon M, Polte T, Yiakouvaki A, Pourzand C, Rice-Evans C, Tyrrell RM. Suppression of UVA-mediated release of labile iron by epicatechin--a link to lysosomal protection. Free Radic Biol Med. 2006;41:1197–1204. doi: 10.1016/j.freeradbiomed.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Codriansky KA, Quintanilla-Dieck MJ, Gan S, Keady M, Bhawan J, Runger TM. Intracellular degradation of elastin by cathepsin K in skin fibroblasts--a possible role in photoaging. Photochem Photobiol. 2009;85:1356–1363. doi: 10.1111/j.1751-1097.2009.00592.x. [DOI] [PubMed] [Google Scholar]
- 50.Cavarra E, Fimiani M, Lungarella G, Andreassi L, de Santi M, Mazzatenta C, Ciccoli L. UVA light stimulates the production of cathepsin G and elastase-like enzymes by dermal fibroblasts: a possible contribution to the remodeling of elastotic areas in sun-damaged skin. Biol Chem. 2002;383:199–206. doi: 10.1515/BC.2002.020. [DOI] [PubMed] [Google Scholar]
- 51.Klose A, Wilbrand-Hennes A, Brinckmann J, Hunzelmann N. Alternate trafficking of cathepsin L in dermal fibroblasts induced by UVA radiation. Exp Dermatol. 2010;19:e117–e123. doi: 10.1111/j.1600-0625.2009.01014.x. [DOI] [PubMed] [Google Scholar]
- 52.Son ED, Shim JH, Choi H, Kim H, Lim KM, Chung JH, Byun SY, Lee TR. Cathepsin G inhibitor prevents ultraviolet B-induced photoaging in hairless mice via inhibition of fibronectin fragmentation. Dermatology. 2012;224:352–360. doi: 10.1159/000339337. [DOI] [PubMed] [Google Scholar]
- 53.Lai W, Zheng Y, Ye ZZ, Su XY, Wan MJ, Gong ZJ, Xie XY, Liu W. Changes of cathepsin B in human photoaging skin both in vivo and in vitro. Chin Med J (Engl) 2010;123:527–531. [PubMed] [Google Scholar]
- 54.Zheng Y, Lai W, Wan M, Maibach HI. Expression of cathepsins in human skin photoaging. Skin Pharmacol Physiol. 2011;24:10–21. doi: 10.1159/000314725. [DOI] [PubMed] [Google Scholar]
- 55.Moysan A, Clement-Lacroix P, Michel L, Dubertret L, Morliere P. Effects of ultraviolet A and antioxidant defense in cultured fibroblasts and keratinocytes. Photodermatol Photoimmunol Photomed. 1996;11:192–197. doi: 10.1111/j.1600-0781.1995.tb00168.x. [DOI] [PubMed] [Google Scholar]
- 56.Marionnet C, Pierrard C, Lejeune F, Sok J, Thomas M, Bernerd F. Different oxidative stress response in keratinocytes and fibroblasts of reconstructed skin exposed to non extreme daily-ultraviolet radiation. PLoS One. 2010;5:e12059. doi: 10.1371/journal.pone.0012059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diment S, Eidelman M, Rodriguez GM, Orlow SJ. Lysosomal hydrolases are present in melanosomes and are elevated in melanizing cells. J Biol Chem. 1995;270:4213–4215. doi: 10.1074/jbc.270.9.4213. [DOI] [PubMed] [Google Scholar]
- 58.Felbor U, Kessler B, Mothes W, Goebel HH, Ploegh HL, Bronson RT, Olsen BR. Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc Natl Acad Sci U S A. 2002;99:7883–7888. doi: 10.1073/pnas.112632299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagaoka Y, Otsu K, Okada F, Sato K, Ohba Y, Kotani N, Fujii J. Specific inactivation of cysteine protease-type cathepsin by singlet oxygen generated from naphthalene endoperoxides. Biochem Biophys Res Commun. 2005;331:215–223. doi: 10.1016/j.bbrc.2005.03.146. [DOI] [PubMed] [Google Scholar]
- 60.Shao B, Belaaouaj A, Verlinde CL, Fu X, Heinecke JW. Methionine sulfoxide and proteolytic cleavage contribute to the inactivation of cathepsin G by hypochlorous acid: an oxidative mechanism for regulation of serine proteinases by myeloperoxidase. J Biol Chem. 2005;280:29311–29321. doi: 10.1074/jbc.M504040200. [DOI] [PubMed] [Google Scholar]
- 61.Sommerburg O, Ullrich O, Sitte N, von Zglinicki D, Siems W, Grune T. Dose- and wavelength-dependent oxidation of crystallins by UV light--selective recognition and degradation by the 20S proteasome. Free Radic Biol Med. 1998;24:1369–1374. doi: 10.1016/s0891-5849(98)00012-4. [DOI] [PubMed] [Google Scholar]
- 62.Grune T, Jung T, Merker K, Davies KJ. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and 'aggresomes' during oxidative stress, aging, and disease. Int J Biochem Cell Biol. 2004;36:2519–2530. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 63.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28alphabeta regulator are required for adaptation to oxidative stress. J Biol Chem. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Silva GM, Netto LE, Simoes V, Santos LF, Gozzo FC, Demasi MA, Oliveira CL, Bicev RN, Klitzke CF, Sogayar MC, Demasi M. Redox control of 20S proteasome gating. Antioxid Redox Signal. 2012;16:1183–1194. doi: 10.1089/ars.2011.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bivik CA, Larsson PK, Kågedal KM, Rosdahl IK, Ollinger KM. UVA/B-induced apoptosis in human melanocytes involves translocation of cathepsins and Bcl-2 family members. J Invest Dermatol. 2006;126:1119–1127. doi: 10.1038/sj.jid.5700124. [DOI] [PubMed] [Google Scholar]