Abstract
The retinoblastoma protein (Rb)/E2F pathway links cellular proliferation control to apoptosis and is critical for normal development and cancer prevention. Here we define a transcription-mediated pathway in which deregulation of E2F1 by ectopic E2F expression or Rb inactivation by E7 of human papillomavirus type 16 signals apoptosis by inducing the expression of Chk2, a component of the DNA damage response. E2F1- and E7-mediated apoptosis are compromised in cells from patients with the related disorders ataxia telangiectasia and Nijmegen breakage syndrome lacking functional Atm and Nbs1 gene products, respectively. Both Atm and Nbs1 contribute to Chk2 activation and p53 phosphorylation following deregulation of normal Rb growth control. E2F2, a related E2F family member that does not induce apoptosis, also activates Atm, resulting in phosphorylation of p53. However, we found that the key commitment step in apoptosis induction is the ability of E2F1, and not E2F2, to upregulate Chk2 expression. Our results suggest that E2F1 plays a central role in signaling disturbances in the Rb growth control pathway and, by upregulation of Chk2, may sensitize cells to undergo apoptosis.
Multiple signaling pathways activated by cellular stresses converge on the p53 tumor suppressor. As a result of activating p53, a cell will either undergo a growth arrest or commit programmed cell death. Loss or mutation of p53 or the components that regulate p53 can predispose cells to neoplastic transformation (2, 40).
Stressors known to activate p53 include hypoxia, DNA damage, and the expression of cellular or viral oncoproteins (24, 69, 85). In response to these cellular stressors, p53 is covalently modified, including phosphorylation at numerous N- and C-terminal serine residues and acetylation on C-terminal lysine residues (4). Several cellular kinases that play critical roles in the activation of p53 following DNA damage have been identified. These kinases include Atm, the kinase mutated in ataxia telangiectasia (AT), Atr, the Atm- and Rad3-related kinase, and their downstream kinase substrates, checkpoint kinase 1 (Chk1) and checkpoint kinase 2 (Chk2) (1, 78). The role of Atr in regulating the p53 response to DNA damage is not well understood due to the early embryonic lethality of atr−/− mouse embryos (12, 20). However, it has been proposed that Atr is activated in response to certain types of DNA damage and can phosphorylate p53 on serine 15 (14, 26, 45, 48). Atr is also able to phosphorylate and activate Chk1 (25, 30, 64), which can subsequently phosphorylate the N terminus of p53 (77).
The role of Atm in activating p53 following DNA damage is better understood. In response to gamma-irradiation or genotoxic drugs that induce DNA double-strand breaks, Atm is activated and can directly phosphorylate p53 at serine 15 (44). In cells from AT patients, there is a delay in activation of p53 following gamma-irradiation (79). In addition to directly phosphorylating p53, Atm can phosphorylate and activate the human checkpoint kinase Chk2 (11, 16, 55-57). Chk2 is able to further phosphorylate p53 at additional N-terminal serine residues, including serine 15 and serine 20, causing increased p53 stability and transcriptional activation (17, 33, 77). The importance of Chk2 in this pathway has been demonstrated in dominant negative Chk2-expressing cells (17) and Chk2-deficient mice, which exhibit a defect in apoptosis and a decrease in p53 stabilization in response to gamma-irradiation (82).
While the pathways resulting in p53 activation following DNA damage are beginning to become clear, the pathways leading to p53 activation and apoptosis following cellular or viral oncogene expression remain somewhat elusive. Expression of cellular or viral oncoproteins that promote proliferation, such as c-myc or adenovirus E1A, results in p53-dependent apoptosis (18, 31) and, in the case of c-myc expression, appears to be largely dependent on E2F1 (49). E2F1 is a member of the E2F family of transcription factors that modulate expression of many genes involved in the transition from G1 to S phase of the cell cycle (63). Ectopic expression of E2F1 induces p53-dependent apoptosis in both tissue culture (47, 70, 88) and mouse models (54, 66, 68). Like that of E2F1, expression of the E2F family members E2F2 or E2F3 will also induce quiescent cells to enter S phase, but unlike E2F1 expression, E2F2 and E2F3 expression does not induce apoptosis in fibroblasts (19, 41, 46).
E2F1 signaling to p53 was thought to be through the p19ARF/Mdm2 pathway. p19ARF encodes a protein that modulates the activity of Mdm2, an E3-like ubiquitin ligase that regulates p53 stability by promoting its degradation via the proteasome (22, 23, 28, 35). It has been hypothesized that E2F1 activates p53 by transactivation of p19ARF, thereby alleviating MDM2-promoted degradation of p53 (8, 37, 43, 72) and subsequently committing a cell to apoptosis (61, 67). However, E2F1 has been shown to induce p53-dependent apoptosis in mouse models and in mouse embryo fibroblasts (MEFs) that lack p19ARF (73, 74, 83, 84). Additionally, E2F1 has been shown to induce covalent modification of p53 in the presence or absence of p19ARF (73, 74), and these modifications are associated with E2F1-mediated apoptosis (73).
Having found that E2F1 can induce p53-dependent apoptosis in the absence of p19ARF, we wanted to determine the pathway(s) by which E2F1 activates p53 to induce cell death. Here we define a pathway in which deregulation of E2F1 either by ectopic expression of E2F1 or inactivation of retinoblastoma protein (Rb) family members by human papillomavirus type 16 (HPV-16) E7 signals apoptosis by inducing the expression of Chk2. Additionally, E2F1- and E7-induced apoptosis are compromised in cells from patients with the related disorders AT and Nijmegen breakage syndrome (NBS), diseases involving the lack of functional Atm and Nbs1 gene products, respectively. This loss of apoptosis is coincident with a decrease in the ability of E2F1 and E7 to promote p53 phosphorylation. E2F2, an E2F family member that induces S phase but not apoptosis, also activates Atm, resulting in phosphorylation of p53. However, we find that the key commitment step in apoptosis induction is the ability of E2F1, and not E2F2, to induce Chk2 expression.
MATERIALS AND METHODS
Cell culture.
Primary human dermal fibroblasts GM00316B and GM02270A (normal), GM03395C and GM05823C (AT), and GM07166A (NBS) were obtained from Coriell Cell Repositories, Camden, N.J. Human embryonic lung (HEL) fibroblasts were obtained from the American Type Culture Collection, Manassas, Va. atm−/− and genetically matched wild-type mice were purchased from The Jackson Laboratory, Bar Harbor, Maine, and MEFs were isolated from mouse embryos as described previously (73). Human cells were cultured as recommended by Coriell or the American Type Culture Collection, and MEFs were cultured as described previously (73).
Adenoviral vectors.
Recombinant adenoviral vectors encoding E2F1 and E2F2 have been described previously (19, 47, 75). The Chk1, DN-Chk1, Chk2, DN-Chk2, and HPV-16 E7 recombinant adenoviruses were created by homologous recombination in Escherichia coli (29). DN-Chk1 contains an aspartic acid-to-alanine substitution at position 330. Plasmids encoding Chk2 and DN-Chk2 constructs were generously provided by David Johnson (M. D. Anderson Cancer Center, Smithville, Tex.). DN-Chk2 contains a serine-to-alanine substitution at position 347. A plasmid encoding HPV-16 E7 was generously provided by Karl Munger (Harvard Medical School, Boston, Mass.). Control viruses encode either an empty expression cassette or green fluorescent protein (GFP). Infection with control virus had no effect on the parameters tested relative to mock infection (data not shown). Viruses were propagated in 293 cells and purified by centrifugation through cesium-chloride gradients (73) and titered as described previously (15). All viruses were infected at a multiplicity of infection (MOI) of 1,000 unless otherwise noted. The viral inoculum was then removed and replaced with Dulbecco's modified Eagle medium containing the appropriate serum concentrations and cultured under the conditions described previously (73).
Analysis of apoptosis.
Cells were plated in 10-cm-diameter dishes at 6,000 cells per cm2 or in 24-well plates at 104 cells per well. Virus infections were performed 24 h after plating. At 96 h postinfection, cells were centrifuged at 500 × g for 10 min at 4°C and lysed, and the cell death detection ELISAplus assay was performed as described by the manufacturer (Roche).
Western blot analysis.
Whole-cell extracts were harvested from recombinant adenovirus-infected cells at 24 h postinfection (hpi). Cells were washed twice with cold phosphate-buffered saline and lysed in whole-cell extract buffer (50 mM HEPES, 2 mM magnesium chloride, 250 mM sodium chloride, 0.1 mM EDTA, 1 mM EGTA, 0.1% Nonidet P-40, 1 mM dithiothreitol, 1× mammalian protease inhibitor cocktail [Sigma], 1× phosphatase inhibitor cocktails I and II [Sigma]) by incubation for 30 min on ice followed by sonication. Soluble proteins were separated by centrifugation at 13,000 × g in a microcentrifuge, and supernatants were stored at −70°C. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis and transferred to a polyvinylidene difluoride membrane (Perkin-Elmer) by electroblotting. E2F1 was detected using monoclonal antibody KH-95 (Santa Cruz Biotechnology), and E2F2 was detected using polyclonal antiserum C-20 (Santa Cruz Biotechnology). p53 protein was detected using monoclonal antibody D0-1 (Oncogene Research Products), and the phospho-serine 15 and -serine 20 forms of p53, the phospho-threonine 68 form of Chk2, and the phospho-serine 345 form of Chk1 were detected using polyclonal antisera specific for each modification (Cell Signaling Technologies). Chk1 was detected using polyclonal antiserum FL-476 (Santa Cruz Biotechnology), and Chk2 was detected using polyclonal antiserum H-300 (Santa Cruz Biotechnology) or monoclonal antibody clone no. 7 (Lab Vision Corp.). Atm was detected using polyclonal antiserum Ab-3 (Oncogene Research Products), and the phospho-serine 1981 form of Atm was detected using polyclonal antiserum (Rockland). Actin was detected using polyclonal antiserum I-19 (Santa Cruz Biotechnology). Immunoreactive proteins were detected with a chemiluminescence kit (Perkin-Elmer) according to the manufacturer's recommendations. Actin blots are shown in the figures as protein loading controls. Relative changes in the levels of p53 were estimated from scanned images of Western blots by using Multianalyst software (Bio-Rad).
Northern blot analysis.
Poly(A) RNA was isolated from cells by using a Micro-FastTrack mRNA isolation kit as described by the manufacturer (Invitrogen). Total cellular RNA was isolated using Trizol as described by the manufacturer (Invitrogen). Biotinylated Chk2, Atm, and GAPDH probes were generated by PCR using primers Chk2F (5′-ATGTCTCGGGAGTCGGATGTTG-3′), Chk2R (5′-GCACCACTTCCAAGAGTTTTTGAC-3′), ATMAF (5′-ACGATGCCTTACGGAAGTTGC-3′), ATMAR (5′-GGACAGAGAAGCCAATACTGGACTG-3′), GAPDHF (5′-CAAGGTCATCCATGACAAC-3′), and GAPDHR (5′-TGGTCGTTGAGGGCAATG-3′) as described by the manufacturer (KPL). Hybridized probes were visualized with a chemiluminescence kit as described by the manufacturer (KPL). Blots were sequentially probed and stripped.
RNA interference.
The small interfering RNAs (siRNAs) used in this study were generated by Xeragon, Germantown, Md. siRNA oligonucleotides were transfected into cells at a concentration of 100 nM by using Lipofectamine 2000 (Invitrogen) as described by the manufacturer. All experiments shown were done using siE2F1c, siChk2b, siE2F2a, or siE2F3a. Similar results (data not shown) were obtained using the other siRNAs described below. Control siRNAs (siCon) recognize either GFP or retrovirus long terminal repeat and had no effect on the parameters tested relative to mock transfection: siGFP (5′-CGUAAACGGCCACAAGUUC-3′), siLTR (5′-GAUCCAGCAUAUAAGCAGC-3′), siE2F1a (5′-GGCCCGAUCGAUGUUUUCC-3′), siE2F1b (5′-CUGACCAUCAGUACCUGGC-3′), siE2F1c (5′-GUCACGCUAUGAGACCUCA-3′), siChk2a (5′-CUCCAGCCAGUCCUCUCAC-3′), siChk2b (5′-GAACCUGAGGACCAAGAAC-3′), siE2F2a (5′-GUGCAUCAGAGUGGAUGGC-3′), siE2F2b (5′-CAAGAGGCUGGCCUAUGTG-3′), siE2F3a (5′-AGCGGUCAUCAGUACCUCU-3′), siE2F3b (5′-CUGUUAACCGAGGAUUCAG-3′).
RESULTS
Roles for Atm and Nbs1 in apoptosis induction.
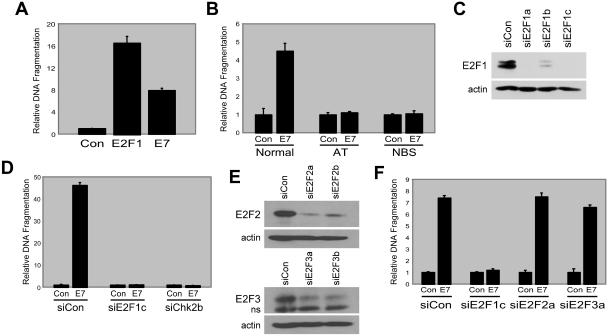
Atm kinase activity is often induced in response to cellular stress, leading to phosphorylation of many substrates, including serine 15 on p53. Given that ectopic E2F1 expression also results in phosphorylation of serine 15 on p53 (73), we determined whether Atm was required for E2F1-mediated apoptosis and p53 phosphorylation. We found that E2F1-mediated apoptosis was compromised in fibroblasts isolated from an AT patient that were ectopically expressing E2F1 (Fig. 1A). Similar results were obtained with AT fibroblasts isolated from a different donor (data not shown). In addition, apoptosis was also reduced following expression of E2F1 in atm−/− MEFs (Fig. 1B). The apoptosis observed in fibroblasts ectopically expressing E2F1 appears to be specific to E2F1 because the related E2F family member, E2F2, was unable to induce apoptosis (Fig. 1A).
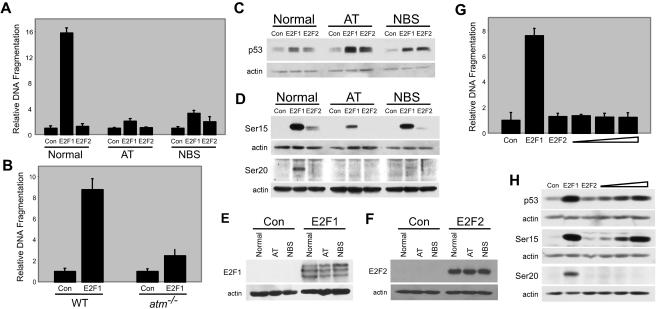
FIG. 1.
Atm and Nbs1 are required for apoptosis induction and p53 phosphorylation. (A) Apoptosis induction in normal human dermal fibroblasts, AT fibroblasts, and NBS fibroblasts. Cells were infected with recombinant adenovirus encoding E2F1 (AdE2F1), E2F2 (AdE2F2), or a control virus (AdCon). In panels A, B, and G, the ordinate axis represents DNA fragmentation relative to control, which is defined as 1, and error bars represent standard deviations calculated from experiments performed in triplicate. (B) Apoptosis induction in wild-type and atm−/− MEFs. Cells were infected with AdE2F1 or AdCon. Cells were harvested and apoptosis was detected at 72 hpi. (C) Western blot analysis of p53 protein levels following infection with AdCon, AdE2F1, or AdE2F2. Cells were harvested and lysates were generated at 24 hpi. (D) Western blot analysis for phospho-serine 15 and phospho-serine 20 forms of p53 in extracts of cells infected as in panel C. (E) Western blot analysis for E2F1 in normal human dermal fibroblasts, AT fibroblasts, and NBS fibroblasts infected with AdCon or AdE2F1. (F) Western blot analysis for E2F2 in normal human dermal fibroblasts, AT fibroblasts, and NBS fibroblasts infected with AdCon or AdE2F2. (G) Apoptosis analysis in normal human fibroblasts infected with AdCon or AdE2F1 at an MOI of 1,000 or with AdE2F2 at MOIs of 1,000, 1,500, 2,000, or 2,500. (H) Western blot analysis of p53, phospho-serine 15, and phospho-serine 20 p53 levels following infection with AdCon, AdE2F1, or increasing doses of AdE2F2 as in panel G.
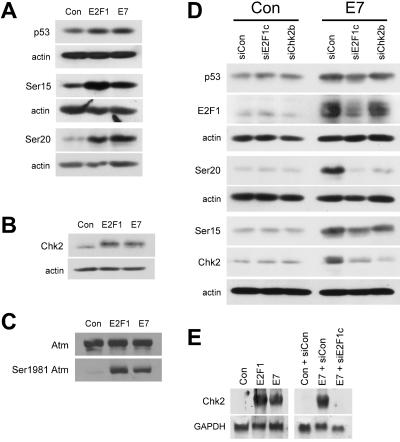
We next examined the ability of E2F1 and E2F2 to induce the phosphorylation of serine 15 and serine 20 residues on p53 in the absence of Atm. We found that in cells lacking Atm, both E2F1 and E2F2 were still able to induce total p53 protein levels (Fig. 1C), likely due to induction of p14ARF (73). However, phosphorylation of p53 at serine 15 was reduced and phosphorylation at serine 20 was absent in AT cells following E2F1 expression (Fig. 1D). An increase in the phospho-serine 15 form of p53 was also observed following E2F2 expression in normal, but not AT, cells (Fig. 1D). Because E2F2 expression led to lower levels of the phospho-serine 15 form of p53 than did E2F1 expression, it was possible that E2F2 was not inducing apoptosis because it is not as efficient at inducing Atm activity as is E2F1. To control for the differences in activation by E2F1 and E2F2 of the kinase(s) that phosphorylates p53 on serine 15, we expressed E2F2 at doses that resulted in levels of serine 15 phosphorylation and p53 accumulation that were similar to levels observed following E2F1 expression (Fig. 1H). Even at these elevated doses, E2F2 still did not induce apoptosis (Fig. 1G) or phosphorylation of p53 at serine 20 (Fig. 1H). Because E2F2 can also activate Atm, resulting in p53 phosphorylation at serine 15 in the absence of apoptosis, these results suggest that while E2F1 requires Atm to signal apoptosis, Atm activation is not the commitment step for apoptosis induction.
In addition to directly phosphorylating p53 on serine 15 (6, 14), Atm also activates other kinases that lead to p53 phosphorylation on serine 20 (17, 33, 56, 77, 89). Since downstream kinase activation by Atm can require Nbs1 (13, 51, 90), we investigated whether functional Nbs1 protein was necessary for E2F1-induced apoptosis. We found that E2F1-induced apoptosis was compromised in fibroblasts from NBS patients, similar to the reduction observed in AT cells (Fig. 1A). Although expression of E2F1 was found to be slightly less in AT cells than in normal cells (Fig. 1E), increased amounts of E2F1 still did not induce apoptosis in AT cells (data not shown). Although E2F1 was able to induce total p53 protein levels in NBS cells (Fig. 1C), we observed a modest decrease in the levels of the phospho-serine 15 form and a large decrease in the levels of the phospho-serine 20 form of p53 in NBS cells following E2F1 expression (Fig. 1D), demonstrating that functional Nbs1 protein is required for E2F1-mediated apoptosis and for signaling of p53 phosphorylation at the serine 20 residue. Ectopic E2F2 expression, found to be similar in all three cell types (Fig. 1F), failed to induce apoptosis in NBS cells (Fig. 1A) but did cause an increase in both total p53 levels and the levels of the phospho-serine 15 form of p53 (Fig. 1C and D). These results are consistent with a mechanism whereby E2F2 alters the phospho-serine 15 form of p53 in NBS cells through its ability to activate Atm.
Chk2 is required for E2F1-mediated apoptosis.
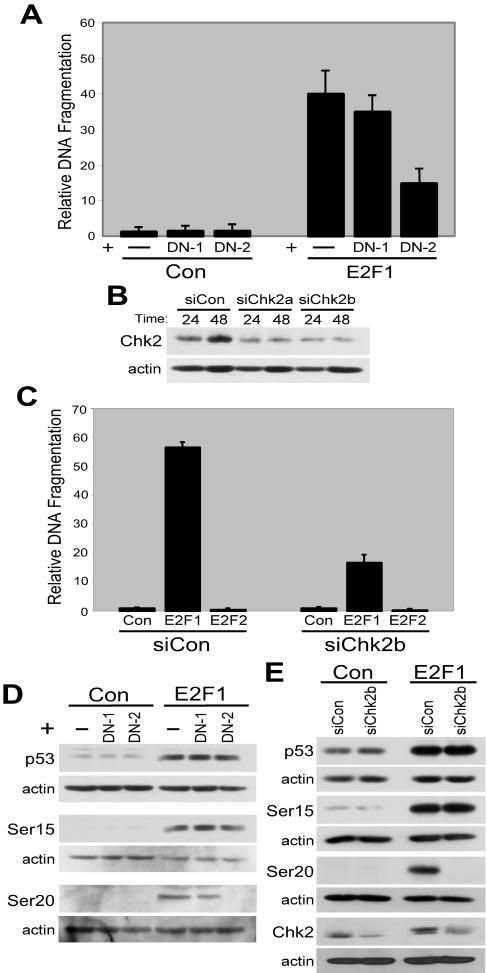
Given that phosphorylation of p53 on serine 20 correlates with apoptosis, we proceeded to use this as a marker to identify any additional kinase(s) that may contribute to E2F1-mediated apoptosis. Among the Atm-induced kinases that require functional Nbs1 protein for activation and that directly phosphorylate p53 on serine 20 is the human checkpoint kinase Chk2 (13, 51, 77). To examine the role of Chk2 in E2F1-induced apoptosis, we coexpressed E2F1 with a kinase-defective form of Chk2 (DN-Chk2) to inhibit Chk2 kinase activity in fibroblasts. We observed a reduction in apoptosis when E2F1 was coexpressed with DN-Chk2. Apoptosis levels did not appreciably change with a dominant negative form of Chk1 (DN-Chk1) (Fig. 2A), another DNA damage-responsive kinase that is capable of phosphorylating p53 on serine 20 (77). Expression of DN-Chk1 or DN-Chk2 alone did not alter levels of E2F1 protein (data not shown). To confirm the involvement of Chk2 in E2F1-mediated apoptosis, we used siRNAs to reduce the levels of Chk2 in cells (Fig. 2B). We observed a reduction in apoptosis following E2F1 expression in cells transfected with siChk2b (Fig. 2C) and no effect of this siRNA following expression of E2F2 (Fig. 2C). Similar results were obtained using siChk2a (data not shown).
FIG. 2.
Role for Chk2 in apoptosis induction. (A) Apoptosis induction in normal human fibroblasts infected with AdCon or AdE2F1 alone or coinfected with AdDN-Chk1 or AdDN-Chk2. In panels A and C, the ordinate axis represents DNA fragmentation relative to control, which is defined as 1, and error bars represent standard deviations calculated from experiments performed in triplicate. (B) Western blot analysis of Chk2 protein levels in HEL fibroblasts transfected with multiple siRNAs targeted to Chk2. Cells were harvested and lysates were generated at the indicated times posttransfection. (C) Analysis of apoptosis in HEL fibroblasts. Cells were transfected with siCon or siChk2b at 24 h prior to infection with AdCon, AdE2F1, or AdE2F2. (D) Western blot analysis of p53 protein levels and the levels of the phospho-serine 15 and phospho-serine 20 forms of p53 in normal human fibroblasts infected with AdCon or AdE2F1 alone or coinfected with AdDN-Chk1 or AdDN-Chk2. (E) Western blot analysis of p53 protein levels, the levels of the phospho-serine 15 and phospho-serine 20 forms of p53, and the levels of Chk2 protein in cells transfected with siCon or siChk2b 24 h prior to infection with AdCon or AdE2F1.
We next determined the involvement of Chk2 in E2F1-induced p53 accumulation and modification. Since Chk2 directly phosphorylates p53 on serine 20, we investigated whether DN-Chk2 expression or Chk2 siRNA transfection could block E2F1-induced p53 phosphorylation. We found that DN-Chk2 expression and the Chk2 siRNA reduced the levels of the phospho-serine 20 form of p53 following E2F1 expression but had no effect on either total p53 levels or the levels of the phospho-serine 15 form of p53 (Fig. 2D and E). Coexpression of DN-Chk1 was unable to inhibit E2F1-induced p53 accumulation and had only a modest effect on p53 phosphorylation (Fig. 2D).
E2F1 specifically induces Chk2 expression.
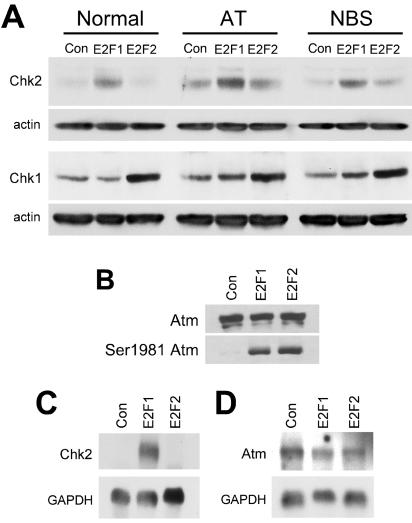
We have shown that E2F1 requires Atm, Nbs1, and Chk2 to efficiently induce apoptosis. However, E2F2 is also able to activate Atm, resulting in phosphorylation of p53 at serine 15, but it does so without inducing apoptosis. We confirmed the activation state of Atm by using an antibody that recognizes a modified form of Atm observed following DNA damage that correlates with Atm activation (5). Expression of either E2F1 or E2F2 led to an increase in the levels of the phospho-serine 1981 form of Atm, while leaving the total Atm protein levels unchanged (Fig. 3B). The difference between E2F1 signaling and E2F2 signaling appears to be the ability of E2F1 to stimulate Chk2 activity, which results in an increase in the phospho-serine 20 form of p53 and correlates with E2F1-induced apoptosis. Because Atm activation is upstream of Chk2 in signaling to p53, E2F1 expression must have an additional effect(s) downstream or independent of Atm that is specific to E2F1 for apoptosis induction. We found that expression of E2F1, but not E2F2, led to an increase in the levels of Chk2 protein, and this increase occurred in the absence of Atm or functional Nbs1 (Fig. 3A). E2F1 expression also results in accumulation of the phospho-threonine 68 form of Chk2 (data not shown), a modification observed following DNA damage that may be associated with Chk2 activation (56, 57). The phospho-threonine 68 modification of Chk2 may not be a reliable marker of Chk2 activation (3, 76, 87). Instead, we examined the Chk2 substrate p53 serine 20 residue as a marker for Chk2 activation. While expression of E2F1 resulted in an increase in Chk2 protein levels in the absence of functional Atm or Nbs1 (Fig. 3A), we did not observe an increase in the phospho-serine 20 form of p53 in these cells (Fig. 1D). Induction and activation of Chk2 by E2F1 appeared to be specific because E2F1 expression did not result in an increase in Chk1 protein (Fig. 3A) or in an increase in the phospho-serine 345 form of Chk1 (data not shown). Chk2 protein accumulation appeared to result from an increase in Chk2 mRNA levels following E2F1 expression (Fig. 3C). E2F2 expression did not lead to an increase in Chk2 protein levels (Fig. 3A) or Chk2 mRNA (Fig. 3C). Since neither E2F1 nor E2F2 induced the expression of Atm mRNA (Fig. 3D), induction of Chk2 expression appears to be specific to E2F1 and correlates with the induction of apoptosis.
FIG. 3.
E2F1 specifically induces Chk2 protein and mRNA. (A) Western blot analysis for Chk1 and Chk2 protein levels. Normal, AT, and NBS fibroblasts were infected with AdCon, AdE2F1, or AdE2F2. (B) Western blot analysis for Atm and the phospho-serine 1981 form of Atm. (C) Northern blot analysis for Chk2 mRNA isolated from normal human fibroblasts infected with AdCon, AdE2F1, or AdE2F2. GAPDH was used as a loading control. (D) Northern blot analysis for Atm mRNA isolated from normal human fibroblasts and infected as in panel C. GAPDH was used as a loading control.
Chk2 and E2F cooperate in apoptosis induction.
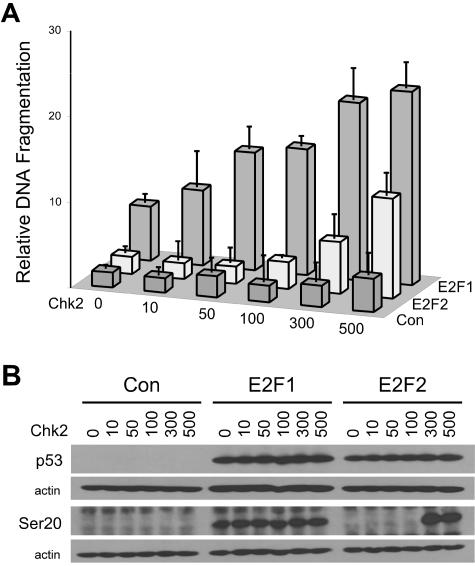
Given that the induction of Chk2 mRNA and protein levels following expression of E2F1 is associated with E2F1-specific apoptosis, we next examined whether Chk2 cooperates with E2F1 to signal apoptosis. We found that coexpression of Chk2 with a reduced amount of E2F1-encoding virus resulted in enhanced levels of apoptosis, whereas expression of Chk2 alone had a nominal effect on apoptosis levels (Fig. 4A). Thus, it appears that Chk2 is limiting for E2F1-induced apoptosis. This observation raises the possibility that the different abilities of E2F1 and E2F2 to activate the apoptosis program lies in the capacity of E2F1 to induce Chk2 expression. Given this possibility, we determined whether Chk2 could cooperate with E2F2 to induce apoptosis if provided in trans. We found that coexpression of Chk2 with E2F2 permitted E2F2 to induce apoptosis (Fig. 4A) and led to an increase in the phospho-serine 20 form of p53 (Fig. 4B). Coexpression of E2F1 or E2F2 with Chk1 had no effect on apoptosis (data not shown). The sudden onset of serine 20 phosphorylation at the point where cells are undergoing apoptosis in response to coexpression of E2F2 and Chk2 may mean that additional events are required to lead to phosphorylation of p53 through Chk2. These data suggest that an E2F1-specific increase in Chk2 expression is essential for p53 activation and apoptosis induction.
FIG. 4.
Chk2 cooperates with E2F1 and E2F2 in apoptosis induction. (A) Analysis of apoptosis in normal human fibroblasts infected with AdCon, AdE2F1, or AdE2F2 at an MOI of 500 and coinfected with the indicated MOIs of AdChk2. The ordinate axis represents DNA fragmentation relative to control, which is defined as 1, and error bars represent standard deviations calculated from experiments performed in triplicate. (B) Western blot analysis for p53 and the phospho-serine 20 form of p53 in normal human fibroblasts infected as in panel A.
Apoptosis induction by HPV-16 E7 is dependent on E2F1 and Atm/Nbs1/Chk2.
We next examined the role of Chk2 in apoptosis resulting from deregulation of endogenous E2F activity. The HPV-16 E7 protein binds to and inactivates Rb family members, resulting in the release of Rb-associated factors, including E2F proteins (59). We found that expression of the HPV-16 E7 protein resulted in apoptosis induction in human fibroblasts plated at low density (Fig. 5A). Similar to the apoptosis observed following ectopic E2F1 expression, apoptosis resulting from Rb family inactivation by HPV-16 E7 required functional Atm and Nbs1 proteins (Fig. 5B). Since Rb inactivation by E7 results in release of five E2F family members from Rb proteins, we used siRNAs targeted to E2F1 to address the requirement for E2F1 in E7-induced apoptosis. We screened three siRNAs targeted to E2F1 for their ability to inhibit E2F1 expression (Fig. 5C). siE2F1c was used for the remainder of the experiments presented, but similar results were obtained with the other E2F1 siRNAs (data not shown). We found that pretreatment of cells with siE2F1 blocked the ability of E7 to induce apoptosis (Fig. 5D and data not shown). Having observed that E7 induces apoptosis through E2F1, we next determined whether E7-induced apoptosis requires Chk2. We found that reducing Chk2 levels with an siRNA decreased the ability of E7 to induce apoptosis (Fig. 5D). To confirm the requirement of and determine the specificity for E2F1 in E7-mediated apoptosis, we used siRNAs targeted to E2F1, E2F2, or E2F3 (Fig. 5C and E). siE2F2a and siE2F3a were used for the remainder of the experiments presented, but similar results were obtained with the other E2F2 and E2F3 siRNAs (data not shown). We found that neither siE2F2 nor siE2F3 were able to block apoptosis induced by E7 expression (Fig. 5F). These data suggest that apoptosis resulting from deregulation of endogenous E2F activity occurs specifically through E2F1 and the Atm/Nbs/Chk2 pathway.
FIG. 5.
HPV E7 induces apoptosis dependent on E2F1, Atm, Nbs1, and Chk2. (A) Analysis of apoptosis in HEL fibroblasts infected with AdCon at an MOI of 1,000, AdE2F1 at an MOI of 250, or AdE7 at an MOI of 1,000. In panels A, B, D, and F, the ordinate axis represents DNA fragmentation relative to control, which is defined as 1, and error bars represent standard deviations calculated from experiments performed in triplicate. (B) Apoptosis induction in normal human dermal fibroblasts, AT fibroblasts, and NBS fibroblasts infected with AdCon or AdE7. (C) Western blot analysis of E2F1 in HEL fibroblasts transfected with siRNAs targeted to E2F1. Cells were infected with AdE2F1 at an MOI of 100 at 24 h posttransfection. Cells were harvested and lysates were generated at 24 hpi. (D) Analysis of apoptosis in cells transfected with siCon, siE2F1c, or siChk2b prior to infection with AdCon or AdE7. (E) Western blot analysis for E2F2 or E2F3 in HEL fibroblasts transfected with the labeled siRNA and infected with AdE2F2 or AdE2F3 at an MOI of 100. (F) Analysis of apoptosis in cells transfected with siCon, siE2F1c, siE2F2a, or siE2F3a prior to infection with AdCon or AdE7.
HPV-16 E7 induces Chk2 expression and p53 modification.
We next examined whether E7 expression induces modifications to p53 similar to those induced by E2F1. We found that E7 expression resulted in an increase in p53 levels and the levels of the phospho-serine 15 and phospho-serine 20 forms of p53 (Fig. 6A). Additionally, Chk2 protein levels were elevated following either E2F1 or E7 expression (Fig. 6B). Similar to that of E2F1, expression of E7 resulted in an increase in the phospho-serine 1981 form of Atm while leaving total Atm protein levels unchanged (Fig. 6C). Expression of E7 also resulted in an increase in the phospho-threonine 68 form of Chk2 (data not shown). These observations suggest that E7 is able to activate Atm kinase activity, resulting in an increase in active Chk2 kinase.
FIG. 6.
E7 induces E2F1-dependent p53 modifications and Chk2 induction. (A) Western blot analysis for p53 and the phospho-serine 15 and phospho-serine 20 forms of p53 in HEL fibroblasts infected with AdCon, AdE2F1, or AdE7. (B) Western blot analysis for Chk2 in cells infected with AdCon, AdE2F1, or AdE7. (C) Western blot analysis for Atm and the phospho-serine 1981 form of Atm in cells infected with AdCon, AdE2F1, or AdE7. (D) Western blot analysis for p53, the phospho-serine 15 and phospho-serine 20 forms of p53, Chk2, and E2F1 in cells transfected with siE2F1c or siChk2b prior to infection with AdCon or AdE7. The blot for p53 and E2F1 and that for serine 20 and Chk2 were sequentially stripped and reprobed. (E) Northern blot analysis for Chk2 in cells infected with AdCon, AdE2F1, or AdE7 and transfected with the marked siRNA. RNA was isolated at 24 hpi. GAPDH was used as a loading control.
We next determined whether E2F1 was required for E7 to increase Chk2 and p53 levels and induce modifications to p53. We found that E2F1 is not required for much of the observed increase in p53 or the phospho-serine 15 form of p53 following E7 expression (Fig. 6D). This increase in p53 protein was likely due to activation of the p14ARF/Mdm2 pathway by E2F2 and/or E2F3, while the increase in the phospho-serine 15 form of p53 is likely due to the ability of E2F2, and possibly other E2Fs, to activate Atm (Fig. 1D and 3B). However, we found that E2F1 was required for E7 to induce the phosphorylation of p53 at serine 20 and to increase the levels of Chk2 protein (Fig. 6D). Additionally, we found that E7 expression resulted in an increase in Chk2 mRNA levels (Fig. 6E) and this could be attributed to E2F1 (Fig. 6E). Having found that E2F1 is required for E7 to induce Chk2 expression and phosphorylation of p53 at serine 20, we next determined whether Chk2 was required for E7 to induce this modification. We found that an siRNA directed against Chk2 was able to block E7-induced phosphorylation of p53 at serine 20, while total p53 levels and the levels of the phospho-serine 15 form of p53 remained unaffected by the addition of this siRNA (Fig. 6D). These results demonstrate a requirement for E2F1 in Chk2 induction and kinase activation following Rb inactivation by HPV-16 E7 as measured by an increase in the phospho-serine 20 form of p53. Taken together, these results show that apoptosis resulting from inactivation of Rb family members is dependent specifically on E2F1 and its ability to induce Chk2 expression.
DISCUSSION
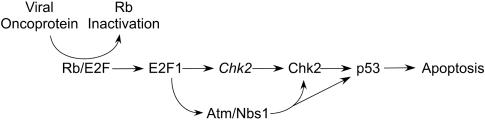
Here we describe a pathway involving the E2F1-specific induction of Chk2 expression that links loss of proliferation control to an apoptotic pathway with some similarity to the apoptosis pathway induced by DNA double-strand breaks. Unlike the DNA damage signals that activate p53 in the absence of de novo gene expression (39), E2F1-mediated apoptosis requires induction of Chk2 expression and possibly other components of apoptosis signaling (62) to fully activate p53 and kill cells. The results presented here suggest that deregulated E2F1 function induces apoptosis by activation of an Atm/Nbs1/Chk2/p53 pathway following disruptions in the Rb/E2F proliferation pathway (Fig. 7).
FIG. 7.
Model of p53 activation following deregulation of E2F1.
The search for E2F target genes has been complicated by the fact that E2F family members can regulate gene expression by transcriptional activation, promoter repression, and derepression of promoters (27). In addition to regulating genes involved in cell cycle regulation, DNA replication, and chromatin remodeling, E2F family members are also found at the promoters of genes involved in DNA repair and checkpoint activation (71, 86). Our finding that E2F1 specifically induces expression of the gene encoding the human checkpoint kinase, Chk2, as a requirement for apoptosis ascribes biological function to the data emerging from E2F gene expression profiles (38, 42, 53, 60, 71, 80), although Chk2 induction by E2F was not tested in these studies. While Chk2 may not be a direct transcriptional target of E2F1, our data confirm that Chk2 is specifically regulated by E2F1. We speculate that the induction of Chk2 expression by E2F1 in vivo sensitizes cells to undergo apoptosis in the event of DNA damage or loss of proliferation control due to Rb mutation or inactivation. This induction of Chk2 by E2F1 is not a direct consequence of promoting the G1-to-S-phase transition since both E2F1 and E2F2 are adept at inducing S phase. Similarly, Chk2 induction is not a result of simply increasing cellular E2F transcriptional activity and thus upregulating many E2F responsive genes involved in apoptosis signaling, like Apaf1, caspase 3, and caspase 7 (60, 62). In fact, we found that neither E2F1 nor E2F2 induces Atm mRNA in human fibroblasts, which could, in principle, be a simple way to lower the activation threshold of this signaling pathway.
The finding that Atm and Nbs1 are required for apoptosis associated with deregulated E2F1 is similar to the requirement for p53 activation following DNA damage from gamma-irradiation and certain genotoxic agents (9). How Atm is activated following expression of E2F1, E2F2, or HPV-16 E7 remains unclear. We speculate that expression of E2F proteins or E7 results in chromatin changes associated with induction of S phase or activation of DNA damage response proteins. In the case of HPV-16 E7, activation of Atm may be a result of chromosomal structural changes and DNA breaks that occur following E7 expression (21). However, since activation of Atm by E2F1 results in apoptosis, we cannot rule out the possibility that the different E2F family members activate Atm by distinct mechanisms.
Activation of Atm can result in phosphorylation of Chk2 at threonine 68 (11, 16, 55-57), and this Chk2 modification requires functional Nbs1 (13, 51, 90). However, the phospho-threonine 68 modification of Chk2 may not be a reliable marker of Chk2 activation due to the complexity of Chk2 regulation and the importance of individual phosphorylation events on Chk2 activation status (3, 76, 87). Instead, we examined phosphorylation of the serine 20 residue on p53 as a reliable marker for Chk2 activation. While we observed an increase in total p53 levels in normal, AT, and NBS cells, only in normal cells did we observe an increase in the phospho-serine 20 form of p53, a substrate for active Chk2 kinase. Inhibition of Chk2 activity by a dominant negative construct or by siRNA targeting resulted in a failure to phosphorylate the serine 20 residue following expression of either E2F1 or E7, demonstrating the specificity of this modification by Chk2.
Interestingly, Atm was found not to be required for apoptosis resulting from Rb inactivation in murine brain choroid plexus epithelium (50). While it is not apparent why this apoptosis is Atm independent in the choroid plexus epithelium, p53-dependent apoptosis that is Atm independent has been described in certain cell types (7, 32). Alternatively, these observations suggest that there may be a species-specific bias for Atm in the E2F1-mediated apoptosis pathway. Indeed, the reduction in apoptosis observed in atm−/− MEFs is not as dramatic as that seen in human dermal fibroblasts following E2F1 expression. We speculate that in the murine system other signaling pathways such as Atr/Chk1 may compensate for the loss of Atm, whereas a more stringent requirement for Atm in human dermal fibroblasts is observed. Therefore, there may be both cell-type and species-specific requirements for Atm in apoptosis induction, and it is possible that other Atm-related kinases may compensate for the loss of Atm function in some cells.
Although we have defined a pathway linking deregulated E2F1 activity to p53 and apoptosis, integration of E2F1 signaling and activation of the Atm/Chk2/p53 pathway also offer a mechanism for the proposed involvement of E2F1 in apoptosis resulting from DNA damage. Following treatment of cells with DNA-damaging agents, E2F1 protein accumulates (10, 34, 36, 52, 58, 65) and is phosphorylated at an N-terminal Atm recognition sequence that is unique to E2F1 among the E2F family members (52). This phosphorylation of E2F1 is largely dependent on Atm and is required for efficient E2F1 stabilization following DNA damage (52). Chk2 has also been shown to phosphorylate and stabilize E2F1 following DNA damage, and this modification has been shown to be required for E2F1-dependent apoptosis following DNA damage by altering its promoter specificity (81). Additionally, DNA damage-induced apoptosis is compromised in thymocytes from E2F1−/− mice (52), suggesting that E2F1 has multiple roles in DNA damage signaling. We speculate that activation of E2F1 by DNA damage leads to increased p14ARF expression, resulting in increased pools of p53 protein. E2F1 is also able to activate Atm kinase activity and induce Chk2 expression, leading to increased p53 activation and E2F1 activity. E2F1 activation following DNA damage would therefore act to amplify DNA damage signals converging at p53 to result in apoptosis.
Acknowledgments
We thank Jonathan Castillo and Bradford Stadler for their assistance, Michael Brodsky, Michelle Kelliher, Roger Johnson, Nick Rhind, and Dario Altieri for commenting on the manuscript, and David Johnson for sharing unpublished observations.
This work was supported by National Institutes of Health (NIH) grants CA86038 (T.F.K.) and CA77735 (S.J.). H.A.R. was supported by an NIH training grant (5T32 AI07349).
The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
REFERENCES
- 1.Abraham, R. T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 15:2177-2196. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal, M. L., W. R. Taylor, M. V. Chernov, O. B. Chernova, and G. R. Stark. 1998. The p53 network. J. Biol. Chem. 273:1-4. [DOI] [PubMed] [Google Scholar]
- 3.Ahn, J., and C. Prives. 2002. Checkpoint kinase 2 (Chk2) monomers or dimers phosphorylate Cdc25C after DNA damage regardless of threonine 68 phosphorylation. J. Biol. Chem. 277:48418-48426. [DOI] [PubMed] [Google Scholar]
- 4.Appella, E., and C. W. Anderson. 2001. Post-translational modifications and activation of p53 by genotoxic stresses. Eur. J. Biochem. 268:2764-2772. [DOI] [PubMed] [Google Scholar]
- 5.Bakkenist, C. J., and M. B. Kastan. 2003. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421:499-506. [DOI] [PubMed] [Google Scholar]
- 6.Banin, S., L. Moyal, S. Shieh, Y. Taya, C. W. Anderson, L. Chessa, N. I. Smorodinsky, C. Prives, Y. Reiss, Y. Shiloh, and Y. Ziv. 1998. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science 281:1674-1677. [DOI] [PubMed] [Google Scholar]
- 7.Barlow, C., K. D. Brown, C. X. Deng, D. A. Tagle, and A. Wynshaw-Boris. 1997. Atm selectively regulates distinct p53-dependent cell-cycle checkpoint and apoptotic pathways. Nat. Genet. 17:453-456. [DOI] [PubMed] [Google Scholar]
- 8.Bates, S., A. C. Phillips, P. A. Clark, F. Stott, G. Peters, R. L. Ludwig, and K. H. Vousden. 1998. p14ARF links the tumour suppressors RB and p53. Nature 395:124-125. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein, C., H. Bernstein, C. M. Payne, and H. Garewal. 2002. DNA repair/pro-apoptotic dual-role proteins in five major DNA repair pathways: fail-safe protection against carcinogenesis. Mutat. Res. 511:145-178. [DOI] [PubMed] [Google Scholar]
- 10.Blattner, C., A. Sparks, and D. Lane. 1999. Transcription factor E2F-1 is upregulated in response to DNA damage in a manner analogous to that of p53. Mol. Cell. Biol. 19:3704-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, A. L., C. H. Lee, J. K. Schwarz, N. Mitiku, H. Piwnica-Worms, and J. H. Chung. 1999. A human Cds1-related kinase that functions downstream of ATM protein in the cellular response to DNA damage. Proc. Natl. Acad. Sci. USA 96:3745-3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown, E. J., and D. Baltimore. 2000. ATR disruption leads to chromosomal fragmentation and early embryonic lethality. Genes Dev. 14:397-402. [PMC free article] [PubMed] [Google Scholar]
- 13.Buscemi, G., C. Savio, L. Zannini, F. Micciche, D. Masnada, M. Nakanishi, H. Tauchi, K. Komatsu, S. Mizutani, K. Khanna, P. Chen, P. Concannon, L. Chessa, and D. Delia. 2001. Chk2 activation dependence on Nbs1 after DNA damage. Mol. Cell. Biol. 21:5214-5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canman, C. E., D. S. Lim, K. A. Cimprich, Y. Taya, K. Tamai, K. Sakaguchi, E. Appella, M. B. Kastan, and J. D. Siliciano. 1998. Activation of the ATM kinase by ionizing radiation and phosphorylation of p53. Science 281:1677-1679. [DOI] [PubMed] [Google Scholar]
- 15.Castillo, J. P., A. D. Yurochko, and T. F. Kowalik. 2000. Role of human cytomegalovirus immediate-early proteins in cell growth control. J. Virol. 74:8028-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaturvedi, P., W. K. Eng, Y. Zhu, M. R. Mattern, R. Mishra, M. R. Hurle, X. Zhang, R. S. Annan, Q. Lu, L. F. Faucette, G. F. Scott, X. Li, S. A. Carr, R. K. Johnson, J. D. Winkler, and B. B. Zhou. 1999. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene 18:4047-4054. [DOI] [PubMed] [Google Scholar]
- 17.Chehab, N. H., A. Malikzay, M. Appel, and T. D. Halazonetis. 2000. Chk2/hCds1 functions as a DNA damage checkpoint in G(1) by stabilizing p53. Genes Dev. 14:278-288. [PMC free article] [PubMed] [Google Scholar]
- 18.Debbas, M., and E. White. 1993. Wild-type p53 mediates apoptosis by E1A, which is inhibited by E1B. Genes Dev. 7:546-554. [DOI] [PubMed] [Google Scholar]
- 19.DeGregori, J., G. Leone, A. Miron, L. Jakoi, and J. R. Nevins. 1997. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc. Natl. Acad. Sci. USA 94:7245-7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Klein, A., M. Muijtjens, R. van Os, Y. Verhoeven, B. Smit, A. M. Carr, A. R. Lehmann, and J. H. Hoeijmakers. 2000. Targeted disruption of the cell-cycle checkpoint gene ATR leads to early embryonic lethality in mice. Curr. Biol. 10:479-482. [DOI] [PubMed] [Google Scholar]
- 21.Duensing, S., and K. Munger. 2002. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosome instability. Cancer Res. 62:7075-7082. [PubMed] [Google Scholar]
- 22.Freedman, D. A., and A. J. Levine. 1998. Nuclear export is required for degradation of endogenous p53 by MDM2 and human papillomavirus E6. Mol. Cell. Biol. 18:7288-7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuchs, S. Y., V. Adler, T. Buschmann, X. Wu, and Z. Ronai. 1998. Mdm2 association with p53 targets its ubiquitination. Oncogene 17:2543-2547. [DOI] [PubMed] [Google Scholar]
- 24.Giaccia, A. J., and M. B. Kastan. 1998. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 12:2973-2983. [DOI] [PubMed] [Google Scholar]
- 25.Guo, Z., A. Kumagai, S. X. Wang, and W. G. Dunphy. 2000. Requirement for Atr in phosphorylation of Chk1 and cell cycle regulation in response to DNA replication blocks and UV-damaged DNA in Xenopus egg extracts. Genes Dev. 14:2745-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall-Jackson, C. A., D. A. Cross, N. Morrice, and C. Smythe. 1999. ATR is a caffeine-sensitive, DNA-activated protein kinase with a substrate specificity distinct from DNA-PK. Oncogene 18:6707-6713. [DOI] [PubMed] [Google Scholar]
- 27.Harbour, J. W., and D. C. Dean. 2000. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14:2393-2409. [DOI] [PubMed] [Google Scholar]
- 28.Haupt, Y., R. Maya, A. Kazaz, and M. Oren. 1997. Mdm2 promotes the rapid degradation of p53. Nature 387:296-299. [DOI] [PubMed] [Google Scholar]
- 29.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hekmat-Nejad, M., Z. You, M. C. Yee, J. W. Newport, and K. A. Cimprich. 2000. Xenopus ATR is a replication-dependent chromatin-binding protein required for the DNA replication checkpoint. Curr. Biol. 10:1565-1573. [DOI] [PubMed] [Google Scholar]
- 31.Hermeking, H., and D. Eick. 1994. Mediation of c-Myc-induced apoptosis by p53. Science 265:2091-2093. [DOI] [PubMed] [Google Scholar]
- 32.Herzog, K. H., M. J. Chong, M. Kapsetaki, J. I. Morgan, and P. J. McKinnon. 1998. Requirement for Atm in ionizing radiation-induced cell death in the developing central nervous system. Science 280:1089-1091. [DOI] [PubMed] [Google Scholar]
- 33.Hirao, A., Y. Y. Kong, S. Matsuoka, A. Wakeham, J. Ruland, H. Yoshida, D. Liu, S. J. Elledge, and T. W. Mak. 2000. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science 287:1824-1827. [DOI] [PubMed] [Google Scholar]
- 34.Hofferer, M., C. Wirbelauer, B. Humar, and W. Krek. 1999. Increased levels of E2F-1-dependent DNA binding activity after UV- or gamma-irradiation. Nucleic Acids Res. 27:491-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Honda, R., H. Tanaka, and H. Yasuda. 1997. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 420:25-27. [DOI] [PubMed] [Google Scholar]
- 36.Huang, Y., T. Ishiko, S. Nakada, T. Utsugisawa, T. Kato, and Z. M. Yuan. 1997. Role for E2F in DNA damage-induced entry of cells into S phase. Cancer Res. 57:3640-3643. [PubMed] [Google Scholar]
- 37.Inoue, K., M. F. Roussel, and C. J. Sherr. 1999. Induction of ARF tumor suppressor gene expression and cell cycle arrest by transcription factor DMP1. Proc. Natl. Acad. Sci. USA 96:3993-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishida, S., E. Huang, H. Zuzan, R. Spang, G. Leone, M. West, and J. R. Nevins. 2001. Role for E2F in control of both DNA replication and mitotic functions as revealed from DNA microarray analysis. Mol. Cell. Biol. 21:4684-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jack, M. T., R. A. Woo, A. Hirao, A. Cheung, T. W. Mak, and P. W. Lee. 2002. Chk2 is dispensable for p53-mediated G1 arrest but is required for a latent p53-mediated apoptotic response. Proc. Natl. Acad. Sci. USA 99:9825-9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jin, S., and A. J. Levine. 2001. The p53 functional circuit. J. Cell Sci. 114:4139-4140. [DOI] [PubMed] [Google Scholar]
- 41.Johnson, D. G., J. K. Schwarz, W. D. Cress, and J. R. Nevins. 1993. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature 365:349-352. [DOI] [PubMed] [Google Scholar]
- 42.Kalma, Y., L. Marash, Y. Lamed, and D. Ginsberg. 2001. Expression analysis using DNA microarrays demonstrates that E2F-1 up-regulates expression of DNA replication genes including replication protein A2. Oncogene 20:1379-1387. [DOI] [PubMed] [Google Scholar]
- 43.Kamijo, T., F. Zindy, M. F. Roussel, D. E. Quelle, J. R. Downing, R. A. Ashmun, G. Grosveld, and C. J. Sherr. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91:649-659. [DOI] [PubMed] [Google Scholar]
- 44.Kastan, M. B., and D. S. Lim. 2000. The many substrates and functions of ATM. Nat. Rev. Mol. Cell Biol. 1:179-186. [DOI] [PubMed] [Google Scholar]
- 45.Kim, S. T., D. S. Lim, C. E. Canman, and M. B. Kastan. 1999. Substrate specificities and identification of putative substrates of ATM kinase family members. J. Biol. Chem. 274:37538-37543. [DOI] [PubMed] [Google Scholar]
- 46.Kowalik, T. F., J. DeGregori, G. Leone, L. Jakoi, and J. R. Nevins. 1998. E2F1-specific induction of apoptosis and p53 accumulation, which is blocked by Mdm2. Cell Growth Differ. 9:113-118. [PubMed] [Google Scholar]
- 47.Kowalik, T. F., J. DeGregori, J. K. Schwarz, and J. R. Nevins. 1995. E2F1 overexpression in quiescent fibroblasts leads to induction of cellular DNA synthesis and apoptosis. J. Virol. 69:2491-2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lakin, N. D., B. C. Hann, and S. P. Jackson. 1999. The ataxia-telangiectasia related protein ATR mediates DNA-dependent phosphorylation of p53. Oncogene 18:3989-3995. [DOI] [PubMed] [Google Scholar]
- 49.Leone, G., R. Sears, E. Huang, R. Rempel, F. Nuckolls, C. H. Park, P. Giangrande, L. Wu, H. I. Saavedra, S. J. Field, M. A. Thompson, H. Yang, Y. Fujiwara, M. E. Greenberg, S. Orkin, C. Smith, and J. R. Nevins. 2001. Myc requires distinct E2F activities to induce S phase and apoptosis. Mol. Cell 8:105-113. [DOI] [PubMed] [Google Scholar]
- 50.Liao, M. J., C. Yin, C. Barlow, A. Wynshaw-Boris, and T. van Dyke. 1999. Atm is dispensable for p53 apoptosis and tumor suppression triggered by cell cycle dysfunction. Mol. Cell. Biol. 19:3095-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim, D. S., S. T. Kim, B. Xu, R. S. Maser, J. Lin, J. H. Petrini, and M. B. Kastan. 2000. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature 404:613-617. [DOI] [PubMed] [Google Scholar]
- 52.Lin, W. C., F. T. Lin, and J. R. Nevins. 2001. Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev. 15:1833-1844. [PMC free article] [PubMed] [Google Scholar]
- 53.Ma, Y., R. Croxton, R. L. Moorer, Jr., and W. D. Cress. 2002. Identification of novel E2F1-regulated genes by microarray. Arch. Biochem. Biophys. 399:212-224. [DOI] [PubMed] [Google Scholar]
- 54.Macleod, K. F., Y. Hu, and T. Jacks. 1996. Loss of Rb activates both p53-dependent and independent cell death pathways in the developing mouse nervous system. EMBO J. 15:6178-6188. [PMC free article] [PubMed] [Google Scholar]
- 55.Matsuoka, S., M. Huang, and S. J. Elledge. 1998. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science 282:1893-1897. [DOI] [PubMed] [Google Scholar]
- 56.Matsuoka, S., G. Rotman, A. Ogawa, Y. Shiloh, K. Tamai, and S. J. Elledge. 2000. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl. Acad. Sci. USA 97:10389-10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melchionna, R., X. B. Chen, A. Blasina, and C. H. McGowan. 2000. Threonine 68 is required for radiation-induced phosphorylation and activation of Cds1. Nat. Cell Biol. 2:762-765. [DOI] [PubMed] [Google Scholar]
- 58.Meng, R. D., P. Phillips, and W. S. El-Deiry. 1999. p53-independent increase in E2F-1 expression enhances the cytotoxic effects of etoposide and of adriamycin. Int. J. Oncol. 14:5-14. [PubMed] [Google Scholar]
- 59.Morris, J. D., T. Crook, L. R. Bandara, R. Davies, N. B. LaThangue, and K. H. Vousden. 1993. Human papillomavirus type 16 E7 regulates E2F and contributes to mitogenic signalling. Oncogene 8:893-898. [PubMed] [Google Scholar]
- 60.Muller, H., A. P. Bracken, R. Vernell, M. C. Moroni, F. Christians, E. Grassilli, E. Prosperini, E. Vigo, J. D. Oliner, and K. Helin. 2001. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 15:267-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muller, H., and K. Helin. 2000. The E2F transcription factors: key regulators of cell proliferation. Biochim. Biophys. Acta 1470:M1-M12. [DOI] [PubMed] [Google Scholar]
- 62.Nahle, Z., J. Polakoff, R. V. Davuluri, M. E. McCurrach, M. D. Jacobson, M. Narita, M. Q. Zhang, Y. Lazebnik, D. Bar-Sagi, and S. W. Lowe. 2002. Direct coupling of the cell cycle and cell death machinery by E2F. Nat. Cell Biol. 4:859-864. [DOI] [PubMed] [Google Scholar]
- 63.Nevins, J. R. 1998. Toward an understanding of the functional complexity of the E2F and retinoblastoma families. Cell Growth Differ. 9:585-593. [PubMed] [Google Scholar]
- 64.Nghiem, P., P. K. Park, Y. Kim, C. Vaziri, and S. L. Schreiber. 2001. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation. Proc. Natl. Acad. Sci. USA 98:9092-9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Connor, D. J., and X. Lu. 2000. Stress signals induce transcriptionally inactive E2F-1 independently of p53 and Rb. Oncogene 19:2369-2376. [DOI] [PubMed] [Google Scholar]
- 66.Pan, H., C. Yin, N. J. Dyson, E. Harlow, L. Yamasaki, and T. Van Dyke. 1998. Key roles for E2F1 in signaling p53-dependent apoptosis and in cell division within developing tumors. Mol. Cell 2:283-292. [DOI] [PubMed] [Google Scholar]
- 67.Phillips, A. C., and K. H. Vousden. 2001. E2F-1 induced apoptosis. Apoptosis 6:173-182. [DOI] [PubMed] [Google Scholar]
- 68.Pierce, A. M., I. B. Gimenez-Conti, R. Schneider-Broussard, L. A. Martinez, C. J. Conti, and D. G. Johnson. 1998. Increased E2F1 activity induces skin tumors in mice heterozygous and nullizygous for p53. Proc. Natl. Acad. Sci. USA 95:8858-8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prives, C. 1998. Signaling to p53: breaking the MDM2-p53 circuit. Cell 95:5-8. [DOI] [PubMed] [Google Scholar]
- 70.Qin, X. Q., D. M. Livingston, W. G. Kaelin, Jr., and P. D. Adams. 1994. Deregulated transcription factor E2F-1 expression leads to S-phase entry and p53-mediated apoptosis. Proc. Natl. Acad. Sci. USA 91:10918-10922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ren, B., H. Cam, Y. Takahashi, T. Volkert, J. Terragni, R. A. Young, and B. D. Dynlacht. 2002. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 16:245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Robertson, K. D., and P. A. Jones. 1998. The human ARF cell cycle regulatory gene promoter is a CpG island which can be silenced by DNA methylation and down-regulated by wild-type p53. Mol. Cell. Biol. 18:6457-6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rogoff, H. A., M. T. Pickering, M. E. Debatis, S. Jones, and T. F. Kowalik. 2002. E2F1 induces phosphorylation of p53 that is coincident with p53 accumulation and apoptosis. Mol. Cell. Biol. 22:5308-5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Russell, J. L., J. T. Powers, R. J. Rounbehler, P. M. Rogers, C. J. Conti, and D. G. Johnson. 2002. ARF differentially modulates apoptosis induced by E2F1 and Myc. Mol. Cell. Biol. 22:1360-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwarz, J. K., C. H. Bassing, I. Kovesdi, M. B. Datto, M. Blazing, S. George, X. F. Wang, and J. R. Nevins. 1995. Expression of the E2F1 transcription factor overcomes type beta transforming growth factor-mediated growth suppression. Proc. Natl. Acad. Sci. USA 92:483-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schwarz, J. K., C. M. Lovly, and H. Piwnica-Worms. 2003. Regulation of the Chk2 protein kinase by oligomerization-mediated cis- and trans-phosphorylation. Mol. Cancer Res. 1:598-609. [PubMed] [Google Scholar]
- 77.Shieh, S. Y., J. Ahn, K. Tamai, Y. Taya, and C. Prives. 2000. The human homologs of checkpoint kinases Chk1 and Cds1 (Chk2) phosphorylate p53 at multiple DNA damage-inducible sites. Genes Dev. 14:289-300. [PMC free article] [PubMed] [Google Scholar]
- 78.Shiloh, Y. 2001. ATM and ATR: networking cellular responses to DNA damage. Curr. Opin. Genet. Dev. 11:71-77. [DOI] [PubMed] [Google Scholar]
- 79.Siliciano, J. D., C. E. Canman, Y. Taya, K. Sakaguchi, E. Appella, and M. B. Kastan. 1997. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 11:3471-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stanelle, J., T. Stiewe, C. C. Theseling, M. Peter, and B. M. Putzer. 2002. Gene expression changes in response to E2F1 activation. Nucleic Acids Res. 30:1859-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stevens, C., L. Smith, and N. B. La Thangue. 2003. Chk2 activates E2F-1 in response to DNA damage. Nat. Cell Biol. 5:401-409. [DOI] [PubMed] [Google Scholar]
- 82.Takai, H., K. Naka, Y. Okada, M. Watanabe, N. Harada, S. Saito, C. W. Anderson, E. Appella, M. Nakanishi, H. Suzuki, K. Nagashima, H. Sawa, K. Ikeda, and N. Motoyama. 2002. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 21:5195-5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tolbert, D., X. Lu, C. Yin, M. Tantama, and T. Van Dyke. 2002. p19(ARF) is dispensable for oncogenic stress-induced p53-mediated apoptosis and tumor suppression in vivo. Mol. Cell. Biol. 22:370-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tsai, K. Y., D. MacPherson, D. A. Rubinson, D. Crowley, and T. Jacks. 2002. ARF is not required for apoptosis in Rb mutant mouse embryos. Curr. Biol. 12:159-163. [DOI] [PubMed] [Google Scholar]
- 85.Vousden, K. H. 2000. p53: death star. Cell 103:691-694. [DOI] [PubMed] [Google Scholar]
- 86.Weinmann, A. S., S. M. Bartley, T. Zhang, M. Q. Zhang, and P. J. Farnham. 2001. Use of chromatin immunoprecipitation to clone novel E2F target promoters. Mol. Cell. Biol. 21:6820-6832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu, X., and J. Chen. 2003. Autophosphorylation of checkpoint kinase 2 at serine 516 is required for radiation-induced apoptosis. J. Biol. Chem. 278:36163-36168. [DOI] [PubMed] [Google Scholar]
- 88.Wu, X., and A. J. Levine. 1994. p53 and E2F-1 cooperate to mediate apoptosis. Proc. Natl. Acad. Sci. USA 91:3602-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xie, S., H. Wu, Q. Wang, J. P. Cogswell, I. Husain, C. Conn, P. Stambrook, M. Jhanwar-Uniyal, and W. Dai. 2001. Plk3 functionally links DNA damage to cell cycle arrest and apoptosis at least in part via the p53 pathway. J. Biol. Chem. 276:43305-43312. [DOI] [PubMed] [Google Scholar]
- 90.Zhao, S., Y. C. Weng, S. S. Yuan, Y. T. Lin, H. C. Hsu, S. C. Lin, E. Gerbino, M. H. Song, M. Z. Zdzienicka, R. A. Gatti, J. W. Shay, Y. Ziv, Y. Shiloh, and E. Y. Lee. 2000. Functional link between ataxia-telangiectasia and Nijmegen breakage syndrome gene products. Nature 405:473-477. [DOI] [PubMed] [Google Scholar]