Abstract
We have previously reported that TLR4 signaling is increased in lipopolysaccharide (LPS) -stimulated Cystic Fibrosis (CF) macrophages (MΦs), contributing to the robust production of pro-inflammatory cytokines. The heme oxygenase (HO-1)/carbon monoxide (CO) pathway modulates cellular redox status, inflammatory responses, and cell survival. The HO-1 enzyme, together with the scaffold protein caveolin 1 (CAV-1), also acts as a negative regulator of TLR4 signaling in MΦs. Here, we demonstrate that in LPS-challenged CF MΦs, HO-1 does not compartmentalize normally to the cell surface and instead accumulates intracellularly. The abnormal HO-1 localization in CF MΦs in response to LPS is due to decreased CAV-1 expression, which is controlled by the cellular oxidative state, and is required for HO-1 delivery to the cell surface. Overexpression of HO-1 or stimulating the pathway with CO-releasing molecules (CORM2)enhancesCAV-1 expression in CF MΦs, suggesting a positive-feed forward loop between HO-1/CO induction and CAV-1 expression. These manipulations reestablished HO-1 and CAV-1 cell surface localization in CF MΦ's. Consistent with restoration of HO-1/CAV-1 negative regulation of TLR4 signaling, genetic or pharmacological (CORM2)-induced enhancement of this pathway decreased the inflammatory response of CF MΦs and CF mice treated with LPS. In conclusion, our results demonstrate that the counter-regulatory HO-1/CO pathway, which is critical in balancing and limiting the inflammatory response, is defective in CF MΦs through a CAV-1-dependent mechanism, exacerbating the CF MΦ's response to LPS. This pathway could be a potential target for therapeutic intervention for CF lung disease.
Introduction
Cystic Fibrosis (CF), the most common lethal genetic disorder in the Caucasian population, is a multiorgan disease affecting the lungs, pancreas, intestine, liver and reproductive tract (1). CF is caused by mutations of the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which encodes a cAMP-dependent chloride channel primarily located at the apical membrane of epithelial cells. CFTR is expressed at high levels in secretory epithelia and at lower levels in other cell types, such as alveolar type II cells (2), immune cells (1, 3-8) and neurons (9). The high morbidity and mortality in CF patients is due to chronic obstructive lung disease characterized by mucous plugging, chronic lung inflammation and recurrent bronchial infections with bacteria such as Pseudomonas aeruginosa (PA). Excessive neutrophilic inflammation, primarily orchestrated by increased production of IL-8 and amplified by chronic bacterial infection, leads to progressive tissue destruction.
Although the imbalance between chloride secretion and sodium absorption in submucosal glands and airway epithelium in the lung is responsible for detrimental airway dehydration, cells lacking functional CFTR display several other abnormalities, such as unregulated activation of the NF-κB pathway (hyper-inflammation), decreased anti-inflammatory responses, defective autophagy, and oxidative stress (10-13). Immune cells lacking functional CFTR also contribute significantly to CF pathology. Human (6, 14) and murine (4, 15-17) CF macrophages (MΦs) are hyper-responsive when exposed to the bacterial endotoxin lipopolysaccharide (LPS), with enhanced MyD88-dependent signal transduction and increased secretion of pro-inflammatory cytokines. The hyper-inflammatory response is, at least in part, due to the altered trafficking of Toll-like receptor (TLR) 4, which leads to a dysregulation of the innate immune response (6). In addition, CF MΦs have reduced bactericidal activity (8, 18-20). Together, these cellular dysfunctions contribute to the irreversible chronic bacterial lung infection and tissue damage.
Clinical trials indicate that effective treatment of CF lung disease requires a combination of interventions that target several aspects of lung pathology, such as ion transport imbalance, hyper-inflammation, and oxidative stress (21). Investigations focused on identifying molecular pathways involved in cellular dysfunctions in CF may help to identify new therapeutic targets that, in combination with current treatments, could improve the life expectancy for CF patients.
Heme oxygenases (HO) are enzymes involved in the catalysis of heme groups, which are the catalytic sites for a variety of hemoproteins (hemoglobin, cytochromes, catalases, peroxidases) involved in aerobic metabolism and are toxic when freely accumulated in cells. HO-1 is abundantly induced in MΦs and other cell types in response to different cellular stresses, including infection. HO-1-dependent heme degradation plays an important cytoprotective role, as the enzymatic degradation of heme leads to the production of mediators (CO, bilirubin and biliverdin) endowed with potent anti-oxidant, anti-inflammatory and anti-apoptotic properties that help to reestablish cellular homeostasis (22). The cytoprotective properties of these mediators would potentially be beneficial in CF lung disease. In addition, HO-1 forms a complex with the transcription factor IRF3, critical for induction of the type-I interferon pathway (23), which was recently shown to be defective in CF airway epithelial cells (24).
CO, which has strong anti-inflammatory properties, is an interesting mediator of the HO-1 pathway (25). Heme degradation is the only mammalian pathway that produces CO. Similar to nitric oxide (NO), CO acts as a signaling mediator by binding to divalent metals of heme-containing proteins (e.g. guanylate cyclase, mitochondrial cytochrome-c oxidase, NADPH oxidase), thus regulating their function. CO modulates several signaling pathways that are implicated in cell protection, anti-inflammation, and homeostasis, such as p38 MAPK, hypoxia-inducible factor 1 (HIF1α), peroxisome proliferator-activated receptor (PPAR)γ, glutathione production and the NOS/NO pathway (26), many of which are altered in CF (12). Exogenous administration of CO by inhalation or by CO-releasing molecules reduces the production of pro-inflammatory cytokines and increases the expression of the anti-inflammatory cytokine IL-10 (27), which is decreased in CF (4, 28, 29). In addition, CO reduces neutrophil migration in septic lungs by suppressing their transendothelial migration (30). Furthermore, the HO-1/CO pathway, together with the scaffold protein caveolin-1 (CAV-1), acts as a negative regulator of TLR4 signaling in MΦs (31).
In this study, we tested whether the hyper-responsiveness to LPS in CF MΦ's is due to unbalanced negative regulation of TLR4 by the HO-1/CAV1 axis and whether modulation of this crucial pathway can ameliorate CF-related innate immune function.
Materials and Methods
Chemicals and Reagents
The following antibodies were used: mouse anti-TLR4 (1:100, Imgenex); rabbit anti-cav-1 (1:100 for IF studies and 1:500 for western blot, Abcam); rabbit anti HO-1 (1:200 for IF studies and 1:2000 for western blot, Abcam); rabbit anti-flotillin-1 (1:1000, Cell Signaling); rabbit anti-actin (1:5000, Santa Cruz); mouse anti-CD68 (1:100, Dako). Secondary antibodies were: anti-mouse Alexa 488 or Alexa 555 and anti-rabbit Alexa 488 or Alexa 555 (1:400, Invitrogen). The 4′,6-diamidino-2-phenylindole (DAPI) was used for nuclei counter staining. Pseudomonas aeruginosa (PA) LPS (Sigma-Aldrich) was prepared in PBS at 100X stock solution. In some experiments, we also used the PA strain PAO1, which has a complement of virulence factors similar to environmental strains likely to be encountered by patients prior to the establishment of chronic infection. PA was prepared as described in ref. (59). CORM-2, which contains a ruthenium metal surrounded by six carbonyl (CO) groups, was dissolved in DMSO (stock solution 20 mM) (Sigma-Aldrich, St Louis MO). As a negative control for CORM-2, an inactive compound, here referred to as iCORM, was used (stock solution 20 mM) (Sigma-Aldrich). This compound [Ru(DMSO)4Cl2) consists of a ruthenium metal where the CO groups have been replaced by DMSO (60) (provided by Dr. Motterlini, Paris, FR). Both drugs were diluted to final concentrations of 50-100 μM, depending on the experiment. Stocks were prepared fresh for each experiment. DMSO alone was included as a vehicle control for each experiment performed. HO-1 was over-expressed in CF MΦs using an adenovirus vector (AdV) expressing HO-1 under the CMV promoter (AdV-HO-1), a kind gift of Dr. MP Soares (39). As control, we used the empty adenovirus (AdV-ctr). For treatment with antioxidants, MΦs were kept overnight with 25μg/ml EUK 134 (synthetic superoxide dismutase-SOD-/catalase mimetic) (Santa Cruz, CAS 81065-76-1). Cells were then treated with LPS (100 ng/ml) for 4h or 6h and analyzed as described in the text. For experiments with the CFTR inhibitor CFTRinh172 (kind gift from Dr. Alan Verkman)(35), WT MΦs cells were pre-treated overnight with 20μM of inhibitor or vehicle alone (DMSO) and refreshed before adding LPS (100 ng/ml). For inhibition of the p38-MAP Kinase (MAPK) pathway, cells were pre-treatment for 1h with 10 μM of the p38-specific inhibitor SB203580 (Cell Signaling).
Mouse Breeding
All procedures were performed in compliance with relevant laws and institutional guidelines, and were approved by the Yale University Institutional Animal Care and Use Committee. Transgenic CFTR-/- (B6.129P2-KOCftrtm1UNC) mice were purchased from the Jackson Laboratory and bred in the Yale University Animal Facility. Mice are fully back-crossed to the black 6 background. Littermates CFTR-/- mice were fed a liquid diet (Peptamen, Nestle, Deerfield, Illinois) as previously described (4). Non-CF littermate control mice used in the experiments were maintained on an identical diet to the CFTR-/- mice to eliminate nutritional status as a potential confounder. The CF mice homozygous for the F508del mutation (abbreviated F508del) in the 129/FVB (ΔF508Cftrtm1eu, FVB/129 background) have been obtained from Bob Scholte, Erasmus MC Rotterdam, The Netherlands (37). F508del mice were bred at the European Institute of Research for Cystic Fibrosis (IERFC). All procedures were conducted at the IERFC were in accordance with Institutional Animal Care and Use Committee (IACUC) regulations/guidelines and approved by the local Ethics Committee for Animal Welfare of the San Raffaele Scientific Institute of Milan (protocol number 382) and also conform to European Community regulations for animal use in research (CEE n°86/609). We use the abbreviations CF-KO and F508del to indicate the CF mice from the knock-out and deltaF508 colonies, respectively. The CAV1-KO mice (B6.Cg-Cav1tm1Mls) are on a B6 background and were originally purchased from Jackson Laboratory and donated to us by Dr. W. Sessa, Yale University School of Medicine (38).
Isolation and culture of macrophages
BM-derived murine macrophages
BM collection was performed as previously described (4). After overnight culture, the non-adherent cells were differentiated for 7 days in 20ng/ml recombinant M-CSF (ConnStem Inc., CT, USA). After 7 days, cells were detached and characterized by flow cytometry (F4-80+/MAC-1+ population). The day before experiments, cells were plated accordantly with the experiments. Cells were challenged with LPS (100 ng/ml) or PA in the presence or absence of drugs at the time indicated. For PA infection, cells were inoculated at a ratio of 1:3 in media without antibiotics. After 40 minutes in culture, unattached planktonic PA were removed by rinsing and replaced with fresh complete media as described in ref. (59). For adenoviral infection, 5 ×105 cells were cultured in 6-well plates and exposed to 2 ×107 PFU of each virus in 1 ml of serum-free medium for 4 h. The cells were then washed and incubated in serum-containing medium for 36 h. The cells were then subjected to treatments as indicated.
Human macrophages
Blood was obtained from healthy donors (HD) or from patients (age range 3-18 yrs, all pancreatic insufficient) with CF carrying at least one deltaF508 allele during their annual check-up with informed consent in accordance with the Yale University Medical School Human Investigation Committee and MΦs differentiated as described in ref. (6). Before LPS treatment, cells were washed extensively with PBS, detached with Accutase (Innovative Cell Technology), and seeded at 0.25×106 cells per well across 24 well plates. The subsequent day, cells were challenged with LPS and lysed for western blot analysis at the times indicated in the text. For time 0h, cells without LPS were harvested after 12h of incubation from the seeding.
Nasal Polyps
Ex-vivo cultures of nasal polyp biopsies from four CF patients and four non-CF patients with non-allergic idiopathic polyposis were performed as described (11, 41, 50, 61). The tissue culture model has been validated and used extensively in studies of intestinal inflammatory conditions (11) and ifor CF airways (11, 41). See ref. (50) for detailed description of the procedure. Patients had a mean age of 21 years (range 16–32) 50% female and 50% males.). Patients in both the CF and non-CF groups suspended the use of corticosteroids at least one month before the surgical intervention and were only treated by saline nasal irrigation. None of the patients had allergic rhinitis, as documented by clinical history. CF patients were homozygous for the deltaF508 mutation and were pancreatic insufficient. Nasal polyp tissues were untreated or treated with LPS and then frozen in cryo-embedding media OCT. Seven μm sections were used for IF studies as described in ref. (11). Informed consent was obtained from all subjects and the ethical committee of Regione Campania Health Authority approved the study.
RT-PCR and expression analysis
Total RNA was isolated from 1×106 cells using QiagenRNAMini Kits™ (Qiagen). After RNase-freeDNaseI treatment (Roche Molecular Biochemical), 2 μg total RNA was reverse-transcribed using Superscript™ II RNaseH- Reverse Transcriptase (Invitrogen) following the manufacturer's specifications. Real-time PCR analysis was performed with a Bio-Rad iCycler using TaqMan technology. Copy number was normalized by 18S expression and the fold increases to 0 (untreated cells) were calculated by ΔΔCt method. All TaqMan primers and probes were purchased from Applied Biosystem (Life Technology).
Cell fractionation and Western Blot
For plasma membrane enrichment, we isolated the detergent-resistant cell fraction. Cells were homogenized in 0.5 ml of MBS buffer with 1% Triton X-100 supplemented with protease inhibitors and subjected to a first centrifugation at 720 G for 5 min; the supernatants were collected and centrifuged at 10,000 G for 5 min. Then, the supernatants from the second centrifugation were subjected to ultracentrifugation at (100,000 G) for 1 h at 4°C using a SW40 Ti rotor (Beckman Ultracentrifuge). The pellet was regarded as the detergent-resistant fraction. An equal amount of protein was separated by electrophoresis on 7% Bis-Tris Gels, transferred to nitrocellulose membrane (Bio-Rad Laboratories, CA) and incubated with first antibodies overnight at 4°C. Horseradish Peroxidase-conjugated to IgG secondary antibodies (1:5000, Santa Cruz) and Amersham ECL Plus Western Blotting System (GE Healthcare Bio-Sciences Corp., NJ) were used for detection. The chemiluminescence imaging system GeneGnome (Syngene) was used for image acquisition band intensity quantification.
Immunofluorescence analysis
WT, CF, and CAV-1 KO MΦs were grown overnight on poly-L-lysine-coated 12mm round cover slips at 70-80% confluence. The following day, cells were challenged with LPS in the presence or absence of drugs or vehicle alone (DMSO). At the times indicated, cells were fixed for 15 min in 4% PFA, permeabilized for 5 min in PBS/0.1% Triton, and stained as indicated. Pictures were taken with a confocal microscope (Leica TCS SP5 Spectral Confocal Microscope) using a 63× objective lens. Each image was subjected to Z-stack analysis for co-localization studies. For each experiment, at least 6 different fields were acquired. Nasal polyp sections were fixed in acetone for twenty minutes, washed in PBS and then stained with indicated antibodies and analyzed as described above.
Cytokine quantification
Cytokine concentrations in the media of MΦs were assessed by Milliplex (Millipore) following the manufacturer's instructions. They were then acquired in a Luminex instrument and analyzed with BeadView Software (Upstate NY). Cytokine concentrations were normalized to protein concentration for each sample analyzed.
Flow Cytometry
For TLR4 plasma membrane flow cytometry, CF MΦs were treated with LPS (100 ng/ml) for 4h and 6h in the absence or presence of 100 μM CORM-2 (or DMSO as vehicle control). CF MΦs were also exposed to CORM2 for 6h in the absence of LPS (No LPS). Cells were then detached with Accutase and washed in wash buffer (PBS/2% serum). After 10 min of incubation with Fc-block, cells were stained on ice for 30 min with rat monoclonal APC-F4-80 (eBioscience) and rat monoclonal FITC-TLR4 (Imgenex). Dead cells were excluded with dead/live staining (Invitrogen, L10119). Appropriate isotype antibodies were used as controls. Plasma membrane TLR4 in the F4-80-positive cell population is expressed as median fluorescence intensity.
Measurement of ROS production
To determine ROS production, WT and CF MΦs (2.5-5.0 × 105) were detached with Accutase, washed in PBS, suspended in DMEM medium without FBS and loaded with 2.5 μM 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA; Molecular Probes) for 20 min at 37°C. In the presence of intracellular ROS and esterases, CM-H2DCFDA is oxidized and deacetylated yielding the fluorescent molecule 2′,7′-dichlorodihydrofluorescein diacetate (DCF). After two washes in 0.1% FBS/PBS, cells were analyzed by flow cytometry. Fluorescence is expressed as median fluorescence intensity.
In vivo experiment and bronchoalveolar lavage (BAL) fluid collection and analysis
WT and CF mice received 3 doses of LPS (Sigma L8643) over 3 days. LPS (12.5 mg) was administered with a nebulizer (Pulmo-Aide Compressor, Natallergy). Five ml of solution were nebulized over 15 minutes as previously described in ref. (4). At the times indicated, mice were sacrificed. BAL fluid was collected using standard methods. The total and differential cell count in the BAL of LPS-treated mice was assessed by counting and by cytospin analysis. Lung tissues were collected and homogenized in lysis buffer for western blot analysis.
Statistical Analysis
Statistical analyses were conducted using one-sided two-sample t-tests or two-sample unequal variance t-tests (in vivo studies). Data are expressed as mean ± standard deviation or standard error of the mean (as indicated). A P value <0.05 was considered statistically significant. All experiments were performed in three biological triplicates (indicated in the figures as ‘n’) unless indicated differently. Statistical significance is labeled as follows: * P <0.05; ** P <0.01.
Results
Defective plasma membrane compartmentalization of HO-1 in CF macrophages challenged with LPS compromises negative regulation of TLR4 signaling
We have reported that regulation of TLR4 signaling is compromised in CF MΦs thus contributing to overproduction of pro-inflammatory cytokines in response to LPS isolated from PA (6). HO-1 activity negatively regulates TLR4 signaling. The mechanism by which HO-1 mitigates TLR4 signaling relies on production of CO at the level of the TLR4-MyD88 complex, which is assembled at the plasma membrane during signaling. HO-1/CO-dependent TLR4 negative regulation requires expression of the scaffold protein caveolin 1 (CAV-1), which has binding motifs for both HO-1 and the TLR4 TIR domain. HO-1/CAV-1 binds to the TLR4 complex and promotes detachment of the MyD88 adaptor from TLR4, thus terminating the signal (31).
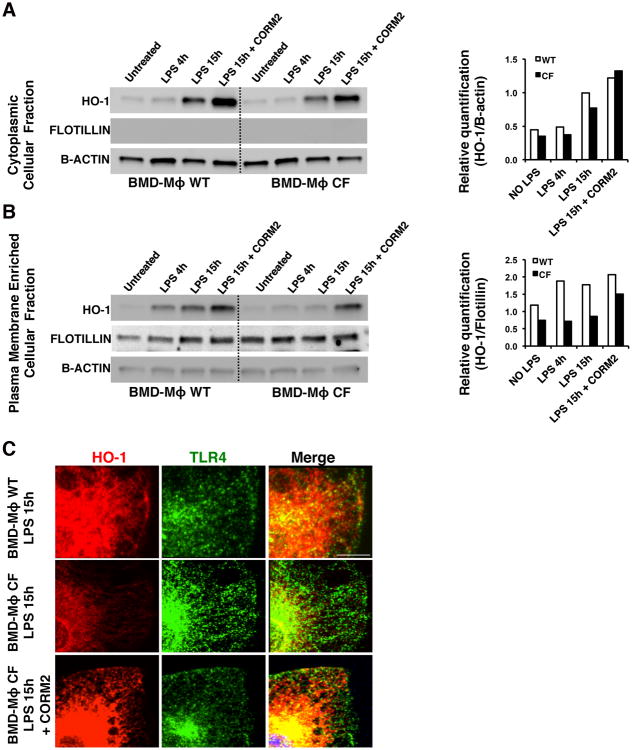
We hypothesized that the hyper-TLR4 signaling in CF MΦs during LPS stimulation is due to defective HO-1/CO-dependent negative TLR4 regulation. First, we examined whether HO-1 in LPS-challenged CFTR knockout (CF-KO) MΦs could efficiently compartmentalize to the plasma membrane. WT and CF bone marrow-derived (BMD) MΦs, either unchallenged or challenged with LPS for 4h or 15h were subjected to cellular protein fractionation. Flotillin, which is a protein absent from intracellular membranes and abundant in the lipid raft regions of the plasma membrane, was used to validate the enrichment of plasma membrane proteins after fractionation. We observed that upon LPS treatment, HO-1 protein increased in the cytosolic fraction of both WT and CF-KOMΦs although HO-1 protein levels are slightly less abundant in CF-KO than WT MΦs at each time point after LPS challenge (Fig. 1A). Analysis of the plasma membrane-enriched fraction revealed strong enrichment of HO-1 protein in WT cells, as early as after 4 h after LPS, whereas in CF-KOMΦs, HO-1 failed to translocate to the plasma membrane fraction after either 4h or 15h of LPS stimulation (Fig.1B). These data were corroborated by immunofluorescence (IF) studies, in which WT and CF-KO MΦs were treated with LPS for 15h and then stained for TLR4 and HO-1 (Fig. 1C and Supplementary Fig. S1A-C). In untreated cells, HO-1 was expressed at a low level and the fluorescence signal was even fainter in CF-KO MΦs (Supplementary Fig. S1A). After 15h of LPS stimulation, the expression of HO-1 was induced in both WT and CF-KO cells. HO-1 was abundant at the cell surface of WT MΦs (Fig. 1C and Supplementary Fig. S1B) in areas enriched in TLR4 (Supplementary Fig. S1D), while in CF-KOMΦs, HO-1 was mostly peri-nuclear, and failed to localize to the plasma membrane despite its increased expression level. Lower HO-1 protein levels were also observed in the total protein lysates of human peripheral blood-derived MΦs isolated from CF patients as compared to healthy controls (Supplementary Fig. S1E).
Figure 1. CF MΦs fail to compartmentalize HO-1 to the cell surface during LPS stimulation.
WT and CF bone marrow-derived (BMD) MΦs unchallenged or LPS challenged (100 ng/ml) for 4 and 15 hours (h) were subjected to detergent-resistant fraction isolation. The cytosolic (A) and the plasma membrane (PM)-enriched fractions (B) were immunoblotted for HO-1, flotillin and B-actin. CF MΦs were also challenged with LPS for 15h in the presence of CORM2 (100μM). Representative western blots are shown on the left panels and the relative HO-1 quantification normalized to B-actin (cytosolic fractions) or flotillin (PM-enriched fractions), is shown on the right. (C) Representative immunofluorescence (IF) staining for HO-1 (red) and TLR4 (green) on WT (top panels), CF (middle panels) and CF+CORM2 (lower panels) MΦs challenged with LPS for 15 h. Images were acquired by confocal microscopy. Scale bars = 3μm. In both the biochemical and IF studies, unchallenged and LPS challenged samples were exposed to DMSO as vehicle control for CORM2, and showed no differences from no DMSO controls (omitted from image for simplicity).
CO, the main HO-1 catabolic product, which acts as a signaling mediator and activates a positive feedback to the HO-1 pathway (32), has strong anti-inflammatory proprieties (33). Therefore, we tested whether exogenous delivery of CO influencesHO-1 protein levels and cellular distribution. CO was delivered with the CO-releasing molecule CORM2 (100μM) (34). When WT and CF-KO MΦs were exposed to LPS for 15h, CORM-2 (100μM) increased HO-1 protein levels in the cytosol of both WT and CF-KO MΦs (Fig. 1A). Treatment with CORM2 was effective in partially reestablishing the plasma membrane pool of HO-1 in CF-KO MΦs buy Western blot (Fig.1B) as well as in partially restoring HO-1 localization at the surface of CF-KOMΦs by IF (Fig. 1C and Supplementary Fig. S1C). Treatment with CORM2 did not alter TLR4 expression in CF-KO MΦs treated with LPS (Fig. S1F), but slightly decreased TLR4 plasma membrane localization as assessed by flow cytometry analysis (Fig. S1G). These data support the hypothesis that HO-1 fails to accumulate at the site of TLR4 signaling at cell surface following LPS challenge in CF-KO MΦs and that CO can modulate this process.
Reduced CAV-1 in CF macrophages impairs HO-1 plasma membrane compartmentalization
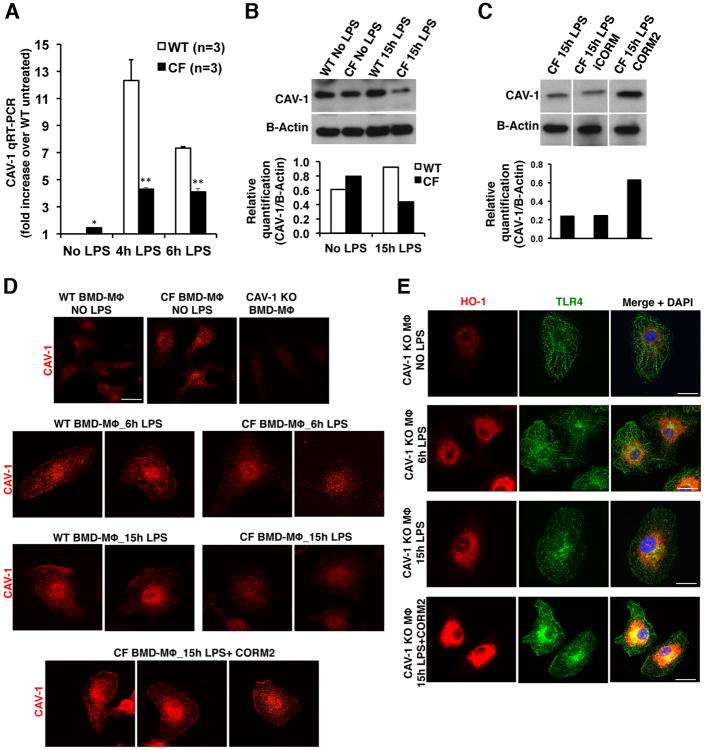
HO-1/CO compartmentalization to the TLR4-complex relies on binding to the scaffold protein CAV-1 (31). Therefore, we investigated whether CAV-1 expression was altered in CF-KO MΦs. Compared to WT cells, CF-KO MΦs at steady state had 2-fold increased CAV-1 mRNA expression, which, however, at 4h and 6h LPS stimulation, was not induced to the same extent, in CF-KO as in WT cells (p<0.001) (Fig. 2A). Consistent with the expression data, CAV-1 protein was 2-fold less abundant in CF-KO MΦs after 15h of LPS challenge (Fig. 2B).
Figure 2. Reduced CAV-1 expression in CF MΦs decreases HO-1 cell surface localization.
(A) Mean ± standard deviation of CAV-1 expression in WT and CF untreated (steady state) or challenged with LPS for 4h and 6h as assessed by qRT-PCR. Data are shown as CAV-1 fold increase over unchallenged WT cells. Representative western blot of CAV-1 from (B) WT and CF MΦs unchallenged or challenged with LPS for 15h; and from (C) CF MΦs challenged with LPS for 15h in the absence or presence of the control molecule iCORM (100μM) or of the CO-releasing molecule CORM2 (100μM). (D) Representative IF staining for CAV-1 (red) on unchallenged (first row) and LPS-challenged (6h and 15h) WT, CF, and CF+CORM2 MΦs, as indicated. CAV-1 antibody specificity is demonstrated by the lack of specific signal in MΦs isolated from CAV-1 KO mice (CAV-1 KO MΦs; first row, right panel). (E) Representative IF staining for HO-1 (red) and TLR4 (green) on CAV-1 KO and CAV-1 KO+CORM2 MΦs unchallenged or challenged with LPS (6h and 15h). The merged images (right) also include DAPI staining (blue). All images were acquired by confocal microscopy. Scale bars = 3.00 μm. In both biochemical and IF studies, unchallenged or LPS-challenged samples were exposed to DMSO as vehicle control for CORM2 (omitted from image for simplicity).
To investigate whether the inability of CF-KO MΦs to up-regulate CAV-1 expression in response to LPS stimulation is a consequence of defective CFTR function, we treated WT MΦs with the specific CFTR inhibitor CFTRinh172 (35). We show that CFTRinh172 reduced CAV-1 expression in WT cells in response to LPS stimulation (p<0.01). (Supplementary Figure S2A), which is consistent with our previous observation showing cross-talk between CFTR function and increased TLR4 signaling (6).
CORM2 treatment induced CAV-1 expression in both untreated and LPS treated CF-KO MΦs (Fig. 2C and Supplementary Figure S2B)while iCORM (a nonfunctional control molecule with the same CORM2 structure, but with DMSO replacing the CO groups) did not (Supplementary Figure S2B). IF analysis confirmed that CAV-1 localized intracellularly in untreated CF-KO MΦs and was not recruited to the cell surface after 6h and 15hof LPS stimulation, as it was in WT MΦs (Fig. 2D; second and third rows). The specificity of the CAV-1 antibody in MΦs, was validated using cells derived from CAV-1 KO mice (Fig. 2D; first row, right panel). Consistent with the expression (Supplementary Figure S2B) and biochemical data shown in Figure 2C, CORM2 increased CAV-1 expression and induced CAV-1 localization to the surface of CF-KO MΦs (Fig. 2D; fourth row; see also magnification in Supplementary Fig. S2C). Confocal merged images revealed that CAV-1 colocalizes with TLR4 in WT but not in CF-KO MΦs in response to LPS (Supplementary Fig. S2D).
Deletion of the phenylalanine in position 508 of CFTR (F508del) is the most common mutation in the CF population (36). F508del-CFTR encodes a misfolded protein that is prematurely degraded and fails to traffic to the plasma membrane, leading to defective channel function. We therefore extended our studies to MΦs isolated from CF mice homozygous for the F508del-CFTR (37). As was observed for CF-KO cells, less CAV1 upregulation coupled with altered HO-1 cellular localization in response to LPS stimulation, was observed in F508del-MΦs, andCORM2 enhances HO-1 protein expression and favors CAV-1 plasma membrane distribution (Supplementary Figure S2E-G). These results are in agreement with those obtained in CF-KO cells, supporting the hypothesis that the alterations of HO-1/CAV-1 axis are due to lack of a functional CFTR. Together, these data indicate that increasing HO-1 signaling by CO-releasing molecules restores CAV-1 expression and its localization to the cell surface, thus highlighting a putative feed forward loop between CAV-1 and HO-1 at the MΦ cell surface.
To determine whether CAV-1 could orchestrate HO-1 localization at the cell surface during LPS stimulation, we used MΦs isolated from CAV-1 KO mice (38). In spite of HO-1 expression, CAV-1 KO MΦs did not show any HO-1 localization at the cell surface after 6h or 15 h of LPS challenge (Fig. 2E). Accordingly, CORM2 increased HO-1 protein expression, but failed to restore HO-1 compartmentalization to the cell surface (Fig. 2E; fourth row). These results indicate that CAV-1 is required for orchestrating HO-1 compartmentalization in response to LPS stimulation.
Activation of the HO-1/CO pathway regulates TLR4 signaling and inflammation in CF macrophages
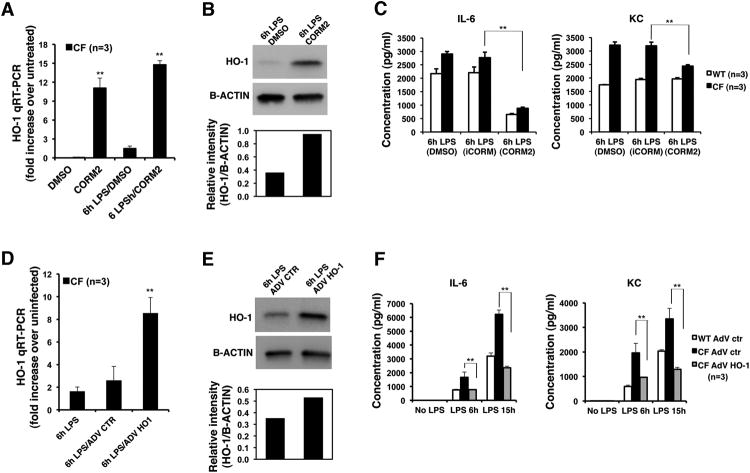
Besides its strong anti-inflammatory proprieties (33), CO induces both HO-1 and CAV-1 expression and rescues HO-1 cell surface localization in CF MΦs, as described above. Therefore, we hypothesized that exogenous delivery of CO could modulate TLR4 signaling, thus reducing the hyper-secretion of pro-inflammatory cytokines observed inmurine (4) and human (6) CF MΦs. Both WT and CF MΦs (either KO or F508del) were exposed to LPS and simultaneously treated with CORM2 (100μM), vehicle alone or iCORM. In addition to the ability to increase HO-1 levels in KO and F508del CF MΦs (Fig. 3A and 3B and Supplementary Figure S3A), CORM2, but not iCORM, decreasedIL-6 secretion in both WT and CF LPS-challenged MΦs, and decreased keratinocyte-derived cytokine (KC) secretion only in CF cells (Fig. 3C and Supplementary Figure S3B). Negligible cytokine levels were detected in untreated or CORM2 treated cells in the absence of LPS stimulation. Moreover, CORM-2 decreased IL-6 and KC secretion in Pseudomonas aeruginosa (PA) treated CF-KO MΦs (Supplementary Fig. S3C).
Figure 3. HO-1/CO pathway modulates production of pro-inflammatory cytokines in CF MΦs treated with LPS.
The HO-1 pathway was induced in CF-BMD MΦs by CORM2 (100 μM) (or iCORM control) or by infection with adenoviral vector (AdV) expressing HO-1 (or empty AdV control). Cells were then challenged with LPS (100 ng/ml) and pro-inflammatory cytokine secretion was measured at the times indicated. (A) Mean ± standard deviation of HO-1 fold increase expression over unchallenged CF cells in comparison to CF MΦs in steady state and during LPS stimulation, in the absence or presence of CORM2 as assessed by qRT-PCR. (B) Representative western blot of HO-1 and B-actin from CF MΦs challenged with LPS in the presence of vehicle control (DMSO) or CORM2; the panel below shows the relative HO-1 quantification. (C) Mean ± standard error of the mean (SEM) of IL-6 (left panel) and KC (right panel) concentrations in the supernatant of WT and CF MΦs treated with LPS in the absence or presence of CORM2 or iCORM. (D) Mean ± standard deviation of HO-1 fold increase expression over unchallenged CF MΦs treated with AdV-control (ctr) or AdV-HO-1 and then challenged with LPS, as assessed by qRT-PCR. (E) Representative western blot of HO-1 and B-actin from AdV ctr or AdV HO-1 unchallenged CF MΦs and challenged with LPS; the panel below shows the relative HO-1 protein quantification. (F) Mean ± SEM of IL-6 (left panel) and KC (right panel) concentrations in the supernatant of WT MΦs treated with AdV ctr (WT AdV ctr) and CF MΦs treated with AdV ctr or AdV HO-1 and then challenged with LPS.
The effects of CO releasing molecules clearly support a defective HO-1 pathway, as CO is a key mediator of HO-1 activity. Therefore, we investigated whether enforced expression of HO-1, with consequently increased CO release, would recapitulate the effects of CORM2. HO-1 was over-expressed in CF-KO MΦs using an adenovirus vector (AdV) expressing HO-1 (AdV-HO-1) under the control of CMV promoter or a mock adenovirus control vector (AdV-Ctr) (39). AdV-HO-1 induced a three-fold increase of HO-1 mRNA (Fig. 3D) and almost two-fold increase of protein (Fig. 3E) together with a two-fold increase in CAV-1 expression (Supplementary Fig. S3D). Accordingly, the infection of CF-KO MΦs with AdV-HO-1significantly reduced both IL-6 and KC secretion as compared to AdV-Ctr (Fig. 3F). Together these data suggest a positive feed forward loop between CAV-1 and HO-1, which contributes to the regulation of TLR4 signaling and inflammatory response.
Defective redox control affects CAV-1 and HO-1 expression in CF MΦs
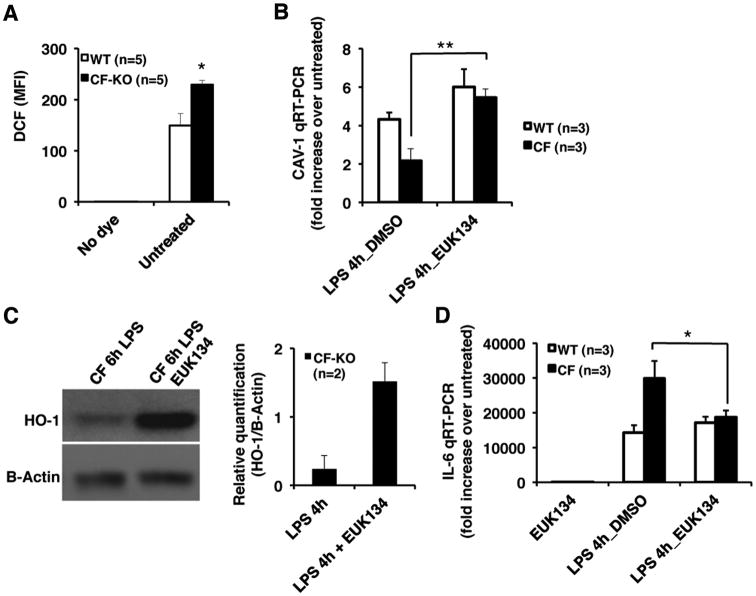
Oxidative stress contributes to the development of CF lung disease (12, 43). Cells lacking functional CFTR are characterized by oxidative stress, which favors hyper-inflammation (40). Increased ROS levels, consequent to defective CFTR function, induce tissue transglutaminase activation (41), leading to defective autophagy and inflammation in human and murine F508del-CFTR homozygous airways (11, 42). Modulation of the manganese superoxide dismutase-SOD (SOD2) is sufficient to re-establish cellular homeostasis as it can rescue many of the ROS-dependent dysfunctions in isolated CF airway epithelial cells and in vivo in mice(11, 42). We observed that CF-KO MΦs have increased levels of reactive oxygen species (ROS) when compared to WT MΦs (Fig. 4A). Therefore, we tested whether modulation of SOD2 activity could restore the unbalanced CAV1 adaptation to LPS stimulation in CF-MΦs. The synthetic superoxide dismutase-SOD-/catalase mimetic EUK134 significantly restored CAV-1 induction in response to LPS to levels comparable to those observed in WT MΦs (Fig. 4B). Notably, EUK134 increased HO-1 protein in response to LPS in CF MΦs(Fig. 4C), supporting the finely tuned connection between CAV-1 and HO-1 during LPS challenge. Accordingly, EUK134 reduced the hyper-inflammatory response of CF MΦs, as assessed by IL-6 expression (Fig. 4D).
Figure 4. The superoxide dismutase activity modulates CAV-1 and HO-1 expression in CF MΦs challenged with LPS.
(A) Reactive oxygen species (ROS) measurements in WT and CF-KO MΦs, as assessed by CM-H2DCFDA staining and flow cytometry analysis;(B) Mean ± standard deviation of CAV-1 fold expression with or without overnight pre-treatments with EUK134 after 4h of LPS stimulation, as assessed by qRT-PCR. (C) Representative western blot of HO-1 and B-actin from CF-KO MΦs challenged for 6h with LPS with or without overnight pre-treatments with EUK134 and relative quantification on the right;(D) Mean ± standard deviation of IL-6 fold increase expression over unchallenged WT MΦs in LPS treated WT e CF-KO MΦs with or without overnight pre-treatments with EUK 134, as assessed by qRT-PCR.
CAV-1is transcriptionally activated by different signaling pathways, among them, p38 MAP kinase (MAPK)/Sp1 activation (44). We demonstrate that CORM2 fails to stimulate CAV-1 expression when the p38 inhibitor SB203580 is added during LPS stimulation (Supplementary Figure S3E). Thus, CORM2-mediated stimulation of CAV-1 expression in CF-KO MΦs treated with LPS requires p38 signaling.
CF mice and human CF nasal polyp biopsies reveal reduced HO-1 induction in CF cells in response to inflammatory stress
To assess these findings in vivo, we used mice homozygous for the ΔF508 (F508del) CFTR mutation (CftrF508del/F508del) and their WT littermate controls. The CF mouse model represents a valuable tool for studying certain aspects of CF lung disease (such as inflammation), albeit not reproducing all aspects of CF lung disease (45-47), as they do not develop spontaneous chronic PA infection. Many laboratories, including ours, have consistently demonstrated that these CF mice display a more prolonged and exuberant inflammatory response than WT mice to bacteria and bacterial products (e.g. LPS and PA) characterized by massive migration of neutrophils in the lung, increased secretion of pro-inflammatory cytokines, and higher rate of mortality after an inflammatory challenge (4, 41, 48). In addition, two different experimental approaches, bone marrow transplantation (4) and CF mice with conditional expression of Cftr crossed with the myeloid-targeted Cre-recombinase LysMCre mice (49), have clearly demonstrated the contribution of immune cells to the exaggerated inflammatory response in CF lungs.
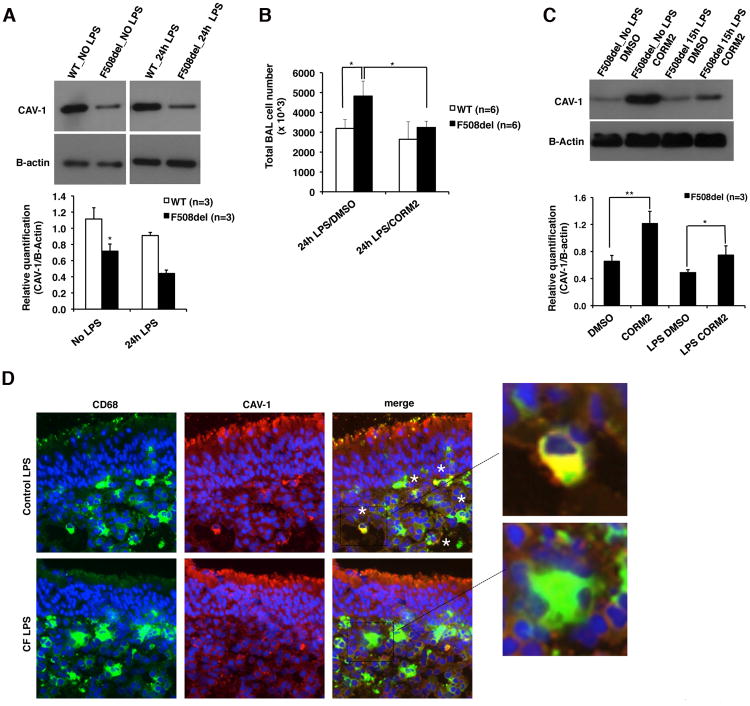
F508del CF and littermate control WT mice (n=3 for each group of treatment) were nebulized with PA-LPS as previously described (4) and 24h later, the lungs were harvested for analysis. Lung lysates from F508del CF mice had reduced CAV-1 protein at steady state and in response LPS compared to WT lungs (Fig. 5A). HO-1 protein was barely detected in WT and CF lungs not treated with LPS. In the lung of F508del CF mice treated with LPS, in spite of the hyper-inflammatory response as revealed by the higher numbers of inflammatory cells in the bronchoalveolar lavage (BAL) compared to WT BAL fluids (4.8×106 vs. 3.2 ×106, p=0.02) (Fig. 5B), there was slightly decreased HO-1 protein expression, which, however, did not reach the statistical significance (Supplementary Figure S4A). This could be attributed to the fact that in the whole lung lysates, there are multiple different cell types expressing HO-1 (e.g. endothelial cells).
Figure 5. Defective CAV-1 induction in murine CF lungs and human CF nasal polyp tissues; and the in vivo immune-modulatory effects of CORM2.
(A) Representative western blots for CAV-1 and B-actin from lung lysates isolated from unchallenged or LPS challenged WT and CF-F508del mice; the panel below shows the relative CAV-1 quantification. (B) Total number of BAL fluid cells harvested from mice treated intraperitoneally with CORM2 or vehicle alone (DMSO) and then nebulized with LPS (12.5mg/5ml). Mice were sacrificed and BAL recovered 24h after last LPS nebulization. (C)Representative western blots for CAV-1 and B-actin from lung lysates isolated from unchallenged or LPS challenged CF-F508del mice with or without CORM2 treatment; the panel below shows the relative CAV-1 quantification. (D)Representative IF staining for CD68 (green), CAV-1 (red) and DAPI (blue) in human nasal polyp biopsies (hNPB) removed from non-CF and CF patients and challenged with LPS for 24h. Merged images and magnifications of interest areas are shown on the right. Yellow staining indicates CD68 and CAV-1 colocalization.
Next, F508del CF mice (n = 3) were treated intraperitoneally (IP) with CORM2 (7.5 mg/Kg) 24 h and 15 minutes before LPS LPS nebulization. In F508del CF mice, CORM2 decreased the number of total BAL fluid cells 24h after LPS challenge (p=0.02), while it had no effect in WT mice (Fig. 5B). CORM2 administration to F508del CF mice also increased steady state and LPS-induced CAV-1lung expression (Fig. 5C) as well as HO-1 protein expression after LPS treatment (Supplementary Figure S4B). Together, these data are consistent with our in vitro studies showing that the anti-inflammatory activity of CORM2 in vivo relies, at least in part, on modulation of the CAV-1/HO-1system.
To confirm these results in a more clinically relevant model of CF, we used ex vivo cultures of primary nasal polyp biopsies fromF508del-CFTR homozygous CF patients (n=4), who had undergone polypectomy for non-allergic nasal polyposis, which often complicates CF (41, 50). As controls, we used nasal polyps isolated from non-CF patients with idiopathic polyposis (n=4). This culture model represents a good approximation to in vivo human studies because local anatomical connections are retained and all cell types still interact with neighboring cells within their natural environment. Nasal polyp biopsies were challenged with PA-LPS for 24h, as previously described (41, 50). As already published (50), there were more CD68 positive cells (in green) in the mucosa of CF than non-CF (control) tissues challenged with LPS for 24h (Supplementary Figure S4C). In contrast, CF nasal polyp mucosae showed fewer CD68-positive cells that also were also positive for CAV-1 (in red), compared to non-CF controls that had several CD68-pos/CAV1-pos cells (colocalization shown in yellow and indicated by white stars, Fig. 5D). In addition, while in non-CF control nasal polyp mucosae CD68-positive cells were always HO-1-positive, only a small subpopulation of CD68-positive cells in CF mucosae were also positive for HO-1 (Supplementary Figure S4D).
Taken together, these data indicate that the unbalanced CAV-1 expression and HO-1 activity may play a role in the innate immune response in CF, and suggest that restoring the CAV-1/HO-1 axis by means of genetic or pharmacological manipulations aimed at increasing CO levels could be an attractive strategy to control lung inflammation in patients with CF.
Discussion
Cells lacking functional CFTR are characterized by constitutive oxidative stress and hyper-inflammation, which alter several mechanisms involved in the maintenance of cellular homeostasis. In this study, we demonstrate that in CF MΦs, constitutive oxidative stress interferes with the function of the regulatory HO-1/CO pathway exacerbating the inflammatory response to LPS. We demonstrate a previously uncharacterized defect of CF MΦs in inducing expression of the scaffold protein CAV-1 in response to LPS. In the absence of CAV-1, HO-1, although expressed, fails to compartmentalize to the cell surface of CF cells, where the complex should act as a negative regulator of the TLR4 signaling (31). This leads to impaired down-regulation of TLR4 signaling, with a resultant increase in the production of pro-inflammatory cytokines (Fig. 6). Experiments on MΦs isolated from CAV-1 KO mice reinforce the hypothesis that CAV-1 is necessary for HO-1 cell surface distribution. It is worthwhile to note that CAV1 KO mice are more susceptible than WT mice to PA lung infection, with higher production of inflammatory cytokines, elevated bacterial burden, and oxidative stress (51, 52), a phenotype that resembles CF pathology. Interestingly, CAV1 also plays important roles in receptor trafficking and degradation (53), which may contribute to the abnormal TLR4 trafficking and reduced degradation that we have previously described in CF MΦs (6).
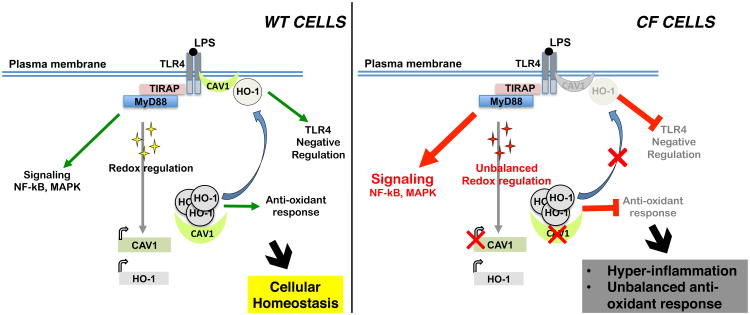
Figure 6. Defective CAV-1/HO-1 axis impairs the cytoprotective response to inflammatory stimuli in CFTR-dysfunctional cells.
In healthy cells, stress signals (e.g. the pathogen-associated molecular pattern LPS that activates TLR4 signaling) induce a physiological inflammatory response with activation of the NF-kB/MAPK and the redox response pathways. Activation of TLR4 induces expression of both CAV-1 and HO-1. The CAV-1-dependent cellular distribution of HO-1 ensures TLR4 signaling negative regulation and the HO-1-mediated anti-inflammatory and anti-oxidant responses, helping to reestablish cellular homeostasis (left panel). In CFTR-dysfunctional cells, activation of the TLR4 signaling induces substantial NF-Kb/MAPK pathways; in addition, the steady-state oxidative stress impairs CAV-1 expression in response to inflammatory stimuli, compromising HO-1 cellular distribution. Thus, CF cells lack the HO-1-mediated counter cytoprotective response, which ultimately exacerbates and prolongs the inflammatory response (right panel).
The decreased expression of CAV-1 in CF MΦs in response to LPS is associated with lack of functional CFTR, since functional CFTR inhibition in WT MΦs is sufficient to recapitulate the defective expression of CAV-1. We had already demonstrated an intimate connection between functional CFTR and TLR4 signaling in MΦs, and the alteration of the HO-1/CAV-1 axis may be part of the dysfunctions previously described by us in CF MΦs(6).
We also demonstrate that either enforced HO expression or stimulation of the HO-1/CO pathway by exogenous CO delivered via CORM2 induces CAV-1 expression as a positive-feedback loop, which is sufficient to restore plasma membrane localized HO-1 and appropriate attenuation of TLR4 signaling, and to reduce lung inflammation in CF mice. Therefore, the HO-1/CAV1 complex has immuno-modulatory effects on CF MΦs. Importantly, stimulation of the SOD2 activity is sufficient to correct CAV-1 expression and HO-1 dysfunction in CF MΦs, decreasing the production of pro-inflammatory cytokines. Thus, attenuation of oxidative stress may be pivotal in restoring HO-1/CAV-1 balance in CF MΦs.
The ERK1/2 (p42/44 MAP kinase) cascade negatively regulates CAV-1 expression (54)and we have already demonstrated that CF MΦs have increased and prolonged ERK1/2 phosphorylation in response to LPS treatment (6). Importantly, the prolonged phosphorylation of ERK1/2 in CF is promoted by sustained ROS levels and its phosphorylation is reduced by genetic or pharmacological up-regulation of SOD2 activity (41). Thus, hyper-activation of the ERK1/2 pathway in conditions of increased oxidative stress may contribute to reduced CAV-1 expression in CF MΦs. CAV-1 expression is also induced by p38 MAPK/Sp1-mediated signaling (44). Although CAV-1 expression can be further decreased by inhibition of the p38 MAPK pathway during LPS stimulation in CF MΦs, CORM2 fails to stimulate LPS-induced CAV-1 expression if the cells are pre-treated with the p38 inhibitorSB203580, suggesting that CORM2 action requires p38 signaling, as also demonstrated in non-CF cells exposed to CO (55).
Exogenous HO-1 expression in human bronchial CF cell lines is associated with reduced expression of inflammatory mediators and with reduced apoptosis/injury during P. aeruginosa challenge (56), which is agreement with our data suggesting that HO-1 is a cytoprotective pathway for CF. As indirect evidence of the importance of this pathway in CF airway epithelial cells, Chen et al. (57)demonstrated that expression of Nrf2, a transcription factor upstream of HO-1, is decreased in CF airway epithelial cells compared to WT cells. Interestingly, other studies have shown that, once expressed, HO-1 facilitates Nrf2 nuclear translocation (in a positive feedback manner), activating the anti-oxidative and anti-inflammatory transcriptional response (58). Lastly, a recent study reported that the gene encoding HO-1 (HMOX1) is a modifier for lung disease severity in pediatric CF patients (24). This further supports the hypothesis that alteration of this pathway in CF may be involved in disease development.
Because of the pleiotropic role of the HO-1/CO pathway in activating cytoprotective pathways and its expression in airway epithelial cells, we anticipate that modulation of this pathway may target different defects associated with dysfunctional CFTR. Modulation of this pathway may also help to reestablish CF epithelial cell homeostasis, which will be assessed in future investigations. Thus, the HO-1/CO pathway may represent an attractive pharmacological target for CF, as it seems to modulate several physiological responses to stress that are altered in the nonfunctional-CFTR background such as exuberant lung neutrophilia, hyper-secretion of pro-inflammatory cytokines and decreased anti-inflammatory response, and redox response. Importantly, therapies aimed at modulating the HO-1/CO pathway via exogenous administration of CO at doses much below (<500ppm) toxic doses associated with its inhalation (which we have mimicked using CO-releasing molecules in our experiments) are currently in phase II trials to treat delayed kidney dysfunction after transplantation (NCT00531856) and is in phase I trials for acute airway inflammation and chronic obstructive pulmonary disease (NCT00094406).
Supplementary Material
Acknowledgments
We thank S. Donaldson (Laboratory Medicine, Yale University School of Medicine, USA), C. Caputo C. (Pediatrics, Yale University School of Medicine), K. Konstantina (Pediatrics, Yale University School of Medicine), Dr. S. Kassmer (Laboratory Medicine, Yale University School of Medicine), Dr. R. Foresti (INSERM U955 University Paris-Est, France), Alessandro Luciani and Manuela Gavina (previous members of the European Institute for Research in Cystic Fibrosis, San Raffaele Scientific Institute, Milan, Italy) for their contributions to these studies. Dr. L. Cohn(Yale University School of Medicine), Dr. P. Lee(Yale University School of Medicine), and Dr. A. Choi (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) for helpful discussions. We also thank Dr. R. Motterlini(INSERM U955 University Paris-Est, France) for providing the CORM-2 control molecule (iCORM) and critical discussion; Dr. M.P. Soares and S. Rebelo (Instituto Gulbenkian de Ciência, Portugal) for AdV-HO-1 vector, Dr. F. Giordano for the (AdV-ctr) (Yale University School of Medicine), Dr. W. Sessa and Dr. J. Chidlow (Yale University School of Medicine) for caveolin-1 knock-out mice.
Supporting Agencies:This work was supported by National Institutes of Health Grants: RO1 HL093004 to MEE and EMB, NIAID K08 AI071074 to TSM, RO1 HL073742 to DSK; The European Institute for Research in Cystic Fibrosis and Italian Cystic Fibrosis Association to LM; The Italian Cystic Fibrosis Research Foundation, grant FFC #15/2012 with the contribution of VR, EMB, LM and MEE; and Telethon grant #GGP12128to LM.
Abbreviation
- CFTR
Cystic fibrosis transmembrane conductance regulator
- CF
Cystic Fibrosis
- HO-1
heme oxygenase 1
- CO
carbon monoxide
- CAV-1
caveolin 1
- LPS
lipopolysaccharide
- CORMs
CO-releasing molecule 2
- WT
wild-type
- AdV
adenoviral vector
- PA
Pseudomonas aeruginosa
- MΦs
macrophages
- HD
healthy donors
- hNPB
human nasal polyp biopsies
- IF
immunofluorescence
- ctr
control
- TLR
toll like receptor
Footnotes
Author Contributions: P.Z., V.R.V., F.E., S.E., A.D.S., performed experiments; T.M.helped in developing assays and reviewed the manuscript; V.R. collected and cultured nasal polyp biopsies from patients; L.M., D.S.K. and M.E.E. contributed designing and writing the manuscript. E.M.B. designed the study, developed assays, performed experiments, analyzed data and wrote the manuscript.
Competing Interests Statement: The authors declare no competing financial interests.
References
- 1.Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med. 2006;173:475–482. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- 2.Bove PF, Grubb BR, Okada SF, Ribeiro CM, Rogers TD, Randell SH, O'Neal WK, Boucher RC. Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J Biol Chem. 2010;285:34939–34949. doi: 10.1074/jbc.M110.162933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Painter RG, Valentine VG, Lanson NA, Jr, Leidal K, Zhang Q, Lombard G, Thompson C, Viswanathan A, Nauseef WM, Wang G, Wang G. CFTR Expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry. 2006;45:10260–10269. doi: 10.1021/bi060490t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruscia EM, Zhang PX, Ferreira E, Caputo C, Emerson JW, Tuck D, Krause DS, Egan ME. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator-/- mice. Am J Respir Cell Mol Biol. 2009;40:295–304. doi: 10.1165/rcmb.2008-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller C, Braag SA, Keeler A, Hodges C, Drumm M, Flotte TR. Lack of cystic fibrosis transmembrane conductance regulator in CD3+ lymphocytes leads to aberrant cytokine secretion and hyperinflammatory adaptive immune responses. Am J Respir Cell Mol Biol. 2011;44:922–929. doi: 10.1165/rcmb.2010-0224OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruscia EM, Zhang PX, Satoh A, Caputo C, Medzhitov R, Shenoy A, Egan ME, Krause DS. Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. J Immunol. 2011;186:6990–6998. doi: 10.4049/jimmunol.1100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sorio C, Buffelli M, Angiari C, Ettorre M, Johansson J, Vezzalini M, Viviani L, Ricciardi M, Verze G, Assael BM, Melotti P. Defective CFTR Expression and Function Are Detectable in Blood Monocytes: Development of a New Blood Test for Cystic Fibrosis. PLoS One. 2011;6:e22212. doi: 10.1371/journal.pone.0022212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abuarqoub H, Foresti R, Green CJ, Motterlini R. Heme oxygenase-1 mediates the anti-inflammatory actions of 2'-hydroxychalcone in RAW 264.7 murine macrophages. Am J Physiol Cell Physiol. 2006;290:C1092–1099. doi: 10.1152/ajpcell.00380.2005. [DOI] [PubMed] [Google Scholar]
- 9.Kanno T, Nishizaki T. CFTR mediates noradrenaline-induced ATP efflux from DRG neurons. Mol Pain. 2011;7:72. doi: 10.1186/1744-8069-7-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilliard JB, Konstan MW, Davis PB. Inflammatory mediators in CF patients. Methods Mol Med. 2002;70:409–431. doi: 10.1385/1-59259-187-6:409. [DOI] [PubMed] [Google Scholar]
- 11.Luciani A, Villella VR, Esposito S, Brunetti-Pierri N, Medina D, Settembre C, Gavina M, Pulze L, Giardino I, Pettoello-Mantovani M, D'Apolito M, Guido S, Masliah E, Spencer B, Quaratino S, Raia V, Ballabio A, Maiuri L. Defective CFTR induces aggresome formation and lung inflammation in cystic fibrosis through ROS-mediated autophagy inhibition. Nat Cell Biol. 2010;12:863–875. doi: 10.1038/ncb2090. [DOI] [PubMed] [Google Scholar]
- 12.Galli F, Battistoni A, Gambari R, Pompella A, Bragonzi A, Pilolli F, Iuliano L, Piroddi M, Dechecchi MC, Cabrini G. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim Biophys Acta. 2012;1822:690–713. doi: 10.1016/j.bbadis.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Hartl D, Gaggar A, Bruscia E, Hector A, Marcos V, Jung A, Greene C, McElvaney G, Mall M, Doring G. Innate immunity in cystic fibrosis lung disease. J Cyst Fibros. 2012 doi: 10.1016/j.jcf.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Ollero M, Junaidi O, Zaman MM, Tzameli I, Ferrando AA, Andersson C, Blanco PG, Bialecki E, Freedman SD. Decreased expression of peroxisome proliferator activated receptor gamma in cftr-/- mice. J Cell Physiol. 2004;200:235–244. doi: 10.1002/jcp.20020. [DOI] [PubMed] [Google Scholar]
- 15.Manson ME, Corey DA, Rymut SM, Kelley TJ. beta-arrestin-2 regulation of the cAMP response element binding protein. Biochemistry. 2011;50:6022–6029. doi: 10.1021/bi200015h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersson C, Zaman MM, Jones AB, Freedman SD. Alterations in immune response and PPAR/LXR regulation in cystic fibrosis macrophages. J Cyst Fibros. 2008;7:68–78. doi: 10.1016/j.jcf.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Meyer M, Huaux F, Gavilanes X, van den Brule S, Lebecque P, Lo Re S, Lison D, Scholte B, Wallemacq P, Leal T. Azithromycin Reduces Exaggerated Cytokine Production by M1 Alveolar Macrophages in Cystic Fibrosis. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2008-0155OC. [DOI] [PubMed] [Google Scholar]
- 18.Deriy LV, Gomez EA, Zhang G, Beacham DW, Hopson JA, Gallan AJ, Shevchenko PD, Bindokas VP, Nelson DJ. Disease-causing mutations in the cystic fibrosis transmembrane conductance regulator determine the functional responses of alveolar macrophages. J Biol Chem. 2009;284:35926–35938. doi: 10.1074/jbc.M109.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Li X, Grassme H, Doring G, Gulbins E. Alterations in ceramide concentration and pH determine the release of reactive oxygen species by Cftr-deficient macrophages on infection. J Immunol. 2010;184:5104–5111. doi: 10.4049/jimmunol.0902851. [DOI] [PubMed] [Google Scholar]
- 20.Del Porto P, Cifani N, Guarnieri S, Di Domenico EG, Mariggio MA, Spadaro F, Guglietta S, Anile M, Venuta F, Quattrucci S, Ascenzioni F. Dysfunctional CFTR alters the bactericidal activity of human macrophages against Pseudomonas aeruginosa. PLoS One. 2011;6:e19970. doi: 10.1371/journal.pone.0019970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belcher CN, Vij N. Protein processing and inflammatory signaling in Cystic Fibrosis: challenges and therapeutic strategies. Curr Mol Med. 10:82–94. doi: 10.2174/156652410791065408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilban M, Haschemi A, Wegiel B, Chin BY, Wagner O, Otterbein LE. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med. 2008;86:267–279. doi: 10.1007/s00109-007-0276-0. [DOI] [PubMed] [Google Scholar]
- 23.Koliaraki V, Kollias G. A new role for myeloid HO-1 in the innate to adaptive crosstalk and immune homeostasis. Adv Exp Med Biol. 2011;780:101–111. doi: 10.1007/978-1-4419-5632-3_9. [DOI] [PubMed] [Google Scholar]
- 24.Parker D, Cohen TS, Alhede M, Harfenist BS, Martin FJ, Prince A. Induction of Type I Interferon Signaling by Pseudomonas aeruginosa is Diminished in Cystic Fibrosis Epithelial Cells. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2011-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motterlini R, Otterbein LE. The therapeutic potential of carbon monoxide. Nat Rev Drug Discov. 2010;9:728–743. doi: 10.1038/nrd3228. [DOI] [PubMed] [Google Scholar]
- 26.Tsoyi K, Ha YM, Kim YM, Lee YS, Kim HJ, Seo HG, Lee JH, Chang KC. Activation of PPAR-gamma by carbon monoxide from CORM-2 leads to the inhibition of iNOS but not COX-2 expression in LPS-stimulated macrophages. Inflammation. 2009;32:364–371. doi: 10.1007/s10753-009-9144-0. [DOI] [PubMed] [Google Scholar]
- 27.Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 28.Moss RB, Bocian RC, Hsu YP, Dong YJ, Kemna M, Wei T, Gardner P. Reduced IL-10 secretion by CD4+ T lymphocytes expressing mutant cystic fibrosis transmembrane conductance regulator (CFTR) Clin Exp Immunol. 1996;106:374–388. doi: 10.1046/j.1365-2249.1996.d01-826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saadane A, Soltys J, Berger M. Role of IL-10 deficiency in excessive nuclear factor-kappaB activation and lung inflammation in cystic fibrosis transmembrane conductance regulator knockout mice. J Allergy Clin Immunol. 2005;115:405–411. doi: 10.1016/j.jaci.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 30.Mizuguchi S, Stephen J, Bihari R, Markovic N, Suehiro S, Capretta A, Potter RF, Cepinskas G. CORM-3-derived CO modulates polymorphonuclear leukocyte migration across the vascular endothelium by reducing levels of cell surface-bound elastase. Am J Physiol Heart Circ Physiol. 2009;297:H920–929. doi: 10.1152/ajpheart.00305.2009. [DOI] [PubMed] [Google Scholar]
- 31.Wang XM, Kim HP, Nakahira K, Ryter SW, Choi AM. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol. 2009;182:3809–3818. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- 32.Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 33.Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 34.Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 35.Sonawane ND, Verkman AS. Thiazolidinone CFTR inhibitors with improved water solubility identified by structure-activity analysis. Bioorg Med Chem. 2008;16:8187–8195. doi: 10.1016/j.bmc.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: a worldwide analysis of CFTR mutations--correlation with incidence data and application to screening. Human mutation. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 37.van Doorninck JH, French PJ, Verbeek E, Peters RH, Morreau H, Bijman J, Scholte BJ. A mouse model for the cystic fibrosis delta F508 mutation. EMBO J. 1995;14:4403–4411. doi: 10.1002/j.1460-2075.1995.tb00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin MI, Yu J, Murata T, Sessa WC. Caveolin-1-deficient mice have increased tumor microvascular permeability, angiogenesis, and growth. Cancer Res. 2007;67:2849–2856. doi: 10.1158/0008-5472.CAN-06-4082. [DOI] [PubMed] [Google Scholar]
- 39.Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, Kolls JK, Alam J, Ritter T, Volk HD, Farmer DG, Ghobrial RM, Busuttil RW, Kupiec-Weglinski JW. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104:1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bartling TR, Drumm ML. Oxidative stress causes IL8 promoter hyperacetylation in cystic fibrosis airway cell models. Am J Respir Cell Mol Biol. 2009;40:58–65. doi: 10.1165/rcmb.2007-0464OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luciani A, Villella VR, Vasaturo A, Giardino I, Raia V, Pettoello-Mantovani M, D'Apolito M, Guido S, Leal T, Quaratino S, Maiuri L. SUMOylation of tissue transglutaminase as link between oxidative stress and inflammation. J Immunol. 2009;183:2775–2784. doi: 10.4049/jimmunol.0900993. [DOI] [PubMed] [Google Scholar]
- 42.Luciani A, Villella VR, Esposito S, Gavina M, Russo I, Silano M, Guido S, Pettoello-Mantovani M, Carnuccio R, Scholte B, De Matteis A, Maiuri MC, Raia V, Luini A, Kroemer G, Maiuri L. Targeting autophagy as a novel strategy for facilitating the therapeutic action of potentiators on DeltaF508 cystic fibrosis transmembrane conductance regulator. Autophagy. 2012;8 doi: 10.4161/auto.21483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Villella VR, Esposito S, Bruscia EM, Maiuri MC, Raia V, Kroemer G, Maiuri L. Targeting the Intracellular Environment in Cystic Fibrosis: Restoring Autophagy as a Novel Strategy to Circumvent the CFTR Defect. Frontiers in pharmacology. 2013;4:1. doi: 10.3389/fphar.2013.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dasari A, Bartholomew JN, Volonte D, Galbiati F. Oxidative stress induces premature senescence by stimulating caveolin-1 gene transcription through p38 mitogen-activated protein kinase/Sp1-mediated activation of two GC-rich promoter elements. Cancer Res. 2006;66:10805–10814. doi: 10.1158/0008-5472.CAN-06-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clarke LL, Grubb BR, Yankaskas JR, Cotton CU, McKenzie A, Boucher RC. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ-level disease in Cftr(-/-) mice. Proc Natl Acad Sci U S A. 1994;91:479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Heeckeren A, Walenga R, Konstan MW, Bonfield T, Davis PB, Ferkol T. Excessive inflammatory response of cystic fibrosis mice to bronchopulmonary infection with Pseudomonas aeruginosa. J Clin Invest. 1997;100:2810–2815. doi: 10.1172/JCI119828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bragonzi A. Murine models of acute and chronic lung infection with cystic fibrosis pathogens. International journal of medical microbiology: IJMM. 2010;300:584–593. doi: 10.1016/j.ijmm.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 48.van Heeckeren AM, Schluchter MD, Drumm ML, Davis PB. Role of Cftr genotype in the response to chronic Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol. 2004;287:L944–952. doi: 10.1152/ajplung.00387.2003. [DOI] [PubMed] [Google Scholar]
- 49.Bonfield TL, Hodges CA, Cotton CU, Drumm ML. Absence of the cystic fibrosis transmembrane regulator (Cftr) from myeloid-derived cells slows resolution of inflammation and infection. J Leukoc Biol. 2012 doi: 10.1189/jlb.0412188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raia V, Maiuri L, Ciacci C, Ricciardelli I, Vacca L, Auricchio S, Cimmino M, Cavaliere M, Nardone M, Cesaro A, Malcolm J, Quaratino S, Londei M. Inhibition of p38 mitogen activated protein kinase controls airway inflammation in cystic fibrosis. Thorax. 2005;60:773–780. doi: 10.1136/thx.2005.042564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gadjeva M, Paradis-Bleau C, Priebe GP, Fichorova R, Pier GB. Caveolin-1 modifies the immunity to Pseudomonas aeruginosa. J Immunol. 2010;184:296–302. doi: 10.4049/jimmunol.0900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan K, Huang C, Fox J, Gaid M, Weaver A, Li G, Singh BB, Gao H, Wu M. Elevated inflammatory response in caveolin-1-deficient mice with Pseudomonas aeruginosa infection is mediated by STAT3 protein and nuclear factor kappaB (NF-kappaB) J Biol Chem. 2011;286:21814–21825. doi: 10.1074/jbc.M111.237628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Guglielmo GM, Le Roy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 54.Engelman JA, Zhang XL, Razani B, Pestell RG, Lisanti MP. p42/44 MAP kinase-dependent and -independent signaling pathways regulate caveolin-1 gene expression. Activation of Ras-MAP kinase and protein kinase a signaling cascades transcriptionally down-regulates caveolin-1 promoter activity. J Biol Chem. 1999;274:32333–32341. doi: 10.1074/jbc.274.45.32333. [DOI] [PubMed] [Google Scholar]
- 55.Kim HP, Wang X, Nakao A, Kim SI, Murase N, Choi ME, Ryter SW, Choi AM. Caveolin-1 expression by means of p38beta mitogen-activated protein kinase mediates the antiproliferative effect of carbon monoxide. Proc Natl Acad Sci U S A. 2005;102:11319–11324. doi: 10.1073/pnas.0501345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou H, Lu F, Latham C, Zander DS, Visner GA. Heme oxygenase-1 expression in human lungs with cystic fibrosis and cytoprotective effects against Pseudomonas aeruginosa in vitro. Am J Respir Crit Care Med. 2004;170:633–640. doi: 10.1164/rccm.200311-1607OC. [DOI] [PubMed] [Google Scholar]
- 57.Chen J, Kinter M, Shank S, Cotton C, Kelley TJ, Ziady AG. Dysfunction of Nrf-2 in CF epithelia leads to excess intracellular H2O2 and inflammatory cytokine production. PLoS One. 2008;3:e3367. doi: 10.1371/journal.pone.0003367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piantadosi CA, Withers CM, Bartz RR, MacGarvey NC, Fu P, Sweeney TE, Welty-Wolf KE, Suliman HB. Heme oxygenase-1 couples activation of mitochondrial biogenesis to anti-inflammatory cytokine expression. J Biol Chem. 2011;286:16374–16385. doi: 10.1074/jbc.M110.207738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murray TS, Okegbe C, Gao Y, Kazmierczak BI, Motterlini R, Dietrich LEP, Bruscia E. The Carbon Monoxide Releasing Molecule CORM-2 attenuates Pseudomonas aeruginosa biofilm formation. PLoS ONE. 2012 doi: 10.1371/journal.pone.0035499. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Motterlini R, Sawle P, Hammad J, Bains S, Alberto R, Foresti R, Green CJ. CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J. 2005;19:284–286. doi: 10.1096/fj.04-2169fje. [DOI] [PubMed] [Google Scholar]
- 61.Maiuri L, Luciani A, Giardino I, Raia V, Villella VR, D'Apolito M, Pettoello-Mantovani M, Guido S, Ciacci C, Cimmino M, Cexus ON, Londei M, Quaratino S. Tissue transglutaminase activation modulates inflammation in cystic fibrosis via PPARgamma down-regulation. J Immunol. 2008;180:7697–7705. doi: 10.4049/jimmunol.180.11.7697. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.