Abstract
Owing to their properties, dendritic cells (DCs) are often called ‘nature's adjuvants’ and thus have become the natural targets for antigen delivery. DCs provide an essential link between the innate and the adaptive immune responses. DCs are at the center of the immune system owing to their ability to control both tolerance and immunity. DCs are thus key targets for both preventive and therapeutic vaccination. Herein, we will discuss recent progresses in our understanding of DC subsets physiology as it applies to vaccination.
Dendritic cells (DCs) are key regulators of innate and adaptive immune responses [1,2]. The plasticity of DCs in response to extrinsic signals and the existence of distinct DC subsets with distinct functions contribute to the mounting of highly diverse immune responses. DCs are essential in pathogen resistance including different viruses, bacteria and parasites as demonstrated using DC-depleted mice [3]. Vaccine adjuvants primarily act via activation of DCs. Preventive vaccines are designed to initiate protective humoral immune responses. Today, more than 70 preventive vaccines have been licensed for use against approximately 30 microbes, sparing countless lives [4••]. However, effective vaccines remain elusive for diseases such as human immunodeficiency virus (HIV)-induced acquired immune deficiency syndrome, plasmodium-induced malaria, virus-induced hepatitis C, and Mycobacterium-induced tuberculosis, to cite a few [4••]. Most of these are chronic diseases for which it is thought that strong cellular immunity, in particular cytotoxic T cells, is necessary to eliminate the cells that are infected with the causative agent. Therapeutic vaccines have been designed to eliminate existing diseases and cancer represents an important target for such therapeutic vaccines. Early studies also indicate that vaccines might also be developed in noninfectious settings for the treatment of allergy, and autoimmunity. Here we will discuss recent insights and current views on the biology of human DC subsets in the context of vaccination.
Human DC subsets
While there has been considerable progress in understanding the ontogeny of mouse DC subsets [5,6], less is known on the origin of human DCs and their differentiation program. This is due to their rarity in the blood, the poor accessibility of human tissues and the limited experimental approaches that can be applied to humans. Care should be taken, however, in extending the data generated from mouse DC subsets to human DC subsets. The knowledge of human DC subsets came from studies on blood and skin DC subsets. These studies have distinguished human-blood-circulating DC subsets based on three main cell surface markers: CD303 (BDCA-2) on plasmacytoid DCs (pDCs), CD1c (or BDCA-1) expressed on the majority of circulating DCs and CD141 (or BDCA-3) expressed on a minute population [7–9]. Human CD141+CD1c− DCs uniquely express Toll like receptor 3; produce IL-12 and efficiently cross-prime CD8+ T cells when activated with poly I:C [10–16]. However, other human DCs such as epidermal Langerhans cells (LCs) [17,18] and CD1c+ DCs also cross-present antigens to CD8+ T cells [12,14,15]. DCs express numerous nonclonal recognition receptors, including lectins, Toll-like receptors (TLRs), NOD-like receptors (NLRs) and helicases through which they can sense microbes and microbial products as, for example, nucleic acids thereby allowing the launching of protective type I interferon production [19•,20]. Indeed the experimental adjuvants CpG and Imiquimod bind to TLR9 and TLR7/8 respectively [20]. Most recently biochemical approaches revealed novel sensors of nucleic acid function from the DExD/H-box helicase family molecules in DCs [21,22].
Human DC subsets and humoral immune responses
T helper (Th) subsets, specialized for promoting particular types of immune responses and eventually inflammations, function through their secretion of a restricted set of cytokines enabling unique immune responses (reviewed in [23]). Among those, T follicular helper (Tfh) cells help B cells to differentiate into antibody-secreting cells and govern the germinal center reaction, the main site of Immunoglobulin somatic mutation and isotype switching [24,25]. Human blood CXCR5+ CD4+ T cells represent circulating memory Tfh cells. Blood CXCR5+ CD4+ T cells comprise three subsets: T helper 1 (Tfh1), Tfh2, and Tfh17 cells. Tfh2 and Tfh17 cells efficiently induced naive B cells to produce immunoglobulins via interleukin-21 (IL-21) [26]. In contrast, Tfh1 cells lacked the capacity to help naïve B cells [26]. In vitro studies, permitted us to conclude that Tfh development is regulated by a specific dermal DC subset, interstitial CD14+ DCs [17] and requires IL-12 both in vitro [27] and in vivo as IL-12Rb1 deficient humans displayed substantially less circulating memory Tfh and memory B cells than control subjects [28]. Importantly in the context of vaccination, expansion of Tfh1 cells at day 7 correlates with protective antibody titers at day 28 after influenza vaccination in healthy adults and children [29]. Whether the induction of Tfh differentiation depends on the same mechanisms in mice remains to be established. In vivo DC targeting tools will facilitate delineation of specific subset function in antigen responses as discussed later.
Human DC subsets and cellular immune responses
CD8+ T cells recognize peptide-MHC (pMHC) class I molecules expressed by DC and develop into cytotoxic T lymphocytes (CTLs) able to kill cells presenting a specific pMHC complex [30]. As such CD8+ T cells represent the goal of therapeutic vaccination in cancer and chronic infections. The ideal properties of vaccine-elicited CD8+ T cells include: (i) high avidity for pMHC on tumor cells; (ii) high levels of granzyme and perforin, molecules essential for cytotoxic activity against cancer/infected cells; (iii) expression of surface molecules allowing trafficking into the tumor; and (iv) resistance to regulatory mechanisms present in the tumor [17,31]. At least four components of the immune response are necessary for that ideal response to happen: (1) the presence of antigen presenting DCs; (2) the quality of induced CD4+ helper T cells; (3) the elimination of Tregs; and (4) the breakdown of the immunosuppressive tumor microenvironment. Earlier studies of human cutaneous DCs have demonstrated their phenotypic and functional heterogeneity with regards to cellular immunity and priming of highly efficient CTLs [32]. Our studies with human Langerhans cells and interstitial DCs, showed their specialization in priming CD8+ T cell immunity and humoral immunity, respectively [17,33]. Skin LC efficiency in priming naïve CD8+ T can be at least partially explained by their surface expression of IL-15 [34,35] and/or upregulation of CD70 upon viral exposure [36]. Furthermore, interstitial DCs play a major role in generation of suppressor CD8+ T cells [37]. Here again the mouse and the human seem to differ under some circumstances as suggested by murine studies using a Candida albicans skin infection model. There, direct presentation of antigen by LC is necessary for Th17 responses whereas Langerin-expressing dermal DCs are required for the generation of antigen specific CTLs [38]. Recent studies have further analyzed lymph-node-resident and skin-migratory DC subsets in the human [16,39]. Both CD1c+ and CLEC9A-expressing CD141+ DCs isolated from human lymph nodes were able to cross-present long peptides (requiring processing) of melanoma-tissue-derived antigen (MART-1) to T cell lines [39] whereas blood DCs can cross-present when activated via Toll-like receptor ligands [11,12] (see Figure 1).
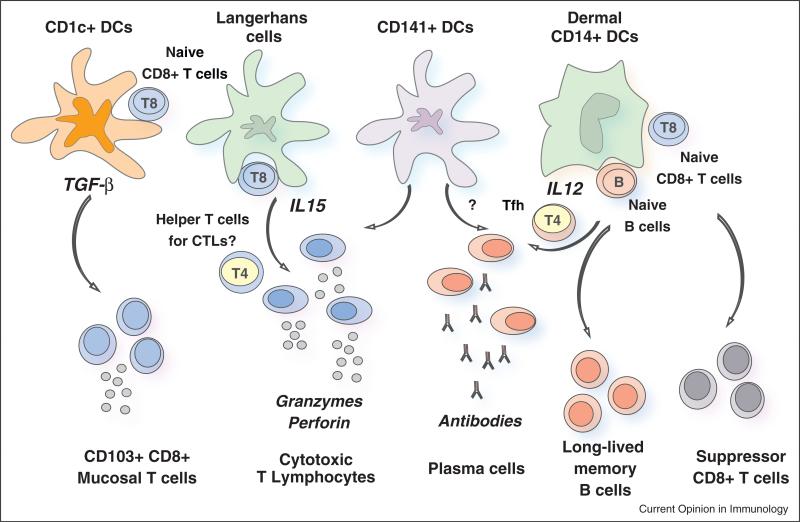
Figure 1.
The two arms of the adaptive immune response — humoral and cellular — are regulated by different subsets of dendritic cells (DCs) in humans. Humoral immunity is preferentially regulated by CD14+ dermal DCs, which produce interleukin-12 (IL-12). IL-12, in turn, acts directly on B cells and promotes the development of T follicular helper (TFH) cells. In the mouse, CD141+ DCs seem to be able to promote humoral immunity upon CLEC9A engagement. In the human this function remains to be established. CD141+ DCs might also be involved in the development of humoral responses through IL-12 secretion. Cellular immune responses in the blood, skin and peripheral tissues are differentially regulated by human DC subsets. Thus, Langerhans cells prime highly efficient cytotoxic T lymphocytes (CTLs), possibly via IL-15. It is also possible that Langerhans cells can preferentially activate a dedicated subset of CD4+ T cells that are specialized to help CD8+ CTLs, though this remains to be established. Given their capacity to cross-present antigens to CD8+ T cells, CD141+ DCs are also involved in the development of CTL-mediated responses. CD1c+ DCs can cross-present antigens as well and can equip the primed CD8+ T cells with the expression of CD103 which allows them to reside in mucosa. Finally, CD14+ dermal DCs generate suppressor CD8+ T cells with ILT-2 and ILT-4 among possible mediators. Much remains to be learned about other immune phenotypes that these DC subsets can elicit.
T cell immunity has long been described in terms of two circulating memory populations. Central memory T cells migrate between the secondary lymphoid organs and are capable of mounting a recall proliferative response on pathogen re-encounter, whereas effector memory T cells traffic between blood and extralymphoid compartments for effective peripheral immune surveillance. A third category of memory cells, that is, tissue-resident memory T cells are phenotypically distinct from other T cells [40••,41]. Studies in mice [42,43] and humans [44] have revealed that these tissue-resident memory T cells can be superior to circulating central memory T cells at providing rapid long-term protection against re-infection. Therefore, an active mechanism of T cell retention in the periphery likely exists not only to facilitate the clearance of infected cells but also to promote the accumulation noted at sites that have cleared an infectious virus. Among relevant molecules is CD103/β7 integrin that endows peripheral CD8+ T cells with a unique capacity to access the epithelial compartments [45,46]. The expression of CD103 on CTLs that mediates adherence to E-cadherin appears to be an important factor in the final cancer lysis and rejection [47]. The role of DC subsets residing in the tissue in the regulation and maintenance of tissue residing T cells remains to be characterized [48]. Studies using humanized mice and human lung tissues revealed that lung CD1c+ DCs were uniquely able to drive the differentiation of CD103+CD8+ mucosal T cells while both lung CD1c+ and CD141+ DC subsets DC subsets could acquire viral antigens and drive anti-viral effector CD8+ T cell responses (Yu et al., in press). These findings have important implications for our understanding of protective immune memory at epithelial interfaces with the environment, and suggest novel strategies for vaccines that protect against tissue tropic organisms. They are also important for cancer vaccines as recently demonstrated in the context of mucosal cancers in the mouse [49••].
Vaccination via DCs: cell-based therapy
Therapeutic vaccines in humans have been mostly developed in the context of cancer. DCs can be engaged indirectly as for example with GVAX [50] or Listeria-based vaccines [51] to name a few. DC can also be used directly following their generation ex vivo and injection to patients [52]. These studies concluded that DC-based vaccines are safe and can induce the expansion of circulating CD4+ and CD8+ T cells that are specific for tumor antigens [52–55]. Objective clinical responses have been observed in some patients. A recent study focused on optimizing vaccine immunogenicity and demonstrated in phase I/II clinical trials that provision of MHC class II epitopes from defined melanoma tumor antigens results in improved immunogenicity [56]. Furthermore, novel approaches are being developed including the preoperative vaccination of patients with her2+ breast cancer [57] as well as combination therapies in ovarian cancer utilizing autologous DC vaccines and adoptive T cell transfer to enhance vaccine efficacy [58]. More recent studies have utilized another DC subset, plasmacytoid DCs, which are the main source of type I interferon upon viral infection [59•]. Patients with metastatic melanoma received intranodal injections of pDCs activated and loaded with tumor antigen-associated peptides ex vivo. Several patients mounted vaccine antigen-specific CD4+ and CD8+ T cell responses. Despite the limited number of administered pDCs, an IFN signature was observed after each vaccination [59•]. Whereas the clinical efficacy of elicited immunity will need to be determined in larger cohorts and long-term follow up, type IFN response is highly desirable in melanoma [60,61]. All in all, the field is active as evidenced by 122 open and ongoing trials with known status, identified by searching the clinical trial database (clinicaltrials.gov) with the term ‘dendritic cells’.
Vaccination with DCs has also been used for other medical conditions including HIV infection and autoimmune diseases. Numerous approaches have been taken to vaccinate HIV infected individuals, including peptides, inactivated virus, viral vectors, and ex vivo generated DCs [62–64]. The latter represents an approach to optimize the induction of immune responses in patients. Different DC preparations loaded with different HIV antigens have been tested [65]. Two studies, where DCs have been loaded with a high dose of chemically inactivated autologous virus, have reported a decrease in viral load. One study reported a decrease by 90% in eight out of 18 untreated patients lasting at least one year [66] and the other study reported a decrease of plasma viral load set point ≥1 log in 12 of 22 (55%) at 12 weeks after analytical treatment interruption [67•]. Another approach to load HIV antigens onto DCs is to transfect them with RNA isolated from the autologous virus [68]. This approach has yielded some immune response in patients but has not clearly been associated with a control of viral replication. Whereas further studies are needed to achieve the functional cure, DC-based vaccines represent an essential component of modern therapeutic strategies in HIV.
Autoimmune diseases are the result of an imbalanced immune regulatory network [69]. Tolerogenic DCs are key players of this network by inducing and maintaining both central and peripheral tolerance [70]. Therefore, ex vivo generated tolerogenic DCs are considered as therapeutic vaccines to re-establish antigen-specific tolerance in autoimmune disorders such as rheumatoid arthritis [71] or multiple sclerosis [72]. Studies in the mouse using antigen targeting approach demonstrated that migratory DCs have a superior ability to generate antigen-specific Tregs in vivo, leading to improved outcomes in experimental autoimmune encephalomyelitis [73••]. Furthermore, targeting of DCs via DEC-25 with beta cell antigens led to deletion of autoreactive CD8+ T cells even in the context of ongoing autoimmunity in NOD mice [74••]. These results provide support for the development of DC targeting of self antigens for treatment of chronic T cell-mediated autoimmune diseases.
Vaccination via DCs: in vivo DC targeting
Following the pioneering studies from Ralph Steinman and Michel Nussenzweig labs with anti-DEC 205 antibodies [75–77], numerous studies performed in mouse models and in human in vitro systems demonstrated the efficacy of targeting DCs [2]. Most particularly, targeting antigens through the DC surface lectins DCIR [18,78], DC-SIGN [79], Dectin [80], Clec9A [81], and Langerin [82], results in humoral and/or cellular CD4+ and/or CD8+ T-cell responses. In the absence of adjuvants, targeting DEC205+ DCs in vivo can induce tolerance [75]. Provision of adjuvants such as TLR3 or TLR7/8 agonists or DC activation signal via CD40 enables the concomitant maturation of vaccine engulfing DCs [83]. Furthermore, targeting different DC receptors generate quantitatively and qualitatively different immune responses [84••,85••]. Injection of antigens coupled to antibodies against DC surface molecule Clec9A results in production of strong antibody responses even without co-administration of adjuvants [86]. That happens via antigen presentation by DC on MHC class II and consequent Tfh expansion [87]. These results in the mouse are in line with prior studies showing the essential role of DCs in the generation of antibody responses and show that these can be amplified by targeting antigen to DC surface receptors in vivo. Importantly CLEC9a is also a receptor for necrotic cells and has been shown to facilitate cross-presentation [88]. As opposed to antibody response, CLEC9A dependent generation of CD8+ T cell responses requires adjuvant. Generation of different responses by targeting distinct DC receptors is further exemplified by recent studies targeting DC-asialoglycoprotein receptor (DC-ASGPR), a lectin-like receptor, which is a known scavenger receptor. Targeting antigens to human DCs via DC-ASGPR in vitro but not lectin-like oxidized-LDL receptor, Dectin-1, or DC-specific ICAM-3-grabbing nonintegrin favored the generation of antigen-specific suppressive CD4+ T cells that produce interleukin 10 (IL-10) [89]. These findings apply to both self-antigens and foreign antigens, as well as memory and naive CD4+ T cells both in vitro in the human system and in vivo in nonhuman primates [89]. Furthermore, comparing the cross presentation of identical antigens conjugated with antibodies against different DC receptors that are targeted to early or late endosomes at distinct efficiencies revealed remarkable differences [90]. Thus, in human BDCA1+ and monocyte-derived DCs, CD40 and mannose receptor targeted antibody conjugates to early endosomes, whereas DEC205 targeted antigen primarily to late compartments. Surprisingly, the receptor least efficient at internalization, CD40, was the most efficient at cross presentation. This did not reflect DC activation by CD40, but rather its relatively poor uptake or intraendosomal degradation compared with mannose receptor or DEC205 [90]. DC targeting-based vaccination studies in nonhuman primates demonstrated robust T cell immunity in prime-boost design with HIV gag-DEC205 targeting vaccine [91]. Early clinical trials in the human analyzed the delivery of gonadotropin [hCG-b] to APCs by antibody-mediated targeting of a mannose receptor [92]. Delivery of this product with GM-CSF and TLR3/TLR7/8 agonists induced consistent humoral and cellular immune responses to hCG-b [92]. Several studies are ongoing testing the immune efficacy of HIV antigens or NY-ESO1 cancer antigen targeted via DEC-205 in healthy individuals and in cancer patients (clinicaltrials.gov).
Conclusions
DCs are composed of subsets that differ in their localization, phenotype, and functions. DCs regulate cell types critical to generation of protective and therapeutic immunity including Tfh cells which dictate the quality of humoral immunity and CD8+ T cells that give rise to CTLs able to eliminate infected/transformed cells. Targeting antigens and adjuvants to distinct DC subsets in vivo can be used to generate specific type of immune response. Thus, ever increasing understanding of DC biology along with the generation of tools allowing their direct manipulation in vivo will enable the development of next generation improved vaccines.
Acknowledgements
We thank all the patients and volunteers who participated in our studies and clinical trials. We thank former and current members of the Institute for their contributions to our progresses. Our studies have been supported by the NIH (P01 CA084514, U19 AIO57234, R01 CA089440, CA078846 and CA140602), the Dana Foundation, the Susan Komen Foundation, the Baylor Health Care System; the Baylor Health Care System Foundation, the ANRS and the INSERM. KP holds the Michael A. Ramsay Chair for Cancer Immunology Research. Due to space limits we could cite only a fraction of the vast number of publications.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
of special interest
of outstanding interest
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu Rev Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 3.Bar-On L, Jung S. Defining dendritic cells by conditional and constitutive cell ablation. Immunol Rev. 2010;234:76–89. doi: 10.1111/j.0105-2896.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- 4••.Nabel GJ. Designing tomorrow's vaccines. N Engl J Med. 2013;368:551–560. doi: 10.1056/NEJMra1204186. [Outstanding review of challenges and needs for improved vaccines.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reis e Sousa C. Harnessing dendritic cells. Semin Immunol. 2011;23:1. doi: 10.1016/j.smim.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 8.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, et al. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 10.Lauterbach H, Bathke B, Gilles S, Traidl-Hoffmann C, Luber CA, Fejer G, Freudenberg MA, Davey GM, Vremec D, Kallies A, et al. Mouse CD8alpha+ DCs and human BDCA3+ DCs are major producers of IFN-lambda in response to poly IC. J Exp Med. 2010;207:2703–2717. doi: 10.1084/jem.20092720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre CA, Ventre E, Vu Manh TP, Baranek T, Storset AK, Marvel J, et al. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V, et al. Human CD141+ (BDCA-3)+ dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachem A, Guttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+ CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poulin LF, Salio M, Griessinger E, Anjos-Afonso F, Craciun L, Chen JL, Keller AM, Joffre O, Zelenay S, Nye E, et al. Characterization of human DNGR-1+ BDCA3+ leukocytes as putative equivalents of mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1261–1271. doi: 10.1084/jem.20092618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittag D, Proietto AI, Loudovaris T, Mannering SI, Vremec D, Shortman K, Wu L, Harrison LC. Human dendritic cell subsets from spleen and blood are similar in phenotype and function but modified by donor health status. J Immunol. 2011;186:6207–6217. doi: 10.4049/jimmunol.1002632. [DOI] [PubMed] [Google Scholar]
- 16.Haniffa M, Shin A, Bigley V, McGovern N, Teo P, See P, Wasan PS, Wang XN, Malinarich F, Malleret B, et al. Human tissues contain CD141(hi) cross-presenting dendritic cells with functional homology to mouse CD103(+) nonlymphoid dendritic cells. Immunity. 2012;37:60–73. doi: 10.1016/j.immuni.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klechevsky E, Flamar AL, Cao Y, Blanck JP, Liu M, O'Bar A, Agouna-Deciat O, Klucar P, Thompson-Snipes L, Zurawski S, et al. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood. 2010;116:1685–1697. doi: 10.1182/blood-2010-01-264960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Barber GN. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr Opin Immunol. 2011;23:10–20. doi: 10.1016/j.coi.2010.12.015. [Outstanding work unraveling the basic molecular pathways controlling type I IFN production.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desmet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12:479–491. doi: 10.1038/nri3247. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, Kim T, Bao M, Facchinetti V, Jung SY, Ghaffari AA, Qin J, Cheng G, Liu YJ. DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity. 2011;34:866–878. doi: 10.1016/j.immuni.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Z, Bao M, Lu N, Weng L, Yuan B, Liu YJ. The E3 ubiquitin ligase TRIM21 negatively regulates the innate immune response to intracellular double-stranded DNA. Nat Immunol. 2013;14:172–178. doi: 10.1038/ni.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bluestone JA, Mackay CR, O'Shea JJ, Stockinger B. The functional plasticity of T cell subsets. Nat Rev Immunol. 2009;9:811–816. doi: 10.1038/nri2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma CS, Deenick EK, Batten M, Tangye SG. The origins, function, and regulation of T follicular helper cells. J Exp Med. 2012;209:1241–1253. doi: 10.1084/jem.20120994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinuesa CG, Cyster JG. How T cells earn the follicular rite of passage. Immunity. 2011;35:671–680. doi: 10.1016/j.immuni.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–121. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmitt N, Bustamante J, Bourdery L, Bentebibel SE, Boisson-Dupuis S, Hamlin F, Tran MV, Blankenship D, Pascual V, Savino DA, et al. IL-12 receptor beta1 deficiency alters in vivo T follicular helper cell response in humans. Blood. 2013;121:3375–3385. doi: 10.1182/blood-2012-08-448902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, et al. Induction of ICOS+ CXCR3+ CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med. 2013;5:176ra132. doi: 10.1126/scitranslmed.3005191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 32.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 33.Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 34.Banchereau J, Thompson-Snipes L, Zurawski S, Blanck JP, Cao Y, Clayton S, Gorvel JP, Zurawski G, Klechevsky E. The differential production of cytokines by human Langerhans cells and dermal CD14(+) DCs controls CTL priming. Blood. 2012;119:5742–5749. doi: 10.1182/blood-2011-08-371245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romano E, Cotari JW, Barreira da Silva R, Betts BC, Chung DJ, Avogadri F, Fink MJ, St Angelo ET, Mehrara B, Heller G, et al. Human Langerhans cells use an IL-15R-alpha/IL-15/pSTAT5-dependent mechanism to break T-cell tolerance against the self-differentiation tumor antigen WT1. Blood. 2012;119:5182–5190. doi: 10.1182/blood-2011-09-382200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Aar AM, de Groot R, Sanchez-Hernandez M, Taanman EW, van Lier RA, Teunissen MB, de Jong EC, Kapsenberg ML. Cutting edge: virus selectively primes human langerhans cells for CD70 expression promoting CD8+ T cell responses. J Immunol. 2011;187:3488–3492. doi: 10.4049/jimmunol.1101105. [DOI] [PubMed] [Google Scholar]
- 37.Banchereau J, Zurawski S, Thompson-Snipes L, Blanck JP, Clayton S, Munk A, Cao Y, Wang Z, Khandelwal S, Hu J, et al. Immunoglobulin-like transcript receptors on human dermal CD14+ dendritic cells act as a CD8-antagonist to control cytotoxic T cell priming. Proc Natl Acad Sci U S A. 2012;109:18885–18890. doi: 10.1073/pnas.1205785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segura E, Valladeau-Guilemond J, Donnadieu MH, Sastre-Garau X, Soumelis V, Amigorena S. Characterization of resident and migratory dendritic cells in human lymph nodes. J Exp Med. 2012;209:653–660. doi: 10.1084/jem.20111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40••.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [Groundbreaking demonstration of the importance and function of tissue-residing T cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2012;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 42.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 43.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010;16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark RA, Watanabe R, Teague JE, Schlapbach C, Tawa MC, Adams N, Dorosario AA, Chaney KS, Cutler CS, Leboeuf NR, et al. Skin effector memory T cells do not recirculate and provide immune protection in alemtuzumab-treated CTCL patients. Sci Transl Med. 2012;4:117ra117. doi: 10.1126/scitranslmed.3003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shaw SK, Brenner MB. The beta 7 integrins in mucosal homing and retention. Semin Immunol. 1995;7:335–342. doi: 10.1016/1044-5323(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 46.Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Floc'h A, Jalil A, Vergnon I, Le Maux Chansac B, Lazar V, Bismuth G, Chouaib S, Mami-Chouaib F. Alpha E beta 7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J Exp Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seneschal J, Clark RA, Gehad A, Baecher-Allan CM, Kupper TS. Human epidermal Langerhans cells maintain immune homeostasis in skin by activating skin resident regulatory T cells. Immunity. 2012;36:873–884. doi: 10.1016/j.immuni.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G, et al. Mucosal imprinting of vaccine-induced CD8+ T cells is crucial to inhibit the growth of mucosal tumors. Sci Transl Med. 2013;5:172ra120. doi: 10.1126/scitranslmed.3004888. [Outstanding demonstration of how critically important it is to elicit mucosal T cells in cancer vaccination.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le DT, Pardoll DM, Jaffee EM. Cellular vaccine approaches. Cancer J. 2010;16:304–310. doi: 10.1097/PPO.0b013e3181eb33d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Le DT, Dubenksy TW, Jr, Brockstedt DG. Clinical development of Listeria monocytogenes-based immunotherapies. Semin Oncol. 2012;39:311–322. doi: 10.1053/j.seminoncol.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuler G. Dendritic cells in cancer immunotherapy. Eur J Immunol. 2010;40:2123–2130. doi: 10.1002/eji.201040630. [DOI] [PubMed] [Google Scholar]
- 54.Kalinski P, Edington H, Zeh HJ, Okada H, Butterfield LH, Kirkwood JM. Bartlett DL: Dendritic cells in cancer immunotherapy: vaccines or autologous transplants? Immunol Res. 2011;50:235–247. doi: 10.1007/s12026-011-8224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galluzzi L, Senovilla L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012;1:1111–1134. doi: 10.4161/onci.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH, Haanen JB, et al. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res. 2013;73:19–29. doi: 10.1158/0008-5472.CAN-12-1127. [DOI] [PubMed] [Google Scholar]
- 57.Sharma A, Koldovsky U, Xu S, Mick R, Roses R, Fitzpatrick E, Weinstein S, Nisenbaum H, Levine BL, Fox K, et al. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer. 2012;118:4354–4362. doi: 10.1002/cncr.26734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kandalaft LE, Powell DJ, Jr, Chiang CL, Tanyi J, Kim S, Bosch M, Montone K, Mick R, Levine BL, Torigian DA, et al. Autologous lysate-pulsed dendritic cell vaccination followed by adoptive transfer of vaccine-primed ex vivo co-stimulated T cells in recurrent ovarian cancer. Oncoimmunology. 2013;2:e22664. doi: 10.4161/onci.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59•.Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJ, van Rossum M, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res. 2013;73:1063–1075. doi: 10.1158/0008-5472.CAN-12-2583. [The first demonstration of immunogenicity and potential clinical relevance of pDCs as cell based vaccines.] [DOI] [PubMed] [Google Scholar]
- 60.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13:5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 61.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Levy Y. Preparation for antiretroviral interruption by boosting the immune system. Curr Opin HIV AIDS. 2008;3:118–123. doi: 10.1097/COH.0b013e3282f5122a. [DOI] [PubMed] [Google Scholar]
- 63.Johnston MI, Fauci AS. HIV vaccine development — improving on natural immunity. N Engl J Med. 2011;365:873–875. doi: 10.1056/NEJMp1107621. [DOI] [PubMed] [Google Scholar]
- 64.Saunders KO, Rudicell RS, Nabel GJ. The design and evaluation of HIV-1 vaccines. AIDS. 2012;26:1293–1302. doi: 10.1097/QAD.0b013e32835474d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garcia F, Routy JP. Challenges in dendritic cells-based therapeutic vaccination in HIV-1 infection Workshop in dendritic cell-based vaccine clinical trials in HIV-1. Vaccine. 2011;29:6454–6463. doi: 10.1016/j.vaccine.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 66.Lu W, Arraes LC, Ferreira WT, Andrieu JM. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med. 2004;10:1359–1365. doi: 10.1038/nm1147. [DOI] [PubMed] [Google Scholar]
- 67•.Garcia F, Climent N, Guardo AC, Gil C, Leon A, Autran B, Lifson JD, Martinez-Picado J, Dalmau J, Clotet B, et al. A dendritic cell-based vaccine elicits T cell responses associated with control of HIV-1 replication. Sci Transl Med. 2013;5:166ra162. doi: 10.1126/scitranslmed.3004682. [Very elegant randomized study demonstrating the immune efficacy of DC vaccines in HIV and the impact on viral replication.] [DOI] [PubMed] [Google Scholar]
- 68.Van Gulck E, Vlieghe E, Vekemans M, Van Tendeloo VF, Van De Velde A, Smits E, Anguille S, Cools N, Goossens H, Mertens L, et al. mRNA-based dendritic cell vaccination induces potent antiviral T-cell responses in HIV-1-infected patients. AIDS. 2012;26:F1–F12. doi: 10.1097/QAD.0b013e32834f33e8. [DOI] [PubMed] [Google Scholar]
- 69.Banchereau J, Pascual V. Type I: interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 70.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 71.Gross CC, Wiendl H. Dendritic cell vaccination in autoimmune disease. Curr Opin Rheumatol. 2013;25:268–274. doi: 10.1097/BOR.0b013e32835cb9f2. [DOI] [PubMed] [Google Scholar]
- 72.Gross CC, Jonuleit H, Wiendl H. Fulfilling the dream: tolerogenic dendritic cells to treat multiple sclerosis. Eur J Immunol. 2012;42:569–572. doi: 10.1002/eji.201242402. [DOI] [PubMed] [Google Scholar]
- 73••.Idoyaga J, Fiorese C, Zbytnuik L, Lubkin A, Miller J, Malissen B, Mucida D, Merad M, Steinman RM. Specialized role of migratory dendritic cells in peripheral tolerance induction. J Clin Invest. 2013;123:844–854. doi: 10.1172/JCI65260. [Targeting DCs for tolerance.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74••.Mukhopadhaya A, Hanafusa T, Jarchum I, Chen YG, Iwai Y, Serreze DV, Steinman RM, Tarbell KV, DiLorenzo TP. Selective delivery of beta cell antigen to dendritic cells in vivo leads to deletion and tolerance of autoreactive CD8+ T cells in NOD mice. Proc Natl Acad Sci U S A. 2008;105:6374–6379. doi: 10.1073/pnas.0802644105. [Targeting DCs for tolerance.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hawiger D, Inaba K, Dorsett Y, Guo K, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–780. doi: 10.1084/jem.194.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM. subset of dendritic cells induces CD4+ T cells to produce IFN-{gamma} by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meyer-Wentrup F, Cambi A, Joosten B, Looman MW, de Vries IJ, Figdor CG, Adema GJ. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J Leukoc Biol. 2009;85:518–525. doi: 10.1189/jlb.0608352. [DOI] [PubMed] [Google Scholar]
- 79.Dakappagari N, Maruyama T, Renshaw M, Tacken P, Figdor C, Torensma R, Wild MA, Wu D, Bowdish K, Kretz-Rommel A. Internalizing antibodies to the C-type lectins, L-SIGN and DC-SIGN, inhibit viral glycoprotein binding and deliver antigen to human dendritic cells for the induction of T cell responses. J Immunol. 2006;176:426–440. doi: 10.4049/jimmunol.176.1.426. [DOI] [PubMed] [Google Scholar]
- 80.Ni L, Gayet I, Zurawski S, Duluc D, Flamar AL, Li XH, O'Bar A, Clayton S, Palucka AK, Zurawski G, et al. Concomitant activation and antigen uptake via human dectin-1 results in potent antigen-specific CD8+ T cell responses. J Immunol. 2010;185:3504–3513. doi: 10.4049/jimmunol.1000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sancho D, Mourao-Sa D, Joffre OP, Schulz O, Rogers NC, Pennington DJ, Carlyle JR, Reis e Sousa C. Tumor therapy in mice via antigen targeting to a novel. DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–2110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flacher V, Sparber F, Tripp CH, Romani N, Stoitzner P. Targeting of epidermal Langerhans cells with antigenic proteins: attempts to harness their properties for immunotherapy. Cancer Immunol Immunother. 2009;58:1137–1147. doi: 10.1007/s00262-008-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tacken PJ, Figdor CG. Targeted antigen delivery and activation of dendritic cells in vivo: steps towards cost effective vaccines. Semin Immunol. 2011;23:12–20. doi: 10.1016/j.smim.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 84••.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, Cheong C, Liu K, Lee HW, Park CG, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [Targeting DC subsets in vivo demonstrates their unique functions.] [DOI] [PubMed] [Google Scholar]
- 85••.Soares H, Waechter H, Glaichenhaus N, Mougneau E, Yagita H, Mizenina O, Dudziak D, Nussenzweig MC, Steinman RM. A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med. 2007;204:1095–1106. doi: 10.1084/jem.20070176. [Targeting DC subsets in vivo demonstrates their unique functions.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caminschi I, Vremec D, Ahmet F, Lahoud MH, Villadangos JA, Murphy KM, Heath WR, Shortman K. Antibody responses initiated by Clec9A-bearing dendritic cells in normal and Batf3(–/–) mice. Mol Immunol. 2012;50:9–17. doi: 10.1016/j.molimm.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 87.Lahoud MH, Ahmet F, Kitsoulis S, Wan SS, Vremec D, Lee CN, Phipson B, Shi W, Smyth GK, Lew AM, et al. Targeting antigen to mouse dendritic cells via Clec9A induces potent CD4 T cell responses biased toward a follicular helper phenotype. J Immunol. 2011;187:842–850. doi: 10.4049/jimmunol.1101176. [DOI] [PubMed] [Google Scholar]
- 88.Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li D, Romain G, Flamar AL, Duluc D, Dullaers M, Li XH, Zurawski S, Bosquet N, Palucka AK, Le Grand R, et al. Targeting self- and foreign antigens to dendritic cells via DC-ASGPR generates IL-10-producing suppressive CD4+ T cells. J Exp Med. 2012;209:109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chatterjee B, Smed-Sorensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, Widger J, Keler T, Delamarre L. Mellman I: Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood. 2012;120:2011–2020. doi: 10.1182/blood-2012-01-402370. [DOI] [PubMed] [Google Scholar]
- 91.Flynn BJ, Kastenmuller K, Wille-Reece U, Tomaras GD, Alam M, Lindsay RW, Salazar AM, Perdiguero B, Gomez CE, Wagner R, et al. Immunization with HIV Gag targeted to dendritic cells followed by recombinant New York vaccinia virus induces robust T-cell immunity in nonhuman primates. Proc Natl Acad Sci U S A. 2011;108:7131–7136. doi: 10.1073/pnas.1103869108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Morse MA, Chapman R, Powderly J, Blackwell K, Keler T, Green J, Riggs R, He LZ, Ramakrishna V, Vitale L, et al. Phase I: study utilizing a novel antigen-presenting cell-targeted vaccine with Toll-like receptor stimulation to induce immunity to self-antigens in cancer patients. Clin Cancer Res. 2011;17:4844–4853. doi: 10.1158/1078-0432.CCR-11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]