Abstract
To serve in its function as an assembly machine for spliceosomal small nuclear ribonucleoprotein particles (snRNPs), the survival of motor neurons (SMN) protein complex binds directly to the Sm proteins and the U snRNAs. A specific domain unique to U1 snRNA, stem-loop 1 (SL1), is required for SMN complex binding and U1 snRNP Sm core assembly. Here, we show that each of the major spliceosomal U snRNAs (U2, U4, and U5), as well as the minor splicing pathway U11 snRNA, contains a domain to which the SMN complex binds directly and with remarkable affinity (low nanomolar concentration). The SMN-binding domains of the U snRNAs do not have any significant nucleotide sequence similarity yet they compete for binding to the SMN complex in a manner that suggests the presence of at least two binding sites. Furthermore, the SMN complex-binding domain and the Sm site are both necessary and sufficient for Sm core assembly and their relative positions are critical for snRNP assembly. These findings indicate that the SMN complex stringently scrutinizes RNAs for specific structural features that are not obvious from the sequence of the RNAs but are required for their identification as bona fide snRNAs. It is likely that this surveillance capacity of the SMN complex ensures assembly of Sm cores on the correct RNAs only and prevents illicit, potentially deleterious, assembly of Sm cores on random RNAs.
Pre-mRNA splicing is carried out by the spliceosome, a macromolecular complex in the nucleus of eukaryotic cells. The small nuclear ribonucleoprotein particles (snRNPs) U1, U2, U5, and U4/U6 are major components of the spliceosome. Each U snRNP contains the corresponding snRNA (U1, U2, U5, or U4/U6), seven common Sm proteins, and a set of proteins that are specific to individual snRNAs (reviewed in references 25, 26, and 51). The Sm proteins B/B', D1, D2, D3, E, F, and G are common to all spliceosomal snRNPs and are arranged into a seven-membered ring on the Sm site of the U snRNA (2, 19, 48). The process of bringing these components together (snRNP assembly) takes place in the cytoplasm of vertebrate cells shortly after the nuclear export of nascent U snRNAs. The formation of the Sm core is required for the hypermethylation of the 7-methyl guanosine (m7G) cap of these snRNAs to convert it into a 2,2,7-trimethyl guanosine (m3G or TMG) (27, 45). Proper assembly of the Sm core, cap hypermethylation, and 3′-end processing of the U snRNAs are prerequisites for the subsequent nuclear import of the U snRNPs, which then go on to function in nuclear pre-mRNA splicing (7, 8, 15, 16, 27, 29, 51).
Important and unexpected insights into the process of U snRNP assembly came from studies on the function of the survival of motor neurons (SMN) protein (6, 21, 22, 28). Reduced levels of SMN due to a genetic defect cause degeneration of motor neurons in the spinal cord and result in spinal muscular atrophy (20, 34). SMN is part of a large multiprotein complex which contains Gemin2 (22), the DEAD box RNA helicase Gemin3 (4), Gemin4 (5), Gemin5 (13), Gemin6 (39), and Gemin7 (1). Previous studies suggested that the SMN complex plays a role in the assembly and metabolism of various ribonucleoprotein particles (RNPs) (including snRNPs, snoRNPs, and miRNPs) and the machineries that carry out transcription and pre-mRNA splicing (3, 6, 9, 18, 22, 30, 36, 37, 38, 40, 41, 42, 43). Several of the components of the SMN complex interact directly with Sm proteins (1, 3, 4, 5, 9, 13, 22, 39, 40). Symmetric dimethylarginine modification of the Sm proteins by the 20S methylosome containing an arginine methyltransferase (JBP1/PRMT5) enhances the interaction with the SMN complex (10, 11, 12, 32, 46).
Experiments with Xenopus oocytes and mammalian somatic cells revealed an essential role for the SMN complex in the process of U snRNP assembly (3, 5, 6, 33, 42). Further evidence that the SMN complex is necessary for assembly of Sm site-containing U snRNPs as well as the mixed, Sm-Lsm-containing, U7 snRNP was provided using cell extracts (31, 33, 43, 44). Importantly, a critical role for the SMN complex in determining the specificity of U snRNP assembly has been recently demonstrated (43).
To facilitate snRNP assembly the SMN complex must bring together the Sm proteins and the U snRNAs. An RNA binding activity for SMN was first indicated by the recombinant SMN binding to ribohomopolymers (23, 24). The SMN complex binds directly and with sequence specificity to the stem-loop 1 (SL1) of U1 snRNA, and disruption of this interaction impairs the assembly of U1 snRNP in the cytoplasm of Xenopus oocytes (52). Furthermore, we demonstrated that the SMN complex has an essential role in determining the specificity of U snRNP assembly. In these studies, the SMN complex was shown to be critical for the selection of the specific RNA targets and for allowing Sm core assembly on these RNAs only, thus preventing promiscuous and deleterious binding of Sm proteins to various RNAs (43).
Other Sm site-containing spliceosomal snRNAs, however, do not contain the U1 SL1 sequence, and yet SMN mediates their assembly with Sm proteins (43). Here, we studied the interaction of the SMN complex with the other spliceosomal U snRNAs. We show that the SMN complex binds to major spliceosomal U snRNAs directly and with high affinity and we delineate the binding domains of each U snRNA that are necessary and sufficient for the direct interaction with the SMN complex. These domains (mini U snRNA fragments) are sufficient for SMN-dependent assembly of Sm cores. We further demonstrate that each of the various U snRNAs contains a domain designed to mediate its interaction with the SMN complex and show that this interaction is crucial for U snRNP biogenesis.
MATERIALS AND METHODS
Plasmids for in vitro transcription.
Plasmids for in vitro transcription of U snRNAs and their mutations are described elsewhere (8, 15, 17, 27). Construction of cDNAs for deletion mutants of U snRNAs was described previously (52). A cDNA clone for U1Swap RNA was constructed by swapping SL1 (nucleotides 17 to 47) and SL4 (nucleotides 140 to 164) of U1 snRNA. A cDNA clone for U1A3Swap RNA was constructed by swapping SL1A3 and SL4 of U1A3 RNA.
Labeling of RNAs.
In vitro transcription and [32P]UTP labeling of RNAs were carried out as described previously (52). [32P]UTP-labeled RNAs were purified by electrophoresis on 7 M urea-6% acrylamide gels and precipitated with ethanol. RNAs were resuspended in deionized distilled water. 5′- or 3′-end labeling of U snRNAs was carried out as described elsewhere (53).
Xenopus oocyte microinjections and immunoprecipitations.
Injections were carried out as described previously (52). Briefly, oocytes were harvested and incubated for 2 h in modified Barth's solution containing 0.2% collagenase type II (Sigma). Defolliculated stage V and VI oocytes were collected and used the next day for microinjection. In a typical injection experiment, 20 nl of [32P]-labeled RNA (usually approximately 106 cpm/μl for each RNA) was injected into the cytoplasm of oocytes. After incubation, oocytes were homogenized to prepare extracts for further analysis.
Immunoprecipitation of RNA-protein complexes was carried out as described previously (52). For a typical immunoprecipitation experiment, cytoplasmic fractions were homogenized in 300 μl of ice-cold RSB-150 buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 2.5 mM MgCl2) and the insoluble material was pelleted by centrifugation. The cleared supernatant was incubated with antibodies bound to protein A-Sepharose (Pharmacia). Immunoprecipitation was performed for 30 min at 4°C with constant rotation, and the reaction mixture was subsequently washed five times with 1 ml of ice-cold RSB-150 buffer. The immunoprecipitated RNAs were isolated by proteinase-K treatment followed by phenol-chloroform extraction and ethanol precipitation. RNAs were analyzed by electrophoresis on 7 M urea-8% polyacrylamide gels and autoradiography.
Limited alkaline hydrolysis and minimal binding analysis.
Limited alkaline hydrolysis was carried out as described previously (53), with the following modification: 5′- or 3′-end-labeled full-length U snRNA transcripts (100,000 cpm total) in 5 μl were treated with 0.5 μl of alkaline buffer (0.5 M NaOH, 10 mM EDTA) at 94°C for 40 s and immediately neutralized by the addition of 0.5 μl of acid buffer (0.5 M NaOAc [pH 5.2]). After ethanol precipitation, the hydrolyzed RNA pieces were incubated with the Flag-purified SMN complex or control purification. The bound RNAs were isolated and analyzed using 8% polyacrylamide gel electrophoresis. RNase T1 and alkali digestion ladders of the end-labeled U snRNA transcripts were used as molecular markers.
Preparation of HeLa cell cytoplasmic extracts.
HeLa cell extracts competent for snRNP assembly were prepared as described previously (43). HeLa S3 cells were resuspended in equal volumes of reconstitution buffer containing 50 μg of digitonin/ml and passed five times through a 25-gauge needle on ice. Following centrifugation for 1 min at 4,000 rpm (7,000 × g), nuclei were discarded and NP-40 was added to supernatants to achieve a final concentration of 0.01%. Following centrifugation for 15 min at 10,000 rpm (17,000 × g) and 4°C, supernatants were collected and stored in aliquots at −80°C.
Purification of native SMN complex.
Flag-Gemin2 (SMN complex) or HeLa Tet-ON cells (control) were grown in the presence of doxycycline (5 μg/ml). Total cell extracts in RSB-100 buffer containing 0.1% NP-40 and protease inhibitors were incubated with anti-Flag beads (Sigma) for 2 h at 4°C. Supernatants were discarded and the beads were extensively washed with RSB-100 containing 0.02% NP-40. Three washes were performed for 15 min at 4°C with 10 bead volumes of RSB-500 containing 0.02% NP-40. The bound proteins were either equilibrated with 10 bead volumes of RSB-100 containing 0.01% NP-40 for further experiments or eluted for 1 h at 4°C with 3× Flag peptides (Sigma) at a final concentration of 0.5 mg/ml. Purified SMN complex was analyzed by sodium dodecyl sulfate-12.5% PAGE and silver staining.
In vitro binding of RNAs.
A total of 10,000 cpm of [32P]UTP-labeled RNAs was mixed with the binding buffer (10 mM Tris [pH 7.4], 100 mM NaCl, 2.5 mM MgCl2, 0.01% NP-40, 10 μM tRNA) and added directly to the purified SMN complex on the Flag beads. The binding was carried out for 1 h at 4°C. The beads were then washed five times with 1 ml of binding buffer. For control experiments, the same procedure was carried out using the beads previously incubated with extracts from HeLa Tet-On cells. The bound RNAs were isolated and analyzed by electrophoresis on 7 M urea-8% polyacrylamide gels.
Equilibrium binding experiments.
Binding constants were determined by an equilibrium binding assay. The SMN complex was prepared as described above from a stable cell line expressing Flag-Gemin2. The SMN complex immobilized on anti-Flag beads at a concentration of <100 pM was incubated with increasing amounts of nonradioactive U4 snRNA supplemented with trace amounts of U4 snRNA transcribed in the presence of [32P]UTP. Reactions were carried out for 2 h at 25°C in a buffer containing 10 mM Tris (pH 7.4), 100 mM NaCl, 2.5 mM MgCl2, 0.01% NP-40, and 1 mg of Escherichia coli tRNA/ml. It was found that the standard approach of washing the beads by resuspension and centrifugation broke equilibrium, allowing RNA to elute off of the protein during the wash procedure. Also, eluting the RNA from the beads to quantify the signal on a polyacrylamide gel introduced error. To overcome these problems, a filter-binding approach was adapted. After incubation, the reaction mixture was passed through a nitrocellulose filter using a multiwell vacuum manifold, immobilizing the beads with SMN complex and bound RNA on the filter. The beads in each well of the filter apparatus were washed 2× in 200 μl of wash buffer (10 mM Tris (pH 7.4), 100 mM NaCl, 2.5 mM MgCl2, 0.01% NP-40) and allowed to air dry for 30 min. In this procedure the beads could be washed for only 5 to 10 s, which was too short a time for RNA to significantly elute from the complex. In confirmation, binding was found to be independent of the wash volume (data not shown). tRNA also did not contribute to the equilibrium, as the binding was found to be independent of tRNA concentration. The saturation of SMN with RNA was determined by directly quantifying the radiolabeled RNA remaining on the beads after washing. To confirm that the RNA was not degraded, a control experiment was performed in which the RNA was eluted from the beads by proteinase K treatment and phenol-chloroform extraction followed by visualization of the RNA on a 7 M urea-8% polyacrylamide gel.
The filters were imaged using a Molecular Dynamics PhosphorImager and ImageQuant software. The resulting data were analyzed using Microsoft Excel and SigmaPlot software. Background intensities from a control experiment using a cell line that does not express Flag-Gemin2 were subtracted from the values for each spot. A least-squares fit for a single binding site was obtained using the equation Y = Bmax[RNA]/(Kd + [RNA]), where Bmax is the maximum SMN complex saturation with RNA (normalized to 1) and Kd(apparent) is the apparent equilibrium dissociation constant. The Kd(apparent) value reported is the average of the results from three independent experiments.
Assay for in vitro assembly of snRNPs.
In vitro Sm core assembly and electrophoretic mobility shift assays were carried out as described previously (43). For the anti-Sm (Y12) monoclonal antibody inhibition experiment, 3 μg of purified anti-Sm (Y12) monoclonal antibody was preincubated with HeLa cytoplasmic extract for 20 min on ice and used immediately for in vitro assembly.
Immunodepletion of the SMN complex.
100 μl of Flag beads (Sigma) were divided into four aliquots. Cytoplasmic extracts (50 μl) from HeLa cell lines expressing Flag-Gemin2 were incubated with the first aliquot of these beads for 1 h at 4°C, and the supernatant was transferred to the next aliquot of beads and again incubated for 1 h at 4°C. This procedure was repeated four times in total, and the supernatant after the final incubation was stored in aliquots at −80°C. Western blotting using the anti-SMN (2B1) monoclonal antibody was performed to verify the immunodepletion of the SMN complex in the extracts.
RESULTS
Binding of the SMN complex to the major Sm site-containing spliceosomal U snRNAs.
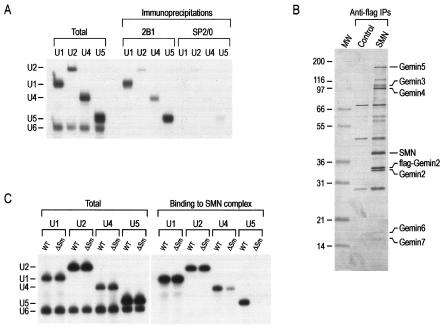
To determine whether the SMN complex interacts with the Sm site-containing spliceosomal U snRNAs, U2, U4, and U5 snRNAs were labeled by transcription in vitro in the presence of [32P]UTP and each snRNA was mixed with similarly labeled U6 snRNA as an internal control. These RNAs were injected into the cytoplasm of Xenopus oocytes, which were then incubated for 3 h. The oocytes were homogenized, and immunoprecipitations were carried out using anti-SMN (2B1) or control (SP2/0) antibodies. Consistent with our previous observations, U1 and U5 snRNAs and, to a lesser extent, U4 snRNA were efficiently immunoprecipitated with 2B1 (5, 6). In addition, a small amount of U2 snRNA was reproducibly immunoprecipitated by 2B1 above the background level of the control immunoprecipitation (Fig. 1A). The discrepancy with respect to U snRNA immunoprecipitation in this experiment comes from the epitope recognition of the 2B1 antibody (unpublished data). These data show that all the major Sm site-containing spliceosomal U snRNAs associate with the SMN complex in vivo.
FIG. 1.
The SMN complex associates with major spliceosomal U snRNAs in vivo and in vitro. (A) [32P]UTP-labeled U1, U2, U4, or U5 snRNA was mixed with U6 snRNA, and each RNA mixture was injected into the cytoplasm of Xenopus oocytes. After incubation for 3 h, oocytes were homogenized and immunoprecipitations were carried out using anti-SMN (2B1) monoclonal antibody and control (SP2/0) antibody. The RNAs were isolated and analyzed by electrophoresis on 7 M urea-8% polyacrylamide gels. Lanes labeled “Total” represent 10% of input. (B) Native SMN complexes were purified from stable cell lines expressing Flag-Gemin2 (as described in Materials and Methods) and were analyzed by sodium dodecyl sulfate-12.5% PAGE and silver staining. Immunoprecipitation using anti-Flag antibody from the parental HeLa cell line (Tet ON) was carried out as a control (Control). Components of the SMN complex are indicated on the basis of molecular weight and Western blotting (data not shown). Half of the total amount of the SMN complex shown in this gel was used for direct RNA-binding experiments. (C) The same mixtures of RNAs used as described for panel A were added to the Flag-purified SMN complex as shown in Fig. 1B (SMN complex) and incubated for 1 h. Subsequently, bound RNAs were isolated after washing and analyzed by electrophoresis on 7 M urea-8% polyacrylamide gels.
To examine whether the interaction of the SMN complex with U snRNAs is direct, native SMN complexes were purified from stably transfected cell lines expressing a Flag-Gemin2 construct under stringent conditions (500 mM NaCl) as described previously (1, 39). The complexes isolated using the Flag epitope under these conditions (as shown in Fig. 1B) contained all the known integral components of the SMN complex, including SMN, Gemin2, Gemin3, Gemin4, Gemin5, Gemin6, and Gemin7 but not the Sm proteins (1, 39, 43). To test direct binding, purified SMN complexes on anti-Flag beads were incubated with [32P]UTP-labeled U snRNAs, U1, U2, U4, U5, and U6. Substitution mutations of the Sm sequences of each U snRNA (ΔSm) were produced and tested similarly. After a 1 h incubation, the beads were precipitated and washed with the binding buffer and the RNAs bound to the purified SMN complexes on beads were isolated and analyzed by electrophoresis on 7 M urea-8% polyacrylamide gels. As shown in Fig. 1C, wild-type (WT) U1, U2, U4, and U5 snRNAs bound to the SMN complex efficiently. When the Sm site was mutated, however, the binding of U4ΔSm was significantly reduced and the binding of U5ΔSm was abolished. The binding of U1ΔSm and U2ΔSm was as efficient as that of the corresponding WT snRNAs. Because the SMN complex purified under these stringent conditions does not contain the Sm proteins, it is not likely that the Sm proteins mediate the binding to WT U snRNAs (39, 43, 52). The reduced binding of the SMN complexes to U4ΔSm and U5ΔSm suggests that the Sm sites of these RNAs play a role in the interaction with the SMN complex (see below). Nonetheless, these experiments demonstrate that the SMN complex binds to the U snRNAs directly and that the interaction does not require Sm proteins.
Specific domains of the U snRNAs mediate binding to the SMN complex.
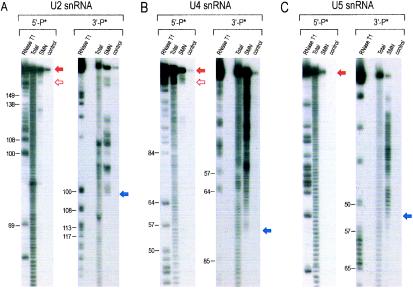
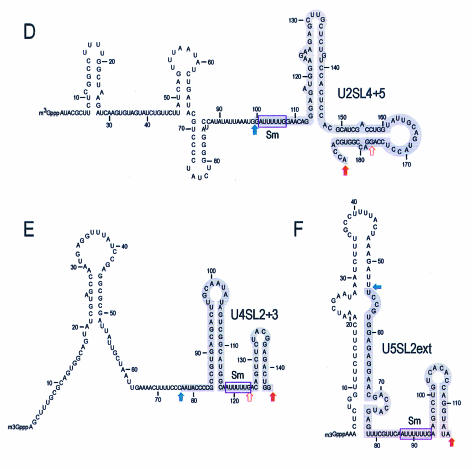
We have previously shown that SL1 of U1 snRNA is necessary and sufficient for a specific interaction of U1 snRNA with the SMN complex. However, the other major Sm site-containing U snRNAs do not contain sequences similar to that of SL1 of U1 snRNA. We used limited alkaline hydrolysis to map the binding domains of U snRNAs necessary for the interaction with purified SMN complexes. The 5′- or 3′-end-labeled U2, U4, or U5 snRNAs were subjected to partial alkaline hydrolysis (53), and the resulting hydrolyzed RNA ladders were incubated with the SMN complex immobilized on anti-Flag beads. Bound RNA fragments were purified and analyzed by denaturing polyacrylamide gel electrophoresis. These experiments allowed a rough delineation of the subdomains of each U snRNA that are required or dispensable for binding to SMN complexes. As shown in Fig. 2A and D, the domain of U2 snRNA necessary for SMN complex binding encompasses at most the region between nucleotide 100 and the 3′ end of U2. This U2 snRNA fragment is designated U2SL4+5, as it contains stem-loops 4 and 5 of U2 (Fig. 2D). In the case of U4 snRNA (Fig. 2B), the SMN complex-binding domain resides between nucleotide 77 and the 3′ end of U4 and is designated U4SL2+3 (Fig. 2E), although it is possible that a few nucleotides at the extreme 3′ end are also dispensable. The SMN complex binds to U5 snRNA from nucleotide 54 to the 3′ end of U5; this domain is designated U5SL2ext (Fig. 2C and 2F). All SMN complex-binding domains of the U snRNAs (except U1) contain the Sm site, interestingly, and in the cases of U4 and U5 snRNAs, the Sm sites are located in the middle of the binding domains (Fig. 2E and 2F). These findings may explain why the substitution mutations in the Sm sites of U4 and U5 snRNAs described above (U4ΔSm and U5ΔSm) affect the binding to the SMN complex.
FIG. 2.
Mapping of U snRNA domains binding to the SMN complex. (A) The SMN complex-binding domain of Xenopus laevis U2 snRNA. The 5′ (5′-P*)- and 3′ (3′-P*)-end-labeled U2 snRNA was subjected to limited alkaline hydrolysis in the presence of tRNA (10 μg). The resulting hydrolyzed RNA ladders were incubated with the SMN complex. The RNA fragments bound to the SMN complex were isolated and analyzed by electrophoresis on 7 M urea-8% acrylamide gels. RNase T1-digested RNA ladders of the same RNAs were used as size markers. Solid red arrows indicate the largest extent of the SMN complex-binding domains. Open red arrows indicate the smallest possible binding fragments. Total, 5% input; control, binding in control purification. (B) The SMN complex-binding domain of chicken U4 snRNA. The same experiment was performed using 5′ and 3′-end-labeled U4 snRNAs as described for panel A. (C) The SMN complex-binding domain of X. laevis U5 snRNA. The same experiment was performed using 5′- and 3′-end-labeled U5 snRNAs as described for panel A. (D) The secondary structure of X. laevis U2 snRNA and its domain for SMN complex binding. The region denoted by the gray box (from nucleotide 100 to the 3′ end) is sufficient for the interaction with the SMN complex and is designated U2SL4 + 5. (E) The secondary structure of chicken U4 snRNAs and its domain for SMN complex binding. The region denoted by the gray box (nucleotide 77 to the 3′ end) is sufficient for the interaction with the SMN complex and is designated U4SL2 + 3. (F) The secondary structure of X. laevis U5 snRNA and its domain for SMN complex binding. The region denoted by the gray box (nucleotide 55 to the 3′ end) is sufficient for the interaction with the SMN complex and is designated U5SL2ext.
The SMN complex-binding domains and the Sm sites are necessary and sufficient for the Sm core assembly.
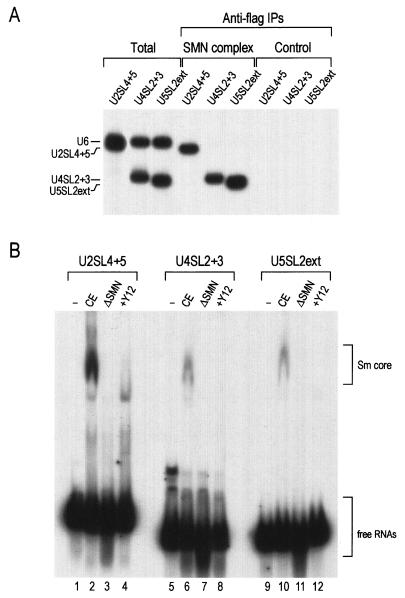
To further examine the binding of these domains to the SMN complex, U2SL4+5, U4SL2+3, and U5SL2ext (mini U snRNA fragments) were transcribed in the presence of [32P]UTP and mixed with radiolabeled U6 snRNA as an internal control. These RNA mixtures were incubated with the purified SMN complex on beads or with nonspecific proteins purified from HeLa cells as a control (Fig. 1B). As shown in Fig. 3A, U2SL4+5, U4SL2+3, and U5SL2ext bind to the SMN complex efficiently. RNA fragments derived from domains excluding mini U snRNA fragments did not bind to the SMN complex (data not shown). These data demonstrate that the mini U snRNA fragments are sufficient for the binding to the SMN complex. Because the SMN complex-binding domains of U2, U4, and U5 snRNAs contain the Sm site, we asked whether the SMN complex-binding domains and the Sm sites of these RNAs are sufficient for Sm core assembly. U2SL4+5, U4SL2+3, and U5SL2ext were transcribed in the presence of [32P]UTP, and in vitro snRNP assembly was carried out in HeLa cytoplasmic extracts. Subsequently, assembly reaction products were analyzed by electrophoresis on 6% native gels. Figure 3B shows that the mini snRNA fragments from U2, U4, and U5 snRNAs assemble Sm cores (lanes 2, 6, and 10). To further confirm that the RNA-protein complexes causing the shifts on a native gel are genuine Sm cores, HeLa cell cytoplasmic extracts were preincubated with anti-Sm (Y12) monoclonal antibodies and used for in vitro snRNP assembly. As shown in Fig. 3B, lanes 4, 8, and 12, anti-Sm (Y12) monoclonal antibody inhibits the assembly of Sm cores on these mini U snRNA fragments, confirming that the high-molecular-weight RNA-protein complexes contain assembled Sm cores. In addition, immunodepletion of the SMN complex prior to the assembly reaction results in the inhibition of Sm core formation, demonstrating that the Sm core assembly on mini U snRNA fragments is mediated by the SMN complex (lanes 3, 7 and 11). However, immunodepletion of SMN complexes did not affect the amount of Sm proteins in the cell extracts, as described previously (data not shown and reference 43). These results demonstrate that SMN complex binding to Sm site-containing target RNAs is necessary and sufficient for Sm core assembly.
FIG. 3.
The SMN complex-binding domains and Sm sequences of U snRNAs are required for the formation of Sm cores in cell extracts. (A) Mini snRNA fragments from each U snRNA mapped by minimal binding analysis using alkaline hydrolysis were transcribed in the presence of [32P]UTP and mixed with radiolabeled U6 as an internal control. RNA mixtures were incubated with the SMN complex or nonspecific proteins purified from HeLa cells (Control) for 1 h. The bound RNAs were isolated and analyzed by electrophoresis on 7 M urea-8% polyacrylamide gels. Total, 10% input; IPs, immunoprecipitations. (B) U2SL4 + 5, U4SL2 + 3 and U5SL2ext were transcribed in the presence of [32P]UTP and incubated with buffer (−), HeLa extracts (CE), the SMN complex-depleted HeLa extracts (ΔSMN), or HeLa extracts preincubated with anti-Sm (Y12) monoclonal antibody (+Y12) for 30 min at 30°C. After assembly reactions, the products were analyzed by electrophoresis on 6% native polyacrylamide gels and autoradiography. Sm cores and free RNAs are indicated by brackets.
U snRNAs bind with high affinity to the SMN complex.
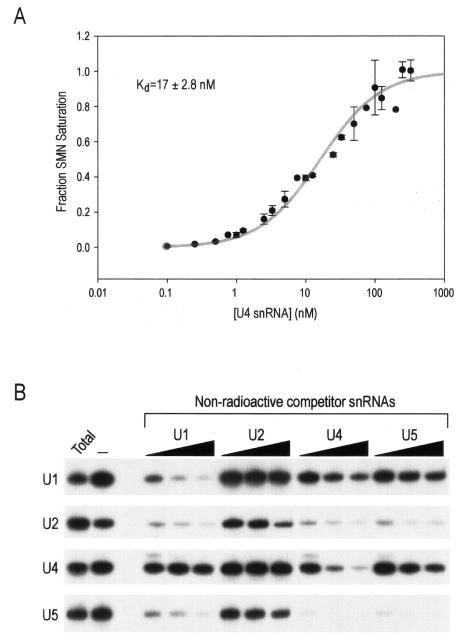
Equilibrium binding experiments were carried out to determine the affinity of the SMN complex-U snRNA interaction (Fig. 4A). Purified SMN complexes on anti-Flag beads were incubated with various amounts of [32P]UTP-labeled U4 snRNA, and binding was allowed to reach equilibrium by incubation for 2 h. The complexes with bound U4 snRNA were isolated by trapping the beads on a nitrocellulose filter and rapidly washing away the free RNA. The fraction of immobilized SMN complexes saturated with RNA was determined by quantification of the radioactivity remaining bound to the beads and was corrected for nonspecific weak interactions with the beads alone. The bound RNA was confirmed to be a single full-length species by polyacrylamide gel electrophoresis (data not shown). Although we were unable to rule out the possibility of multiple classes of SMN complexes contributing unequally to the overall binding (or the possibility that multiple, unequal U4 binding sites are present on each SMN complex), the data fit well to a model for a single saturable U4 binding site. We therefore determined an apparent equilibrium dissociation constant [Kd(apparent)] of 17 ± 2.8 nM, corresponding to this proposed single type of high-affinity U4 binding site.
FIG. 4.
U snRNAs bind with high affinity to the SMN complex. (A) The affinity of U4 snRNA to the SMN complex was determined by a nitrocellulose filter binding assay. The SMN complex was incubated under equilibrium conditions with increasing amounts of U4 snRNA. A plot of the fraction of SMN complex saturation as a function of U4 snRNA concentration is shown. Error bars represent standard deviations from three independent experiments. (B) Purified SMN complexes were preincubated with nonradioactive U1, U2, U4, or U5 snRNA at a concentration of 10, 50, or 250 nM for 30 min at 4°C; subsequently, 10,000 cpm of [32P]UTP-labeled U1, U2, U4, or U5 snRNA, respectively, was added to the preincubated mixtures and further incubated for 1 h at 4°C. Bound RNAs were isolated and analyzed by electrophoresis on 7 M urea-8% polyacrylamide gels. Total, 10% input.
The fact that the SMN complex is able to recognize several U snRNAs suggests that the U snRNAs might share a common binding domain on the SMN complex. To investigate how the binding of the SMN complex is affected by the presence of one or more other U snRNAs, as well as to examine the relative affinities for the various U snRNAs, competition binding experiments were performed. The SMN complex was incubated with trace amounts of [32P]UTP-labeled U1, U2, U4, or U5 snRNA and three different concentrations (10, 50, and 250 nM) of nonradioactive U1, U2, U4, and U5 snRNAs. After incubation, the bound RNAs were purified and analyzed by denaturing polyacrylamide gel electrophoresis. As shown in Fig. 4B, nonradioactive U1 snRNA effectively competes with labeled U1, U2, and U5 snRNAs, but not with U4 snRNA, for SMN complex binding. Similarly, U4 snRNA effectively competes with each of the labeled U2, U4, and U5 snRNAs for binding to the SMN complex but is unable to fully compete with U1 even at very high concentrations. U2 snRNA does not compete with labeled U1 and U4 snRNAs, but it slightly affects the binding of itself and U5 snRNA at a high concentration (250 nM). U5 snRNA significantly competes with labeled U2 and U5 snRNAs but not with U1 and U4 snRNAs. These data suggest that U4 and U1 might bind in separate or only partially overlapping sites on the SMN complex, although since they both can fully compete U2 and U5, the arrangement of RNA binding sites is likely more complex. Despite the possibly complex arrangement of binding sites, the data suggest that there are at least two distinct high-affinity binding sites, one for U1 and one for U4, while U2 and U5 snRNAs bind less avidly.
SL1 of U1 snRNA can inhibit the assembly of the various spliceosomal U snRNPs.
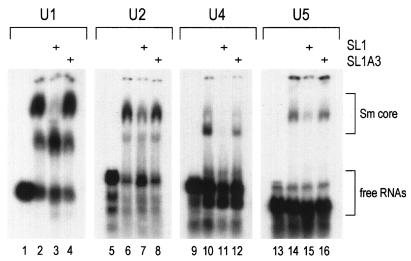
Microinjection of excess SL1 of U1 snRNA, but not SL1A3 (which does not interact efficiently with the SMN complex), into the cytoplasm of Xenopus oocytes inhibits the binding of the SMN complex to U1 snRNA and U1 snRNP assembly (52). To determine whether SL1 can interfere with the assembly of other U snRNPs in vitro, SL1 and SL1A3 were transcribed in vitro without radioactive labeling. HeLa cell cytoplasmic extracts were incubated with an excess of either SL1 or SL1A3 (∼2.5 μM), and these extracts were used to assay snRNP assembly in vitro on [32P]UTP-labeled U snRNAs. Untreated HeLa cytoplasmic extracts were used as a control. As shown in Fig. 5, lanes 2, 6, 10, and 14, all U snRNAs tested formed the Sm cores in the extracts. In the presence of excess SL1, Sm core assembly is inhibited (albeit to a different extent for each U snRNA) (Fig. 5, lanes 3, 7, 11, and 15). However, preincubation of extracts with SL1A3 did not inhibit Sm core assembly at all (in the cases of U1, U2, and U5) or inhibited it only slightly (in the case of U4). These results indicate that the SMN complex can be saturated with SL1 of U1 snRNA, leaving no additional SMN complex available for assembly in the extract. Taken together, these data demonstrate that interactions of SMN complex with Sm site-containing major U snRNAs are essential for the assembly of snRNPs.
FIG. 5.
SL1 of U1 snRNA inhibits the assembly of Sm core in vitro. HeLa extracts were added (+) to nonradioactive SL1 or SL1A3 and incubated for 30 min on ice. Subsequently, [32P]UTP-labeled U1, U2, U4, or U5 snRNA was added to the preincubated extracts and the mixtures were further incubated for 30 min at 30°C; as a control, the extracts were also mixed with [32P]UTP-labeled U1, U2, U4, or U5 snRNA, respectively, in the absence of nonradioactive RNAs (lanes 2, 6, 10, and 14). The formation of Sm cores was analyzed by electrophoresis on native 6% polyacrylamide gels and autoradiography.
The relative positions of the SMN complex-binding domain and Sm site are important for snRNP assembly.
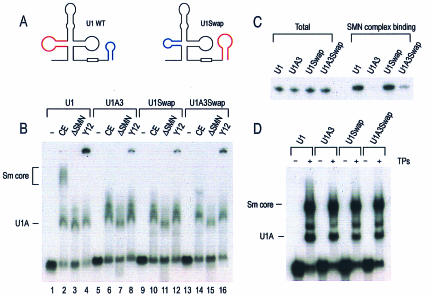
To further understand the requirements for SMN complex-mediated Sm core assembly, we asked whether the position of the SMN complex-binding site relative to that of the Sm site of the U snRNAs affects snRNP assembly. For this purpose, U1Swap RNA (in which SL1 and SL4 of U1 snRNA were swapped) (Fig. 6A) and U1A3Swap RNA (in which SL1 of U1Swap was changed to the corresponding A3 mutation) were constructed. U1, U1A3, U1Swap, and U1A3Swap RNAs were transcribed in the presence of [32P]UTP, and in vitro snRNP assembly was carried out using HeLa extracts. Assembled RNA-protein complexes were analyzed by electrophoresis on 6% native gels. As shown in Fig. 6B, Sm cores assemble on U1 snRNA (but not on U1A3) in a SMN complex-dependent manner (lanes 2, 3, and 6) (as previously reported); a Y12 monoclonal antibody supershift (lane 4) was used to confirm the presence of Sm cores. Interestingly, the assembly of Sm cores on U1Swap RNA was impaired, as was the case for the corresponding A3 mutant, U1A3Swap (Fig. 6B lanes 10 and 14).
FIG. 6.
The correct arrangement of the SMN complex-binding domain and Sm site is required for snRNP assembly. (A) U1Swap RNA was constructed by swapping SL1 (nucleotides 17 to 47) and SL4 (nucleotides 140 to 164) of U1 snRNA. SL1 of U1 snRNA is highlighted in red, and SL4 is highlighted in blue. (B) U1, U1A3, U1 Swap, and U1A3 Swap RNAs were transcribed in the presence of [32P]UTP and incubated with buffer (−), HeLa extracts (CE), or the SMN complex-depleted HeLa extracts (ΔSMN) for 30 min at 30°C. Additional assembly reactions were performed using HeLa extracts in the presence of Y12 monoclonal antibody for antibody supershifting (Y12). The products were analyzed by electrophoresis on 6% native polyacrylamide gels and autoradiography. Assembled snRNPs are indicated by brackets. (C) The SMN complexes were purified and incubated with [32P]UTP-labeled U1, U1A3, U1 Swap, and U1A3 Swap RNAs. Immunoprecipitations were performed using anti-Flag antibodies, and immunoprecipitated RNAs were analyzed by electrophoresis on 7 M urea-8% polyacrylamide gels. Total, 10% input. (D) snRNP TPs were purified as described previously by Raker et al. (47). Purified TPs were mixed with [32P]UTP-labeled U1, U1A3, U1 Swap, and U1A3 Swap RNAs and further incubated for 30 min at 30°C. Assembled snRNPs were analyzed by electrophoresis on 6% native polyacrylamide gels and autoradiography.
Next, we asked whether the swapping of domains reduces the binding efficiency of the SMN complex to the RNA and whether this subsequently results in impaired Sm core assembly. For this purpose, [32P]UTP-labeled U1Swap and U1A3Swap RNAs were incubated with purified SMN complex for direct binding. As controls, [32P]UTP-labeled U1 and U1A3 snRNAs were used. Figure 6C shows that U1Swap RNA binds to the SMN complex as efficiently as WT U1 snRNA. The A3 mutation of both U1 and U1Swap almost impaired the binding of these RNAs to the SMN complex. These data suggest that domain swapping of U1 snRNA does not affect the affinity of this RNA to the SMN complex.
Since swapping of SL1 and SL4 of U1 snRNA may inhibit the interaction between the Sm proteins and the Sm site, we examined whether purified snRNP total proteins (TPs) are able to associate with these RNAs. For this experiment, [32P]UTP-labeled U1, U1A3, U1Swap, and U1A3Swap RNAs were incubated with TPs and the assembled products were analyzed by electrophoresis on 6% native gels. As shown in Fig. 6D, TPs assemble on these RNAs irrespective of swapping and mutation in SL1. Taken together, these results indicate that in addition to the binding of the SMN complex to the target RNA sequences, correct spatial arrangement of SMN complex binding on target RNAs is necessary for snRNP assembly.
The assembly of minor splicing pathway U11 snRNP is also mediated by the SMN complex.
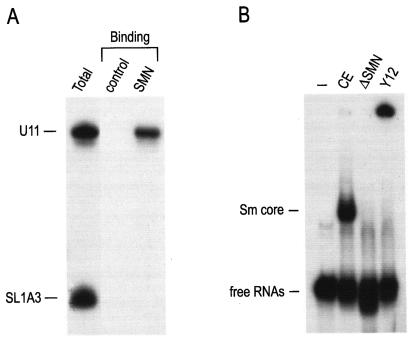
In addition to the class of the major snRNPs that are required for the splicing of most introns, there is a class of low-abundance snRNPs that are required for splicing of ATAC introns (also referred to as the minor spliceosome pathway) (14, 35, 49). These include U11, U12, and U4atac snRNPs, all of which contain Sm cores (14, 35, 50). To examine whether the assembly of minor snRNPs is also mediated by the SMN complex, we tested the direct binding of the SMN complex to U11 snRNA. To do so, U11 snRNA was transcribed in the presence of [32P]UTP and incubated with purified SMN complex. Radiolabeled SL1A3 was used as an internal control. As shown in Fig. 7A, the SMN complex binds directly to U11 snRNA. To test whether minor U11 snRNP assembly is mediated by the SMN complex, in vitro snRNP assembly was carried out in HeLa extracts or in SMN complex-depleted HeLa extracts. The products were analyzed by electrophoresis on 6% native polyacrylamide gels. As shown in Fig. 7B, the assembly of U11 snRNP is mediated by the SMN complex. These results demonstrate that the SMN complex binds directly to U11 snRNA and carries out Sm core assembly on U11 snRNA and indicate that the SMN complex also has a role in the assembly of the minor pathway U snRNPs.
FIG. 7.
The assembly of U11 snRNP is mediated by the SMN complex. (A) The SMN complexes were purified and incubated with [32P]UTP-labeled U11 snRNA. [32P]UTP-labeled SL1A3 was used as an internal control (Control). Immunoprecipitations were performed using anti-Flag antibodies, and immunoprecipitated RNAs were analyzed by electrophoresis on 7 M urea-8% polyacrylamide gels. Total, 10% input. (B) U11 snRNA was transcribed in the presence of [32P]UTP and incubated with buffer (−), HeLa extracts (CE), or SMN complex-depleted HeLa extracts (ΔSMN) for 30 min at 30°C. Additional assembly reactions were performed using HeLa extracts in the presence of Y12 monoclonal antibody for antibody supershifting (Y12). The products were analyzed by electrophoresis on 6% native polyacrylamide gels and autoradiography.
DISCUSSION
To perform its essential function in snRNP biogenesis (specifically in the assembly of the Sm core), the SMN complex must have the capacity to interact with and bring together the Sm proteins and the U snRNAs. The recognition of the Sm proteins is accomplished by binding to the unique RG domains found in three of these, SmB, SmD1, and SmD3, and this association is strongly enhanced by the methylation of specific arginines in these domains, a process that is carried out by the methylosome/PRMT5 complex (10, 11, 12, 32, 46). The binding to the snRNAs needs to occur after the SMN complex has already been bound with at least some of the methylated Sm proteins, and that raised the possibility that the Sm proteins might be able to bridge the binding of the snRNAs to the SMN complex. However, SMN complexes washed at high salt concentrations, a condition that removes all detectable Sm proteins, still bind snRNAs efficiently (39, 43). Experiments with U1 snRNA have demonstrated that the deletion of the Sm site does not reduce the binding of U1 snRNA to the SMN complex, indicating that sequences in U1 snRNA other than that of the Sm site, which we subsequently identified as SL1, are necessary and sufficient for binding to the SMN complex (52). SL1 was further found to be critical for the assembly of the Sm core on U1 snRNA. Mindful of the fact that the other major Sm site-containing snRNAs that do not contain sequences that bear obvious similarity to sequences of SL1 also assemble Sm cores mediated by the SMN complex, we wished to determine which domains in these snRNAs provide the recognition for the SMN complex.
We show here that the SMN complex binds directly to all Sm site-containing major spliceosomal U snRNAs, and we further delineate the sequence elements of the U snRNAs that are responsible for SMN complex binding. Unlike U1 snRNA, the SMN complex binds to U2, U4, and U5 snRNAs via domains near their 3′ ends. All of these snRNAs contain at least one well-defined stem-loop structure and also include the Sm site. Although there is no extensive nucleotide sequence similarity or obvious consensus RNA sequence among these SMN complex-binding domains, the SMN complex binds to all of these with remarkable affinity. The mapping and deletion analysis could not separate the Sm site sequence from the minimal recognition domain for SMN complex binding. Further mutagenesis and more detailed binding experiments will be needed to determine whether it is the specific sequence of the Sm sites that is important and required for binding to SMN complex or whether the Sm sites are important for the overall structure and presentation of the adjacent stem-loop. With these data taken together, we envision that the interaction between the SMN complex and the U snRNAs occurs through specific recognition of stem-loop structure(s) in an orientation-dependent and/or sequence-specific interaction. The binding competition experiments suggest that the affinities of the various SMN complex-binding domains for the SMN complex are not the same, although all appear to be in the low nanomolar range and to exhibit a clear order of affinities: U4∼U1 > U5 > U2. The binding data suggest that there are at least two binding sites on the SMN complex for which the snRNAs compete to various degrees, although the actual arrangement of RNA binding sites might be more complex.
Early studies using purified snRNP TPs suggested that the minimal sequence requirement of Sm core assembly in vitro is simply a region of 6 to 10 single-stranded uridine-rich nucleotides (47). These studies left unanswered the question of how the Sm proteins distinguish their targets specifically among the myriads of uridine-rich RNA sequences in cells. In contrast, microinjection experiments with Xenopus oocytes showed that the Sm sites of each U snRNA are not functionally interchangeable in Sm protein binding (17). These studies suggested that the Sm site, in spite of being the common binding site for Sm proteins, might cooperate specifically with other elements of U snRNAs for snRNP assembly (17). These studies imply that the assembly of U snRNPs, although they share the common Sm proteins and the Sm site, is not a simple process but is rather a strictly regulated and coordinated process involving many factors. We demonstrate here that the assembly of snRNPs requires the SMN complex and Sm proteins as well as an RNA containing recognition elements for both the SMN complex and Sm proteins.
The results seen with mutants of U1 snRNA with swapped domains show that the SMN-mediated snRNP assembly is more stringent than can be explained with a simple RNA-protein association model. The SMN complex recognizes specific sequence elements within an RNA and scrutinizes the RNA to ensure that these elements are arranged correctly in the context of Sm core assembly. The evidence we provide here strongly suggests that the SMN complex not only provides the platform for binding both Sm proteins and RNAs and brings all components together into close spatial proximity for assembly but also confers stringent specificity to the assembly pathway by distinguishing the positions of the target sequences relative to those of the Sm sequences. In this way, the SMN complex functions as an assemblyosome to ensure that Sm core assembly occurs only on correct RNA targets.
The observations we present here further expand the repertoire of RNA substrates for the SMN complex to include the minor pathway spliceosomal snRNPs. It is likely, given the numerous RNA-binding proteins with which the SMN complex interacts, that there are numerous RNA targets of the SMN complex in cells and that the SMN complex is involved also in the assembly of other classes of RNPs. It remains possible that reduced levels of the SMN protein not only affect the biogenesis of U snRNPs but also impair other RNP assembly processes, including those that might be specific to motor neurons.
Acknowledgments
We are grateful to Iain W. Mattaj and Joan Steitz for providing plasmids and Y12 antibody. We thank the members of our laboratory, especially Amelie Gubitz, for helpful discussions and comments on the manuscript. We are also grateful to Gina Daly for secretarial assistance.
This work was supported by the Association Française Contre les Myopathies (AFM) and by a grant from the National Institute of Health. L.P. is a Telethon Assistant Scientist and an EMBO Young Investigator. G.D. is an Investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Baccon, J., L. Pellizzoni, J. Rappsilber, M. Mann, and G. Dreyfuss. 2002. Identification and characterization of Gemin7, a novel component of the survival of motor neuron complex. J. Biol. Chem. 277:31957-31962. [DOI] [PubMed] [Google Scholar]
- 2.Branlant, C., A. Krol, J. P. Ebel, E. Lazar, B. Haendler, and M. Jacob. 1982. U2 RNA shares a structural domain with U1, U4, and U5 RNAs. EMBO J. 1:1259-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhler, D., V. Raker, R. Luhrmann, and U. Fischer. 1999. Essential role for the tudor domain of SMN in spliceosomal U snRNP assembly: implications for spinal muscular atrophy. Hum. Mol. Genet. 8:2351-2357. [DOI] [PubMed] [Google Scholar]
- 4.Charroux, B., L. Pellizzoni, R. A. Perkinson, A. Shevchenko, M. Mann, and G. Dreyfuss. 1999. Gemin3: A novel DEAD box protein that interacts with SMN, the spinal muscular atrophy gene product, and is a component of gems. J. Cell Biol. 147:1181-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charroux, B., L. Pellizzoni, R. A. Perkinson, J. Yong, A. Shevchenko, M. Mann, and G. Dreyfuss. 2000. Gemin4. A novel component of the SMN complex that is found in both gems and nucleoli. J. Cell Biol. 148:1177-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer, U., Q. Liu, and G. Dreyfuss. 1997. The SMN-SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell 90:1023-1029. [DOI] [PubMed] [Google Scholar]
- 7.Fischer, U., and R. Luhrmann. 1990. An essential signaling role for the m3G cap in the transport of U1 snRNP to the nucleus. Science 249:786-790. [DOI] [PubMed] [Google Scholar]
- 8.Fischer, U., V. Sumpter, M. Sekine, T. Satoh, and R. Luhrmann. 1993. Nucleocytoplasmic transport of U snRNPs: definition of a nuclear location signal in the Sm core domain that binds a transport receptor independently of the m3G cap. EMBO J. 12:573-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friesen, W. J., and G. Dreyfuss. 2000. Specific sequences of the Sm and Sm-like (Lsm) proteins mediate their interaction with the spinal muscular atrophy disease gene product (SMN). J. Biol. Chem. 275:26370-26375. [DOI] [PubMed] [Google Scholar]
- 10.Friesen, W. J., S. Massenet, S. Paushkin, A. Wyce, and G. Dreyfuss. 2001. SMN, the product of the spinal muscular atrophy gene, binds preferentially to dimethylarginine-containing protein targets. Mol. Cell 7:1111-1117. [DOI] [PubMed] [Google Scholar]
- 11.Friesen, W. J., S. Paushkin, A. Wyce, S. Massenet, G. S. Pesiridis, G. Van Duyne, J. Rappsilber, M. Mann, and G. Dreyfuss. 2001. The methylosome, a 20S complex containing JBP1 and pICln, produces dimethylarginine-modified Sm proteins. Mol. Cell. Biol. 21:8289-8300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friesen, W. J., A. Wyce, S. Paushkin, L. Abel, J. Rappsilber, M. Mann, and G. Dreyfuss. 2002. A novel WD repeat protein component of the methylosome binds Sm proteins. J. Biol. Chem. 277:8243-8247. [DOI] [PubMed] [Google Scholar]
- 13.Gubitz, A. K., Z. Mourelatos, L. Abel, J. Rappsilber, M. Mann, and G. Dreyfuss. 2002. Gemin5, a novel WD repeat protein component of the SMN complex that binds Sm proteins. J. Biol. Chem. 277:5631-5636. [DOI] [PubMed] [Google Scholar]
- 14.Hall, S. L., and R. A. Padgett. 1996. Requirement of U12 snRNA for in vivo splicing of a minor class of eukaryotic nuclear pre-mRNA introns. Science 271:1716-1718. [DOI] [PubMed] [Google Scholar]
- 15.Hamm, J., E. Darzynkiewicz, S. M. Tahara, and I. W. Mattaj. 1990. The trimethylguanosine cap structure of U1 snRNA is a component of a bipartite nuclear targeting signal. Cell 62:569-577. [DOI] [PubMed] [Google Scholar]
- 16.Jarmolowski, A., W. C. Boelens, E. Izaurralde, and I. W. Mattaj. 1994. Nuclear export of different classes of RNA is mediated by specific factors. J. Cell Biol. 124:627-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarmolowski, A., and I. W. Mattaj. 1993. The determinants for Sm protein binding to Xenopus U1 and U5 snRNAs are complex and non-identical. EMBO J. 12:223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones, K. W., K. Gorzynski, C. M. Hales, U. Fischer, F. Badbanchi, R. M. Terns, and M. P. Terns. 2001. Direct interaction of the spinal muscular atrophy disease protein SMN with the small nucleolar RNA-associated protein fibrillarin. J. Biol. Chem. 276:38645-38651. [DOI] [PubMed] [Google Scholar]
- 19.Kambach, C., S. Walke, R. Young, J. M. Avis, E. de la Fortelle, V. A. Raker, R. Luhrmann, J. Li, and K. Nagai. 1999. Crystal structures of two Sm protein complexes and their implications for the assembly of the spliceosomal snRNPs. Cell 96:375-387. [DOI] [PubMed] [Google Scholar]
- 20.Lefebvre, S., L. Burglen, S. Reboullet, O. Clermont, P. Burlet, L. Viollet, B. Benichou, C. Cruaud, P. Millasseau, M. Zeviani, et al. 1995. Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80:155-165. [DOI] [PubMed] [Google Scholar]
- 21.Liu, Q., and G. Dreyfuss. 1996. A novel nuclear structure containing the survival of motor neurons protein. EMBO J. 15:3555-3565. [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, Q., U. Fischer, F. Wang, and G. Dreyfuss. 1997. The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 90:1013-1021. [DOI] [PubMed] [Google Scholar]
- 23.Liu, Q., H. Siomi, M. C. Siomi, U. Fischer, Y. Zhang, L. Wan, and G. Dreyfuss. 1996. Molecular characterization of the protein products of the fragile X syndrome gene and the survival of motor neurons gene. Cold Spring Harbor Symp. Quant. Biol. 61:689-697. [PubMed] [Google Scholar]
- 24.Lorson, C. L., and E. J. Androphy. 1998. The domain encoded by exon 2 of the survival motor neuron protein mediates nucleic acid binding. Hum. Mol. Genet. 7:1269-1275. [DOI] [PubMed] [Google Scholar]
- 25.Luhrmann, R. 1990. Functions of U-snRNPs. Mol. Biol. Rep. 14:183-192. [DOI] [PubMed] [Google Scholar]
- 26.Luhrmann, R., B. Kastner, and M. Bach. 1990. Structure of spliceosomal snRNPs and their role in pre-mRNA splicing. Biochim. Biophys. Acta 1087:265-292. [DOI] [PubMed] [Google Scholar]
- 27.Mattaj, I. W. 1986. Cap trimethylation of U snRNA is cytoplasmic and dependent on U snRNP protein binding. Cell 46:905-911. [DOI] [PubMed] [Google Scholar]
- 28.Mattaj, I. W. 1998. Ribonucleoprotein assembly: clues from spinal muscular atrophy. Curr. Biol. 8:R93-R95. [DOI] [PubMed] [Google Scholar]
- 29.Mattaj, I. W., W. Boelens, E. Izaurralde, A. Jarmolowski, and C. Kambach. 1993. Nucleocytoplasmic transport and snRNP assembly. Mol. Biol. Rep. 18:79-83. [DOI] [PubMed] [Google Scholar]
- 30.Meister, G., D. Buhler, B. Laggerbauer, M. Zobawa, F. Lottspeich, and U. Fischer. 2000. Characterization of a nuclear 20S complex containing the survival of motor neurons (SMN) protein and a specific subset of spliceosomal Sm proteins. Hum. Mol. Genet. 9:1977-1986. [DOI] [PubMed] [Google Scholar]
- 31.Meister, G., D. Buhler, R. Pillai, F. Lottspeich, and U. Fischer. 2001. A multiprotein complex mediates the ATP-dependent assembly of spliceosomal U snRNPs. Nat. Cell Biol. 3:945-949. [DOI] [PubMed] [Google Scholar]
- 32.Meister, G., C. Eggert, D. Buhler, H. Brahms, C. Kambach, and U. Fischer. 2001. Methylation of Sm proteins by a complex containing PRMT5 and the putative U snRNP assembly factor pICln. Curr. Biol. 11:1990-1994. [DOI] [PubMed] [Google Scholar]
- 33.Meister, G., and U. Fischer. 2002. Assisted RNP assembly: SMN and PRMT5 complexes cooperate in the formation of spliceosomal UsnRNPs. EMBO J. 21:5853-5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melki, J. 1997. Spinal muscular atrophy. Curr. Opin. Neurol. 10:381-385. [DOI] [PubMed] [Google Scholar]
- 35.Montzka, K., and J. A. Steitz. 1988. Additional low-abundance human small nuclear ribonucleoproteins: U11, U12, etc. Proc. Natl. Acad. Sci. USA 85:8885-8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mourelatos, Z., L. Abel, J. Yong, N. Kataoka, and G. Dreyfuss. 2001. SMN interacts with a novel family of hnRNP and spliceosomal proteins. EMBO J. 20:5443-5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mourelatos, Z., J. Dostie, S. Paushkin, A. Sharma, B. Charroux, L. Abel, J. Rappsilber, M. Mann, and G. Dreyfuss. 2002. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev. 16:720-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pellizzoni, L., J. Baccon, B. Charroux, and G. Dreyfuss. 2001. The survival of motor neurons (SMN) protein interacts with the snoRNP proteins fibrillarin and GAR1. Curr. Biol. 11:1079-1088. [DOI] [PubMed] [Google Scholar]
- 39.Pellizzoni, L., J. Baccon, J. Rappsilber, M. Mann, and G. Dreyfuss. 2002. Purification of native survival of motor neurons complexes and identification of Gemin6 as a novel component. J. Biol. Chem. 277:7540-7545. [DOI] [PubMed] [Google Scholar]
- 40.Pellizzoni, L., B. Charroux, and G. Dreyfuss. 1999. SMN mutants of spinal muscular atrophy patients are defective in binding to snRNP proteins. Proc. Natl. Acad. Sci. USA 96:11167-11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pellizzoni, L., B. Charroux, J. Rappsilber, M. Mann, and G. Dreyfuss. 2001. A functional interaction between the survival motor neuron complex and RNA polymerase II. J. Cell Biol. 152:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pellizzoni, L., N. Kataoka, B. Charroux, and G. Dreyfuss. 1998. A novel function for SMN, the spinal muscular atrophy disease gene product, in premRNA splicing. Cell 95:615-624. [DOI] [PubMed] [Google Scholar]
- 43.Pellizzoni, L., J. Yong, and G. Dreyfuss. 2002. Essential role for the SMN complex in the specificity of snRNP assembly. Science 298:1775-1779. [DOI] [PubMed] [Google Scholar]
- 44.Pillai, R. S., M. Grimmler, G. Meister, C. L. Will, R. Luhrmann, U. Fischer, and D. Schumperli. 2003. Unique Sm core structure of U7 snRNPs: assembly by a specialized SMN complex and the role of a new component, Lsm11, in histone RNA processing. Genes Dev. 17:2321-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plessel, G., U. Fischer, and R. Lührmann. 1994. m3G cap hypermethylation of U1 small nuclear ribonucleoprotein (snRNP) in vitro: evidence that the U1 small nuclear RNA-(guanosine-N2)-methyltransferase is a non-snRNP cytoplasmic protein that requires a binding site on the Sm core domain. Mol. Cell. Biol. 14:4160-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pu, W. T., G. B. Krapivinsky, L. Krapivinsky, and D. E. Clapham. 1999. pICln inhibits snRNP biogenesis by binding core spliceosomal proteins. Mol. Cell. Biol. 19:4113-4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raker, V. A., K. Hartmuth, B. Kastner, and R. Lührmann. 1999. Spliceosomal U snRNP core assembly: Sm proteins assemble onto an Sm site RNA nonanucleotide in a specific and thermodynamically stable manner. Mol. Cell. Biol. 19:6554-6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stark, H., P. Dube, R. Luhrmann, and B. Kastner. 2001. Arrangement of RNA and proteins in the spliceosomal U1 small nuclear ribonucleoprotein particle. Nature 409:539-542. [DOI] [PubMed] [Google Scholar]
- 49.Tarn, W.-Y., and J. A. Steitz. 1996. A novel spliceosome containing U11, U12, and U5 snRNPs excises a minor class (AT-AC) intron in vitro. Cell 84:801-811. [DOI] [PubMed] [Google Scholar]
- 50.Tarn, W.-Y., and J. A. Steitz. 1996. Highly diverged U4 and U6 small nuclear RNAs required for splicing rare AT-AC introns. Science 273:1824-1832. [DOI] [PubMed] [Google Scholar]
- 51.Will, C. L., and R. Luhrmann. 2001. Spliceosomal UsnRNP biogenesis, structure and function. Curr. Opin. Cell Biol. 13:290-301. [DOI] [PubMed] [Google Scholar]
- 52.Yong, J., L. Pellizzoni, and G. Dreyfuss. 2002. Sequence-specific interaction of U1 snRNA with the SMN complex. EMBO J. 21:1188-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang, A., K. M. Wassarman, J. Ortega, A. C. Steven, and G. Storz. 2002. The Sm-like Hfq protein increases OxyS RNA interaction with target mRNAs. Mol. Cell 9:11-22. [DOI] [PubMed] [Google Scholar]