SUMMARY
Activating the ERK pathway (extracellular signal-regulated kinase pathway) has proven beneficial in several models of Huntington’s disease, and drugs that are protective in HD models have recently been found to activate ERK. Thus, the ERK cascade may be a potential target for therapeutic intervention in this currently untreatable disorder. Huntington’s disease is caused by an expanded polyglutamine repeat in the huntingtin protein that actuates a diverse set of pathogenic mechanisms. In response to mutant huntingtin, ERK is activated and directs a protective transcriptional response and inhibits caspase activation. Paradoxically, Htt also interferes with several signaling events of the ERK pathway. Mutant huntingtin compromises the ERK dependent transcriptional response to corticostriatal BDNF signaling. Mutant huntingtin also hinders glutamate uptake from the synaptic cleft by down-regulating ERK dependent expression of glutamate transporters leaving cells vulnerable to excitotoxicity. Some of this cellular complexity can be capitalized on to achieve selective activation of ERK which can be protective.
Keywords: ERK, Huntington’s disease, MAP kinase, neurodegenerative disorders, polyglutamine
INTRODUCTION
Polyglutamine disorders are a group of currently untreatable, inherited neurodegenerative diseases caused by a CAG trinucleotide repeat expansion in the affected genes. The repeats translate to an elongated form of an endogenous polyglutamine (polyQ) stretch in the diseased proteins that induces aggregation and causes aberrant protein interactions. Although gene specific effects in polyglutamine disorders cannot be ruled out [1], numerous studies show that the primary cause of pathogenesis is not the loss of function of the affected proteins but the new functions gained by the expansion of the polyQ domain [2]. This theory suggests that although the proteins affected in these diseases are diverse, many of the pathogenic processes are common and therapeutic approaches that are beneficial in one disease might be successfully applied in other polyQ or protein misfolding disorders.
Huntington’s disease (HD), which is characterized most strikingly by the loss of striatal neurons, is the most studied polyglutamine disorder. The wild-type huntingtin (Htt) protein is involved in intracellular vesicular trafficking, transcriptional regulation and is required for neuronal development [3]. Mutant Htt carries an elongated polyQ domain in the N-terminus of the protein and is believed to undergo proteolytic processing that results in one or more toxic N-terminal fragments [4]. Expression of mutant Htt leads to disruption of various vital cellular functions, including mitochondrial metabolism, transcription and protein clearance [3]. Recent studies have identified the mitogen-activated protein kinase (MAPK), namely extracellular signal-regulated kinase (ERK or MAPK1) as a cellular effector that is affected by mutant Htt and is able to modulate HD pathology [5-10] and drugs that affect ERK activation levels are able to impact pathogenesis [5, 8], all implicating the MAP kinases in HD.
MAP kinases, such as c-Jun N-terminal kinases (JNK), ERK and p38, are components of intracellular signal transduction pathways that communicate extracellular signals to cellular effector molecules through a multi-step protein kinase relay. In these pathways, the extracellular signal, usually a growth factor, activates a membrane bound receptor tyrosine kinase that after autophosphorylation binds and activates a small GTPase. The activated GTPase then activates a sequential phosphorylation cascade of MAPK-kinase-kinases (MAP3K), MAPK-kinases (MAP2K) and MAP kinases [11]. The activated MAP kinases then phosphorylate downstream effector molecules, such as transcription factors [11]. MAPKs also respond to intracellular stimuli such as endoplasmic reticulum stress or oxidative stress, which suggest that they might play a crucial role in the integration of extracellular and intracellular signals both in healthy tissues and in complex diseases [12]. The ERK pathway (Fig. 1), which is activated most notably by growth factors, is composed of the Ras small GTPase, and the Raf, MEK and ERK kinases. ERK has two highly homologous isoforms (ERK1/2) that are activated by the dual-specificity kinase, MEK, through phosphorylation of specific tyrosine and threonine residues on the conserved TEY motif in its activation loop [11, 13]. ERK can be inactivated by removal of either of the two phosphate groups. Therefore, several phosphatases with different substrate specificities are able to inactivate ERK, including serine/threonine specific phosphatases, tyrosine specific phosphatases and dual specificity (tyrosine/threonine) phosphatases [13].
Figure 1.
The ERK pathway, its upstream activators and downstream targets. The signaling branches affected in Huntington’s disease are shown; not all components are indicated.
Experimental evidence accumulated in recent years suggests that activation of the ERK pathway is beneficial in Huntington’s disease [5, 8]. This positive effect can be partially attributed to its involvement in transducing Brain-derived neurotrophic factor (BDNF) and glutamate signaling, but cell autonomous effects that do not involve these signaling pathways also seem to contribute significantly.
ERK MODULATES BDNF SIGNALING
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin protein family expressed in the cortex and the hippocampus, is implicated in several neuropsychiatric disorders [14]. BDNF signaling is transmitted through two distinct receptor molecules, the low-affinity p75 neurotrophin (p75NTR) receptors, which activate pro-apoptotic mechanisms upon stress conditions [15], and the high-affinity tropomyosin receptor kinase B (TrkB), which promotes neuronal survival and differentiation by activating phosphoinositol-3 kinase (PI3K), phospholipase C-gamma (PLCγ) and the Ras/ERK pathway (Fig. 1) [16-17]. Both temporal and chronic application of BDNF results in the activation of the ERK pathway in primary neurons [18], which had been found necessary for protection against oxidative stress [6] and for BDNF induced transcriptional response in striatal neurons [19]. Mutant huntingtin interferes with BDNF signaling at several levels, both upstream and downstream of BDNF.
BDNF expression is suppressed by mutant Htt
Normal huntingtin has a positive impact on the transcriptional control [20] and intracellular trafficking of BDNF [21]. In contrast, mutant Htt has a detrimental effect on BDNF production and signal transmission [6, 20, 22-24]. The BDNF gene is transcribed in both the cortex and in the striatum of mice [20] and its protein level is decreased in these brain regions in transgenic HD mouse models [20, 23] and HD patient samples [20, 22, 24]. Thus, two mechanisms could contribute to reduced BDNF in the striatum, namely, reduced supply through corticostriatal afferents and/or decreased expression in the striatum itself. However, the finding that BDNF mRNA levels were not affected in the striatum by either wild-type or mutant Htt in a transgenic mouse model [20] suggests that a decreased supply of cortical BDNF might be primarily responsible for the diminished striatal BDNF protein levels. Notably, both the mRNA and protein levels of BDNF are decreased in the cortex of HD patients [20, 24]. Meanwhile, in the caudate nucleus of the striatum, the expression level of full-length TrkB is reduced and that of p75NTR is elevated [24], suggesting that, in addition to the decreased cortical level of BDNF, dysregulated expression of its receptors may also impact corticostriatal BDNF signal transmission. In accordance with its supposed role in HD pathogenesis, lowered BDNF levels exacerbated cognitive impairment in transgenic HD mice [25], while overexpression of BDNF in the forebrain reduced neuronal loss in the striatum and ameliorated motor symptoms in mouse models [26-27]. Thus, BDNF activity is reduced in HD and its restoration is protective.
Mutant Htt inhibits signaling events downstream of the TrkB receptor
The response to BDNF signaling is complex with multiple branches of intracellular signal transduction pathways affected and with different cell types or cell stages having different transduction pathways at their disposal which likely account for the fact that responses to perturbation are often tissue specific. In a recent article, Ginés et al. reported that BDNF dependent activation of the MEK/ERK pathway was significantly decreased in striatal STHdhQ111 cells, while the activation of other TrkB targets, Akt and PLCγ was unaffected [6]. The activation of the TrkB receptor was not seriously affected by BDNF treatment, however, the level of the p52/p46 Shc docking protein, which binds to phosphorylated TrkB and participates in Ras activation, was decreased, suggesting that Htt insult impacts events downstream of the TrkB receptor by selectively inhibiting Ras activation which may be responsible for the selective reduction of ERK phosphorylation (Figs. 1; 2). Accordingly, while Ras-GTP is normally detected in BDNF treated cells, no Ras-GTP could be detected in striatal STHdhQ111 cells upon BDNF treatment, and transfection of the cells with constitutively active Ras restored ERK activation [6] further underscoring the selective effect of Htt insult on the ERK pathway in striatal cells.
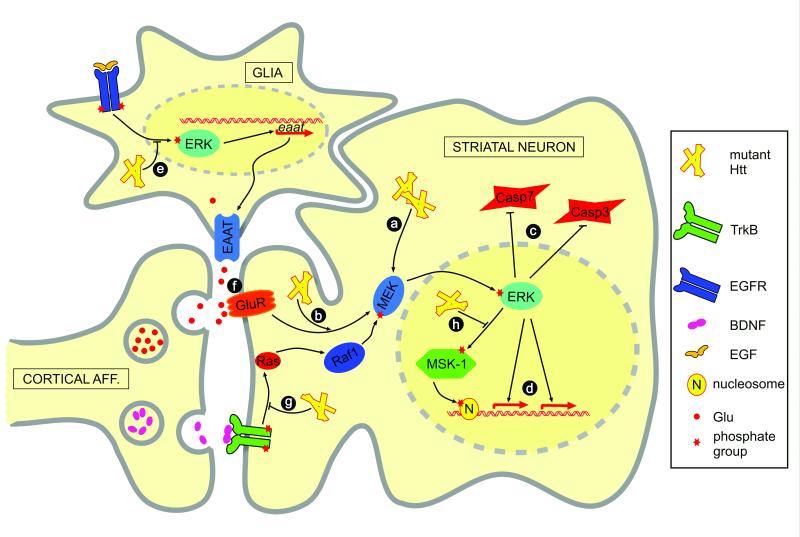
Figure 2.
The ERK pathway is implicated in Htt pathogenesis in a complex manner. Mutant Htt may upregulate the ERK pathway either cell autonomously (a) or by enhancing the activating effect of glutamate signaling (b). Activated ERK contributes to cell survival by inhibiting caspase activation (c) and directing a transcriptional response (d). In glial cells, mutant Htt downregulates EGF and ERK dependent expression of EAAT glutamate transporters (e), which might lead to increased glutamate signaling in neuronal cells and eventual potential excitotoxicity (f). The protective transcriptional effects of corticostriatal BDNF signaling are mediated by the ERK pathway. Mutant Htt compromises the BDNF response both upstream and downstream of ERK, by inhibiting the activation of the Ras GTPase (g) or the MSK-1 kinase (h) that phosphorylates H3S10 in the chromatin and contributes to c-fos expression, respectively. Thus, the response to Htt challenge is mixed and involves both activation and dampening of different up- or downstream branches of the ERK cascade. Pharmacologic intervention with compounds that can selectively activate or enhance desirable responses while avoiding the non-desirable ones would provide attractive agents for treatment.
In another example, a study performed on the hippocampi of 5 week old transgenic R6/1 mice, which already have decreased levels of BDNF protein and mRNA compared to wild-type mice [25] could not detect changes in the level of p-ERK, although, the abundance of phosphorylated PLCγ, another transduction response to the activation of the TrkB receptor by BDNF (Fig. 1.), was significantly reduced in these tissues with respect to wild-type. Presumably, the different response of striatal and hippocampal cells reflects different constellations of signaling receptors and transducers. The basis for the different responses might lie in the diverse cellular history as a particular tissue responds to the various inputs it receives to mobilize or extinguish certain transduction components.
ERK AND GLUTAMATE SIGNALING ARE MUTUALLY MISREGULATED IN HUNTINGTON’S DISEASE
L-glutamate is a major excitatory neurotransmitter that activates two main types of glutamate receptors on the postsynaptic surface: the ionotropic receptors, which form ion channels, and the G-protein coupled metabotropic receptors that activate ion channels indirectly [28]. Released glutamate is cleared form the synaptic cleft by glutamate transporters, also known as excitatory amino acid transporters (EAAT). Failing to remove excess glutamate leads to excitotoxicity, i.e. cell damage and death by overstimulation of glutamate receptors [29]. Excitotoxicity is implicated in several neurodegenerative disorders, including HD [30].
Data from different model systems indicate that ERK is involved in excitotoxicity in HD on at least two levels: first, at the intercellular level, where ERK dependent expression of glutamate transporters (EAATs) is decreased by mutant Htt leading to increased glutamate signaling that can affect neighboring cells [31]; second, at the intracellular level, where the presence of mutant Htt seems to disturb glutamate induced ERK signaling in a complex manner by acting both upstream of ERK to enhance its activation [7, 9] and by acting downstream of ERK to repress the ability of p-ERK to activate the MSK-1 kinase necessary for the activation of genes such as c-fos [10] (Fig. 2).
Reduced expression of glial glutamate transporters by mutant Htt may lead to neuronal excitotoxicity
dEAAT1 is the only high-affinity glutamate transporter in Drosophila [32-33] and it is expressed in glial cells of the CNS [34] in an EGFR/Ras/ERK dependent manner [31]. Expression of mutant Htt in dEAAT1 producing glial cells resulted in early adult lethality without significant loss of glial cells suggesting that the reduced lifespan was due to disturbed glial function not cell death [31]. EGFR-mediated phosphorylation of ERK was strongly inhibited by expressing mutant Htt or a Q48 protein in glia, which led to a progressive decrease in dEAAT1 transcription (Fig. 2.). Mutant Htt also abolished dEAAT1 upregulation by a constitutively active EGFR, while active Ras and ERK could still increase dEAAT1 expression [31]. These results show that, like the inhibition downstream of TrkB signaling above [6], expanded polyQ proteins in Drosophila glia disrupt the EGFR signaling pathway upstream of Ras activation, and imply the involvement of mutant Htt in excitotoxicity induced non-cell-autonomous neuronal dysfunction [31]. In its simplest form, mutant Htt can block the production of EAATs by preventing EGFR activation of ERK in glia, thus leading to buildup of synaptic glutamate and toxicity to nearby neurons (Fig 2).
Mutant Htt enhances glutamate induced activation of ERK but inhibits downstream signaling events
In addition to the non-cell-autonomous events discussed above, ERK also modulates excitotoxicity in a cell-autonomous manner. The ERK pathway is induced by glutamate through both metabotropic and NMDA type ionotropic receptors [35-36]. In vitro and in vivo data show that mutant Htt enhances the glutamate-induced phosphorylation of ERK (Fig. 2.). Activation of ERK by stimulation of the metabotropic mGluR5 receptor was enhanced by the expression of mutant Htt-120Q in HEK293 cells [7], and a similar effect was observed in primary striatal cells from Hdh-Q111 mice [9].
Glutamate signaling affects several ERK dependent processes in the nucleus, including phosphorylation of serine 10 on histone H3 (H3S10) [37], activation of transcription factors and the transcription of ERK target genes [11]. Roze et al. found that in the striatum of symptomatic transgenic R6/2 mice the number of p-ERK immunoreactive cells was significantly increased compared to wild-type controls, and phosphorylated forms of the ERK activated transcription factors Elk-1 (Ets like gene 1) and CREB (cAMP response element binding) were also more abundant [10]. Elk-1 and CREB activate the transcription of the c-fos gene in a glutamate and p-ERK dependent manner [38]. Underscoring the need to look at the system as a whole, an unexpected observation was that in the striatum of R6/2 mice, the transcriptional activity of c-fos was down-regulated, and glutamate induced phosphorylation of H3S10 was not detected [10]. The explanation for this apparent paradox was found in the observation that mutant Htt also led to the down-regulation of mitogen- and stress-activated kinase-1 (MSK-1) [10], which is an H3S10 kinase downstream of ERK [39] (Fig. 2). MSK-1 overexpression restored both glutamate-induced H3S10 phosphorylation and c-fos transcription, and protected 103Q-Htt-transfected striatal neurons from cell death [10]. Importantly, MSK-1 expression was decreased in postmortem HD patient samples in the caudate nucleus, but not in the cerebral cortex [10].
The data presented above indicate that mutant Htt can affect events both up- and downstream of ERK activation. These processes can enhance or suppress one another, which makes predicting the final outcome more challenging. For example, blocking of EGFR signaling by mutant Htt in glia might lead to decreased glutamate transporter levels [31] and hence increased glutamate dependent activation of ERK in mutant Htt challenged neurons [7, 9]. However, the consequences of this activation could be partially dampened by mutant Htt [10] (Fig. 2). These studies strongly suggest that cell type is a major determinant of ERK dependent processes induced by mutant Htt. Furthermore, it must be taken into account that there are multiple members of the ERK protein family that can respond to the same stimuli and/or be sensitive to the same inhibitors and which may contribute differentially to the nuanced responses to cellular insult discussed here [40].
ERK IS ACTIVATED BY MUTANT HTT AND MEDIATES PRO-SURVIVAL RESPONSES
Cell autonomous activation of ERK upon Htt expression is protective
It is an interesting question, whether mutant Htt only interferes with the normal functioning of MAP kinase signaling pathways or if it also activates some of these pathways by itself. Data derived from cell culture experiments, not involving stimulation with extracellular signaling molecules, show that ERK is activated in response to expression of mutant huntingtin, and this cell autonomous activation increases cell survival. Expression of mutant Htt leads to prolonged activation of ERK in a rat pheochromocytoma (PC12) cell line, Htt14A2.5, and similar results were obtained from immortalized striatal ST14A cells [5]. Mutant Htt provokes upregulation of several transcriptional targets of the ERK, JNK and p38 kinases in PC12 cells [5], suggesting that accumulation of mutant Htt induces the MAP kinase dependent activation of various stress response and pro-survival mechanisms. One of the protective mechanisms that ERK promotes is inhibition of apoptotic caspases. Caspase-3 and 7 are both implicated in promoting apoptotic functions [41] and are activated in several cell culture models of HD [5, 42]. ERK signaling seems to dampen this process, as suppression of ERK activation by a MEK inhibitor resulted in increased activation of Caspase-3, while MEK overexpression had the opposite effect [5]. Similarly, rescue of mutant Htt induced cell death by rotenone, an inhibitor of mitochondrial complex I, is partially dependent on the activity of the Akt and ERK kinases and correlated with the inhibition of Caspase-3 and 7 activation in immortalized striatal cells [42].
The ability of stressing agents such as Htt to alter the cellular response and especially to alter the response of cells to pharmacologic agents has recently been reported by Varma et al. who describe that microtubule depolymerizing agents, which are typically detrimental to normal cells, activate a series of protective events in cultured and primary neurons if they are expressing mutant Htt. This somewhat unusual rescue was linked to selective activation of ERK through the RhoA/ROCK pathway but notably only in mutant Htt expressing cells [43]. This finding highlights the possibility that mutant Htt might modulate certain activation mechanisms of ERK in a way that enables the pharmacological upregulation of ERK only in those cells that are impacted by the disease.
Late activation of ERK in HD animal models suggests a compensatory role
The ERK pathway is upregulated in several transgenic animal models of HD. Significant ERK activation could not be detected in the hippocampus [25] or in the striatum [10] of mutant Htt expressing mice before the onset of symptoms, but a robust increase in the number of p-ERK immunoreactive cells was observed in the dorsal striatum of 12-week-old R6/2 mouse [10]. The timing of ERK activation in HD mice supports the hypothesis suggested by cell culture experiments, i.e. that the ERK pathway might not be involved in a primary pathological process but rather that it is a compensatory mechanism that transduces stress responses provoked by mutant Htt. Neuronal expression of Htt also increases the level of p-ERK in transgenic Drosophila [8]. However, in glial cells of transgenic Drosophila, expression of either mutant Htt fragments or expanded polyQ proteins inhibited ERK phosphorylation indicating that, in this cell type, polyQ peptides disrupt the signaling pathway upstream of ERK [31]. These results underscore the fact that the modulation of ERK signaling upon Htt challenge is highly dependent on cell type and thus might contribute to the tissue specificity of HD pathology.
MODULATION OF ERK ACTIVITY IN HUNTINGTON’S DISEASE
Different MAP kinases have different impacts on HD pathology
The data accumulated to date imply that ERK activation is protective in Huntington’s disease. However, in addition to ERK phosphorylation, c-Jun N-terminal kinase (JNK) activation was also detected in several cell culture models of HD [5, 7], and an antagonistic relationship between ERK and JNK upon glutamate receptor stimulation has been observed in Htt expressing cells, as inhibition of either kinase led to the enhanced activation of the other [7]. Importantly, in these experiments, ERK was shown to confer protection against mGluR5 mediated excitotoxicity, while JNK had opposite effect [7]. These findings were further supported by genetic interaction data from fruit flies. A recent study by Maher et al. described phenotypic responses elicited by altered levels of Drosophila JNK (dJNK/basket) or ERK (dERK/rolled) in mutant Htt expressing flies that clearly demonstrate the antagonistic role of these proteins in HD pathogenesis [8]. Flies heterozygous for a loss of function allele of dJNK had better viability and less severe neurodegeneration than control siblings with two normal dJNK alleles. Reducing the level of dERK by a loss of function mutation did not have a phenotypic effect. However, heterozygosity of Dsor1, encoding the Drosophila MEK kinase, enhanced the Htt induced lethality. In contrast, mutations in the ERK deactivating genes mts (microtubule star)[44] or PTP-ER (Protein Tyrosine Phosphatase-ERK/Enhancer of Ras1)[45], which encode serine/threonine specific and tyrosine specific phosphatases, respectively, led to increased viability and neuronal survival in Htt challenged flies [8]. Further, pERK was found to be hyperactivated by polyphenolic compounds (e.g. resveratrol; fisetin) that have been shown to be protective in fly, cell and rodent models of HD [8, 46]. These findings raise the possibility that treatments leading to increased levels of phospho-ERK may ameliorate HD, but also underscore the importance of finding drugs that are selective for ERK and do not activate other MAP kinases.
The polyphenols fisetin and resveratrol ameliorate HD phenotypes and promote ERK activation
ERK activation can be accomplished by either selective activation of the Raf/MEK/ERK pathway or by selective inhibition of ERK phosphatases. Finding suitable small molecule candidates to activate ERK is complicated by the fact that overactivation of the ERK pathway can lead to tumor transformation [12]. Therefore, it is especially significant, that fisetin and resveratrol, two plant polyphenols that exert their neurological effect, at least partially, by upregulating the ERK pathway [8, 46], also provide protection against cancer [47, 48]. Fisetin increased both ERK phosphorylation and cell survival in mutant Htt expressing PC12 cells, while it led to decreased activation of JNK and Caspase-3 [8]. Both the activation of ERK and the suppression of Htt induced cell death were abolished by inhibition of the MEK kinase, suggesting that activation of the ERK kinase pathway plays a central role in the rescue. In Htt expressing flies, treatment with either fisetin or resveratrol led to increased p-ERK levels, while JNK phosphorylation was unaffected and similar observations were made with fisetin in mammalian cells [8]. Further, flies challenged by expression of mutant huntingtin exhibited significantly more ERK activation than unchallenged control flies consistent with the suggestion that cellular responses can be affected by the disease process itself [8, 43]. Also, consistent with this, both compounds have been described as inhibitors of ERK activation in other systems [49-51], suggesting that their effect on ERK phosphorylation is likely indirect and further underscoring the importance of cellular context for any of these responses. Concurrently with its effects on pERK, fisetin also ameliorated the Htt induced phenotypes of transgenic animals: it reduced neurodegeneration in flies and enhanced the rotarod performance of transgenic R6/2 mice, and also increased the median lifespan of both flies and mice challenged with mutant Htt expression [8]. The efficacy of these polyphenols in ameliorating pathology in multiple models of HD coupled with the observation that they appear to selectively target cellular events that uniquely lead to increased activation of ERK while not triggering competing events such as JNK activation raises the possibility that a class of drugable compounds with the properties of these polyphenols could be particularly promising for therapeutic intervention in neurodegenerative and possibly other diseases where ERK activation is beneficial.
Resveratrol is claimed to provide several favorable health benefits, including protection against cardiovascular disease [52], cancer [47] and neurodegenerative disorders [53, 54]. The surprisingly diverse therapeutic effects might be explained by its pleiotropic action on molecular targets. Its reported ability to delay aging and activate sirtuins has been controversial and perhaps deflected attention from its other potential benefits [55-57, 63]. However, studies have found that the neuroprotective effect of resveratrol is independent of Sir2 in flies [53], and that the apparent activation of sirtuins by resveratrol is specific for the artificial fluorescent substrate commonly used in sirtuin enzyme assays [58-63]. Nevertheless, resveratrol and other polyphenols are beneficial in HD models suggesting that at least some of the benefits of resveratrol and other plant polyphenols like fisetin can be attributed to mechanisms not involving sirtuin activation - ERK activation being one of them [8, 46].
CONCLUSIONS
Mutant huntingtin leads to neurodegeneration via its pleiotropic effects on several cellular functions. Recent studies demonstrated that mutant huntingtin activates ERK, and impacts intercellular BDNF and glutamate signaling events that are transmitted through the ERK kinase pathway. Thus, ERK might serve as an integration point of these signaling events.
The effects of mutant Htt on BDNF and glutamate signaling are complex. Mutant Htt down-regulates BDNF production and perturbs its signal transmission by dysregulating the level of BDNF receptors and inhibiting the activation of the Ras/ERK pathway. In glutamate signaling, mutant Htt inhibits ERK dependent expression of glutamate transporters in glial cells, which might lead to an increase in glutamate activation of ERK in neurons. However, this effect can be partially dampened by inhibition of MSK-1 and consequent reduction of ERK dependent transcription, which might lead to imbalanced activation of ERK dependent processes.
Pharmacologic intervention that promotes ERK activation could suppress the adverse effects of mutant Htt by activating pro-survival mechanisms (including some of which naturally decline with age) and suppressing apoptotic responses. As suggested in Figure 3, ERK does stand at the crossroads of signaling events especially in relation to the cellular response to challenges such as mutant Htt. Given the multiple points of impact of pathogenic Htt and the multiple points of regulation and output of a target such as ERK, it can seem almost bewilderingly complex to try and effectively target ERK as a therapeutic strategy. However, it may be possible to capitalize on these complexities. For example, some polyphenols show modest if any activation of ERK in normal cells but the levels of activation are significantly increased in cells challenged by mutant Htt. Seeking additional agents that capitalize on the unique cellular environment associated with a particular disease state may lead to productive and specific therapeutic strategies.
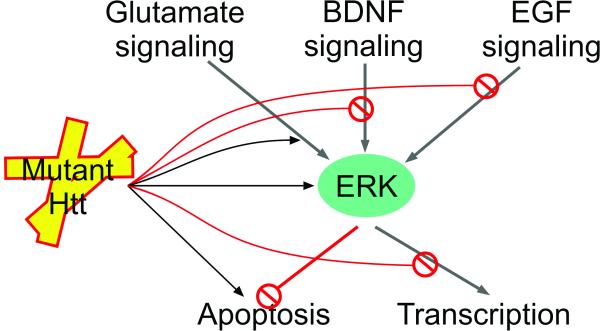
Figure 3.
Htt affects ERK signaling in multiple ways. The ERK cascade potentiates pro-survival mechanisms, i.e. inhibition of apoptosis and transcriptional activation of pro-survival genes, in response to various stimuli. Mutant huntingtin activates the ERK pathway in a cell-autonomous manner but also interferes with both upstream and downstream signaling events. The points at which mutant Htt affects ERK signaling both positively and negatively are indicated.
ACKNOWLEDGEMENTS
This work was supported in part by the National Institutes of Health NS045283 to J.L.M.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- ERK
extracellular signal-regulated kinase
- HD
Huntington’s disease
REFERENCES
- 1.Robertson AL, Bottomley SP. Towards the treatment of polyglutamine diseases: the modulatory role of protein context. Curr Med Chem. 2010;17:3058–68. doi: 10.2174/092986710791959800. [DOI] [PubMed] [Google Scholar]
- 2.Rubinsztein DC. Lessons from animal models of Huntington’s disease. Trends Genet. 2002;18:202–9. doi: 10.1016/s0168-9525(01)02625-7. [DOI] [PubMed] [Google Scholar]
- 3.Ross CA, Tabrizi SJ. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 4.Sawa A, Nagata E, Sutcliffe S, Dulloor P, et al. Huntingtin is cleaved by caspases in the cytoplasm and translocated to the nucleus via perinuclear sites in Huntington’s disease patient lymphoblasts. Neurobiol Dis. 2005;20:267–74. doi: 10.1016/j.nbd.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Apostol BL, Illes K, Pallos J, Bodai L, et al. Mutant huntingtin alters MAPK signaling pathways in PC12 and striatal cells: ERK1/2 protects against mutant huntingtin-associated toxicity. Hum Mol Genet. 2006;15:273–85. doi: 10.1093/hmg/ddi443. [DOI] [PubMed] [Google Scholar]
- 6.Gines S, Paoletti P, Alberch J. Impaired TrkB-mediated ERK1/2 activation in huntington disease knock-in striatal cells involves reduced p52/p46 Shc expression. J Biol Chem. 2010;285:21537–48. doi: 10.1074/jbc.M109.084202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang SS, He J, Zhao DM, Xu XY, et al. Effects of mutant huntingtin on mGluR5-mediated dual signaling pathways: implications for therapeutic interventions. Cell Mol Neurobiol. 2010;30:1107–15. doi: 10.1007/s10571-010-9543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maher P, Dargusch R, Bodai L, Gerard PE, et al. ERK activation by the polyphenols fisetin and resveratrol provides neuroprotection in multiple models of Huntington’s disease. Hum Mol Genet. 2011;20:261–70. doi: 10.1093/hmg/ddq460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ribeiro FM, Paquet M, Ferreira LT, Cregan T, et al. Metabotropic glutamate receptor-mediated cell signaling pathways are altered in a mouse model of Huntington’s disease. J Neurosci. 2010;30:316–24. doi: 10.1523/JNEUROSCI.4974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roze E, Betuing S, Deyts C, Marcon E, et al. Mitogen- and stress-activated protein kinase-1 deficiency is involved in expanded-huntingtin-induced transcriptional dysregulation and striatal death. FASEB J. 2008;22:1083–93. doi: 10.1096/fj.07-9814. [DOI] [PubMed] [Google Scholar]
- 11.Krishna M, Narang H. The complexity of mitogen-activated protein kinases (MAPKs) made simple. Cell Mol Life Sci. 2008;65:3525–44. doi: 10.1007/s00018-008-8170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim EK, Choi EJ. Pathological roles of MAPK signaling pathways in human diseases. Biochim Biophys Acta. 2010;1802:396–405. doi: 10.1016/j.bbadis.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Pouyssegur J, Volmat V, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem Pharmacol. 2002;64:755–63. doi: 10.1016/s0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- 14.Chao MV, Rajagopal R, Lee FS. Neurotrophin signalling in health and disease. Clin Sci (Lond) 2006;110:167–73. doi: 10.1042/CS20050163. [DOI] [PubMed] [Google Scholar]
- 15.Kenchappa RS, Tep C, Korade Z, Urra S, et al. p75 neurotrophin receptor-mediated apoptosis in sympathetic neurons involves a biphasic activation of JNK and up-regulation of tumor necrosis factor-alpha-converting enzyme/ADAM17. J Biol Chem. 2010;285:20358–68. doi: 10.1074/jbc.M109.082834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugimoto T, Kuroda H, Horii Y, Moritake H, et al. Signal transduction pathways through TRK-A and TRK-B receptors in human neuroblastoma cells. Jpn J Cancer Res. 2001;92:152–60. doi: 10.1111/j.1349-7006.2001.tb01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuen EC, Mobley WC. Early BDNF, NT-3, and NT-4 signaling events. Exp Neurol. 1999;159:297–308. doi: 10.1006/exnr.1999.7148. [DOI] [PubMed] [Google Scholar]
- 18.Rohe M, Synowitz M, Glass R, Paul SM, et al. Brain-derived neurotrophic factor reduces amyloidogenic processing through control of SORLA gene expression. J Neurosci. 2009;29:15472–8. doi: 10.1523/JNEUROSCI.3960-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gokce O, Runne H, Kuhn A, Luthi-Carter R. Short-term striatal gene expression responses to brain-derived neurotrophic factor are dependent on MEK and ERK activation. PLoS One. 2009;4:e5292. doi: 10.1371/journal.pone.0005292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293:493–8. doi: 10.1126/science.1059581. [DOI] [PubMed] [Google Scholar]
- 21.Gauthier LR, Charrin BC, Borrell-Pages M, Dompierre JP, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell. 2004;118:127–38. doi: 10.1016/j.cell.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Ferrer I, Goutan E, Marin C, Rey MJ, et al. Brain-derived neurotrophic factor in Huntington disease. Brain Res. 2000;866:257–61. doi: 10.1016/s0006-8993(00)02237-x. [DOI] [PubMed] [Google Scholar]
- 23.Gines S, Seong IS, Fossale E, Ivanova E, et al. Specific progressive cAMP reduction implicates energy deficit in presymptomatic Huntington’s disease knock-in mice. Hum Mol Genet. 2003;12:497–508. doi: 10.1093/hmg/ddg046. [DOI] [PubMed] [Google Scholar]
- 24.Zuccato C, Marullo M, Conforti P, MacDonald ME, et al. Systematic assessment of BDNF and its receptor levels in human cortices affected by Huntington’s disease. Brain Pathol. 2008;18:225–38. doi: 10.1111/j.1750-3639.2007.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giralt A, Rodrigo T, Martin ED, Gonzalez JR, et al. Brain-derived neurotrophic factor modulates the severity of cognitive alterations induced by mutant huntingtin: involvement of phospholipaseCgamma activity and glutamate receptor expression. Neuroscience. 2009;158:1234–50. doi: 10.1016/j.neuroscience.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 26.Gharami K, Xie Y, An JJ, Tonegawa S, et al. Brain-derived neurotrophic factor over-expression in the forebrain ameliorates Huntington’s disease phenotypes in mice. J Neurochem. 2008;105:369–79. doi: 10.1111/j.1471-4159.2007.05137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie Y, Hayden MR, Xu B. BDNF overexpression in the forebrain rescues Huntington’s disease phenotypes in YAC128 mice. J Neurosci. 2010;30:14708–18. doi: 10.1523/JNEUROSCI.1637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Platt SR. The role of glutamate in central nervous system health and disease--a review. Vet J. 2007;173:278–86. doi: 10.1016/j.tvjl.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 29.O’Shea RD. Roles and regulation of glutamate transporters in the central nervous system. Clin Exp Pharmacol Physiol. 2002;29:1018–23. doi: 10.1046/j.1440-1681.2002.03770.x. [DOI] [PubMed] [Google Scholar]
- 30.Dong XX, Wang Y, Qin ZH. Molecular mechanisms of excitotoxicity and their relevance to pathogenesis of neurodegenerative diseases. Acta Pharmacol Sin. 2009;30:379–87. doi: 10.1038/aps.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lievens JC, Rival T, Iche M, Chneiweiss H, et al. Expanded polyglutamine peptides disrupt EGF receptor signaling and glutamate transporter expression in Drosophila. Hum Mol Genet. 2005;14:713–24. doi: 10.1093/hmg/ddi067. [DOI] [PubMed] [Google Scholar]
- 32.Seal RP, Daniels GM, Wolfgang WJ, Forte MA, et al. Identification and characterization of a cDNA encoding a neuronal glutamate transporter from Drosophila melanogaster. Receptors Channels. 1998;6:51–64. [PubMed] [Google Scholar]
- 33.Besson MT, Soustelle L, Birman S. Selective high-affinity transport of aspartate by a Drosophila homologue of the excitatory amino-acid transporters. Curr Biol. 2000;10:207–10. doi: 10.1016/s0960-9822(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 34.Soustelle L, Besson MT, Rival T, Birman S. Terminal glial differentiation involves regulated expression of the excitatory amino acid transporters in the Drosophila embryonic CNS. Dev Biol. 2002;248:294–306. doi: 10.1006/dbio.2002.0742. [DOI] [PubMed] [Google Scholar]
- 35.Kurino M, Fukunaga K, Ushio Y, Miyamoto E. Activation of mitogen-activated protein kinase in cultured rat hippocampal neurons by stimulation of glutamate receptors. J Neurochem. 1995;65:1282–9. doi: 10.1046/j.1471-4159.1995.65031282.x. [DOI] [PubMed] [Google Scholar]
- 36.Wang JQ, Fibuch EE, Mao L. Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. [DOI] [PubMed] [Google Scholar]
- 37.Brami-Cherrier K, Lavaur J, Pages C, Arthur JS, et al. Glutamate induces histone H3 phosphorylation but not acetylation in striatal neurons: role of mitogen- and stress-activated kinase-1. J Neurochem. 2007;101:697–708. doi: 10.1111/j.1471-4159.2006.04352.x. [DOI] [PubMed] [Google Scholar]
- 38.Vanhoutte P, Barnier JV, Guibert B, Pages C, et al. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol Cell Biol. 1999;19:136–46. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deak M, Clifton AD, Lucocq LM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–41. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimoto S, Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006;7:782–6. doi: 10.1038/sj.embor.7400755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lakhani SA, Masud A, Kuida K, Porter GA, Jr., et al. Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science. 2006;311:847–51. doi: 10.1126/science.1115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varma H, Cheng R, Voisine C, Hart AC, et al. Inhibitors of metabolism rescue cell death in Huntington’s disease models. Proc Natl Acad Sci USA. 2007;104:14525–30. doi: 10.1073/pnas.0704482104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varma H, Yamamoto A, Sarantos MR, Hughes RE, et al. Mutant huntingtin alters cell fate in response to microtubule depolymerization via the GEF-H1-RhoA-ERK pathway. J Biol Chem. 2010;285:37445–57. doi: 10.1074/jbc.M110.125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silverstein AM, Barrow CA, Davis AJ, Mumby MC. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc Natl Acad Sci USA. 2002;99:4221–6. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karim FD, Rubin GM. PTP-ER, a novel tyrosine phosphatase, functions downstream of Ras1 to downregulate MAP kinase during Drosophila eye development. Mol Cell. 1999;3:741–50. doi: 10.1016/s1097-2765(01)80006-x. [DOI] [PubMed] [Google Scholar]
- 46.Maher P, Akaishi T, Abe K. Flavonoid fisetin promotes ERK-dependent long-term potentiation and enhances memory. Proc Natl Acad Sci USA. 2006;103:16568–73. doi: 10.1073/pnas.0607822103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fulda S. Resveratrol and derivatives for the prevention and treatment of cancer. Drug Discov Today. 2010;15:757–65. doi: 10.1016/j.drudis.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Cheng Y, Qu W, Sun Y, et al. Fisetin, a dietary flavonoid, induces cell cycle arrest and apoptosis through activation of p53 and inhibition of NF-kappa B pathways in bladder cancer cells. Basic Clin Pharmacol Toxicol. 2011;108:84–93. doi: 10.1111/j.1742-7843.2010.00613.x. [DOI] [PubMed] [Google Scholar]
- 49.Kim AL, Zhu Y, Zhu H, Han L, et al. Resveratrol inhibits proliferation of human epidermoid carcinoma A431 cells by modulating MEK1 and AP-1 signalling pathways. Exp Dermatol. 2006;15:538–46. doi: 10.1111/j.1600-0625.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 50.Lee EO, Kim SE, Park HK, Kang JL, et al. Extracellular HIV-1 Tat upregulates TNF-alpha dependent MCP-1/CCL2 production via activation of ERK1/2 pathway in rat hippocampal slice cultures: inhibition by resveratrol, a polyphenolic phytostilbene. Exp Neurol. 2011;229:399–408. doi: 10.1016/j.expneurol.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Liao YC, Shih YW, Chao CH, Lee XY, et al. Involvement of the ERK signaling pathway in fisetin reduces invasion and migration in the human lung cancer cell line A549. J Agric Food Chem. 2009;57:8933–41. doi: 10.1021/jf902630w. [DOI] [PubMed] [Google Scholar]
- 52.Das M, Das DK. Resveratrol and cardiovascular health. Mol Aspects Med. 2010;31:503–12. doi: 10.1016/j.mam.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Pallos J, Bodai L, Lukacsovich T, Purcell JM, et al. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington’s disease. Hum Mol Genet. 2008;17:3767–75. doi: 10.1093/hmg/ddn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun AY, Wang Q, Simonyi A, Sun GY. Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol. 2010;41:375–83. doi: 10.1007/s12035-010-8111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–6. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 56.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, et al. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 57.Sinclair D. Sirtuins for healthy neurons. Nat Genet. 2005;37:339–40. doi: 10.1038/ng0405-339. [DOI] [PubMed] [Google Scholar]
- 58.Beher D, Wu J, Cumine S, Kim KW, et al. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des. 2009;74:619–24. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 59.Borra MT, Smith BC, Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–95. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- 60.Kaeberlein M, McDonagh T, Heltweg B, Hixon J, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–45. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- 61.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 2010;285:8340–51. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ledford H. Ageing: Much ado about ageing. Nature. 2010;464:480–1. doi: 10.1038/464480a. [DOI] [PubMed] [Google Scholar]
- 63.Burnett C, Valentini S, Cabreiro F, Goss M, et al. Absence of effects of Sir2 overexpression on lifespan in C. elegans and Drosophila. Nature. 2011;477:482–5. doi: 10.1038/nature10296. [DOI] [PMC free article] [PubMed] [Google Scholar]