Abstract
Using a single-RNA detection technique in live Escherichia coli cells, we measure, for each cell, the waiting time for the production of the first RNA under the control of PBAD promoter after induction by arabinose, and subsequent intervals between transcription events. We find that the kinetics of the arabinose intake system affect mean and diversity in RNA numbers, long after induction. We observed the same effect on Plac/ara-1 promoter, which is inducible by arabinose or by IPTG. Importantly, the distribution of waiting times of Plac/ara-1 is indistinguishable from that of PBAD, if and only if induced by arabinose alone. Finally, RNA production under the control of PBAD is found to be a sub-Poissonian process. We conclude that inducer-dependent waiting times affect mean and cell-to-cell diversity in RNA numbers long after induction, suggesting that intake mechanisms have non-negligible effects on the phenotypic diversity of cell populations in natural, fluctuating environments.
INTRODUCTION
Transcription in E. coli is, at a genome-wide scale, a relatively rare stochastic event (1–3). Further, many genes only become active in response to external stimuli (4–7), via processes that are also stochastic (7). Although much is known on the noise in gene expression at the single-cell level (1–3,7–10), most of our present knowledge concerning the kinetics of response, in terms of gene activity, to external signals concerns the average behaviour of cell populations alone (11). However, to characterize the dynamics and the underlying steps of intake processes, it is necessary to observe their effects in individual live cells (12). This observation should inform also on the robustness of cellular response mechanisms by informing on the degree of change in the responses of a single cell to multiple occurrences of the same stimulus, as well as the difference in responses to different stimuli.
One of the most well-known gene activation mechanisms is the arabinose utilization system of E. coli. This system imports arabinose into the cell by AraFGH, an arabinose-specific high-affinity ABC transporter (11,13–15), and by a low-affinity transporter, AraE, which binds to the inner membrane and makes use of electrochemical potential to intake the arabinose (11,16,17). This system exhibits wide variability in the timing of activation and in the rates of accumulation of inducer molecules (18). It has been hypothesized that this is due to the cell-to-cell variability in the numbers of proteins responsible for the intake of arabinose (18). Interestingly, if the intake gene araE is placed under the control of a constitutive promoter the intake rates become more homogenous (19–21), suggesting that the diversity in the number of intake proteins is a non-negligible source of cell-to-cell variability in the kinetics of the arabinose utilization system (12).
Evidence suggests that when the intracellular concentration of arabinose exceeds a threshold, the dimeric AraC protein activates the genes that code for the proteins responsible for the intake (AraE and AraFGH) and for the catabolism of arabinose (araBAD) (11,22). In the absence of arabinose, AraC binds two half-sites on the DNA (I1 and O2) and promotes the formation of a DNA loop that prevents access of RNA polymerases to the promoters in that region (PC and PBAD). When bound by arabinose, AraC binds instead to the adjacent I1 and I2 half-sites. The resulting configuration promotes transcription initiation at PBAD (11).
Transcription initiation is a complex, multi-stepped process (23,24). In vitro measurements suggest that this process has at least two to three rate limiting steps (25,26). It starts when the RNA polymerase binds to the promoter region of the DNA molecule, forming the closed complex, which is followed by the open complex formation and promoter escape (27,28). The RNA polymerase then elongates the nascent RNA (28). Evidence suggests that, in general, initiation is much longer in duration than elongation (26,29). Recent in vivo measurements of the kinetics of initiation of Plac/ara-1 and PtetA promoters have shown that RNA production under the control of these promoters is a sub-Poissonian process (8–10). These studies also support the existence of multiple steps at the stage of initiation, significantly limiting the rate of RNA production, as suggested by in vitro measurements (30).
Here, we investigate the degree of contribution of the process of intake of arabinose and of the process of transcription under the control of PBAD to the cell-to-cell diversity in RNA production. Namely, we report measurements of the in vivo kinetics of induction and transcript production of PBAD with single-molecule sensitivity, making use of the MS2d-GFP tagging of RNA in E. coli (31). For that, in each cell, we measure the waiting time until the first RNA is produced after induction and the subsequent intervals between consecutive transcript productions. For comparison, we conduct the same measurements for Plac/ara-1 when induced by either of its two inducers, arabinose and IPTG.
MATERIALS AND METHODS
Strains and plasmids
Escherichia coli strain DH5α-PRO was generously provided by I. Golding, University of Illinois and contains the construct PROTET-K133, carrying PLtetO-1-MS2d-GFP (31), along with a new construct, pMK-BAC (PBAD-mRFP1-MS2-96bs), which is a single-copy F-based vector carrying a sequence coding for a monomeric red fluorescent protein (mRFP1) followed by a 96 binding site array under the control of PBAD (cloning information provided in Supplementary Methods) (see also Supplementary Figures S1 and S2). The strain with plasmids PLtetO-1-MS2d-GFP and pIG-BAC (Plac/ara-1-mRFP1-MS2-96bs) (32) was used as well. The DH5α-PRO strain [identical to Z1 (31)] is a genuine producer of AraC (33). No modifications were made to the chromosome of this strain in our experiments.
Media and growth conditions
Cells were grown overnight at 30°C with aeration and shaking in Luria-Bertani (LB) medium, supplemented with antibiotics according to the plasmids. The cells were diluted in fresh M63 medium and allowed to grow until an optical density of OD600 ≈0.3–0.5. To attain full induction of the MS2d-GFP reporter, cells were pre-incubated for 40 min with 100 ng/ml anhydrotetracycline (aTc, IBA GmbH). The same protocol was used for each strain.
Microscopy
For microscopy measurements, cells were pelleted and re-suspended in ∼50 µl of fresh M63 medium. Afterwards, few microlitres of cells were placed between a 3% agarose gel pad made with medium and a glass coverslip before assembling the imaging chamber (FCS2, Bioptechs). Before the starting of the experiment, the chamber was heated to 37°C.
Cells were visualized in a Nikon Eclipse (TE2000-U, Nikon, Japan) inverted microscope with C1 confocal laser-scanning system using a ×100 Apo TIRF objective. A flow of fresh, pre-warmed M63 medium containing the inducer was provided with a peristaltic pump at a rate of 1 ml/min. Images were taken once per minute for 2 h, and the laser shutter was open only during the exposure time to minimize photobleaching. The peristaltic pump was initialized at the same time as the collection of the time series. For image acquisition, we used Nikon EZ-C1 software. GFP fluorescence was measured using a 488 nm argon ion laser (Melles-Griot), 515/30 nm emission filter and a pixel dwell time of 3.36 µs (total image acquisition time of 3.5 s).
An interacting multiple model filter-based autofocus strategy (34) was used to correct focus drift in time series acquisitions. The method estimates the focal drift using an interacting multiple model filter algorithm to predict the focal drift at time t based on the measurement at t-1. It allows reducing the number of required images at different z-planes for drift correction, thus minimizing photobleaching.
Data and image analysis
Data and images were analysed using custom software written in MATLAB 2011b (MathWorks). Cells were detected from fluorescence images by a semi-automatic method described previously (8). In time series, the area occupied by each cell was manually masked. Principal component analysis was used to obtain the dimensions and orientation of the cells within each mask. Fluorescent spots in the cells were automatically segmented using density estimation with a Gaussian kernel (35) and Otsu’s thresholding (36). Finally, background-corrected spot intensities were calculated and summed to produce the total spot intensity in each cell.
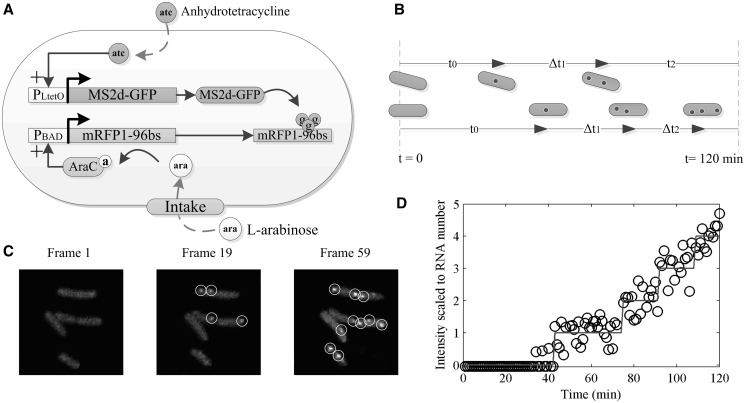
Moments of appearance of novel target RNA molecules in each cell were obtained from time-lapse fluorescence images by fitting the corrected total spots intensity over time in each cell to a monotone piecewise-constant function by least squares (37). The number of terms was selected using the F-test with a P-value of 0.01. Each jump corresponds to the production of a single RNA molecule (37). An example of this procedure is shown in Figure 1D. For more details on the image analysis see (8). Note that, in cells that do not contain target RNA molecules at the start of the measurements, the number of novel RNA molecules detected since the start of the measurements until a given moment equals the total number of RNA molecules in the cell at that moment.
Figure 1.
Measurement system. (A) Components of the detection system. The expression of the tagging protein, MS2d-GFP, is controlled by PLtetO (33) and is inducible by anhydrotetracycline (aTc). The target RNA contains an mRFP1 coding region, followed by an array of 96 MS2d-binding sites. Expression of the target RNA is controlled by PBAD whose activity is regulated by AraC and the inducer l-arabinose. The target construct is on a single-copy F-plasmid. The tagging construct is on a medium-copy vector. (B) Figurative description of the waiting time for the first RNA production (t0) and intervals between subsequent productions (Δt). Images are taken once per minute for 2 h. (C) Example of E. coli cells expressing MS2d-GFP and target RNA. GFP-tagged RNA molecules are marked by circles. (D) Time course of total intensity of spots in a cell (circles) and monotone piecewise-constant fit (line).
Because some cells already contained target RNA molecules at the start of the measurement, the total RNA numbers within cells at a given moment in time is obtained using a different method. Specifically, when comparing measurements using MS2d-GFP tagging and using plate reader (Supplementary Figure S4), the total number of MS2d-GFP–tagged RNA molecules was extracted from the total spot intensity distribution, obtained from all cells in an image obtained at a given moment after induction. For this, the first peak of the obtained distribution is set to correspond to the intensity of a single-RNA molecule. The number of tagged RNAs in each spot can be estimated by dividing its intensity by that of the first peak (32).
RESULTS
Experimental design
To study the kinetics of expression of PBAD, we detect individual RNA molecules, as these are produced in live cells and register when these events occur. For this, we placed the PBAD promoter on a single-copy F-plasmid, followed by a coding region for mRFP1 and an array of 96 binding sites for MS2d-GFP–tagging proteins (32) (Figure 1A). The expression of MS2d-GFP is controlled by PTetO, which is activated before the gene of interest so that sufficient MS2d-GFP proteins are present when target RNA molecules appear. Induction of PBAD and image acquisitions is initialized simultaneously (Figure 1B). For this, we use a temperature-controlled imaging chamber and a peristaltic pump for introducing inducers and fresh media. From the fluorescence images, using semi-automated cell segmentation and tracking (Figure 1C) (8), we measure in each cell the time for the first RNA to appear (named ‘waiting time’, t0), as well as the subsequent intervals between consecutive RNA productions, Δt, until cell division occurs or until the end of the measurement period (Figure 1D).
Given that values of t0 can only be obtained from cells of the first generation (i.e. cells already on the slide when the measurement begins), and as cells that do not divide in the first 2 h will not, in general, divide afterwards, we limited the measurement period to 2 h for simplicity. This was possible, as this period also proved to be sufficient to acquire enough samples of Δt.
From cells born during the measurement period, we only extract intervals between consecutive RNA productions, not waiting times, as these contain inducers by inheritance. We detected no difference in the distributions of intervals obtained from such cells and cells already present when induction is initiated. Finally, we observed ∼0.2 RNA molecules per cell, at the moment preceding induction, because of spurious transcription events. Cells where a target RNA was already present at the start of the measurement were also not used to obtain values of t0.
First, we compared by quantitative polymerase chain reaction the RNA production from the F-plasmid and from the native gene under the control of PBAD (Supplementary Methods). Using 16S rRNA as reference gene, we observe similar trend in activity over time in the native promoter and in the one on the F-plasmid (Supplementary Figure S3).
We next compare expression levels of the target gene, when assessed by independent methods, for two induction levels, namely, 0.1 and 1% l-arabinose (Supplementary Methods). In Supplementary Figure S4A and B, we show the temporal variation after induction in mean numbers of MS2d-GFP–tagged RNA molecules in cell populations and in the fluorescence intensity of RFP measured by plate reader, respectively.
The plate reader measurements of mRFP1 levels, 2 h after induction in liquid culture, show a fold change of 1.67 times when l-arabinose is increased from 0.1 to 1%. The MS2d-GFP in vivo detection method shows a fold change of 1.74 between these same conditions, showing that the results from the two methods are in accordance. From this and the previous experiment, we also conclude that the MS2d-GFP tagging method accurately detects RNA production of the target gene, and that the target gene behaves similarly to the natural system.
We also assessed for what range of inducer concentrations is the target gene under full induction. We measured with the plate reader its expression for varying inducer concentration, 2 h after induction. From Supplementary Figure S5, maximum induction is achieved for 1% arabinose. Here onwards, unless stated otherwise, we use this concentration to assess the kinetics of RNA production under the control of PBAD.
First RNA and intervals between consecutive RNA molecules in individual cells
From the time-lapse images acquired with confocal microscopy, after induction, we measure in each cell both t0 and subsequent values of Δt. t0 is expected to include the time for arabinose to enter the cell via the intake mechanism, the time to find the promoter and release the repressor and also the time for the recruitment of the RNA polymerase and subsequent production of the first target RNA. The latter process includes events such as the closed and the open complex formation at the promoter region, as well as the elongation time. Both the elongation time and the time for MS2d-GFP to bind to a target RNA are expected to be negligible in comparison with the duration of the intake and of transcription initiation (8,12,31). Meanwhile, Δt should depend only on the events in transcription initiation (37).
The distribution of values of the waiting times, t0, is shown in Figure 2A. Cells were induced in the gel with fresh media and 1% arabinose. The distribution is broad, as the waiting times spread through the measurement time and has a mean of 3071 s.
Figure 2.

Kinetics of the intake and production. (A) Probability density distribution of waiting times (µ = 3071 s, σ = 1711 s) for the first RNA to be produced in cells induced by 1% l-arabinose (354 data points). (B) Probability density distribution of intervals between transcription events for PBAD when induced by 1% l-arabinose (µ = 1672 s, σ = 1012 s) (347 data points).
The distribution of intervals between consecutive productions of target RNA molecules (Δt) is shown in Figure 2B. This production is a sub-Poissonian process, as the normalized variance (σ2/µ2) of the distribution is 0.37. Similar conclusions were obtained from measurements of the in vivo kinetics of RNA production under the control of Plac/ara-1 and PtetA (9,10).
The distributions in Figure 2A and B differ significantly. We verified this with a statistical testing of equality of two empirical distributions, the Kolmogorov–Smirnov (K–S) test. We obtained a P-value of 2.8 × 10−18, much smaller than 0.05, which allows rejecting the null hypothesis of similarity. We conclude that in the case of PBAD and the arabinose intake mechanism, the time of intake of inducers affects significantly both mean and standard deviation of RNA numbers in individual cells, long after induction. Finally, note that the difference between the distributions of t0 and Δt provides evidence that the activity of PBAD changes significantly with induction. Otherwise, these two distributions should not differ significantly, as they would both result, e.g. from spurious transcription events alone.
One recent study (12) also focuses on the in vivo induction kinetics of PBAD. This study uses measurements of GFP levels in cell populations, whose expression is controlled by PBAD (inserted into a medium-copy vector) and a model to extrapolate the mean activation time of the promoter, after induction. Assuming a threshold for GFP levels to consider the promoter as active, the mean appearance time of GFP after induction was ∼960 s. By considering several features of the measurement system, including the mean maturation time of GFP, a value was then extrapolated for the expected activation time of the promoter, namely, ∼250 s. This does not include the time for transcription to be completed, once the closed complex is formed. This study thus predicts a faster mean initiation time than what our direct measurements indicate (∼3000 s). Two main reasons exist for this difference. First, in the mutant used previously (12), the chromosomal araBAD operon is deleted, avoiding the negative feedback mechanism, which likely fastens the response time significantly. Additionally, gene expression was assessed from a medium-copy vector, which should respond much faster than the single-copy vector system used here, as its response time depends on the fastest of the response times of several promoter copies. Thus, we find that the results reported previously (12) and ours are in agreement. For example, while observing mean waiting times one order of magnitude longer, we do observe RNA molecules appearing in some of the cells within a time scale of 200–400 s after induction. Therefore, provided the usage of a multi-copy vector instead of the single-copy vector used here, we expect mean waiting times one order of magnitude smaller and thus in agreement with the measurements described previously (12).
Correlations between consecutive processes
To study whether the durations of the processes of intake and of transcription initiation are correlated, we first assessed whether consecutive intervals of Δt in individual cells are correlated. We measured the Pearson correlation from 101 pairs of consecutive intervals, and found it to be 0.16. We obtained a P-value of 0.11, assuming no correlation as the null hypothesis, which implies that we cannot prove that the correlation is significant. This is in agreement with previous studies of Plac/ara-1 kinetics, which also indicate inexistence of correlation between durations of consecutive intervals between RNA productions (8).
We next assessed whether the distributions of t0 and values of Δt (Figure 2A and B) are correlated. Note that t0 and the Δt are of similar order of magnitude as the measurement period. This introduces artificial correlations between t0 and Δt of individual cells, as, e.g. a cell with a large t0 is expected to exhibit smaller than average Δt values, as larger intervals would not be detected during the measurement period as likely as in cells with smaller values of t0. To remove these artificial correlations between t0 and Δt of individual cells, in this assessment, we only considered RNA productions for a certain window size (Supplementary Methods and Supplementary Figure S6). This window is set so as to maximize the number of data points that can be extracted from the measurements.
From the windowed data, we calculated the Pearson correlation between t0 and Δt values in individual cells to be −0.15. We calculated a P-value of 0.18 assuming no correlation as the null hypothesis, which implies that we cannot prove that the correlation is significant. This result is in line with (12), which reports a lack of correlation between initiation of protein expression and subsequent rate of protein synthesis in individual cells.
Dynamics of induction and of transcription initiation under different induction schemes
The distinctiveness of the distributions of t0 and Δt of PBAD, as assessed by the K–S test, suggests that they are, partially, the result of different processes. Although t0 ought to depend on the kinetics of intake of arabinose and on the first transcription initiation event, Δt values ought to depend mostly on the kinetics of transcription initiation events alone.
These assumptions arise from the following. First, in vitro and in vivo measurements (26,38) suggest that transcription initiation (including closed and open complex formation) is a long-duration, multi-step process, usually taking 102–103 s in bacterial promoters (10,25,26,37,38). Other events that need to occur before the appearance of a target RNA because of the tagging of the MS2d-GFP are not expected to affect Δt significantly. These are transcription elongation and the tagging by multiple MS2d-GFP. Elongation of the target RNA was measured to take only tens of seconds (31). Also, the tagging occurs at a rate that makes the RNA visible during elongation or shortly after (31).
To test the two assumptions, we measured the distributions of t0 and Δt for another promoter, Plac/ara-1, in two conditions. Plac/ara-1 can be induced either by IPTG or by arabinose (as PBAD), or by both inducers simultaneously (9). According to our assumption, the distribution of t0 of PBAD is expected to be similar to that of Plac/ara-1 when the latter is induced by arabinose, because of depending on the same intake mechanism, whereas it should differ significantly when Plac/ara-1 is induced by IPTG, given the different intake mechanisms of IPTG.
We measured the distributions of t0 and Δt for Plac/ara-1 when induced by IPTG alone and when induced by arabinose alone (Table 1). We used the same concentration of arabinose as when inducing PBAD. The IPTG concentration used is the one required for maximum induction of Plac/ara-1 (33). Results in Table 1 follow the windowing procedure described earlier in the text. The table shows mean, standard deviation and square of the coefficient of variation (µ2/σ2) of t0 and of Δt for the two promoters, each of which in two induction schemes.
Table 1.
Measurements of t0 and Δt
| Promoter | Inducer | No. of samples (Δt) | µΔt (s) | σΔt (s) | σ2/µ2 | No. samples (t0) | µt0 (s) | σt0 (s) | σ2/µ2 |
|---|---|---|---|---|---|---|---|---|---|
| PBAD | 1% arabinose | 102 | 1440.6 | 532.8 | 0.14 | 84 | 2885.0 | 1159.8 | 0.16 |
| PBAD | 0.1% arabinose | 78 | 1475.4 | 481.2 | 0.11 | 70 | 3519.4 | 1236.2 | 0.12 |
| Plac/ara-1 | 1% arabinose | 149 | 1516.5 | 516.0 | 0.12 | 125 | 2832.5 | 1184.6 | 0.17 |
| Plac/ara-1 | 1 mM IPTG | 485 | 1314.4 | 576.0 | 0.19 | 286 | 2697.0 | 913.6 | 0.11 |
The table shows the mean (µ), the standard deviation (σ) and the normalized variance (σ2/µ2) of the measured distributions of t0 and Δt.
We first assessed the distinctiveness of the distributions of t0 and Δt by the K–S test, for each promoter in each condition (Table 2). In all cases, these two distributions differ in a statistical sense. This is in agreement with the assumption that although both Δt and t0 depend on the kinetics of initiation at the promoter, only t0 depends on the kinetics of intake of inducers.
Table 2.
P-values of the Kolmogorov–Smirnov test between t0 and Δt distributions for each promoter and induction condition
| Promoter | Inducer | P-value |
|---|---|---|
| PBAD | 1% arabinose | 2.83 × 10−18 |
| PBAD | 0.1% arabinose | 4.06 × 10−21 |
| Plac/ara-1 | 1% arabinose | 2.48 × 10−26 |
| Plac/ara-1 | 1 mM IPTG | 3.32 × 10−72 |
For P < 0.05, it is generally accepted that the hypothesis that the two distributions are the same should be rejected.
We next performed statistical tests to assess the distinctiveness between the induction kinetics (t0) of the two promoters (Table 3), when subject to the same inducer and when subject to different inducers. Also, we compared the effects of a different inducer concentration in the case of PBAD. From Table 3, when PBAD and Plac/ara-1 are induced with 1% arabinose, they exhibit distributions of t0 that cannot be distinguished. However, when Plac/ara-1 is induced with IPTG, the resulting t0 distribution is statistically distinguishable from that of PBAD, when induced by either 0.1 or 1% arabinose. It is also distinct from its own t0 distribution when induced by 1% arabinose. This statistically significant difference supports the hypothesis that the distributions of t0 are dependent on the kinetics of the intake system of the inducers, and that these differ for IPTG and arabinose.
Table 3.
P-values of the Kolmogorov–Smirnov test between t0 distributions for each promoter and induction condition
| PBAD 1% arab | PBAD 0.1% arab | Plac/ara-1 1% arab | Plac/ara-1 IPTG | |
|---|---|---|---|---|
| PBAD 1% arab | 1 | |||
| PBAD 0.1% arab | 5.93 × 10−4 | 1 | ||
| Plac/ara-1 1% arab | 0.8533 | 1.10 × 10−4 | 1 | |
| Plac/ara-1 IPTG | 0.0126 | 4.49 × 10–12 | 0.0049 | 1 |
For P < 0.05, it is generally accepted that the hypothesis that the two distributions are the same should be rejected.
Finally, we observed that the distributions of t0 of PBAD, when induced by 0.1% and by 1% arabinose, are distinct. This is expected as the time for inducers to ‘first reach the promoter’ ought to depend on the inducer’s concentration.
Kinetics of the intake process
The intake time of an inducer, here named ‘tdiff’, differs from t0 in that it does not include the time for the first transcription initiation event to occur. Because of this, tdiff cannot be measured directly with the MS2-GFP–tagging method. We thus estimate the mean and variance of the distribution of values of tdiff by subtracting the means and variances of the Δt distribution from the t0 distribution. This method is based on the fact that we were unable to establish the existence of a correlation between the values of t0 and Δt. Given this, and as they are, at most, weakly correlated (Pearson correlation of −0.15), we assume that they are independent so as to be able to estimate the standard deviation of the duration of the intake process alone (note that the mean of this quantity can be estimated as described later in the text, regardless of the existence of dependence).
The estimated mean and a standard deviation of tdiff are similar for PBAD and for Plac/ara-1, when induced with 1% arabinose. Namely, in both cases, we obtained a mean of ∼1400 s and a standard deviation of ∼1100 s. This is expected, given the usage of the same intake mechanism and inducer concentration. Importantly, when Plac/ara-1 is induced by IPTG, the standard deviation of tdiff is much smaller (∼700 s), whereas the mean is similar to when induced by arabinose (∼1400 s). This suggests that the intake of arabinose is a noisier process (concerning the uncertainty of the intake time) than the intake of IPTG. Finally, we find that in the case of PBAD, the concentration of arabinose affects the mean of tdiff significantly, as it equals ∼2000 s for 0.1% arabinose.
Effect of the intake process on the temporal cell-to-cell diversity in RNA numbers
Because of being stochastic and thus variable in duration from one event to the next (i.e. it differs from one cell to the next), the intake process impacts on the diversity in RNA numbers of a cell population. This impact should decrease with time, after induction. We estimated the time during which the effect is tangible for each measurement condition. For this, we assume that values of t0 depend mostly on the intake of arabinose and on the first transcription initiation event at the start site of PBAD. Meanwhile, the distribution of intervals between consecutive RNAs is assumed to depend solely on the kinetics of transcription initiation (8,10,37).
The events determining Δt as well as t0 are modelled as d-step processes, each step with an exponentially distributed duration (Supplementary Methods) (37). From this assumption, it is possible, for a given number of steps, to find the duration of each step that best fits the measurements. We assume transcription initiation to be a three-step process, namely, the closed complex formation, the open complex formation and promoter escape (27,38), as evidence suggests that these are the most rate-limiting steps in normal conditions, i.e. the ones most contributing to the intervals between production of consecutive RNA molecules (26). This assumption also relies on recent studies (37) that indicate that assuming this number of steps suffices to generate distributions that cannot be distinguished, in a statistical sense, from measurements with accuracy and quantity of data similar to the measurements reported here. Finally, we assume the intake to be a two-step process, namely, the binding of extracellular arabinose to an uptake protein and, once bound, its translocation to the cytoplasm (12). The combination of the two processes (intake followed by transcription initiation) is, consequently, assumed to be a five-step process.
Assuming these numbers of steps and stable conditions (e.g. induction level), we searched for models that fit the distributions accurately enough so that the K–S test does not find differences between model and measurements. The P-values of these tests are shown in Supplementary Table S1 and show that in all but one case, it is possible to find a model that cannot be distinguished from the empirical distribution, in a statistical sense.
The case for which we could not find a model that fits the measurements is that of PBAD at 0.1% arabinose induction. This may be due to lack of sufficient data or because the model is unsuitable. Future studies are required to assert this. One explanation may be that, in this case, the distribution of intake times results from two distinct kinetics, one being the productions under induction and the other being spurious productions by promoters in the ‘non-induced’ state.
Given the models aforementioned and provided a rate of RNA degradation, it is possible to estimate the time it takes for the mean RNA numbers of a model cell population to reach equilibrium, as this time depends solely on the rate of degradation of RNAs and t0. We do not have measurements of the degradation rate of the target RNA, as the tagging with MS2d-GFP ‘immortalizes’ it for the duration of the measurements (32). Instead, the models in Figure 3 assume an RNA degradation rate of 5 min−1, which is within realistic intervals for E. coli (1).
Figure 3.

Mean and Fano factor of transient times for different models of intake and subsequent RNA production kinetics. Mean (A) and Fano factor (B) of RNA numbers as obtained by CME models of activation and expression. The models shown are that of Plac/ara-1 with 1 mM IPTG (dashed line), Plac/ara-1 with 1% arabinose (dotted line), PBAD with 1% arabinose (dash-dotted line) and PBAD with 1% arabinose and infinitely fast intake (solid line).
From all of the aforementioned data, we estimated the mean times for RNA numbers to reach near-equilibrium, as well as the Fano factor of this quantity since the start of the simulations. Results are shown in Figure 3, as estimated for each of the models. Also shown is an estimation that assumes the model of transcription initiation of PBAD when induced by 1% arabinose, coupled with an infinitely fast intake.
In all cases, reaching equilibrium in mean RNA numbers takes >1 h, except when assuming infinitely fast intake, in which case the time to reach equilibrium is <0.5 h. Thus, for a time length as long as 1–2 h, the intake process has a non-negligible contribution on the mean and the on the cell-to-cell diversity in RNA numbers of the cell populations. From Figure 3A, one also observes different shapes in the curves of Plac/ara-1 when induced by IPTG (dashed line) and when induced by arabinose (dotted line), because of differing intake kinetics.
From Figure 3B, the contribution of the intake kinetics on the cell-to-cell variability in RNA numbers is also significant. For example, the kinetics of intake causes an increase in the Fano factor in the initial moments not observable in the case of infinitely fast intake.
We also tested models of PBAD induced by 1% arabinose (normal and infinitely fast intake) with other RNA degradation rates (Supplementary Figure S7), within realistic intervals (1). Aside from assessing the degree of dependency on the intake time and degradation rate, one also observes from the figure that although the latter determines the rate at which the system reaches equilibrium, the former acts as a delay towards reaching the numbers at equilibrium. Further, one can see that the intake step adds diversity to the RNA numbers in the cells, during the transient to reach equilibrium.
DISCUSSION
We measured, at the single-cell level, how long it takes for the first RNA under the control of PBAD to be produced, followed the introduction of the inducer in the media. Also, we measured the subsequent intervals between consecutive RNA productions. From the intervals between transcription events, we determined that RNA production under the control of PBAD is a sub-Poissonian process. Two recent studies reached similar conclusions for Plac/ara-1 and PtetA, for all induction conditions tested (9,10). We hypothesize that this may be a common phenomenon because of the kinetic properties of the process of transcription initiation in bacteria, in particular, because of its multi-stepped nature.
From the distributions of the time, it takes for the appearance of the first RNA in each cell when under the control of PBAD and of Plac/ara-1, for different induction conditions, we assessed the effect of the kinetics of the intake process on the mean and cell-to-cell diversity in RNA numbers of cell populations. Relevantly, this effect was found to be tangible for a long period after induction. Also, we verified that different intake mechanisms differ significantly not only in mean but also in the degree of variability of the intake time, and that this has a non-negligible effect on RNA population statistics.
Given the aforementioned data, and considering that natural environments are fluctuating, we expect the kinetics of cellular intake mechanisms to have a significant effect on the degree of phenotypic diversity of cell populations. Finally, we expect the methodology used here to assess the in vivo kinetics of intake of arabinose and of IPTG to be applicable to any gene of interest. Such studies should provide valuable insight into the adaptability of prokaryotic organisms to environmental changes and stress. They should also provide a better understanding of the observed cell-to-cell phenotypic diversity in E. coli when in fluctuating environments.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Table 1, Supplementary Figures 1–7, Supplementary Methods and Supplementary References [39–43].
FUNDING
The Academy of Finland [257603 to A.S.R.]; the Finnish Funding Agency for Technology and Innovation [40226/12 to O.Y.-H.]; the Foundation for Science and Technology, Portugal [PTDC/BBB-MET/1084/2012 to A.S.R.]. Funding for open access charge: Academy of Finland [257603, 2012 to A.S.R.].
Conflict of interest statement. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Material
REFERENCES
- 1.Bernstein JA, Khodursky AB, Lin-Chao S, Cohen SN. Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color fluorescent DNA microarrays. Proc. Natl Acad. Sci. USA. 2002;99:9697–9702. doi: 10.1073/pnas.112318199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu J, Xiao J, Ren X, Lao K, Xie XS. Probing gene expression in live cells, one protein molecule at a time. Science. 2006;311:1600–1603. doi: 10.1126/science.1119623. [DOI] [PubMed] [Google Scholar]
- 3.Taniguchi Y, Choi PJ, Li G-W, Chen H, Babu M, Hearn J, Emili A, Xie XS. Quantifying E. coli proteome and transcriptome with single-molecule sensitivity in single cells. Science. 2010;329:533–538. doi: 10.1126/science.1188308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weickert MJ, Adhya S. The galactose regulon of Escherichia coli. Mol. Microbiol. 1993;10:245–251. doi: 10.1111/j.1365-2958.1993.tb01950.x. [DOI] [PubMed] [Google Scholar]
- 5.Skerra A. Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli. Gene. 1994;151:131–135. doi: 10.1016/0378-1119(94)90643-2. [DOI] [PubMed] [Google Scholar]
- 6.Schleif R. Regulation of the L-arabinose operon of Escherichia coli. Trends. Genet. 2000;16:559–565. doi: 10.1016/s0168-9525(00)02153-3. [DOI] [PubMed] [Google Scholar]
- 7.Choi P, Cai L, Frieda K, Xie X. A stochastic single-molecule event triggers phenotype switching of a bacterial cell. Science. 2008;322:442–446. doi: 10.1126/science.1161427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kandhavelu M, Häkkinen A. Single-molecule dynamics of transcription of the lar promoter. Phys. Biol. 2012;9:026004. doi: 10.1088/1478-3975/9/2/026004. [DOI] [PubMed] [Google Scholar]
- 9.Kandhavelu M, Lloyd-Price J, Gupta A, Muthukrishnan AB, Yli-Harja O, Ribeiro AS. Regulation of mean and noise of the in vivo kinetics of transcription under the control of the lac/ara-1 promoter. FEBS Lett. 2012;586:3870–3875. doi: 10.1016/j.febslet.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 10.Muthukrishnan AB, Kandhavelu M, Lloyd-Price J, Kudasov F, Chowdhury S, Yli-Harja O, Ribeiro AS. Dynamics of transcription driven by the tetA promoter, one event at a time, in live Escherichia coli cells. Nucleic Acids Res. 2012;40:8472–8483. doi: 10.1093/nar/gks583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schleif R. AraC protein, regulation of the l-arabinose operon in Escherichia coli, and the light switch mechanism of AraC action. FEMS Microbiol. Rev. 2010;34:779–796. doi: 10.1111/j.1574-6976.2010.00226.x. [DOI] [PubMed] [Google Scholar]
- 12.Megerle JA, Fritz G, Gerland U, Jung K, Rädler JO. Timing and dynamics of single cell Gene expression in the arabinose utilization system. Biophys. J. 2008;95:2103–2115. doi: 10.1529/biophysj.107.127191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogg RW, Englesberg E. L-arabinose binding protein from Escherichia coli B/r. J. Bacteriol. 1969;100:423–432. doi: 10.1128/jb.100.1.423-432.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schleif R. An l-arabinose binding protein and arabinose permeation in Escherichia coli. J. Mol. Biol. 1969;46:185–196. doi: 10.1016/0022-2836(69)90065-5. [DOI] [PubMed] [Google Scholar]
- 15.Horazdovsky BF, Hogg RW. Genetic reconstitution of the high-affinity L-arabinose transport system. J. Bacteriol. 1989;171:3053–3059. doi: 10.1128/jb.171.6.3053-3059.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Al-Zarban S, Wilcox G. Genetic characterization of the araE gene in Salmonella typhimurium LT2. J. Bacteriol. 1981;146:298–304. doi: 10.1128/jb.146.1.298-304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stoner C, Schleif R. The araE low affinity L-arabinose transport promoter. Cloning, sequence, transcription start site and DNA binding sites of regulatory proteins. J. Mol. Biol. 1983;171:369–381. doi: 10.1016/0022-2836(83)90035-9. [DOI] [PubMed] [Google Scholar]
- 18.Siegele DA, Hu JC. Gene expression from plasmids containing the araBAD promoter at subsaturating inducer concentrations represents mixed populations. Proc. Natl Acad. Sci. USA. 1997;94:8168–8172. doi: 10.1073/pnas.94.15.8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khlebnikov A, Risa O, Skaug T, Carrier TA, Keasling JD. Regulatable arabinose-inducible gene expression system with consistent control in all cells of a culture. J. Bacteriol. 2000;182:7029–7034. doi: 10.1128/jb.182.24.7029-7034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khlebnikov A, Datsenko KA, Skaug T, Wanner BL, Keasling JD. Homogeneous expression of the P-BAD promoter in Escherichia coli by constitutive expression of the low-affinity high-capacity AraE transporter. Microbiology. 2001;147:3241–3247. doi: 10.1099/00221287-147-12-3241. [DOI] [PubMed] [Google Scholar]
- 21.Morgan-Kiss RM, Wadler C, Cronan JE. Long-term and homogeneous regulation of the Escherichia coli araBAD promoter by use of a lactose transporter of relaxed specificity. Proc. Natl Acad. Sci. USA. 2002;99:7373–7377. doi: 10.1073/pnas.122227599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson CM, Schleif RF. In vivo induction kinetics of the arabinose promoters in Escherichia coli. J. Bacteriol. 1995;177:3438–3442. doi: 10.1128/jb.177.12.3438-3442.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walter G, Zillig W, Palm P, Fuchs E. Initiation of DNA-Dependent RNA synthesis and the effect of heparin on RNA polymerase. Eur. J. Biochem. 1967;3:194–201. doi: 10.1111/j.1432-1033.1967.tb19515.x. [DOI] [PubMed] [Google Scholar]
- 24.Chamberlin MJ. The selectivity of transcription. Annu. Rev. Biochem. 1974;43:721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- 25.Buc H, McClure WR. Kinetics of open complex formation between Escherichia coli RNA polymerase and the lac UV5 promoter. Evidence for a sequential mechanism involving three steps. Biochemistry. 1985;24:2712–2723. doi: 10.1021/bi00332a018. [DOI] [PubMed] [Google Scholar]
- 26.Lutz R, Lozinski T, Ellinger T, Bujard H. Dissecting the functional program of Escherichia coli promoters: the combined mode of action of Lac repressor and AraC activator. Nucleic Acids Res. 2001;29:3873–3881. doi: 10.1093/nar/29.18.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu LM. Promoter clearance and escape in prokaryotes. Biochim. Biophys. Acta. 2002;1577:191–207. doi: 10.1016/s0167-4781(02)00452-9. [DOI] [PubMed] [Google Scholar]
- 28.DeHaseth PL, Zupancic ML, Record MT. RNA polymerase-promoter interactions: the comings and goings of RNA polymerase. J. Bacteriol. 1998;180:3019–3025. doi: 10.1128/jb.180.12.3019-3025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greive SJ, Von Hippel PH. Thinking quantitatively about transcriptional regulation. Nat. Rev. Mol. Cell Biol. 2005;6:221–232. doi: 10.1038/nrm1588. [DOI] [PubMed] [Google Scholar]
- 30.McClure WR. Mechanism and control of transcription initiation in prokaryotes. Annu. Rev. Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- 31.Golding I, Cox EC. RNA dynamics in live Escherichia coli cells. Proc. Natl Acad. Sci. USA. 2004;101:11310–11315. doi: 10.1073/pnas.0404443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Golding I, Paulsson J, Zawilski SM, Cox EC. Real-time kinetics of gene activity in individual bacteria. Cell. 2005;123:1025–1036. doi: 10.1016/j.cell.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 33.Lutz R, Bujard H. Independent and tight regulation of transcriptional units in Escherichia coli via the LacR/O, the TetR/O and AraC/I1-I2 regulatory elements. Nucleic Acids Res. 1997;25:1203–1210. doi: 10.1093/nar/25.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chowdhury S, Kandhavelu M, Yli-Harja O, Ribeiro AS. An interacting multiple model filter-based autofocus strategy for confocal time-lapse microscopy. J. Microscopy. 2012;245:265–275. doi: 10.1111/j.1365-2818.2011.03568.x. [DOI] [PubMed] [Google Scholar]
- 35.Chen TB, Lu HH, Lee YS, Lan HJ. Segmentation of cDNA microarray images by kernel density estimation. J. Biomed. Inform. 2008;41:1021–1027. doi: 10.1016/j.jbi.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Otsu N. A threshold selection method from gray-level histograms. IEEE Trans. Sys. Man Cybern. 1979;9:62–66. [Google Scholar]
- 37.Kandhavelu M, Mannerström H, Gupta A, Häkkinen A, Lloyd-Price J, Yli-Harja O, Ribeiro AS. In vivo kinetics of transcription initiation of the lar promoter in Escherichia coli. Evidence for a sequential mechanism with two rate-limiting steps. BMC Syst. Biol. 2011;5:149. doi: 10.1186/1752-0509-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClure WR. Rate-limiting steps in RNA chain initiation. Proc. Natl Acad. Sci. USA. 1980;77:5634–5638. doi: 10.1073/pnas.77.10.5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Totowa, NJ: Humana Press; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 40.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Daruwalla KR, Paxton AT, Henderson PJF. Energization of the transport systems for arabinose and comparison with galactose transport in Escherichia coli. Biochem. J. 1981;200:611–627. doi: 10.1042/bj2000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillespie DT. A general method for numerically simulating the stochastic time evolution of coupled chemical reactions. J. Comput. Phys. 1976;22:403–434. [Google Scholar]
- 43.Munsky B, Khammash M. The finite state projection algorithm for the solution of the chemical master equation. J. Chem. Phys. 2006;124:044104. doi: 10.1063/1.2145882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.