Abstract
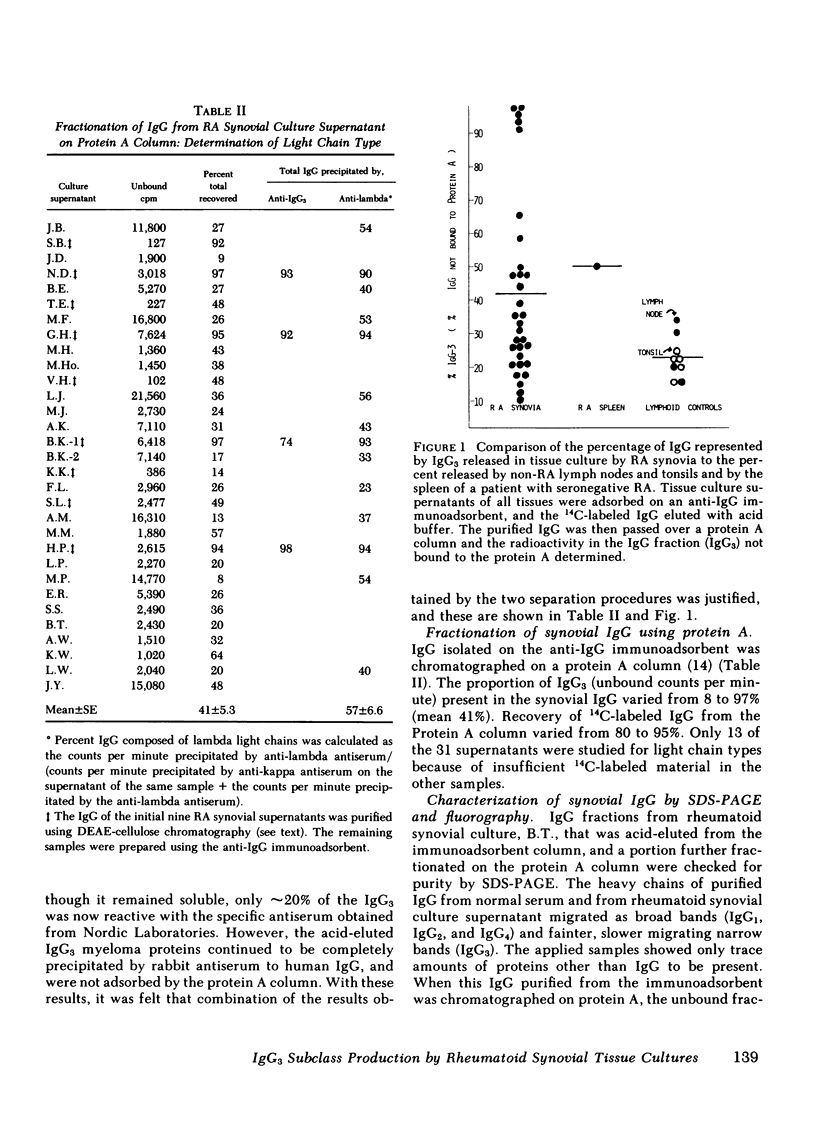
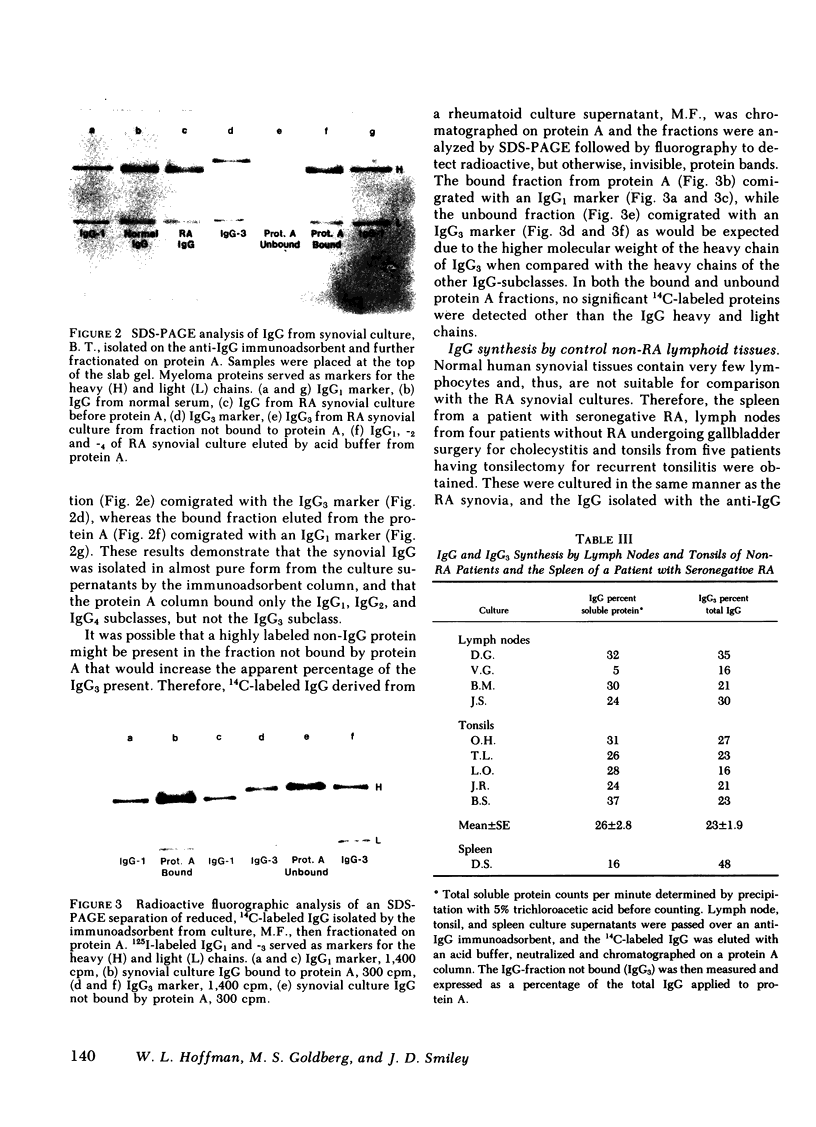
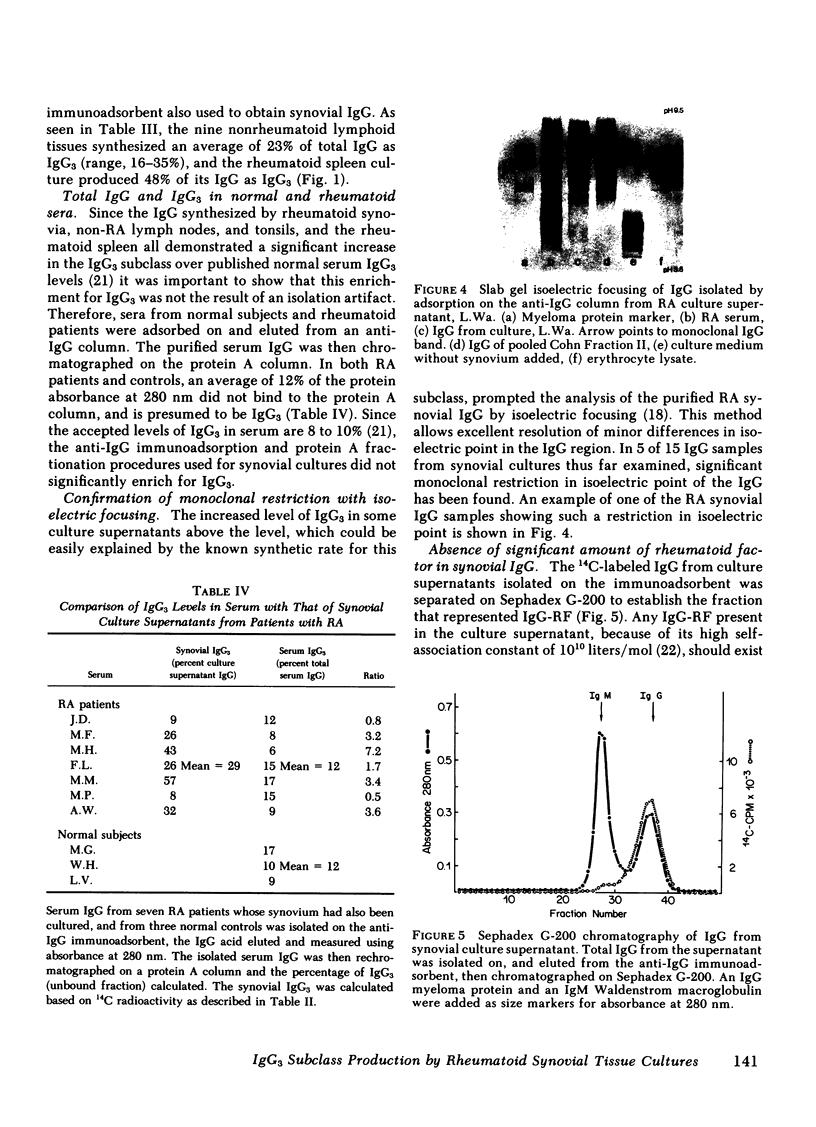
The cellular infiltrate in the deeper layers of the rheumatoid synovium produces a substantial amount of immunoglobulin (Ig)G. Culture supernatants of synovial tissues from 31 patients with rheumatoid arthritis (RA) undergoing joint replacement or synovectomy have been analyzed for the subclass of IgG present. IgG3 was measured by separation with Staphylococcal Protein A chromatography, precipitation with specific anti-IgG3 antibody, and differential separation of IgG3 heavy chains using polyacrylamide gel electrophoresis. IgG from RA synovial cultures contained an average of 41% IgG3 (range, 8-97%) compared with 12% IgG3 (range, 6-17%) in the serum IgG of the same patients. A group of non-RA control lymphoid tissues (four lymph nodes and five tonsils) produced 23% of total IgG as the IgG3 subclass (range, 16-35%). An average of only 9% of the synovial IgG showed aggregation compatible with IgG-rheumatoid factor (IgG-RF). Purified IgG from some of the RA synovial culture supernatants also showed significant restriction when separated by isoelectric focusing. This restriction and the enrichment for the IgG3 subclass in the IgG from RA synovial cultures suggest that either an antigen in the inflamed joint is selectively stimulating an antibody in this subclass, or that significantly differences in the catabolic rate of this subclass are found in cultures of synovial tissue when compared with that occurring in intact patients.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernier G. M., Ballieux R. E., Tominaga K. T., Putnam F. W. Heavy chain subclasses of human gamma G-globulin. Serum distribution and cellular localization. J Exp Med. 1967 Feb 1;125(2):303–316. doi: 10.1084/jem.125.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomo L., Tursi A., Trizio D., Gillardi U., Dammacco F. Immune complexes in rheumatoid synovitis: a mixed staining immunofluorescence study. Immunology. 1970 Apr;18(4):557–563. [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- David G. S. Solid state lactoperoxidase: a highly stable enzyme for simple, gentle iodination of proteins. Biochem Biophys Res Commun. 1972 Jul 25;48(2):464–471. doi: 10.1016/s0006-291x(72)80074-3. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Nutrition needs of mammalian cells in tissue culture. Science. 1955 Sep 16;122(3168):501–514. doi: 10.1126/science.122.3168.501. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Herman J. H., Bradley J., Ziff M., Smiley J. D. Response of the rheumatoid synovial membrane to exogenous immunization. J Clin Invest. 1971 Feb;50(2):266–273. doi: 10.1172/JCI106491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman W. L., Ilan J. Analysis by two-dimensional polyacrylamide gel electrophoresis of liver ribosomal subunnit proteins obtained from free and membrane-bound polysomes of unfasted animals. Biochim Biophys Acta. 1977 Feb 3;474(3):411–424. doi: 10.1016/0005-2787(77)90270-2. [DOI] [PubMed] [Google Scholar]
- KAPUSTA M. A., HALBERSTAM D. PREPARATION OF PURE 7-S GAMMA-GLOBULIN. Biochim Biophys Acta. 1964 Dec 9;93:657–659. doi: 10.1016/0304-4165(64)90352-6. [DOI] [PubMed] [Google Scholar]
- Karpatkin S., Schur P. H., Strick N., Siskind G. W. Heavy chain subclass of human anti-platelet antibodies. Clin Immunol Immunopathol. 1973 Nov;2(1):1–8. doi: 10.1016/0090-1229(73)90030-5. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969 Oct;103(4):828–833. [PubMed] [Google Scholar]
- Kunkel H. G., Fahey J. L., Franklin E. C., Osserman E. F., Terry W. D. Notation for human immunogobulin subclasses. Bull World Health Organ. 1966;35(6):953–953. [PMC free article] [PubMed] [Google Scholar]
- Lindström F. D. Kappa:lambda light chain ratio in IgG eluted from rheumatoid arthritis synovium. Clin Exp Immunol. 1970 Jul;7(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Morell A., Terry W. D., Waldmann T. A. Metabolic properties of IgG subclasses in man. J Clin Invest. 1970 Apr;49(4):673–680. doi: 10.1172/JCI106279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthe E., Natvig J. B. Immunglobulin classes, subclasses and complexes of IgG rheumatoid factor in rheumatoid plasma cells. Clin Exp Immunol. 1972 Sep;12(1):55–70. [PMC free article] [PubMed] [Google Scholar]
- Pope R. M., Teller D. C., Mannik M. The molecular basis of self-association of antibodies to IgG (rheumatoid factors) in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1974 Feb;71(2):517–521. doi: 10.1073/pnas.71.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROPES M. W., BENNETT G. A., COBB S., JACOX R., JESSAR R. A. 1958 Revision of diagnostic criteria for rheumatoid arthritis. Bull Rheum Dis. 1958 Dec;9(4):175–176. [PubMed] [Google Scholar]
- Robboy S. J., Lewis E. J., Schur P. H., Colman R. W. Circulating anticoagulants to factor VIII. Immunochemical studies and clinical response to factor VIII concentrates. Am J Med. 1970 Dec;49(6):742–752. doi: 10.1016/s0002-9343(70)80056-0. [DOI] [PubMed] [Google Scholar]
- Shakib F., Stanworth D. R. IgG subclass composition of rheumatoid arthritic sera and joint fluids. Ann Rheum Dis. 1976 Jun;35(3):263–266. doi: 10.1136/ard.35.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiley J. D., Sachs C., Ziff M. In vitro synthesis of immunoglobulin by rheumatoid synovial membrane. J Clin Invest. 1968 Mar;47(3):624–632. doi: 10.1172/JCI105758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Hurrell J. G., Leach S. J. Elimination of nonspecific adsorption of serum proteins by Sepharose-bound antigens. Anal Biochem. 1978 Jul 1;87(2):299–305. doi: 10.1016/0003-2697(78)90679-6. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]