Abstract
The presence of insulin resistance is increasingly recognized as an important contributor to early stage kidney disease independent of the contribution of diabetes. Important in this relationship is the strong correlation between hyperinsulinemia and low levels of albuminuria (e.g microalbuminuria). Recent work highlight mechanisms for glomerular/tubulointerstitial injury with excess insulin and emerging evidence identifies a unique role for insulin metabolic signaling and altered handling of salt reabsorption at the level of the proximal tubule. Evidence is also emerging for the role of insulin signaling in the glomerulus both epithelial and endothelial. Central to the mechanism of injury is inappropriate activation of the RAAS.
Keywords: Cardiorenal metabolic syndrome, Chronic Kidney Disease, Obesity, Insulin resistance, Hyperinsulinemia, Glomerulus, Proximal Tubule
Introduction
The presence of insulin resistance is central to the cardiorenal metabolic syndrome and is increasingly recognized as a risk factor for both kidney and cardiovascular disease independent of overt diabetes (Pulakat 2011; Sowers 2004; Alberti 1998; NCEP-ATEP III 2006; Grundy 2006; Manhiani 2012; Shephard 1999). The presence of insulin resistance implies an impaired biological and physiological response to insulin-dependent metabolic signaling in traditional insulin sensitive tissues such as skeletal muscle, liver, adipose tissue and pancreas as well as non-traditional cardiovascular tissue such as the heart and kidney (Pulakat 2011; Sowers 2004; Grundy 2006). Much of the work on insulin responses in cardiovascular tissue has focused on NO bioavailability in endothelial function, contractility in vascular smooth muscle cells and relaxation of cardiomyocytes, and regulation of sodium excretion in the kidney as unifying concepts in insulin resistance and cardiovascular disease. In this context, work done in humans and animal models of insulin resistance that display compensatory increases in circulating insulin (e.g hyperinsulinemia) either through endogenous increases in insulin production, through exogenous infusion, or genetic receptor deletion support a direct correlation with endothelial dysfunction, hypertension and kidney disease (DeFronzo 1975; Gesek 1991; Catena 2003; Shephard 1999). Although, a large body of work supported acute insulin-mediated sodium retention, chronic insulin infusion failed to retain sodium in dogs sparking a hot debate over the chronic effects of insulin on sodium retention. Recently, chronic insulin infusion over 6 days during the acute phase of alloxan induced diabetes has shown that prevention of hypoinsulinemia in a hyperglycemic setting can offset natriuresis and diuresis, thereby raising the possibility that insulin is indeed sodium retaining (Manhiani 2012).
Recent work by our group and others implicate inappropriate activation of the renin-angiotensin-aldosterone system (RAAS) with subsequent elevations in angiotensin (Ang) II and aldosterone to alterations in insulin signaling pathways, reactive oxygen species formation and endothelial dysfunction in heart and kidney disease (Manhiani, 2012; Shephard 1999; Whaley-Connell 2011; Sherajee 2012; Hitomi 2011; Wei 2007; Ketsawatsomkaron 2010). Furthermore, all of the above features of insulin resistance correlate well with proteinuria in kidney disease and diastolic dysfunction in the heart, thereby, suggesting a critical role for insulin resistance/hyperinsulinemia as a potential unifying mechanism in the cardiorenal metabolic syndrome. Herein, we review emerging data on the critical role for insulin metabolic signaling, the RAAS, and kidney disease.
The link between insulin resistance and chronic kidney disease (CKD) in the cardiorenal metabolic syndrome
There exists a strong association between insulin resistance and CKD documented through numerous population studies. Data from cohorts such as the national health and nutrition examination survey (NHANES) and the National Kidney Foundation’s Kidney Early Evaluation Program (KEEP), reveal a concurrent increase in prevalence of obesity, the presence of insulin resistance, and incident end-stage renal disease (ESRD) suggesting that insulin resistance independent of diabetes is one of the leading contributors to the recent expansion of CKD persons (USRDS, 2006; Ejerbald, 2006; Iseki, 2004; Yamagata, 2007; Hsu, 2006; Saab, 2001; Gelber, 2005). In this regard, it becomes paramount to distinguish the risk for CKD between earlier stages in the pre-diabetic population (e.g. those with insulin resistance) and overt diabetes with the potential for complications such as progression to ESRD. In this context, data from Kaiser Permanente and the U.S. Renal Data System (USRDS) utilizing more than 320,000 individuals suggest this relationship between insulin resistance and CKD extends beyond early stages of CKD and that after controlling for the presence of diabetes, individuals that are insulin resistant are at an increased risk for progression to ESRD (Fox, 2004).
To extend this notion into prospective evaluation of insulin sensitivity and kidney function, recent work supports a linear correlation with insulin resistance and decline in renal function using clearance rather than estimating equations for glomerular filtration rate (Kobayashi 2005; Emoto 1997; Chen 2003; Chen 2004). In one study, investigators explored glucose disposal in relation to creatinine clearance (Kobayashi 2005). Healthy participants had a higher glucose disposal rate (GDR) compared to patients with CKD. Additionally, this study reported a positive correlation between GDR and creatinine clearance and negative association between GDR and serum creatinine (Cr) level. This holds true in more advanced CKD, a cohort with proteinuria and serum Cr >2.0 mg/dL, demonstrated lower insulin sensitivity than individuals that were normo-albuminuric (Emoto 1997). Following adjustments, increased BMI was an independent contributor to insulin sensitivity in those with CKD. Another analysis of NHANES in non-diabetic participants supports a direct correlation between increasing serum insulin and serum C-peptide levels with CKD.
In order to discern the risk with insulin resistance and earlier stages in the evolution of CKD, investigators have sought to establish correlates with the presence of microalbuminuria. Data from the NHANES and the KEEP support an association between elements of the cardiorenal metabolic syndrome (aka metabolic syndrome) and the respective risks for CKD and microalbuminuria (Chen 2003; Chen 2004; Whaley-Connell 2010). From these studies each component correlates with microalbuminuria. Data from these same studies also suggest there exists a graded relationship between the number of components present and a corresponding increase in the prevalence of microalbuminuria. Data from the Insulin Resistance in Atherosclerosis Study (IRAS) extend this relationship, when adjusted for age and sex there is a decreasing prevalence of microalbuminuria with increasing insulin sensitivity (Mykkanen 1998).
It is clear from population based studies that there exists a strong relationship between insulin resistance and hyperinsulinemia and the presence of early stage CKD in those with microalbuminuria.
Normal insulin signaling in the kidney
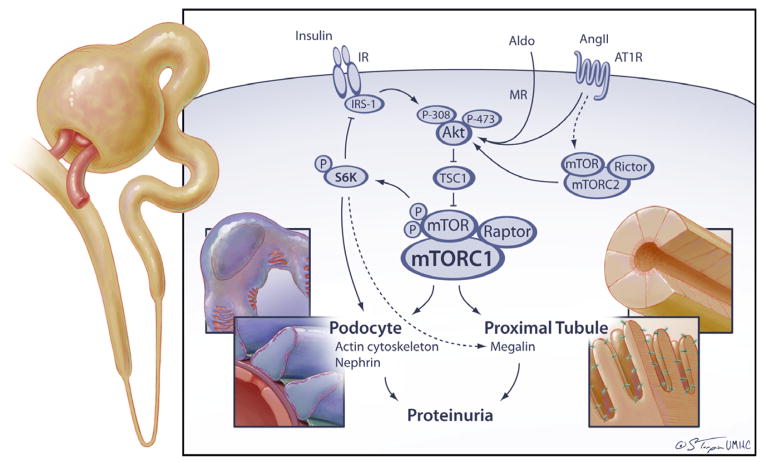
In order to understand the concept of how hyperinsulinemia/insulin resistance can be associated with microalbuminuria one would need to understand how insulin talks to cells in the kidney (Tiwari 2007). Insulin has been shown to bind to both the insulin receptor and insulin-like growth factor receptor to promote its effects in the kidney (Nakamura 1983; Tiwari 2007) (Figure 1). Generally in any tissue, binding to the insulin receptor triggers tyrosine kinase autophosphorylation that then leads to a cascade of events including recruitment of insulin receptor substrate (IRS1/2) which then engages phosphoinositol 3- kinase (PI3-K) to the plasma membrane (Gual 2005; Tiwari 2007). Docking of PI3-K requires tyrosine phosphorylation of IRS-1 at positions 608 and 628 (rat IRS-1 nomenclature) (Gual 2005). PI3-K catalyzes PIP2 to PIP3 that then activates PDK1 that in turn recruits and activates Akt via Ser308 and Thr473 phosphorylation. It should be noted that PI3-K and Akt activation are not insulin specific and can be mediated by multiple pathways including RAS via GPCR activation (Angiotensin II, Ang II) (Figure 1). Activation of Akt is central to insulin signaling in the cells and depending on the context can lead to activation of metabolic pathways such as uptake of glucose via translocation of GLUT4 to the plasma membrane and glycogen synthesis via glycogen synthase kinase (GSK3β) phosphorylation and inhibition. In addition, other pathways such as growth/mitogenic (MAPK/ERK1/2, mTORC1/S6K), NO production (eNOS), cell cycle (overcome G1/G2 arrest), survival (Bcl2, NF-κB), autophagy (ATG, ULK), actin cytoskeleton remodeling (S6K1) are also activated by insulin signaling. How, activation/repression of these pathways leads to albuminuria is the topic of much debate and current work.
Figure 1. Integrative role of the RAAS and insulin in kidney injury.
Akt, protein kinase B; Aldo, aldosterone; Ang II, angiotensin II; AT1R, angiotensin type 1 receptor; IR, insulin receptor; IRS, insulin receptor substrate; mTOR, mammalian Target of Rapamycin, S6K, p70 ribosomal S6 kinase
Abnormal insulin signaling: activation of serine/threonine kinases
Our knowledge about the specifics of insulin signaling derangements in the kidney is obviously limited due to limitations in the understanding of normal insulin signaling. However, some details are likely common and overlap with insulin signaling in other organ systems and cell types (Figure 1). In this section, we will discuss some of the mechanisms by which cells become resistant to the actions of insulin. Broadly, the major players are the insulin receptor, IRS1/2 and Akt while the processes involved are serine/threonine phosphorylation, tyrosine phosphorylation, interaction with SOCS, regulation of expression, cellular localization and degradation (Gual 2005).
In the context of resistance to insulin-dependent activation of metabolic signaling pathways, stimulation by factors such as TNF-α, free fatty acids, cellular stress, amino acids, hyperinsulinemia, Ang II, endothelin-1 leads to increased serine and decreased tyrosine phosphorylation of IRS-1. For example, phosphorylation at ser307 promotes separation of IRS-1 from insulin receptor, decreases tyrosine phosphorylation and promotes proteasomal degradation (Gual 2005). Phosphorylation at ser612 and ser632 decreases the docking ability for PI3-K, thereby leading to decreased insulin metabolic signaling and glucose uptake (Gual 2005). In contrast, phosphorylation at ser302 may promote increased activation of IRS-1 by insulin in vitro. Recent work highlights serine kinases that are redox sensitive in promotion of this excessive serine phosphorylation (Pulakat 2011; Sowers 2004; Gual 2005) and may include known redox-sensitive serine kinases such as JUN NH2-terminal kinase (JNK, ser307), protein kinase C (PKC-θ, ser307 and ser1101), extracellular receptor kinase (ERK, ser612 and ser632), and recently the mammalian target of rapamycin (mTOR, ser612 and ser632) pathway (Pulakat 2011; Sowers 2004; Marmy-Conus 2002; Bhandari 2001). It is thought excess activation of the RAAS with elevations in both Ang II and aldosterone promote activation of these redox-sensitive kinases, i.e., those that are activated by increased reactive oxygen species (ROS) such as ERK1/2, p70S6 kinase (S6K1, ser307 and ser632), or others (Pulakat 2011; Sowers 2004; Gual 2005). This activation leads to increased growth pathway signaling, mitogenesis, hypertrophy, and remodeling in various tissues including those of the kidney.
Insulin metabolic signaling and early kidney injury
The mechanisms ascribed above to insulin metabolic signalling are not fully dissected in the kidney and are at best extrapolated via our understanding of skeletal muscle, liver and vascular biology. To put things in perspective, only recently insulin stimulation of glomeruli and tubules isolated from streptozotocin-diabetic and Zucker fatty rats was done to analyse effects on PI3-K/Akt pathway in the kidney (Mima 2011). Moreover, insulin signalling in the kidney is complicated such that insulin deficiency is as detrimental as insulin excess and insulin actions are milieu specific. To clarify this concept further, recent evidence from glomerular specific insulin receptor knock-out mice support an important protective role for insulin signaling in maintaining the kidney glomerular filtration barrier (Welsh 2010). These mice have normal development of kidneys at birth but later develop podocyte effacement, apoptosis and proteinuria and ultimately develop glomerulosclerosis suggesting that insulin may regulate the actin cytoskeleton and other proteins integral to the filtration barrier. In this regard, earlier in vitro work had demonstrated that the epithelial cell line responsible for filtration, podocytes, are responsive to insulin induced glucose transport via mobilization of GLUT-4 (Coward 2005; Shepard 1999). Furthermore, expression of other insulin-signaling proteins including IRS-1 and 2, vesicular snare, and associated membrane proteins have also been demonstrated in the podocyte (Coward 2005; Shepard 1999). In another model of insulin activation defect (diabetic eNOS knockout mice), advanced changes of diabetic nephropathy develop including hypertension, proteinuria, arterial hyalinosis and KW nodules suggesting the importance of the glomerular endothelium in kidney function (Nakagawa 2007). Glomerular involvement extends into rodent models of insulin resistance wherein evidence from our work and others support that excess Ang II as well as aldosterone with dependent generation of oxidative stress is involved in filtration barrier/podocyte injury (Gloy 1997; Nicholas 2003; Chen 2003; Whaley-Connell 2006) (Figure 1). Resistance to insulin has been shown to induce Ang II in podocytes through increased angiotensinogen expression suggesting an intracrine role (Hayden 2005; Durvasula 2004). In contrast, under conditions of excess insulin, in vitro protein exposure, mechanical stretch, and increased intraglomerular pressure have also been shown to enhance tissue Ang II production which may potentiate the impact of hyperinsulinemia on glomerular injury (Hayden 2005; Durvasula 2004; Han 2005). Further, interruption of the RAAS reverses podocyte effacement and normalizes podocyte-specific protein expression including nephrin, podocin and synaptopodin in animal models of insulin resistance and thereby contributes to decrease in proteinuria that is seen in kidney injury (Whaley-Connell 2006; Bonnet 2001; Mifsud 2003).
In addition to insulin regulation of filtration, excess insulin can contribute to mesangial and tubulointerstitial remodeling that potentiate kidney tissue injury (Shepard 1999; Conti 1988; Nicholas 2003). Insulin contributes to mesangial proliferation as well as synthesis of proteins that regulate extracellular matrix deposition (Conti 1988). Furthermore, insulin in excess may contribute to the development of proteinuria and progression of kidney disease via effects on TGF-β production in the mesangium, the filtration barrier via effects on podocyte effacement, and proximal tubule-dependent interstitial fibrosis (Nicholas 2003; Anderson 1996; Morrisey 2001). In contrast, renal tubule epithelial cell specific knockout of insulin receptor in mice result in impairment of sodium excretion, decreased urinary Nox excretion and hypertension especially in setting of metabolic syndrome (Tiwari 2008). Moreover, in this animal model decrease in Nox excretion was likely the result of decreased NO production and/or increased oxidative stress (Li 2012).
Thereby, insulin has a central and complicated role in kidney tissue remodeling through regulation of tubulointerstitial fibrosis and glomerular filtration barrier injury and in kidney function through its regulation of receptor signaling (Chen 2004; Anderson 1996; Welsh 2010; Li 2012).
Understanding proteinuria in the insulin resistant kidney
As illustrated above, the presence of albuminuria is common in the setting of insulin resistance and the cardiorenal metabolic syndrome independent of the contributions of overt diabetes. Understanding handling of albumin (e.g. proteinuria) provides insight into progression of kidney disease beyond mechanisms of filtration. Recent work has focused on proximal tubule handling of albumin separate to that of glomerular injury (Pavenstadt 2003; Russo 2009). In a recent study utilizing intravital 2-photon microscopy, investigators injected Alexa 568 labeled albumin into a rodent model of insulin resistance and demonstrate that fluorescent albumin was retained at the apical membrane of proximal tubule cells (Pavenstadt 2003). In addition, there was increased excretion of smaller non-immunoreactive albumin fragments (peptiduria) and proteinuria consistent with mishandling of intact albumin at the brush border. Importantly, the glomerular sieving coefficient remained the same for the insulin resistant rats compared to controls, suggesting that albuminuria under conditions of insulin resistance may have a proximal tubular component.
Role of megalin in proteinuria and modulation by insulin
Megalin has been identified as essential for proximal tubular protein reabsorption and transport (Habibi 2010, Leheste 1999) and deletion of megalin in mice manifests as low molecular weight proteinuria including albuminuria (Leheste 1999) (Figure 1). Megalin along with cubilin, forms a heterodimeric receptor complex that internalizes via clathrin-coated endocytic vesicles (Gekle 2005). Decrease in intravesicular pH results in release of albumin to lysosomes where it is broken down into amino acids and reabsorbed via the basolateral membrane into the circulation (Gekle 2005). Megalin is a 660kDa Type 1 transmembrane protein with several LDL(R)-like repeats and EGF motifs in the extracellular domain followed by a transmembrane domain and finally, a smaller intra-cytoplasmic tail of ~200 amino acids (<35kDa, MCTD) (Figure 1). Several adaptor/scaffold proteins bind to the MCTD to regulate apical membrane positioning, shuttling into the endocytic vesicles, trafficking to and from the Golgi/ER and signal transduction (Gotthardt 2000; Gekle 2005).
Recently, interest has grown in the role of megalin as a signal transducer and modulator of proximal tubule cell function. It has been shown to be involved in cell survival (via insulin dependent Akt), auto-regulate self and other metabolic protein expression and function, internalize Ang II and insulin among other roles (Gotthardt 2000; Zou 2004; Gonzalez-Villalobos 2006). Furthermore, a recent genome-wide association scan identified megalin/DAB2 as one of six protein systems associated with decreased eGFR (Chasman 2012). Interestingly, megalin undergoes ectodomain shedding via metalloprotease mediated cleavage and increased shedding is associated with proteinuria in both Type 1 and Type 2 diabetes mellitus, however, the precise mechanisms or conditions needed for this event are not well known (Thrailkill 2009; Ogasawara 2012). Phorbol 12-myristate 13-acetate (PMA) increased megalin C-terminal fragment (MCTF) appearance by 2.5 fold via activation of metalloproteases and this process is likely mediated by protein kinase C (PKC) activation (Zou 2004). Potential candidate metalloproteases have been proposed, however, none have been directly examined. Megalin localizes with γ-secretase (pre-senilin in the apical brush border of the proximal tubules and γ-secretase promotes regulated intramembrane proteolysis (RIP) and release of <35kDa MCTD that is relatively difficult to detect in live cells presumably due to the short half-life from 26S proteasomal degradation which can be inhibited by Lactacystin (Zou 2004). The MCTD has been reported to translocate to the nucleus and regulate gene expression of sodium hydrogen exchanger (NHE-3) and megalin full-length receptor, although the nuclear localization of MCTD has been difficult to prove due to rapid degradation and this is consistent with Notch proteins undergoing RIP (Li 2008).
Recently, insulin has been shown to oppose Ang II-mediated downregulation of megalin in opossum proximal tubule cells (Hosojima 2009). Potential mechanisms include transcriptional regulation and translational regulation via ERK1/2 but not JNK, p38MAPK, PKC and JAK/STAT. We have demonstrated downregulation of megalin correlates with proteinuria in Ren2 rats, a model for insulin resistance (Habibi 2010). Megalin protein expression is restored by blockade of the AT1R (Hosojima 2009, unpublished observations). It is not known if insulin can regulate RIP and what effects it has on megalin and protein transport in conditions of insulin resistance.
Insulin resistance and the mTOR pathway in proteinuria
Recently, the nutrient sensor serine kinase mTOR has been suggested as the link between over-nutrition, insulin resistance, cardiovascular and kidney injury (Pulakat 2011; Whaley-Connell 2011; Nistala 2012) (Figure 1). Data support activation of mTOR pathway to early changes in the glomerulus such as mesangial expansion, basement membrane thickening and collagen deposition in the capillary tuft concurrent with mechanisms of proteinuria described above in insulin resistant models (Nistala 2012; Godel 2011; Inoki 2011). Plausible mechanisms may include IKK-β/NF-κB mediated activation of mTOR by cytokines such as TNF-α (Inoki 2011). Nutrients (amino acids, glucose) may also activate mTOR/raptor (mTORC1) to propagate proliferative signals via S6K1 activation and growth factors/cytokines may signal via mTOR/rictor (mTORC2) to regulate the actin cytoskeleton in proximal tubule cell dependent tubulointerstitial fibrosis (Inoki 2011).
Recent data suggest Ang II or aldosterone signals through mTOR for their proliferative and fibrotic effects in the kidney (Lieberthal 2009; Chen 2009). However, the mechanisms remain to be elucidated. Data so far shows that in coronary and vascular smooth muscle cells, Ang II increased cell growth via activation of PI3-K, downstream phosphorylation of S6K1 and dissociation of eIF-4E from 4EB-P1 (Hafizi 2004; Yamakawa 2003). By contrast, in intestinal epithelial cells, Ang II trans-activated epidermal growth factor receptor (EGFR) to activate Akt/mTOR/S6K1 and cell growth (Chiu 2005). Furthermore, data from S6K1−/− mice demonstrate that the mTORC1 is necessary to facilitate renal hypertrophy in streptozotocin-diabetes animal model (Chen 2009). In summary, evidence for insulin metabolic signaling in mTOR pathway activation and early changes of glomerular and proximal tubular morphology and function is accumulating. The interaction of insulin signaling with RAAS activation in conditions of insulin resistance is a matter of intensive investigation.
Conclusion
The presence of insulin resistance is increasingly recognized as an important contributor to early stage kidney disease independent of the contribution of diabetes. Important in this relationship is the strong correlation between hyperinsulinemia and microalbuminuria. Recent work has demonstrated the mechanism between excess insulin and glomerular injury and emerging evidence highlight a unique role for impairments in insulin metabolic signaling and impaired handling of albumin reabsorption at the level of the glomerulus and proximal tubule. Misregulation of epithelial proteins such as nephrin and megalin and activation of mTOR/S6 Kinase pathway seem to be involved in mediating the pathophysiology of insulin resistance, kidney hypertrophy, hyperfiltration and microalbuminuria. Central to the mechanism of injury appears to be inappropriate activation of the RAAS. However, the role of insulin resistance and hyperinsulinemia and the crosstalk with RAAS needs to be elucidated.
Highlights.
Insulin resistance without diabetes may be a feature of early stage kidney disease in the CardioRenal Metabolic Syndrome
Early proteinuria/microalbuminuria may predict presence and progression of kidney disease
Proteinuria in insulin resistant subjects may have glomerular/tubulointerstitial origins
Insulin may play an important role in salt homeostasis
Inappropriate RAAS activation is central to insulin resistance and kidney dysfunction
Acknowledgments
Source of Support: Our research is supported by Dialysis Clinics, Inc to RN. The NIH AG040638 to AWC. There was also support from the Veterans Affairs CDA-2 BB47 to AWC and the ASN-ASP Junior Development Grant in Geriatric Nephrology to AWC supported by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, the Association of Specialty Professors, and the American Society of Nephrology.
Footnotes
Disclosure: The authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pulakat L, DeMarco VG, Whaley-Connell A, Sowers JR. The Impact of Over-Nutrition on Insulin Metabolic Signaling in the Heart and Kidney. Cardiorenal Med. 2011;1:102–112. doi: 10.1159/000327140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sowers JR. Insulin resistance and hypertension. Am J of Physiol Heart Circ Physiol. 2004;286:H1597–H1602. doi: 10.1152/ajpheart.00026.2004. [DOI] [PubMed] [Google Scholar]
- 3.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 4.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 5.Executive Summary Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21(1):1–6. doi: 10.1097/01.hco.0000200416.65370.a0.
- 6.DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975;55:845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gesek FA, Schoolwerth AC. Insulin increases Na-H+ exchange activity in proximal tubules from normotensive and hypertensive rats. Am J Physiol. 1991;260:F695–F703. doi: 10.1152/ajprenal.1991.260.5.F695. [DOI] [PubMed] [Google Scholar]
- 8.Stenvinkel P, Bolinder J, Alvestrand A. Effects of insulin on renal haemodynamics and the proximal and distal tubular sodium handling in healthy subjects. Diabetologia. 1992;35:1042–1048. doi: 10.1007/BF02221679. [DOI] [PubMed] [Google Scholar]
- 9.Catena C, Cavarape A, Novello M, Giacchetti G, Sechi LA. Insulin receptors and renal sodium handling in hypertensive fructose-fed rats. Kidney Int. 2003;64:2163–2171. doi: 10.1046/j.1523-1755.2003.00313.x. [DOI] [PubMed] [Google Scholar]
- 10.Shepard PR, Kahn BB. Glucose Transporters and Insulin Action: Implications for Insulin Resistance and Diabetes Mellitus. N Eng J Med. 1999;341:248–57. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 11.Manhiani MM, Duggan AD, Wilson H, Brands MW. Chronic intrarenal insulin replacement reverses diabetes mellitus-induced natriuresis and diuresis. Hypertension. 2012;59(2):421–30. doi: 10.1161/HYPERTENSIONAHA.111.185215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whaley-Connell A, Habibi J, Panfili Z, Hayden MR, Schneider R, Bagree S, Nistala R, Parrish A, Sowers JR. Angiotensin II activation of mTOR/S6K contributes to loss of N-cadherin and tubulointerstitial fibrosis. Am J Nephrol. 2011;29;34(2):115–125. doi: 10.1159/000329327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherajee SJ, Fujita Y, Rafiq K, Nakano D, Mori H, Masaki T, Hara T, Kohno M, Nishiyama A, Hitomi H. Aldosterone induces vascular insulin resistance by increasing insulin-like growth factor-1 receptor and hybrid receptor. Arterioscler Thromb Vasc Biol. 2012;32(2):257–63. doi: 10.1161/ATVBAHA.111.240697. [DOI] [PubMed] [Google Scholar]
- 14.Hitomi H, Mehta PK, Taniyama Y, Lassègue B, Seidel-Rogol B, San Martin A, Griendling KK. Vascular smooth muscle insulin resistance, but not hypertrophic signaling, is independent of angiotensin II-induced IRS-1 phosphorylation by JNK. Am J Physiol Cell Physiol. 2011;301(6):C1415–22. doi: 10.1152/ajpcell.00017.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei Y, Whaley-Connell AT, Chen K, Habibi J, Uptegrove GM, Clark SE, Stump CS, Ferrario CM, Sowers JR. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension. 2007;50:384–391. doi: 10.1161/HYPERTENSIONAHA.107.089284. [DOI] [PubMed] [Google Scholar]
- 16.Ketsawatsomkron P, Stepp DW, Fulton DJ, Marrero MB. Molecular mechanism of angiotensin II-induced insulin resistance in aortic vascular smooth muscle cells: roles of Protein Tyrosine Phosphatase-1B. Vascul Pharmacol. 2010;53(3–4):160–8. doi: 10.1016/j.vph.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miettinen H, Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Proteinuria predicts stroke and other atherosclerotic vascular disease events in nondiabetic and non-insulin-dependent diabetic subjects. Stroke. 1996;27:2033–2039. doi: 10.1161/01.str.27.11.2033. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in U.S adults. Ann Intern Med. 2004;140(3):167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 19.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Renal Data System: USRDS. 2006 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesada, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2006. [Google Scholar]
- 21.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyrén O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 22.Iseki K, Ikemiya Y, Kinjo K, Inoue T, Iseki C, Takishita S. Body mass index and the risk of development of end-stage renal disease in a screened cohort. Kidney Int. 2004;65(5):1870–6. doi: 10.1111/j.1523-1755.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 23.Yamagata K, Ishida K, Sairenchi T, Takahashi H, Ohba S, Shiigai T, Narita M, Koyama A. Risk factors for chronic kidney disease in a community-based population: a 10-year follow-up study. Kidney Int. 2007;71:159–166. doi: 10.1038/sj.ki.5002017. [DOI] [PubMed] [Google Scholar]
- 24.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body Mass Index and Risk for End-Stage Renal Disease. Annals of Int Med. 2006;144(1):21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 25.Saab G, Whaley-Connell A, McFarlane SI, Li S, Chen SC, Sowers JR, Bakris GL, McCullough PA. The Association of Parathyroid Hormone Levels and the Cardiorenal Metabolic Syndrome in non-diabetic Chronic Kidney Disease. Cardiorenal Med. 2001;1:123–130. doi: 10.1159/000327149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gelber R, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, Gaziano JM. Association between body mass index and CKD in apparently healthy men. Am J Kidney Dis. 2005;46:871–880. doi: 10.1053/j.ajkd.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 27.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291(7):844–50. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi S, Maesato K, Moriya H, Ohtake T, Ikeda T. Insulin resistance in patients with chronic kidney disease. Am J Kidney Dis. 2005;45(2):275–80. doi: 10.1053/j.ajkd.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 29.Emoto M, Nishizawa Y, Maekawa K, Kawagishi T, Kogawa K, Hiura Y, Mori K, Tanaka S, Ishimura E, Inaba M, Okuno Y, Morii H. Insulin resistance in non-obese, non-insulin-dependent diabetic patients with diabetic nephropathy. Metabolism. 1997;46(9):1013–8. doi: 10.1016/s0026-0495(97)90271-7. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Muntner P, Hamm LL, Fonseca V, Batuman V, Whelton PK, He J. Insulin resistance and risk of chronic kidney disease in non diabetic US adults. J Am Soc Nephrol. 2003;14:469–477. doi: 10.1097/01.asn.0000046029.53933.09. [DOI] [PubMed] [Google Scholar]
- 31.Chen J, Muntner P, Hamm LL, Jones DW, Batuman V, Fonseca V, Whelton PK, He J. The metabolic syndrome and chronic kidney disease in U.S adults. Ann Intern Med. 2004;140:167–174. doi: 10.7326/0003-4819-140-3-200402030-00007. [DOI] [PubMed] [Google Scholar]
- 32.Whaley-Connell A, Pavey BS, McCullough PA, Saab G, Li S, McFarlane SI, Chen SC, Vassalotti JA, Collins AJ, Bakris G, Sowers JR KEEP Investigators. Dysglycemia predicts cardiovascular and kidney disease in the Kidney Early Evaluation Program. J Clin Hypertens (Greenwich) 2010;12(1):51–8. doi: 10.1111/j.1751-7176.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mykkanen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: The insulin resistance atherosclerosis study. Diabetes. 1998;47:793B800. doi: 10.2337/diabetes.47.5.793. [DOI] [PubMed] [Google Scholar]
- 34.Tiwari S, Riazi S, Ecelbarger CA. Insulin’s impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am J Physiol Renal Physiol. 2007;293(4):F974–84. doi: 10.1152/ajprenal.00149.2007. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura R, Emmanouel DS, Katz AI. Insulin binding sites in various segments of the rabbit nephron. J Clin Invest. 1983;72:388–392. doi: 10.1172/JCI110979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gual P, Le Marchand-Brustel Y, Tanti JF. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie. 2005 Jan;87(1):99–109. doi: 10.1016/j.biochi.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Marmy-Conus N, Hannan KM, Pearson RB. Ro 31-6045, the inactive analogue of the protein kinase C inhibitor Ro 31-8220, blocks in vivo activation of p70(s6k)/p85(s6k): implications for the analysis of S6K signalling. FEBS Lett. 2002;519(1–3):135–40. doi: 10.1016/s0014-5793(02)02738-2. [DOI] [PubMed] [Google Scholar]
- 38.Bhandari BK, Feliers D, Duraisamy S, Stewart JL, Gingras AC, Abboud HE, Choudhury GG, Sonenberg N, Kasinath BS. Insulin regulation of protein translation repressor 4E-BP1, an eIF4E-binding protein, in renal epithelial cells. Kidney Int. 2001;59(3):866–75. doi: 10.1046/j.1523-1755.2001.059003866.x. [DOI] [PubMed] [Google Scholar]
- 39.Mima A, Ohshiro Y, Kitada M, Matsumoto M, Geraldes P, Li C, Li Q, White GS, Cahill C, Rask-Madsen C, King GL. Glomerular-specific protein kinase C-β-induced insulin receptor substrate-1 dysfunction and insulin resistance in rat models of diabetes and obesity. Kidney Int. 2011;79:883–896. doi: 10.1038/ki.2010.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welsh GI, Hale LJ, Eremina V, Jeansson M, Maezawa Y, Lennon R, Pons DA, Owen RJ, Satchell SC, Miles MJ, Caunt CJ, McArdle CA, Pavenstädt H, Tavaré JM, Herzenberg AM, Kahn CR, Mathieson PW, Quaggin SE, Saleem MA, Coward RJ. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12(4):329–40. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coward RJ, Welsh GI, Yang J, Tasman C, Lennon R, Koziell A, Satchell S, Holman GD, Kerjaschki D, Tavaré JM, Mathieson PW, Saleem MA. The Human Glomerular Podocyte Is a Novel Target for Insulin Action. Diabetes. 2005;54(11):3095–102. doi: 10.2337/diabetes.54.11.3095. [DOI] [PubMed] [Google Scholar]
- 42.Shepard PR, Kahn BB. Glucose Transporters and Insulin Action: Implications for Insulin Resistance and Diabetes Mellitus. N Eng J Med. 1999;341:248–57. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 43.Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Johnson RJ, Croker B. Diabetic endothelial nitric oxide synthase knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol. 2007;18(2):539–50. doi: 10.1681/ASN.2006050459. [DOI] [PubMed] [Google Scholar]
- 44.Gloy J, Henger A, Fischer KG, Nitschke R, Mundel P, Bleich M, Schollmeyer P, Greger R, Pavenstädt H. Angiotensin II depolarizes podocytes in the intact glomerulus of the rat. J Clin Invest. 1997;99:2772–2781. doi: 10.1172/JCI119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicholas SB. Advances in pathogenetic mechanisms of diabetic nephropathy. Cell Mol Biol. 2003;49(8):1319–25. [PubMed] [Google Scholar]
- 46.Chen S, Jim B, Ziyadeh FN. Diabetic nephropathy and transforming growth beta: transforming our view of glomerulosclerosis and fibrosis build-up. Seminars in Nephrology. 2003;23:532–543. doi: 10.1053/s0270-9295(03)00132-3. [DOI] [PubMed] [Google Scholar]
- 47.Whaley-Connell AT, Chowdhury NA, Hayden MR, Stump CS, Habibi J, Wiedmeyer CE, Gallagher PE, Tallant EA, Cooper SA, Link CD, Ferrario C, Sowers JR. Oxidative stress and glomerular filtration barrier injury: role of the renin-angiotensin system in the Ren2 transgenic rat. Am J Physiol Renal Physiol. 2006;291(6):F1308–14. doi: 10.1152/ajprenal.00167.2006. [DOI] [PubMed] [Google Scholar]
- 48.Hayden MR, Whaley-Connell A, Sowers JR. Renal Redox Stress and Remodeling in Metabolic Syndrome, Type 2 Diabetes mellitus, and Diabetic Nephropathy: Paying Homage to the Podocyte. Am J Nephrol. 2005;25(6):553–569. doi: 10.1159/000088810. [DOI] [PubMed] [Google Scholar]
- 49.Durvasula RV, Petermann AT, Hiromura K, Blonski M, Pippin J, Mundel P, Pichler R, Griffin S, Couser WG, Shankland SJ. Activation of a local tissue angiotensin system in podocytes by mechanical strain. Kidney Int. 2004;65:30–39. doi: 10.1111/j.1523-1755.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 50.Han SY, Kang YS, Jee YH, Han KH, Cha DR, Kang SW, Han DS. High glucose and angiotensin II increase beta1 integrin and integrin-linked kinase synthesis in cultured mouse podocytes. Cell Tissue Res. 2005;28:1–12. doi: 10.1007/s00441-005-0065-4. [DOI] [PubMed] [Google Scholar]
- 51.Bonnet F, Cooper ME, Kawachi H, Allen TJ, Boner G, Cao Z. Irbesartan normalises the deficiency in glomerular nephrin expression in a model of diabetes and hypertension. Diabetologia. 2001;44:874–877. doi: 10.1007/s001250100546. [DOI] [PubMed] [Google Scholar]
- 52.Mifsud SA, Allen TJ, Bertram JF, Hulthen UL, Kelly DJ, Cooper ME, Wilkinson-Berka JL, Gilbert RE. Podocyte foot process broadening in experimental diabetic nephropathy: amelioration with renin-angiotensin blockade. Diabetologia 2001. 2003;44:878–882. doi: 10.1007/s001250100561. [DOI] [PubMed] [Google Scholar]
- 53.Conti FG, Striker LJ, Lesniak MA, MacKay K, Roth J, Striker GE. Studies on binding and mitogenic effect of insulin and insulin-like growth factor I in glomerular mesangial cells. Endocrinology. 1988;122(6):2788–95. doi: 10.1210/endo-122-6-2788. [DOI] [PubMed] [Google Scholar]
- 54.Anderson PW, Zhang XY, Tian J, Correale JD, Xi XP, Yang D, Graf K, Law RE, Hsueh WA. Insulin and angiotensin II are additive in stimulating TGF-beta 1 and matrix mRNAs in mesangial cells. Kidney Int. 1996;50(3):745–53. doi: 10.1038/ki.1996.372. [DOI] [PubMed] [Google Scholar]
- 55.Morrisey K, Evans RA, Wakefield L, Phillips AO. Translational regulation of renal proximal tubular epithelial cell transforming growth factor-beta1 generation by insulin. Am J Pathol. 2001;159(5):1905–15. doi: 10.1016/s0002-9440(10)63037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tiwari S, Sharma N, Gill PS, Igarashi P, Kahn CR, Wade JB, Ecelbarger CM. Impaired sodium excretion and increased blood pressure in mice with targeted deletion of renal epithelial insulin receptor. Proc Natl Acad Sci U S A. 2008;105(17):6469–74. doi: 10.1073/pnas.0711283105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li L, Garikepati RM, Tsukerman S, Tiwari S, Ecelbarger CM. Salt sensitivity of nitric oxide generation and blood pressure in mice with targeted knockout of the insulin receptor from the renal tubule. Am J Physiol Regul Integr Comp Physiol. 2012;303(5):R505–12. doi: 10.1152/ajpregu.00033.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 59.Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol. 2009;20(3):489–94. doi: 10.1681/ASN.2008050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Habibi J, Hayden MR, Sowers JR, Pulakat L, Tilmon RD, Manrique C, Lastra G, Demarco VG, Whaley-Connell A. Nebivolol Attenuates Redox-Sensitive Glomerular and Tubular Mediated Proteinuria in Obese Rats. Endocrinology. 2010;152(2):659–68. doi: 10.1210/en.2010-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, Aucouturier P, Moskaug JO, Otto A, Christensen EI, Willnow TE. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol. 1999;155(4):1361–70. doi: 10.1016/S0002-9440(10)65238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gekle M. Renal tubule albumin transport. Annu Rev Physiol. 2005;67:573–94. doi: 10.1146/annurev.physiol.67.031103.154845. [DOI] [PubMed] [Google Scholar]
- 63.Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J. Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction. J Biol Chem. 2000;275(33):25616–24. doi: 10.1074/jbc.M000955200. [DOI] [PubMed] [Google Scholar]
- 64.Gonzalez-Villalobos R, Klassen RB, Allen PL, Johanson K, Baker CB, Kobori H, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin-(1–7) Am J Physiol Renal Physiol. 2006;290(5):F1270–F1275. doi: 10.1152/ajprenal.00164.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chasman DI, et al. Integration of genome-wide association studies with biological knowledge identifies six novel genes related to kidney function. Hum Mol Genet. 2012;21(24):5329–5343. doi: 10.1093/hmg/dds369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ogasawara S, Hosojima M, Kaseda R, Kabasawa H, Yamamoto-Kabasawa K, Kurosawa H, Sato H, Iino N, Takeda T, Suzuki Y, Narita I, Yamagata K, Tomino Y, Gejyo F, Hirayama Y, Sekine S, Saito A. Significance of urinary full-length and ectodomain forms of megalin in patients with type 2 diabetes. Diabetes Care. 2012;35(5):1112–8. doi: 10.2337/dc11-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thrailkill KM, Nimmo T, Bunn RC, Cockrell GE, Moreau CS, Mackintosh S, Edmondson RD, Fowlkes JL. Microalbuminuria in type 1 diabetes is associated with enhanced excretion of the endocytic multiligand receptors megalin and cubilin. Diabetes Care. 2009;32(7):1266–8. doi: 10.2337/dc09-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou Z, Chung B, Nguyen T, Mentone S, Thomson B, Biemesderfer D. Linking receptor-mediated endocytosis and cell signaling: evidence for regulated intramembrane proteolysis of megalin in proximal tubule. J Biol Chem. 2004;279(33):34302–10. doi: 10.1074/jbc.M405608200. [DOI] [PubMed] [Google Scholar]
- 69.Li Y, Cong R, Biemesderfer D. The COOH terminus of megalin regulates gene expression in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol. 2008;295(2):C529–C537. doi: 10.1152/ajpcell.00037.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hosojima M, Sato H, Yamamoto K, Kaseda R, Soma T, Kobayashi A, Suzuki A, Kabasawa H, Takeyama A, Ikuyama K, Iino N, Nishiyama A, Thekkumkara TJ, Takeda T, Suzuki Y, Gejyo F, Saito A. Regulation of megalin expression in cultured proximal tubule cells by angiotensin II type 1A receptor- and insulin-mediated signaling cross talk. Endocrinology. 2009;150(2):871–8. doi: 10.1210/en.2008-0886. [DOI] [PubMed] [Google Scholar]
- 71.Whaley-Connell A, Habibi J, Panfili Z, Hayden MR, Bagree S, Nistala R, Hyder S, Krueger B, Demarco V, Pulakat L, Ferrario CM, Parrish A, Sowers JR. Angiotensin II activation of mTOR results in tubulointerstitial fibrosis through loss of N-cadherin. Am J Nephrol. 2011;34(2):115–25. doi: 10.1159/000329327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nistala R, Sowers JR, Whaley-Connell A. Over-nutrition contributes to tubulointerstitial fibrosis by targeting nutrient-sensing kinases: Role for the mTOR/S6K pathway. Cell Cycle. 2012;11(5):831–2. doi: 10.4161/cc.11.5.19588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Godel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mór A, Lindenmeyer MT, Rastaldi MP, Hartleben G, Wiech T, Fornoni A, Nelson RG, Kretzler M, Wanke R, Pavenstädt H, Kerjaschki D, Cohen CD, Hall MN, Rüegg MA, Inoki K, Walz G, Huber TB. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J Clin Invest. 2011;121(6):2197–209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Inoki K, Mori H, Wang J, Suzuki T, Hong S, Yoshida S, Blattner SM, Ikenoue T, Rüegg MA, Hall MN, Kwiatkowski DJ, Rastaldi MP, Huber TB, Kretzler M, Holzman LB, Wiggins RC, Guan KL. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J Clin Invest. 2011;121(6):2181–96. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009;20(12):2493–502. doi: 10.1681/ASN.2008111186. [DOI] [PubMed] [Google Scholar]
- 76.Whaley-Connell AT, Habibi J, Nistala R, Demarco VG, Pulakat L, Hayden MR, Joginpally T, Ferrario CM, Parrish AR, Sowers JR. Mineralocorticoid Receptor-Dependent Proximal Tubule Injury Is Mediated by a Redox-Sensitive mTOR/S6K1 Pathway. Am J Nephrol. 2012;35(1):90–100. doi: 10.1159/000335079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hafizi S, Wang X, Chester AH, Yacoub MH, Proud CG. Ang II activates effectors of mTOR via PI3-K signaling in human coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004;287(3):H1232–H1238. doi: 10.1152/ajpheart.00040.2004. [DOI] [PubMed] [Google Scholar]
- 78.Yamakawa T, Tanaka S, Kamei J, Kadonosono K, Okuda K. Phosphatidylinositol 3-kinase in angiotensin II-induced hypertrophy of vascular smooth muscle cells. Eur J Pharmacol. 2003;478(1):39–46. doi: 10.1016/j.ejphar.2003.08.044. [DOI] [PubMed] [Google Scholar]
- 79.Chiu T, Santiskulvong C, Rozengurt E. GF receptor transactivation mediates ANG II-stimulated mitogenesis in intestinal epithelial cells through the PI3-kinase/Akt/mTOR/p70S6K1 signaling pathway. Am J Physiol Gastrointest Liver Physiol. 2005;288(2):G182–G194. doi: 10.1152/ajpgi.00200.2004. [DOI] [PubMed] [Google Scholar]
- 80.Chen JK, Chen J, Thomas G, Kozma SC, Harris RC. S6 kinase 1 knockout inhibits uninephrectomy- or diabetes-induced renal hypertrophy. Am J Physiol Renal Physiol. 2009;297(3):F585–F593. doi: 10.1152/ajprenal.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oroszlan M, Bieri M, Ligeti N, Farkas A, Meier B, Marti HP, Mohacsi P. Sirolimus and everolimus reduce albumin endocytosis in proximal tubule cells via an angiotensin II-dependent pathway. Transpl Immunol. 2010;23(3):125–32. doi: 10.1016/j.trim.2010.05.003. [DOI] [PubMed] [Google Scholar]