Abstract
Biofilms are complex microbial associations anchored to abiotic or biotic surfaces, embedded in extracellular matrix produced by the biofilms themselves where they interact with each other and the environment. One of the main properties of biofilms is their capacity to be more resistant to antimicrobial agents than planktonic cells. Efflux pumps have been reported as one of the mechanisms responsible for the antimicrobial resistance in biofilm structures. Evidence of the role of efflux pump in biofilm resistance has been found in several microorganisms such as Pseudomonas aeruginosa, Escherichia coli and Candida albicans. However, in spite of the studies on the importance of efflux pumps in biofilm growth and about their relevance in antimicrobial resistance forming biofilm, the exact role of these efflux systems has not been determined as yet.
Keywords: biofilm, antimicrobial resistance, efflux pumps, RND family, efflux pump inhibitors
What is a Biofilm?
Biofilms have been defined as complex microbial associations anchored to abiotic or biotic surfaces. This structure may be formed by a single or multiple microbial species. The cells are embedded in extracellular matrix produced by the biofilms themselves by which they interact with each other and the environment. However, a new definition of biofilm has been proposed taking other physiological attributes of the microorganisms forming biofilm into account. Therefore, biofilm is defined as a microbiologically derived sessile community characterized by cells that are irreversibly attached to a substratum or interface or each other, are embedded in a matrix of extracellular polymeric substances that they have produced and exhibit an altered phenotype with respect to growth rate and gene transcription.1 Biofilm formation has been observed by most of the bacteria found in natural, clinical and industrial settings. The matrix contains several substances such as polysaccharides, proteins and DNA from the microorganisms and this matrix provides structural stability to the biofilm.2 The biofilm structure provides protection to the cells against host-defense mechanisms, phagocytosis, biocides, hydrodynamic shear forces and antibiotic treatment.3,4 Biofilm is considered to be responsible for 65% of all bacterial infections.5
Biofilm formation is developed in three main stages (Fig. 1): (1) attachment, the cells arrive to the surface and adhere to this surface; (2) growth and maturation, they begin to produce the exopolysaccharide that constitutes the matrix and mature from microcolonies to multilayered cell clusters; (3) detachment, the cells take on a planktonic state and can thereby form biofilm in other settings.6 It has been proposed that detachment mechanisms can be divided into two categories: active and passive. Active detachment refers to mechanisms initiated by the bacteria themselves, such as enzymatic degradation of the biofilm matrix, quorum sensing, etc. On the other hand, passive detachment refers to that mediated by external forces such as fluid shear, abrasion and human intervention.7 It has also been proposed that the detachment process may be caused by bacteriophage activity within the biofilm.8
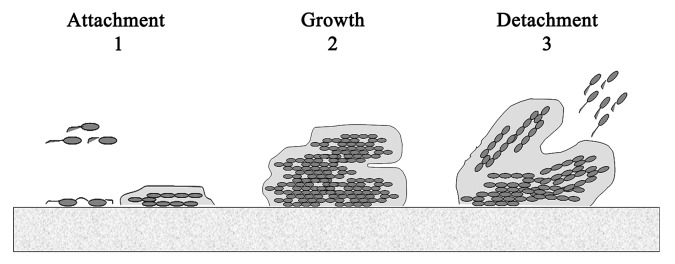
Figure 1. Steps in biofilm formation.
Mechanisms of Antimicrobial Resistance in Bacteria inside the Biofilm Structures
One of the main properties of bacteria in biofilms is their capacity to be more resistant to antimicrobial agents than planktonic cells. This feature makes it difficult to eradicate infections caused by biofilm forming bacteria, constituting a serious clinical problem.9 Biofilm structures show maximum resistance to antibiotics in the mature stage.2
Several mechanisms are reportedly responsible for the antimicrobial resistance in biofilm structures:
(1) poor diffusion of antibiotics through the biofilm polysaccharide matrix, although some antibiotics are able to penetrate the matrix;10
(2) physiological changes due to slow growth rate and starvation responses (oxygen, nutrient deprivation or environmental stress);11,12
(3) phenotypic change of the cells forming the biofilm;4
(4) quorum-sensing, although their exact role is not clear13;
(5) the expression of efflux pumps;14
(6) persister cells: small fractions of persistent bacteria that resist killing when exposed to antimicrobials. The persistent cells are not mutants.15
Drug efflux is a key mechanism of resistance in Gram-negative bacteria. These systems pump solutes out of the cell. Efflux pumps allow the microorganisms to regulate their internal environment by removing toxic substances, including antimicrobial agents, metabolites and quorum sensing signal molecules.16 Efflux pumps may be formed by a single-component or by multiple components, with the latter being found exclusively in Gram-negative bacteria.17
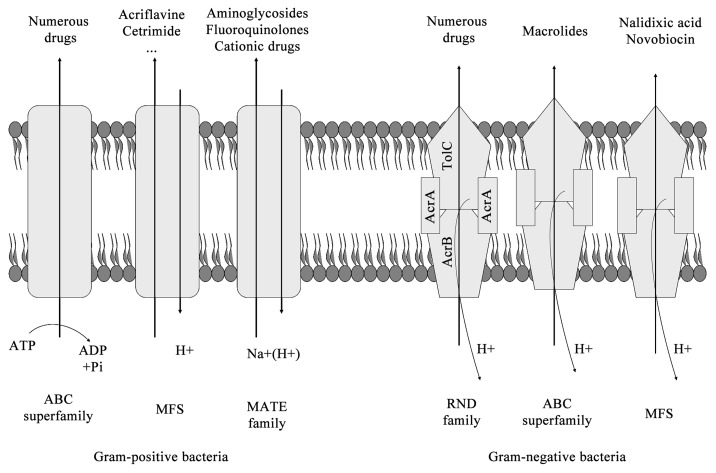
Bacterial drug efflux pumps have been classified into six families by the number of components, the number of transmembrane-spanning regions, the energy source used by the pump and the types of molecules that the pump exports18,19 (Fig. 2):
Figure 2. Efflux pump families.
(1) the ATP-binding cassette (ABC) superfamily;20
(2) the major facilitator superfamily (MFS);21
(3) the multidrug and toxic compound extrusion (MATE);22
(4) the small multidrug resistance (SMR) family;23
(5) the resistance-nodulation-division (RND) superfamily;24-26
(6) the drug metabolite transporter (DMT) superfamily.19
The major clinically relevant efflux systems in Gram-negative bacteria belong to the RND superfamily and are typically composed of a cytoplasmic membrane pump, a periplasmic protein and an outer membrane protein channel. Over the past several years, while further characterizing previously-studied drug efflux pumps including RND systems, novel efflux systems have also been identified in Gram-negative bacteria.27 In the case of Gram-positive bacteria, MFS (for example, Bmr and Blt in Bacillus subtilis and NorA in Staphylococcus aureus) and the ABC transporters are the most frequently efflux pumps systems found.27 For further review, see reference 27.
In spite of the studies related to the importance of efflux pumps in biofilm growth and their relevance in biofilm antimicrobial resistance, the exact role of these efflux systems has yet to be determined.
We present a review of the literature about the role of efflux pumps in biofilm resistance.
The Relationship between Quorum Sensing and Efflux Systems
Quorum sensing (QS), or cell-to-cell signaling, is the controlled expression of specific genes in response to extracellular chemical signals produced by bacteria themselves.16
It is well known that QS plays a role in the development of biofilm.28 The connection between QS and biofilm is known as sociomicrobiology.29
Efflux systems have been implicated in QS regulation, and it is well known that QS controls the expression of a number of virulence factors as well as biofilm differentiation.30 Chan et al.31 demonstrated that QS controlled processes such as biofilm formation were dependent on BpeAB-OprB efflux pump function in Burkholderia pseudomallei. In Pseudomonas aeruginosa, it has been observed that a mutation in a probable RND-like efflux pump transporter downregulates QS-dependent lecA::lux expression.32 Posterior studies have also demonstrated that QS is partly dependent upon efflux. Thus, the signal molecule 3OC12-HSL requires active transport through an efflux pump (through efflux pumps?) to diffuse across the cell membrane in P. aeruginosa.16 Therefore, an increase in efflux pump activity could have several effects on biofilm formation through an increase in the extrusion or intrusion of QS molecules.33
The Role of Efflux Pumps in Pseudomonas aeruginosa Biofilms
P. aeruginosa is one of the main causes of both nosocomial infections in immunocompromised patients and chronic infections in patients with cystic fibrosis.16
The intrinsic resistance of P. aeruginosa to numerous antibiotics is even more pronounced when this organism is found growing in a biofilm.5
Several studies have demonstrated that some antibiotics can diffuse through the biofilm exopolysaccharide matrix while showing a reduced rate of transfer.34,35
It is known that biofilms are constituted by metabolically active and inactive cell subpopulations. These subpopulations show different susceptibility phenotypes against antibiotics since many antibiotics require metabolically active cells to be effective.36 The slow growing cells of a biofilm therefore represent a resistant population.11 It has been observed that active subpopulations show sensitivity to ciprofloxacin, tetracycline and tobramycin, and inactive subpopulations are resistant to these antibiotics.36
Multidrug resistance (MDR) pumps play an important role in the antibiotic resistance of planktonic P. aeruginosa.37 This microorganism presents several putative MDR efflux pump encoding genes belonging to the RND family of bacterial transporters. Among these, MexAB-OprM, MexCD-OprJ, MexEF-OprN and MexXY have been the most widely studied.38-41
P. aeruginosa biofilms exhibit increased resistance to several antibiotics, such as tetracycline, chloramphenicol, quinolones, β-lactams and these resistance patterns are closely related to drug profiles that are actively effluxed by the MexAB-OprM pump. It has been demonstrated that MexAB-OprM plays a role in the resistance of aztreonam, gentamicin, tetracycline and tobramycin in biofilm structures. However, both MexCD-OprJ and MexEF-OprN do not seem to contribute to antibiotic resistance in biofilms.30
The expression of MexAB-OprM differs among the biofilm populations, being maximum in cells located in the substratum.30
Other studies have reported that MexAB-OprM and MexCD-OprJ are involved in biofilm resistance to the macrolide azithromycin.42
Zhang et al.43 described a novel efflux pump in P. aeruginosa named PA1874-1877, the expression of which was higher in biofilm than during planktonic growth. This pump appears to be involved in biofilm resistance to ciprofloxacin, gentamicin and tobramycin.
It has been demonstrated that colistin is able to kill the inactive subpopulation located deep in P. aeruginosa biofilm, whereas the active subpopulation located in the upper layer survives this compound. Studies using colistin as an antimicrobial agent have shown that the mexAB-oprM genes are expressed by the active subpopulation in P. aeruginosa biofilm under colistin exposure and these genes are required for the development of colistin tolerance.36
It has also been demonstrated that resistance to ofloxacin is dependent on the expression of the MexAB-OprM pump at a low range of ofloxacin concentrations.42
In addition, the mexCD-oprJ genes were also induced in the active subpopulation in P. aeruginosa biofilm under colistin exposure and these genes were required for tolerance of the active subpopulation in P. aeruginosa biofilm against colistin.44
The Role of Efflux Pumps in Escherichia coli Biofilms
E. coli is the Enterobacteriaceae most studied worldwide. It can be found as a comensal microorganism in the human gut but can also be found as a pathogen causing several infections such as urinary tract infections (UTIs), sepsis, etc. Several studies have reported that E. coli biofilms have higher antibiotic resistance than planktonic cells and that expression of several gene-encoded efflux pumps was increased in biofilm.45
The AcrAB-TolC system which belongs to the RND family is the best characterized efflux pump in E. coli and has been found to be overexpressed by clinical isolates.46 The substrates that this pump can export include chloramphenicol, fluoroquinolones, fusidic acid, rifampicin, tetracycline, ethidium bromide, bile salts, SDS, etc.47 The AcrAB-TolC efflux pump-encoded genes have been found to be upregulated under growth in biofilms and exposure to several antibiotics.47-49
In uropathogenic E. coli (UPEC), the putative multidrug-resistant pump YhaQ was reported to be involved in antibiotic resistance of bacteria in biofilm. The RapA regulatory protein appears to increase the transcription of the putative multidrug resistance pump gene yhcQ and evidence suggests that this protein also contributes to the biofilm-specific penicillin G resistance.50
Enteroaggregative E. coli (EAEC) is an emerging enteric pathogen in both developing and developed countries.51,52 The main characteristics of this pathogen are its capacity of aggregative adherence to Hep-2 cells in culture53 and to form biofilm in the intestinal mucosa.54
The protein TolC, that acts a channel in the transport of molecules across the outer membrane and in the export of many molecules such as antibiotics,55 is also required for adhesion and biofilm formation in EAEC. Mutants of the tolC gene showed a decrease in adhesion and biofilm formation, related to a reduction in aggregative fimbriae expression.56 This protein, together with the AcrAB proteins, form an efflux pump. The AcrAB-TolC system has been reported to be associated with bacterial colonization and persistence.19 Results from different studies are contradictory. Maira-Litran et al.57 observed that ciprofloxacin resistance in biofilms did not correlate with the expression of AcrAB or MarA in E. coli; however, other studies have shown that the expression of AcrAB only protects E. coli forming biofilm against low concentrations of ciprofloxacin.57
Most of the studies on the relationship between efflux pumps and biofilm formation in E. coli are related to their role in biofilm formation. It has been demonstrated that E. coli mutants lacking the emrD, emrE, emrK, acrD, acrE and mdtE efflux pump encoding genes show extremely lower biofilm formation than the original strain. These six genes belong to the genes encoding the proton motive force (PMF) pump. Therefore, PMF pumps are considered to have important roles in the biofilm formation of E. coli. Indeed, these pumps may contribute to biofilm formation via the export or import of some substances that are necessary for or harmful to biofilm formation.58
The Role of Efflux Pumps in Candida albicans Biofilms
Candida is a pleomorphic fungus that can exist either as a commensal or opportunistic pathogen with the capacity to cause a wide variety of infections.59 Candida biofilms share several characteristics with bacterial biofilms: (1) biofilm growth enhanced resistance to antimicrobial agents and (2) biofilm protects against host-defenses. Both characteristics are the main reasons why biofilm-associated infections are difficult to eradicate by conventional treatment.60
C. albicans possess two types of efflux-pumps: (1) adenosine triphosphate-binding cassette (ABC) transporters encoded by the CDR genes and (2) major facilitators encoded by the MDR genes.61,62 Genes encoding for both types of efflux pumps are upregulated during biofilm formation and development.63,64 The efflux pumps encoded by the CDR1, CDR2 and MDR1 genes are first employed as a means of cellular detoxification exporting toxic substances to the exterior of the cell.64
C. albicans biofilms are resistant to the antifungal agent fluconazole. It has been estimated that biofilms are up to 4,000 times more resistant to fluconazole in comparison with planktonic cells.65,66 Efflux pumps play an additive role in contributing to fluconazole resistance in early-phase biofilms. The expression of the genes encoding these efflux pumps is temporally regulated in C. albicans biofilms at different phases of development.67 Therefore, C. albicans biofilm resistance is a complex and multifactorial phenomenon and cannot be explained by one mechanism alone.
The Role of Efflux Pumps in Other Bacteria Biofilms (Salmonella Typhimurium, Staphylococcus aureus and Listeria monocytogenes)
Salmonella Typhimurium, together with Salmonella Enteritidis, are the two Salmonella serotypes most frequently isolated. These bacteria cause gastroenteritis, and only 1–4% of human infections are caused by invasive salmonellosis. As in the case of E. coli, the AcrAB-TolC system is the efflux pump most frequently studied in Salmonella Typhimurium, being similar to that of E. coli.68 Several studies have observed an increase of relative expression levels of the acrA and acrB genes in biofilm cells of this microorganism.69 Another study observed that the presence of the biocide triclosan upregulates the expression of acrAB pump genes and marA pump activator genes in Salmonella biofilm cells.70
Recently, the role of 9 multidrug-resistant efflux pumps of Salmonella has been demonstrated in biofilm formation. Mutants in these efflux pumps showed differences in curli production, which is an essential component of the Salmonella extracellular matrix of biofilm, and is, therefore, in biofilm.71
S. aureus is an important community- and major hospital-acquired pathogen.72 Six efflux pumps have been described as mechanisms of resistance in S. aureus. For instance, NorA, NorB and NorC are MDR pumps that confer resistance to quinolones and other antimicrobial agents; ant Tet38 is specific for tetracycline resistance.73 In relation to biofilm, one study reported finding a polymicrobial-biofilm-associated multidrug S. aureus isolate carrying a multidrug resistant(?) gene cluster including the macrolide efflux pump msrA.74
Listeria monocytogenes is a Gram-positive bacteria responsible for listeriosis outbreaks and causes severe infections in fetuses, newborns and immunocompromised individuals, with a mortality rate of about 28%.75 Biofilm has been suggested to be the main mechanism of persistence used by L. monocytogenes.76 An ABC-transporter, involved in negative regulation of biofilm formation by L. monocytogenes, has been identified (Lm.G_1771ABC). A mutant in one of the components of this ABC transporter causes a stronger increase in the capacity to form biofilm. This transporter may be an efflux protein exporting signaling molecules that activates a pattern of genome expression characteristic of planktonic growth of L. monocytogenes.75
The Effect of Efflux Pump Inhibitors on Biofilm
Efflux-pump inhibitors (EPIs) are substances that inhibit the flux of substances mediated by efflux pumps. These efflux pumps are considered as important drug targets for the development of combination strategies using antibiotic efflux inhibitors.77,78
EPIs are generally simple, robust and cheap chemicals which are well tolerated by humans.79 EPI compounds must show the following criteria:80
(1) they must enhance the activity of the pump substrates;
(2) they should not show activity in efflux pump mutants;
(3) they must increase the accumulation and decrease the extrusion of efflux pump substrates;
(4) they must not affect the proton gradient across the cytoplasmic membrane.
Inhibition of this efflux activity may be performed in different ways:81
(1) Alternating the regulatory steps in the expression of efflux pumps,
(2) Inhibiting the functional assembly of the multi-component pump,
(3) Blocking the outer membrane channel (TolC, OprM) with a plug,
(4) Collapsing the energy of efflux,
(5) Creating competitive or non-competitive inhibition with a nonantibiotic molecule to the affinity sites of the efflux pump,
(6) Changing the chemical design of previous antibiotics to reduce the affinity for efflux recognition and binding sites.
Some EPIs can also inhibit bacterial biofilm formation. Thus, thioridazine, Phe-Arg β-naphthylamide (PAβN) or the arylpiperazine NMP are some of the compounds categorized as efflux pump inhibitors. It has been observed that the addition of these compounds significantly reduced formation of biofilm in several bacteria, such as E. coli, Klebsiella pneumonia, S. aureus and P. putida.82 PAβN was the first EPI identified and, in combination with fluoroquinolones, showed inhibitory capacity against AcrAB-TolC, MexAB-OprM, MexCD-OprJ and MexEF-OprN pumps.78 PAβN and CCCP (a proton motive force uncouple) had great ability to repress biofilm formation by the inhibition of efflux pumps.71
EPIs from natural sources have also shown antibiofilm activity. Thus, the natural EPI caffeoylquinic acid (CQA) from Artemisa absinthium reduces biofilm viability in combination with subinhibitory concentrations of ethidium bromide and moxifloxacin in S. aureus and Enterococcus faecalis. This EPI acts by enhancing the killing effect of these compounds.83
Therefore, due to their characteristics, EPIs could be used as enhancers of the antibiotics used in the treatment of biofilm.82
In summary, there is currently insufficient knowledge as to the role of efflux pumps in biofilm resistance and, thus, further studies are needed to elucidate the role of these systems in bacterial biofilms. However, the existing studies show that efflux pumps could be an attractive target for antibiofilm drug development.
Acknowledgments
This work was supported by the project PI10/01579 of Fondo de Investigaciones Sanitarias from the Spanish Ministry of Health.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/23724
References
- 1.Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O. Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents. 2010;35:322–32. doi: 10.1016/j.ijantimicag.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Costerton JW. Introduction to biofilm. Int J Antimicrob Agents. 1999;11:217–21, discussion 237-9. doi: 10.1016/S0924-8579(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 4.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 5.Potera C. Forging a link between biofilms and disease. Science. 1999;283:1837–9, 1839. doi: 10.1126/science.283.5409.1837. [DOI] [PubMed] [Google Scholar]
- 6.Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan JB. Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic uses. J Dent Res. 2010;89:205–18. doi: 10.1177/0022034509359403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb JS, Thompson LS, James S, Charlton T, Tolker-Nielsen T, Koch B, et al. Cell death in Pseudomonas aeruginosa biofilm development. J Bacteriol. 2003;185:4585–92. doi: 10.1128/JB.185.15.4585-4592.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoyle BD, Costerton JW. Bacterial resistance to antibiotics: the role of biofilms. Prog Drug Res. 1991;37:91–105. doi: 10.1007/978-3-0348-7139-6_2. [DOI] [PubMed] [Google Scholar]
- 10.Anderl JN, Franklin MJ, Stewart PS. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob Agents Chemother. 2000;44:1818–24. doi: 10.1128/AAC.44.7.1818-1824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown MR, Allison DG, Gilbert P. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother. 1988;22:777–80. doi: 10.1093/jac/22.6.777. [DOI] [PubMed] [Google Scholar]
- 12.Walters MC, 3rd, Roe F, Bugnicourt A, Franklin MJ, Stewart PS. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother. 2003;47:317–23. doi: 10.1128/AAC.47.1.317-323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooun A, Liu S, Lewis K. A dose-response study of antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2000;44:640–6. doi: 10.1128/AAC.44.3.640-646.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert P, Allison DG, McBain AJ. Biofilms in vitro and in vivo: do singular mechanisms imply cross-resistance? J Appl Microbiol. 2002;92(Suppl):98S–110S. doi: 10.1046/j.1365-2672.92.5s1.5.x. [DOI] [PubMed] [Google Scholar]
- 15.Lewis K. Persister cells, dormancy and infectious disease. Nat Rev Microbiol. 2007;5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 16.Pearson JP, Van Delden C, Iglewski BH. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol. 1999;181:1203–10. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee A, Mao W, Warren MS, Mistry A, Hoshino K, Okumura R, et al. Interplay between efflux pumps may provide either additive or multiplicative effects on drug resistance. J Bacteriol. 2000;182:3142–50. doi: 10.1128/JB.182.11.3142-3150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piddock LJ. Multidrug-resistance efflux pumps - not just for resistance. Nat Rev Microbiol. 2006;4:629–36. doi: 10.1038/nrmicro1464. [DOI] [PubMed] [Google Scholar]
- 19.Piddock LJ. Clinically relevant chromosomally encoded multidrug resistance efflux pumps in bacteria. Clin Microbiol Rev. 2006;19:382–402. doi: 10.1128/CMR.19.2.382-402.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubelski J, Konings WN, Driessen AJ. Distribution and physiology of ABC-type transporters contributing to multidrug resistance in bacteria. Microbiol Mol Biol Rev. 2007;71:463–76. doi: 10.1128/MMBR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pao SS, Paulsen IT, Saier MH., Jr. Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuroda T, Tsuchiya T. Multidrug efflux transporters in the MATE family. Biochim Biophys Acta. 2009;1794:763–8. doi: 10.1016/j.bbapap.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Jack DL, Yang NM, Saier MH., Jr. The drug/metabolite transporter superfamily. Eur J Biochem. 2001;268:3620–39. doi: 10.1046/j.1432-1327.2001.02265.x. [DOI] [PubMed] [Google Scholar]
- 24.Li XZ, Nikaido H. Efflux-mediated drug resistance in bacteria. Drugs. 2004;64:159–204. doi: 10.2165/00003495-200464020-00004. [DOI] [PubMed] [Google Scholar]
- 25.Nikaido H, Takatsuka Y. Mechanisms of RNDmultidrug efflux pumps. Bioch Bioph Acta. 2009;1794:769–81. doi: 10.1016/j.bbapap.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeger MA, Diederichs K, Eicher T, Brandstätter L, Schiefner A, Verrey F, et al. The AcrB efflux pump: conformational cycling and peristalsis lead to multidrug resistance. Curr Drug Targets. 2008;9:729–49. doi: 10.2174/138945008785747789. [DOI] [PubMed] [Google Scholar]
- 27.Li XZ, Nikaido H. Efflux-mediated drug resistance in bacteria: an update. Drugs. 2009;69:1555–623. doi: 10.2165/11317030-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–8. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 29.Parsek MR, Greenberg EP. Sociomicrobiology: the connections between quorum sensing and biofilms. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 30.De Kievit TR, Parkins MD, Gillis RJ, Srikumar R, Ceri H, Poole K, et al. Multidrug efflux pumps: expression patterns and contribution to antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2001;45:1761–70. doi: 10.1128/AAC.45.6.1761-1770.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chan YY, Chua KL. The Burkholderia pseudomallei BpeAB-OprB efflux pump: expression and impact on quorum sensing and virulence. J Bacteriol. 2005;187:4707–19. doi: 10.1128/JB.187.14.4707-4719.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diggle SP, Winzer K, Lazdunski A, Williams P, Cámara M. Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J Bacteriol. 2002;184:2576–86. doi: 10.1128/JB.184.10.2576-2586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hocquet D, Berthelot P, Roussel-Delvallez M, Favre R, Jeannot K, Bajolet O, et al. Pseudomonas aeruginosa may accumulate drug resistance mechanisms without losing its ability to cause bloodstream infections. Antimicrob Agents Chemother. 2007;51:3531–6. doi: 10.1128/AAC.00503-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichols WW, Evans MJ, Slack MP, Walmsley HL. The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol. 1989;135:1291–303. doi: 10.1099/00221287-135-5-1291. [DOI] [PubMed] [Google Scholar]
- 35.Darouiche RO, Dhir A, Miller AJ, Landon GC, Raad II, Musher DM. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria. J Infect Dis. 1994;170:720–3. doi: 10.1093/infdis/170.3.720. [DOI] [PubMed] [Google Scholar]
- 36.Pamp SJ, Gjermansen M, Johansen HK, Tolker-Nielsen T. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes. Mol Microbiol. 2008;68:223–40. doi: 10.1111/j.1365-2958.2008.06152.x. [DOI] [PubMed] [Google Scholar]
- 37.Hancock RE. Resistance mechanisms in Pseudomonas aeruginosa and other nonfermentative gram-negative bacteria. Clin Infect Dis. 1998;27(Suppl 1):S93–9. doi: 10.1086/514909. [DOI] [PubMed] [Google Scholar]
- 38.Li XZ, Nikaido H, Poole K. Role of mexA-mexB-oprM in antibiotic efflux in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:1948–53. doi: 10.1128/AAC.39.9.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, et al. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–24. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 40.Köhler T, Michéa-Hamzehpour M, Henze U, Gotoh N, Curty LK, Pechère JC. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–54. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 41.Mine T, Morita Y, Kataoka A, Mizushima T, Tsuchiya T. Expression in Escherichia coli of a new multidrug efflux pump, MexXY, from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:415–7. doi: 10.1128/aac.43.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillis RJ, White KG, Choi KH, Wagner VE, Schweizer HP, Iglewski BH. Molecular basis of azithromycin-resistant Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 2005;49:3858–67. doi: 10.1128/AAC.49.9.3858-3867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Mah TF. Involvement of a novel efflux system in biofilm-specific resistance to antibiotics. J Bacteriol. 2008;190:4447–52. doi: 10.1128/JB.01655-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiang WC, Pamp SJ, Nilsson M, Givskov M, Tolker-Nielsen T. The metabolically active subpopulation in Pseudomonas aeruginosa biofilms survives exposure to membrane-targeting antimicrobials via distinct molecular mechanisms. FEMS Immunol Med Microbiol. 2012;65:245–56. doi: 10.1111/j.1574-695X.2012.00929.x. [DOI] [PubMed] [Google Scholar]
- 45.Ito A, Taniuchi A, May T, Kawata K, Okabe S. Increased antibiotic resistance of Escherichia coli in mature biofilms. Appl Environ Microbiol. 2009;75:4093–100. doi: 10.1128/AEM.02949-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webber MA, Piddock LJ. Absence of mutations in marRAB or soxRS in acrB-overexpressing fluoroquinolone-resistant clinical and veterinary isolates of Escherichia coli. Antimicrob Agents Chemother. 2001;45:1550–2. doi: 10.1128/AAC.45.5.1550-1552.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fernandes P, Ferreira BS, Cabral JM. Solvent tolerance in bacteria: role of efflux pumps and cross-resistance with antibiotics. Int J Antimicrob Agents. 2003;22:211–6. doi: 10.1016/S0924-8579(03)00209-7. [DOI] [PubMed] [Google Scholar]
- 48.Bailey AM, Webber MA, Piddock LJ. Medium plays a role in determining expression of acrB, marA, and soxS in Escherichia coli. Antimicrob Agents Chemother. 2006;50:1071–4. doi: 10.1128/AAC.50.3.1071-1074.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White DG, Maneewannakul K, von Hofe E, Zillman M, Eisenberg W, Field AK, et al. Inhibition of the multiple antibiotic resistance (mar) operon in Escherichia coli by antisense DNA analogs. Antimicrob Agents Chemother. 1997;41:2699–704. doi: 10.1128/aac.41.12.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lynch SV, Dixon L, Benoit MR, Brodie EL, Keyhan M, Hu P, et al. Role of the rapA gene in controlling antibiotic resistance of Escherichia coli biofilms. Antimicrob Agents Chemother. 2007;51:3650–8. doi: 10.1128/AAC.00601-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang DB, Nataro JP, DuPont HL, Kamat PP, Mhatre AD, Okhuysen PC, et al. Enteroaggregative Escherichia coli is a cause of acute diarrheal illness: a meta-analysis. Clin Infect Dis. 2006;43:556–63. doi: 10.1086/505869. [DOI] [PubMed] [Google Scholar]
- 52.Okeke IN, Nataro JP. Enteroaggregative Escherichia coli. Lancet Infect Dis. 2001;1:304–13. doi: 10.1016/S1473-3099(01)00144-X. [DOI] [PubMed] [Google Scholar]
- 53.Nataro JP, Kaper JB, Robins-Browne R, Prado V, Vial P, Levine MM. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J. 1987;6:829–31. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Wakimoto N, Nishi J, Sheikh J, Nataro JP, Sarantuya J, Iwashita M, et al. Quantitative biofilm assay using a microtiter plate to screen for enteroaggregative Escherichia coli. Am J Trop Med Hyg. 2004;71:687–90. [PubMed] [Google Scholar]
- 55.Koronakis V. TolC--the bacterial exit duct for proteins and drugs. FEBS Lett. 2003;555:66–71. doi: 10.1016/S0014-5793(03)01125-6. [DOI] [PubMed] [Google Scholar]
- 56.Imuta N, Nishi J, Tokuda K, Fujiyama R, Manago K, Iwashita M, et al. The Escherichia coli efflux pump TolC promotes aggregation of enteroaggregative E. coli 042. Infect Immun. 2008;76:1247–56. doi: 10.1128/IAI.00758-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maira-Litrán T, Allison DG, Gilbert P. Expression of the multiple antibiotic resistance operon (mar) during growth of Escherichia coli as a biofilm. J Appl Microbiol. 2000;88:243–7. doi: 10.1046/j.1365-2672.2000.00963.x. [DOI] [PubMed] [Google Scholar]
- 58.Matsumura K, Furukawa S, Ogihara H, Morinaga Y. Roles of multidrug efflux pumps on the biofilm formation of Escherichia coli K-12. Biocontrol Sci. 2011;16:69–72. doi: 10.4265/bio.16.69. [DOI] [PubMed] [Google Scholar]
- 59.Pfaller MA, Messer SA, Hollis RJ, Jones RN, Doern GV, Brandt ME, et al. In vitro susceptibilities of Candida bloodstream isolates to the new triazole antifungal agents BMS-207147, Sch 56592, and voriconazole. Antimicrob Agents Chemother. 1998;42:3242–4. doi: 10.1128/aac.42.12.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–94. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White TC, Holleman S, Dy F, Mirels LF, Stevens DA. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother. 2002;46:1704–13. doi: 10.1128/AAC.46.6.1704-1713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wirsching S, Moran GP, Sullivan DJ, Coleman DC, Morschhäuser J. MDR1-mediated drug resistance in Candida dubliniensis. Antimicrob Agents Chemother. 2001;45:3416–21. doi: 10.1128/AAC.45.12.3416-3421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, Douglas LJ, et al. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res. 2001;80:903–8. doi: 10.1177/00220345010800031101. [DOI] [PubMed] [Google Scholar]
- 64.Ramage G, Bachmann S, Patterson TF, Wickes BL, López-Ribot JL. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J Antimicrob Chemother. 2002;49:973–80. doi: 10.1093/jac/dkf049. [DOI] [PubMed] [Google Scholar]
- 65.Hawser SP, Douglas LJ. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother. 1995;39:2128–31. doi: 10.1128/AAC.39.9.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramage G, Wickes BL, Lopez-Ribot JL. Biofilms of Candida albicans and their associated resistance to antifungal agents. Am Clin Lab. 2001;20:42–4. [PubMed] [Google Scholar]
- 67.Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun. 2003;71:4333–40. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eaves DJ, Ricci V, Piddock LJ. Expression of acrB, acrF, acrD, marA, and soxS in Salmonella enterica serovar Typhimurium: role in multiple antibiotic resistance. Antimicrob Agents Chemother. 2004;48:1145–50. doi: 10.1128/AAC.48.4.1145-1150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zou Y, Woo J, Ahn J. Cellular and molecular responses of Salmonella Typhimurium to antimicrobial-induced stresses during the planktonic-to-biofilm transition. Lett Appl Microbiol. 2012;55:274–82. doi: 10.1111/j.1472-765X.2012.03288.x. [DOI] [PubMed] [Google Scholar]
- 70.Tabak M, Scher K, Hartog E, Romling U, Matthews KR, Chikindas ML, et al. Effect of triclosan on Salmonella typhimurium at different growth stages and in biofilms. FEMS Microbiol Lett. 2007;267:200–6. doi: 10.1111/j.1574-6968.2006.00547.x. [DOI] [PubMed] [Google Scholar]
- 71.Baugh S, Ekanayaka AS, Piddock LJ, Webber MA. Loss of or inhibition of all multidrug resistance efflux pumps of Salmonella enterica serovar Typhimurium results in impaired ability to form a biofilm. J Antimicrob Chemother. 2012;67:2409–17. doi: 10.1093/jac/dks228. [DOI] [PubMed] [Google Scholar]
- 72.Perl TM. The threat of vancomycin resistance. Am J Med. 1999;106(5A):26S–37S, discussion 48S-52S. doi: 10.1016/S0002-9343(98)00354-4. [DOI] [PubMed] [Google Scholar]
- 73.Ding Y, Onodera Y, Lee JC, Hooper DC. NorB, an efflux pump in Staphylococcus aureus strain MW2, contributes to bacterial fitness in abscesses. J Bacteriol. 2008;190:7123–9. doi: 10.1128/JB.00655-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weigel LM, Donlan RM, Shin DH, Jensen B, Clark NC, McDougal LK, et al. High-level vancomycin-resistant Staphylococcus aureus isolates associated with a polymicrobial biofilm. Antimicrob Agents Chemother. 2007;51:231–8. doi: 10.1128/AAC.00576-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu X, Long F, Chen Y, Knøchel S, She Q, Shi X. A putative ABC transporter is involved in negative regulation of biofilm formation by Listeria monocytogenes. Appl Environ Microbiol. 2008;74:7675–83. doi: 10.1128/AEM.01229-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frank JF, Koffi RA. Surface-adherent growth of Listeria monocytogenes is associated with increased resistance to surfactant sanitizers and heat. J Food Prot. 1990;53:550–4. doi: 10.4315/0362-028X-53.7.550. [DOI] [PubMed] [Google Scholar]
- 77.Mahamoud A, Chevalier J, Alibert-Franco S, Kern WV, Pagès JM. Antibiotic efflux pumps in Gram-negative bacteria: the inhibitor response strategy. J Antimicrob Chemother. 2007;59:1223–9. doi: 10.1093/jac/dkl493. [DOI] [PubMed] [Google Scholar]
- 78.Lomovskaya O, Watkins WJ. Efflux pumps: their role in antibacterial drug discovery. Curr Med Chem. 2001;8:1699–711. doi: 10.2174/0929867013371743. [DOI] [PubMed] [Google Scholar]
- 79.Marquez B. Bacterial efflux systems and efflux pumps inhibitors. Biochimie. 2005;87:1137–47. doi: 10.1016/j.biochi.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 80.Schweizer HP. Understanding efflux in Gram-negative bacteria: opportunities for drug discovery. Expert Opin Drug Discov. 2012;7:633–42. doi: 10.1517/17460441.2012.688949. [DOI] [PubMed] [Google Scholar]
- 81.Pagès JM, Amaral L. Mechanisms of drug efflux and strategies to combat them: challenging the efflux pump of Gram-negative bacteria. Biochim Biophys Acta. 2009;1794:826–33. doi: 10.1016/j.bbapap.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 82.Kvist M, Hancock V, Klemm P. Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl Environ Microbiol. 2008;74:7376–82. doi: 10.1128/AEM.01310-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fiamegos YC, Kastritis PL, Exarchou V, Han H, Bonvin AM, Vervoort J, et al. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS One. 2011;6:e18127. doi: 10.1371/journal.pone.0018127. [DOI] [PMC free article] [PubMed] [Google Scholar]