Abstract
Recent data suggest that ghrelin exerts its orexigenic action through regulation of hypothalamic AMP-activated protein kinase pathway, leading to a decline in malonyl-CoA levels and desinhibition of carnitine palmitoyltransferase 1A (CPT1A), which increases mitochondrial fatty acid oxidation and ultimately enhances the expression of the orexigenic neuropeptides agouti-related protein (AgRP) and neuropeptide Y (NPY). However, it is unclear whether the brain-specific isoform CPT1C, which is located in the endoplasmic reticulum of neurons, may play a role in this action. Here, we demonstrate that the orexigenic action of ghrelin is totally blunted in CPT1C knockout (KO) mice, despite having the canonical ghrelin signaling pathway activated. We also demonstrate that ghrelin elicits a marked upregulation of hypothalamic C18:0 ceramide levels mediated by CPT1C. Notably, central inhibition of ceramide synthesis with myriocin negated the orexigenic action of ghrelin and normalized the levels of AgRP and NPY, as well as their key transcription factors phosphorylated cAMP-response element–binding protein and forkhead box O1. Finally, central treatment with ceramide induced food intake and orexigenic neuropeptides expression in CPT1C KO mice. Overall, these data indicate that, in addition to formerly reported mechanisms, ghrelin also induces food intake through regulation of hypothalamic CPT1C and ceramide metabolism, a finding of potential importance for the understanding and treatment of obesity.
Ghrelin is a hormone produced by the stomach that induces food intake through the growth hormone segretagogue receptor 1a in the hypothalamus (1,2). Ghrelin and the proteins that are involved in the downstream signaling pathway are clear targets for the treatment of obesity and food intake disorders. Recently, much effort has been invested in studying the molecular mechanism by which ghrelin enhances the expression of the orexigenic neuropeptides agouti-related protein (AgRP) and neuropeptide Y (NPY) in the arcuate nucleus of the hypothalamus (ARC). It has been described that ghrelin binding to its receptor induces intracellular calcium release, which activates hypothalamic calmodulin-dependent protein kinase kinase 2 and the phosphorylation of the energy sensor AMP-activated protein kinase (AMPK) (3–5). It also has been described that ghrelin specifically triggers a hypothalamic Sirtuin1/p53 pathway that is essential for AMPK phosphorylation (6,7). One of the main effects of AMPK activation in the hypothalamus is the modulation of fatty acid metabolism; when activated, phosphorylated AMPK (pAMPK) further phosphorylates and inactivates acetyl-CoA carboxylase (ACC), causing a decrease in malonyl-CoA levels and the disinhibition of carnitine palmitoyltransferase 1 (CPT1) A enzyme (4,5). The overall outcome of that effect is increased fatty acid oxidation and accumulation of reactive oxygen species, which are mainly buffered by uncoupling protein 2 (UCP2) (5). All these metabolic changes ultimately activate transcriptional events in the cell nucleus by eliciting increased levels or activation of key transcription factors, such as cAMP-response element–binding protein (CREB) and its phosphorylated isoform (pCREB), forkhead box O1 (FoxO1) and its phosphorylated isoform, and brain-specific homeobox transcription factor. These are responsible, in part, for the increase of the orexigenic neuropeptides AgRP and NPY (8). The physiological relevance of hypothalamic AMPK signaling on the orexigenic effect of ghrelin stems from the finding that genetic or pharmacological inhibition of calmodulin-dependent protein kinase kinase 2, AMPK, CPT1A, or UCP2, as well as increased concentrations of malonyl-CoA levels in the hypothalamus block ghrelin-induced feeding (3–5). Nevertheless, despite that compelling evidence, the exact molecular mechanism through which changes in fatty acid metabolism modulate AgRP and NPY expression is not completely understood.
Carnitine palmitoyltransferase 1C (CPT1C) is a brain-specific CPT1 isoform that, quite opposite to mitochondrial CPT1A, localizes in the endoplasmic reticulum (ER) of neurons (9). CPT1C has very low CPT1 activity but has been demonstrated to bind malonyl-CoA (the physiological inhibitor of CPT1 enzymes) with a Kd within the dynamic range of hypothalamic malonyl-CoA concentration in fasted and refed states (10,11). Consequently, CPT1C has been proposed to be a sensor of malonyl-CoA levels in hypothalamic neurons (12,13). At the physiological level, it is well-established that hypothalamic CPT1C is involved in the control of energy homeostasis because CPT1C knockout (KO) mice show reduced food intake and impaired peripheral metabolism (11,14). However, the hypothalamic molecular pathway through which CPT1C regulates food intake remains unclear. We recently have demonstrated that overexpression of CPT1C in ARC blocks the anorectic effects of leptin through a mechanism involving increased hypothalamic ceramide levels (15). Considering that ceramide levels in the mediobasal hypothalamus (MBH) are increased in response to fasting (15), a state in which circulating ghrelin levels are elevated, we hypothesized that CPT1C might be involved in the hypothalamic ghrelin signaling pathway. Here, we demonstrate that CPT1C mediates a short-term increase in hypothalamic ceramide levels in response to ghrelin and, notably, that this effect is critical for the effects of ghrelin on AgRP and NPY expression, as well as on feeding.
RESEARCH DESIGN AND METHODS
Animal preparations.
All animal procedures were performed in accordance with the guidelines of European Community Directive 86/609/EEC (European Union directive 86/609, European Union decree 2001–486) and Standards for Use of Laboratory Animals A5388–01 (National Institutes of Health) and were approved by the Local Ethics Committee. We used adult (25–30 g) CPT1C KO male mice and their wild-type (WT) littermates. They were housed in a controlled (12-h light/12-h dark) environment. The animals were fed ad libitum with standard laboratory chow and water.
Cannulation surgery.
Mice were anesthetized by an intraperitoneal (IP) injection of ketamine/xylazine (ketamine 75 mg/kg body weight plus xylazine 10 mg/kg body weight). Brain infusion cannulae were stereotaxicaly placed in the lateral cerebral ventricle using the following coordinates: 0.58 mm posterior to bregma; 1 mm lateral to the midsagittal suture and to a depth of 2.2 mm; and with bregma and lambda at the same vertical dimension. Former studies of our group have demonstrated that ghrelin administration by using this route does not affect fatty acid metabolism in other brain areas apart from MBH, such as amygdala, striatum, habenula, fields CA1, CA2, and CA3 of the hippocampus, hippocampus dentate gyrus, motor cortex, pyriform cortex, sensory cortex, substantia nigra, and zona incerta (thalamus) (4). Animals were individually caged and allowed to recover for 1 week before experiments.
Intracerebroventricular and IP treatments and sample recollection.
For the ghrelin (Bachem, Bubendorf, Switzerland) experiments, mice received an intracerebroventricular (ICV) administration of 5 µg (dissolved in 2 µL of physiological serum) or an IP administration of 10 µg (dissolved in 20 µL of physiological serum) ghrelin. We have previously demonstrated that ICV ghrelin exerts a dose-dependent effect on food intake and hypothalamic fatty acid metabolism, with the dose of 5 µg being the one that results in a greater response (4). The dose of 10 µg ghrelin IP has been previously used in the literature to induce food intake and produces serum ghrelin levels in the range observed in fasted mice (16). For the myriocin (Sigma-Aldrich, St. Louis, MO) experiments, mice received an ICV administration of 4 μg myriocin (dissolved in 1:3 DMSO:saline). For the C6:0 ceramide (N-hexanoyl-d-sphingosine; Sigma-Aldrich) experiments, mice received an ICV administration of 2.5 µg C6:0 ceramide (dissolved in 1:3 DMSO:saline). Ghrelin and C6:0 ceramide were administered at the beginning of the light cycle when mice were satiated. When indicated, myriocin was administrated 1 h before ghrelin administration. Mice were killed by cervical dislocation and tissue was collected. The whole brain was used for in situ hybridization analysis, the hypothalami were used for Western blotting, and the MBH was used for real-time PCR analysis and ceramide measurements. To dissect the MBH, brains were placed in a coronal brain matrix (Roboz Surgical Instrument, Gaithersburg, MD) and were sectioned from bregma −1 mm to −2.5 mm. Then, a 1-mm-diameter tissue collector was used to obtain the MBH from each section.
Ceramide quantification.
Ceramides were extracted and analyzed via the LC-ESI-MS/MS System (API 3000 PE Sciex; Spectralab Scientific, Markham, Ontario, Canada) in positive ionization, as described previously (17). Their concentrations were measured by multiple reaction monitoring experiments using N-heptadecanoyl-d-erythro-sphingosine (C17 ceramide) as internal standard (50 ng⋅mL−1). The method was linear over the range from 2 to 600 ng⋅mL−1.
Western blotting.
Hypothalamic total protein lysates (30 µg) were subjected to SDS-PAGE, electrotransferred on a polyvinylidene fluoride membrane, and probed with the following antibodies: phosphorylated ACC (pACC)α-Ser79 1:1,000; pAMPKα1 1:1,000; BiP 1:1,000; FoxO1 1:1,000 (Cell Signaling, Danvers, MA); ATF-6β 1:1,000; CHOP 1:500; pCREB-Ser129 1:500; peIF2α 1:2,000; pIKKα/β 1:1,000; IKKβ 1:1,000; nuclear factor-κB 1:1,000; pPERK 1:500; TLR4 1:1,000 (Santa Cruz Biotechnology, Santa Cruz, CA); anti-β-actin 1:10,000; ATF4 1:1,000; and 0.2 µg/mL anti-β-tubulin III (Sigma-Aldrich). Values are expressed relative to β-actin or β-tubulin levels. The blots were developed using the ECL Western blotting system (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK).
Real-time quantitative PCR.
We performed real-time PCR (TaqMan; Applied Biosystems, Carlsbad, CA) as described (18) using primers designed by Applied Biosystems (AgRP Mm00475829_g1, NPY Mm00445771_m1, and glyceraldehyde 3-phosphate dehydrogenase 4352339E) or IDT Integrated Technologies (UCP2 63705740). Values were expressed in relation to glyceraldehyde 3-phosphate dehydrogenase levels.
In situ hybridization.
Coronal brain sections (16 µm) were probed with specific oligonucleotides for AgRP (5′-CGA CGC GGA GAA CGA GAC TCG CGG TTC TGT GGA TCT AGC ACC TCT GCC-3′) and NPY (5′-AGA TGA GAT GTG GGG GGA AAC TAG GAA AAG TCA GGA GAG CAA GTT TCA TT-3′) as previously published (4,18–21).
Statistical analysis.
We used 8–10 animals per group in all experiments except for real-time PCR analysis, for which we used six animals. Data are expressed as mean ± SEM in relation (%) to vehicle-treated mice. Statistical significance was determined by Student t test when two groups were compared and by ANOVA with post hoc two-tailed Bonferroni test when more than two groups were compared. P < 0.05 was considered significant.
RESULTS
Ghrelin administration did not increase either food intake or the expression of orexigenic neuropeptides in CPT1C KO mice.
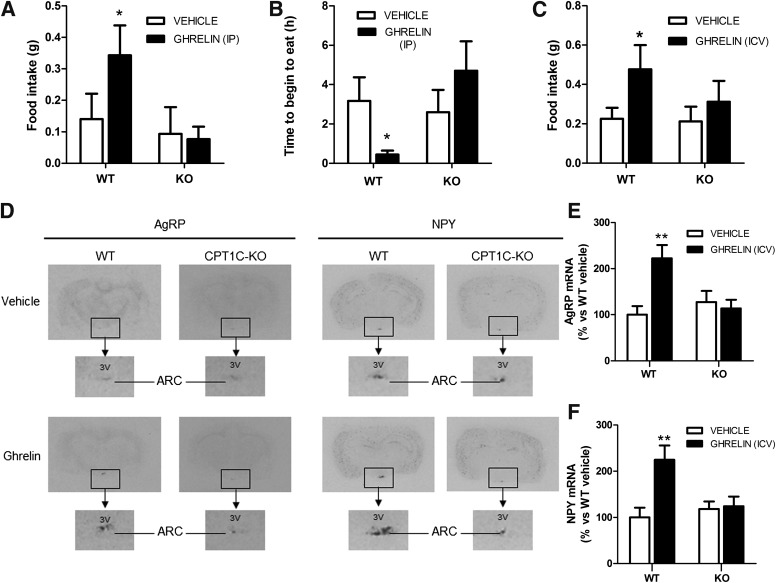
To establish whether CPT1C was part of the hypothalamic ghrelin signaling pathway, we analyzed the orexigenic effect of ghrelin in CPT1C KO mice. We injected ghrelin IP and analyzed food intake and the food-seeking behavior. We found that ghrelin injection to WT mice increased food intake two fold and highly reduced the time to begin eating. Both effects were completely blocked in CPT1C KO mice (Fig. 1A and B). Then, we repeated the experiment ICV injection of ghrelin and again found that CPT1C KO mice failed to respond to ghrelin treatment (Fig. 1C). Next, we analyzed the expression of the orexigenic neuropeptides AgRP and NPY by in situ hybridization. The ghrelin-induced increase in AgRP and NPY levels present in WT mice was completely blunted in CPT1C KO mice (Fig. 1D and E), which correlates with the lack of the orexigenic effect of ghrelin in those mice. These results indicate that CPT1C is involved in the ghrelin orexigenic effect.
FIG. 1.
Ghrelin does not induce orexigenic effects in CPT1C KO mice. A: The 2-h food intake in WT and CPT1C KO mice treated IP with vehicle (white bars) or with 10 μg ghrelin (black bars). B: Time to begin to eat after IP injection of vehicle (white bars) or 10 μg ghrelin (black bars). C: The 2-h food intake in WT and CPT1C KO mice treated with ICV vehicle (white bars) or with 5 μg ICV ghrelin (black bars). ARC mRNA levels of AgRP (D and E) and NPY (D and F) of WT and CPT1C KO mice treated with ICV vehicle (white bars) or 5 μg ghrelin (black bars). Samples were obtained 2 h after the treatment. *P < 0.05, **P < 0.01 vs. WT mice treated with vehicle. 3V, third ventricle.
The canonical ghrelin signaling pathway is impaired in CPT1C KO mice.
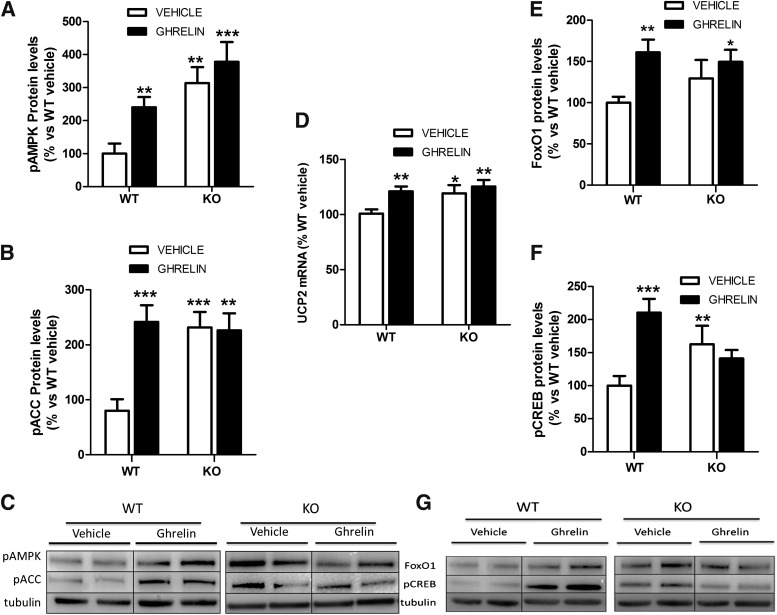
Next, we analyzed the ghrelin signaling pathway in hypothalamus from CPT1C KO mice. Our data showed that central ghrelin treatment induced a marked stimulatory effect on the levels of pAMPK, pACC, UCP2, and the transcription factors FoxO1 and pCREB in WT mice. Those effects were impaired in CPT1C KO mice (Fig. 2A–G). Basal levels of pAMPK and pACC, putative upstream factors of CPT1C, were significantly increased in CPT1C KO mice when compared with WT mice, suggesting that AMPK pathway is constitutively activated in the hypothalamus of CPT1C KO mice (Fig. 2A–C). Notably, in keeping with the altered levels of pAMPK and pACC, the expression of UCP2, a downstream factor of this pathway related to mitochondrial fatty acid oxidation, and the levels of the transcription factors FoxO1 and pCREB also were increased in the hypothalamus of CPT1C KO mice when compared with vehicle-injected WT mice (Fig. 2D–G). Therefore, these data suggest that even the canonical signaling pathway of ghrelin is activated in CPT1C KO mice. The lack of CPT1C blocks the ghrelin induction of orexigenic neuropeptides and food intake.
FIG. 2.
The ghrelin signaling pathway in WT and CPT1C KO mice. Hypothalamic protein levels of pAMPK (A and C) and pACC (B and C), MBH mRNA levels of UCP2 measured by real-time PCR (D), and the hypothalamic protein levels of FoxO1 (E and G) and pCREB (F and G) in WT and CPT1C KO mice after 2 h of ICV injection of vehicle (white bars) or 5 μg ICV injection of ghrelin (black bars). *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT mice treated with vehicle.
Central administration of ghrelin increased ceramide levels in hypothalamus of WT but not CPT1C KO mice.
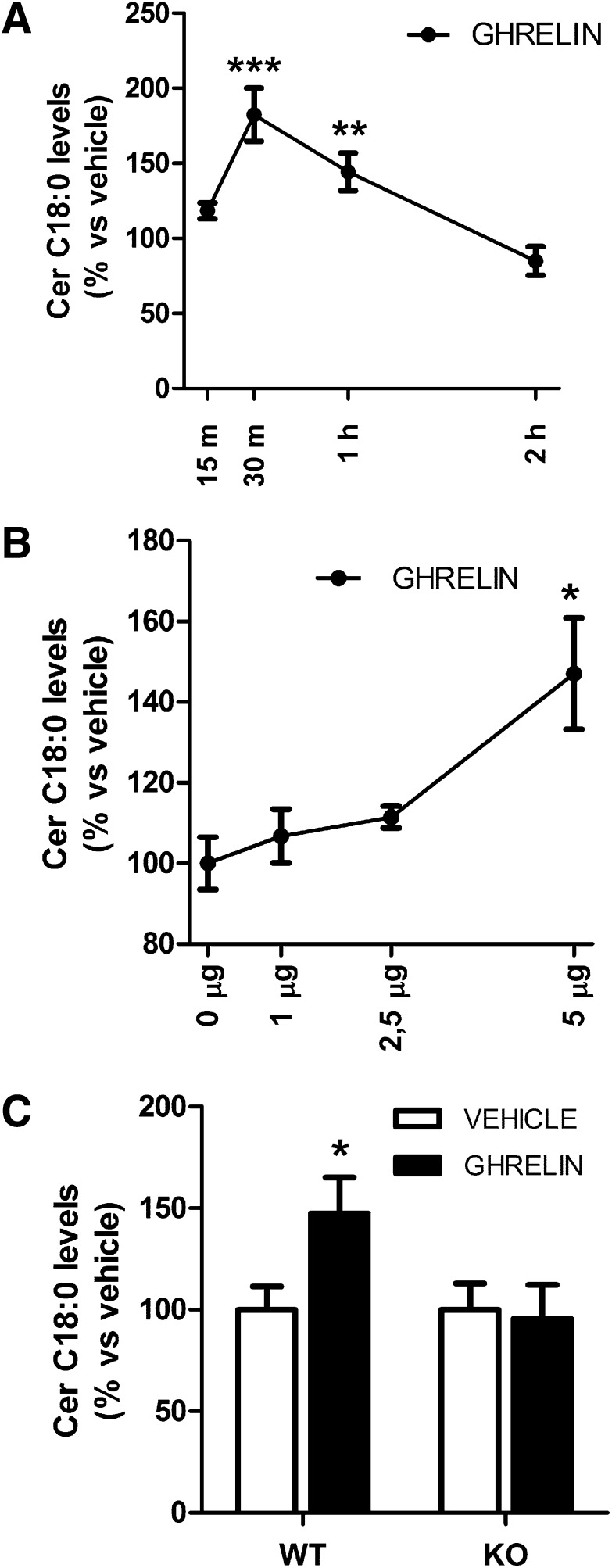
Current evidence from our group has identified CPT1C as a key regulator of ceramide levels in neurons (22). To investigate whether ghrelin had any impact on hypothalamic ceramide concentration, we measured the levels of C18:0 ceramide, the most abundant ceramide in brain and neurons (23), in WT mice at different times after ghrelin administration. Our data showed that central ghrelin promoted a marked stimulatory and transitory action in C18:0 ceramide levels in the MBH, evident from the maximal effect detected at 30 and 60 min after ghrelin injection (Fig. 3A). Then, we performed a dose-response curve and found that MBH ceramide levels at 30 min after ghrelin administration increased progressively with the dose of ghrelin, with the increase statistically significant at the dose of 5 μg (Fig. 3B). Next, we analyzed what happened in CPT1C KO mice. Opposite to WT mice, 5 μg ghrelin failed to induce any effect in the C18:0 ceramide levels of CPT1C KO mice 30 min after its administration (Fig. 3C), indicating a requirement of CPT1C for the stimulatory effect of ghrelin on ceramide content in the MBH.
FIG. 3.
MBH ceramide levels in response to ghrelin. A: Time course of MBH C18:0 ceramide levels in WT mice after ghrelin administration (ICV, 5 μg). Percentage of respective increase in vehicle-treated mice is represented. B: MBH C18:0 ceramide levels in WT after 30 min of ICV administration of different doses of ghrelin. C: MBH C18:0 ceramide levels of WT and CPT1C KO mice after 30 min of ICV administration of vehicle (white bars) or 5 μg ICV administration of ghrelin (black bars). *P < 0.05, **P < 0.01, ***P < 0.001 vs. WT vehicle. Cer, ceramide.
Inhibition of hypothalamic ceramide synthesis blocked the orexigenic effect of ghrelin.
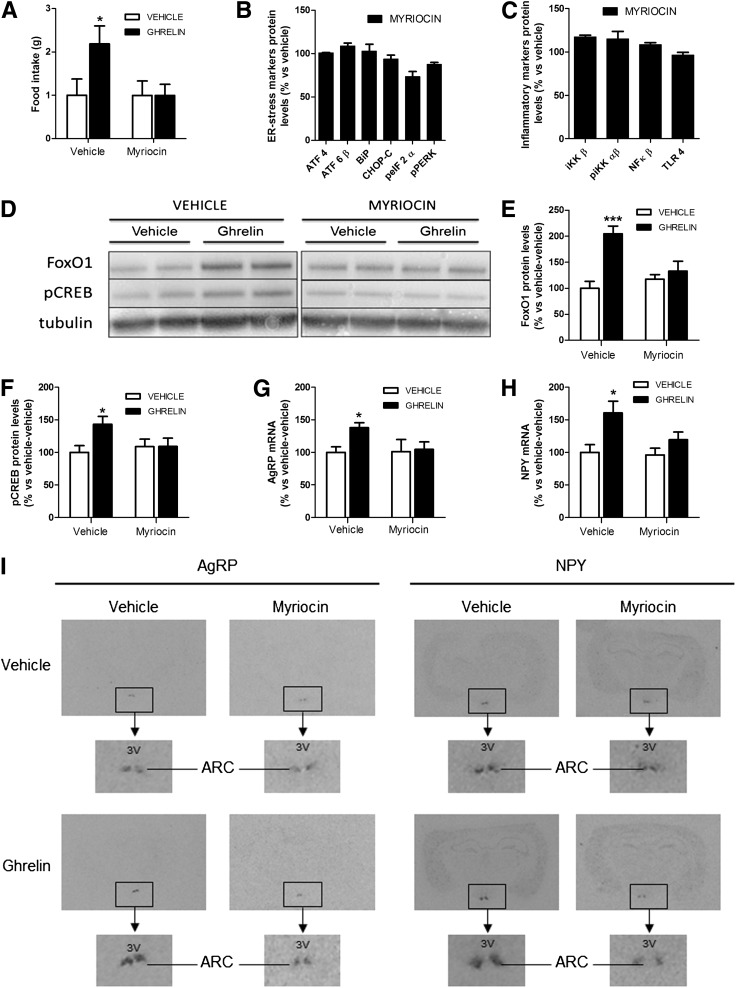
To investigate the existence of any mechanistic link between the orexigenic effect of ghrelin and the activation of ceramide synthesis, we investigated the effects of 4 μg myriocin, a specific inhibitor of the de novo synthesis of ceramide (24), on ghrelin action. The selected dose of myriocin induced neither an anorectic effect per se at any evaluated time (Fig. 4A and data not shown) nor illness or malaise (data not shown), nor hypothalamic inflammation (measured as protein levels), nor ER stress (measured as protein levels), an indirect indicator of inflammation (Fig. 4B and C). Although this dose of myriocin was subeffective when injected alone, our data showed that ICV injection of myriocin 1 h before ghrelin administration decreased the orexigenic effect of ghrelin (Fig. 4A) and its stimulatory effect on transcription factors FoxO1 and pCREB and on neuropeptides AgRP and NPY (Fig. 4D–I). These results indicate that ceramide synthesis also is a required component of the ghrelin hypothalamic signaling pathway.
FIG. 4.
Myriocin injection blocks the orexigenic effect of ghrelin. WT mice were pretreated with ICV vehicle or 4 μg ICV myriocin 1 h before ICV administration of vehicle (white bars) or 5 μg ICV administration of ghrelin (black bars). A: Food intake after 2 h of ghrelin injection. ICV injection of myriocin does not cause ER stress (B) or inflammation (C) in hypothalamus. Hypothalamic ER stress and inflammatory markers were measured by Western blot. ATF4, activating transcription factor 4; ATF6β, activating transcription factor 6β; BiP, ER chaperone-binding immunoglobulin protein, also known as glucose-regulated protein 78 kDa, GRP78; CHOP-C, C/EBP homologous protein C; pEIF2α, phosphorylated eukaryotic initiator factor 2α; pPERK, phosphorylated RNA–dependent protein kinase-like ER kinase; IKKβ, IκB kinase β; pIKK αβ, phosphorylated IκB kinase αβ; NFκB, nuclear factor-κB; TLR4, Toll-like receptor 4. Hypothalamic protein levels (D–F) of FoxO1 and pCREB measured by Western blot. ARC mRNA levels (G–I) of AgRP and NPY measured by in situ hybridization. *P < 0.05, ***P < 0.001 vs. vehicle-vehicle–treated mice. 3V, third ventricle.
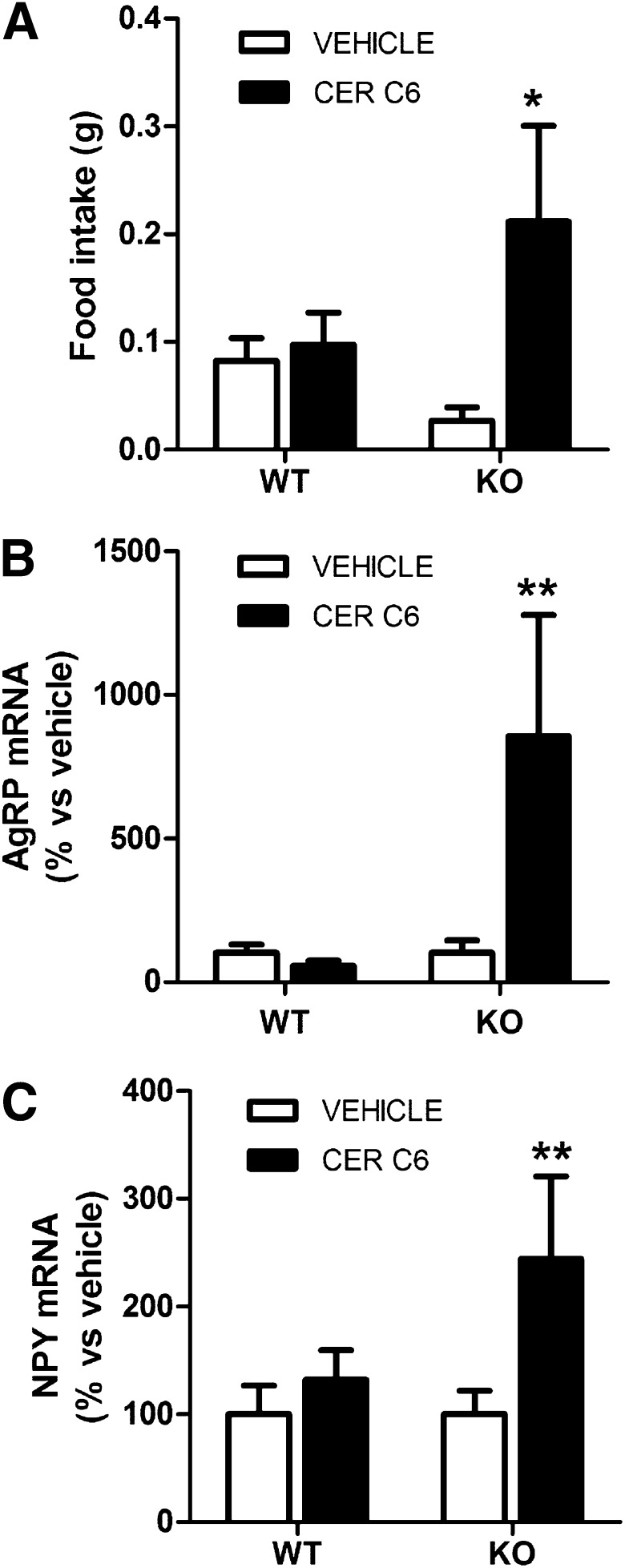
Ceramide administration increases food intake in CPT1C KO mice.
Bearing in mind that CPT1C has been involved in ceramide synthesis, we next investigated whether ceramide injection had any orexigenic effect in CPT1C KO mice; we used C6:0-ceramide, a cell-penetrating ceramide that is converted to long chain ceramides inside the cell (25). We used the submaximal dose of 2.5 μg, which has been reported to block the satiating effects of leptin but lacks of orexigenic effect per se in WT animals (15). Of note, 2.5 μg C6:0 ceramide via ICV injection produced MBH C18:0 ceramide levels in the range observed in ghrelin-treated mice (ICV ceramide: 0.53 ± 0.10 ng C18:0 ceramide/mg protein; ICV ghrelin: 0.41 ± 0.04 ng C18:0 ceramide/mg protein); in both cases, hypothalamic ceramide levels were significantly higher than those found in controls (vehicle-treated). Ceramide, which was injected at the beginning of the light cycle when animals were satiated, increased food intake and the expression of AgRP and NPY in CPT1C KO mice but, as expected, had no effect in WT mice (Fig. 5). These results indicate that ceramide is able to rescue feeding patterns when the canonical ghrelin signaling pathway (pAMPK/pACC/CPT1A/UCP2) is previously activated, as it happens in CPT1C KO mice (Fig. 2). By contrast, in fed WT mice, which have the ghrelin canonical pathway not activated, ceramide alone is unable to induce orexigenic neuropeptides expression and food intake. These results argue for two parallel signaling pathways for ghrelin, with the involvement of CPT1C and ceramide in one of the branches (Fig. 6). We propose that ghrelin must activate two parallel pathways, the mitochondrial pathway (with the activation of CPT1A and fatty acid oxidation) and the ER pathway (with the activation of CPT1C and ceramide synthesis), for its orexigenic effect to be effective.
FIG. 5.
Ceramide induces food intake in CPT1C KO mice. WT and CPT1C KO mice were treated with ICV vehicle (white bars) or 2.5 μg ICV ceramide C6:0 (black bars) at the beginning of the light cycle. Food intake (A) was measured 3 h after the injection. AgRP (B) and NPY (C) mRNA levels in MBH were measured by real-time PCR in samples obtained 3 h after the treatment. *P < 0.05, **P < 0.01 vs. vehicle-treated mice. CER, ceramide.
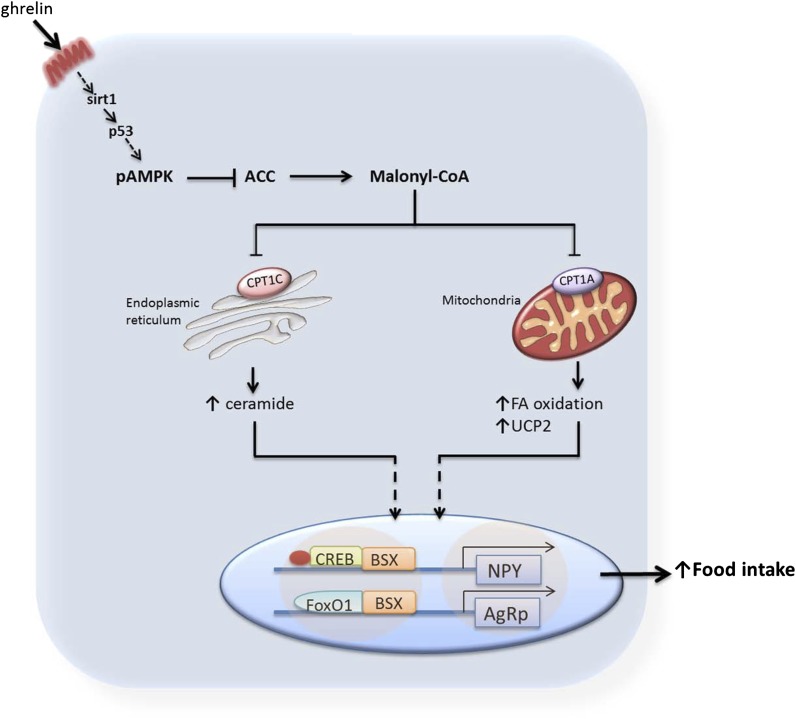
FIG. 6.
Schematic diagram showing the involvement of CPT1C and ceramides in the hypothalamic ghrelin signaling pathway. Ghrelin stimulates the hypothalamic SIRT1/p53/AMPK axis, leading to decreased levels of hypothalamic malonyl-CoA, the physiological inhibitor of CPT1 enzymes. In the “classical” mechanism of ghrelin action, this effect promotes disinhibition of CPT1A, increased fatty acid (FA) oxidation, and altered reactive oxygen species levels. These metabolic changes ultimately activate the nuclear transcription machinery (pCREB, FoxO1, and brain-specific homeobox transcription factor [BSX]), increasing mRNA expression of Agrp and Npy genes. Here, we demonstrate the existence of a parallel downstream pathway involving CPT1C, a specific brain isoform located in the ER, which triggers a short-term increase in ceramide synthesis after ghrelin treatment. This new pathway is of physiological importance because the orexigenic action of ghrelin is totally blunted in CPT1C KO mice or in mice treated with an inhibitor of ceramide synthesis. The fact that central ceramide treatment induces food intake and triggers orexigenic neuropeptides expression in CPT1C KO mice, which have the canonical ghrelin signaling pathway activated during satiating conditions, but not in WT mice, indicates that both branches need to be activated for ghrelin to exert its orexigenic effect.
DISCUSSION
In this study we demonstrate that the orexigenic effect of ghrelin is coupled to an ability of ghrelin to regulate hypothalamic CPT1C and ceramide synthesis, and that this action is required for the subsequent increase in levels of AgRP and NPY mRNA expression in the ARC via modulation of the transcription factors pCREB and FoxO1. Recent data have demonstrated that the orexigenic effect of ghrelin is mediated by the selective modulation of hypothalamic SIRT1/p53/AMPK and fatty acid metabolism pathways, as well as UCP2 levels, which culminate in increased AgRP and NPY expression in the ARC (4–7). Although it is clear that the modulation of hypothalamic fatty acid metabolism is a bona fide component of ghrelin signaling, it is unclear whether complex species might be involved in that action. In fact, this is a major constraint in our current knowledge about hypothalamic lipids and energy balance, which is restricted to a small corner of lipid pathways, namely de novo fatty acid synthesis (regulated by AMPK, ACC, fatty acid synthase, and malonyl-CoA decarboxylase) and fatty acid oxidation (regulated by CPT1A).
Current evidence from our group has implicated hypothalamic ceramides and CPT1C in the actions of leptin on food intake (15). Quite opposite to CPT1A, which is located in the mitochondria, CPT1C resides in the ER of neurons (9). At the cellular level, CPT1C is involved in ceramide metabolism, which is demonstrated by the increased ceramide levels detected after CPT1C overexpression in vitro and the reduced ceramide concentration detected in neurons from CPT1C KO mice (22). Furthermore, our recent data also show that genetic (adenoviral-driven) overexpression of CPT1C in the ARC increases feeding through a mechanism involving increased ceramide levels and that this effect antagonizes the anorectic actions of leptin at central level (15). However, so far no evidence has linked the orexigenic effect of ghrelin to alterations in ceramide metabolism or hypothalamic CPT1C function. Thus, all the evidence led us to investigate the possible involvement of hypothalamic CPT1C and ceramides on the action of ghrelin.
Here, we demonstrate that central ghrelin administration promotes a marked short-term increase in the MBH C18:0 ceramide concentration mediated by CPT1C, and that this increase in ceramide levels is necessary to induce hyperphagia and AgRP and NPY expression. In fact, inhibition of hypothalamic ceramide synthesis with myriocin negated the orexigenic action of ghrelin treatment and normalized AgRP and NPY expression in the ARC. Having shown that central inhibition of ceramide synthesis blocked the orexigenic action of ghrelin, we aimed to investigate whether ceramide treatment induced food intake in animals fed ad libitum. Our data show that central injection of ceramide increased the levels of AgRP and NPY and induced food intake in CPT1C KO mice, which had the ghrelin canonical pathway constitutively activated. Quite opposite, ceramide had no effect in fed WT mice, indicating that ceramide is necessary but not sufficient to induce food intake. Altogether, these results indicate that, besides the canonical SIRT1/p53/AMPK/ACC/CPT1A/UCP2 pathway, ghrelin-induced food intake is mediated by specific modulation of CPT1C and ceramide concentration in the MBH (Fig. 6). Therefore, the reduction of hypothalamic malonyl-CoA levels after ghrelin treatment (4) would activate two parallel routes, fatty acid oxidation–mediated by CPT1A and ceramide synthesis mediated by CPT1C, and both routes require triggering for ghrelin to exert its orexigenic effects. As described previously, blocking fatty acid oxidation by inhibition of CPT1A (4) or by deletion of UCP2 (5) blunts the orexigenic effects of ghrelin. The evidence presented here demonstrates that the deletion of CPT1C or the inhibition of ceramide synthesis itself also blunts food intake after ghrelin administration, indicating that both parallel routes are required for an appropriate orexigenic response of ghrelin. Considering that ghrelin and leptin are conceptualized as the "yin and yang" in the hypothalamic regulation of feeding (26,27), our data about ghrelin and ceramide are in agreement with those of our previous report showing that the anorectic action of leptin is associated with decreased ceramide concentration and CPT1C function in the hypothalamus (15).
The cellular implications of our findings are multiple. First, this is the first evidence linking the effect of an orexigenic hormone, such as ghrelin, with a molecular mechanism involving the normal function of the ER, i.e., ceramide synthesis. In this regard, ceramides and their derivate sphingomyelin are one of the major lipids in plasmatic membranes of neurons, traditionally having been considered as structural lipids. However, current data are challenging that view, demonstrating that ceramides can act as signaling molecules in a bulk of processes, such as differentiation, proliferation, apoptosis, and neuronal plasticity, and can regulate the function of various kinases, phosphatases, deacetylases, and others (28). In this sense, ceramides could regulate the expression of orexigenic neuropeptides AgRP and NPY by modulating the activity of the transcription factors involved. An alternative hypothesis might be a mechanism involving hypothalamic ER stress. Ceramides are one of the most reactive lipid species at the peripheral level, and impaired ceramide content in pancreatic β-cells and hepatocytes induces lipotoxicity and subsequently ER stress (29–31). Taking into account that hypothalamic ER stress also has been recently proposed as a central mechanism modulating energy homeostasis and particularly leptin resistance (32–35), it would be reasonable to hypothesize that CPT1C, ceramide-induced lipotoxicity, and ER stress might play a role in the effects of ghrelin at the hypothalamic level.
In summary, our study shows that CPT1C and ceramides are part of a new hypothalamic mechanism mediating the action of ghrelin on feeding through increased Agrp and Npy gene expression. Our data also describe activation of hypothalamic CPT1C and ceramides as mediators of food intake, which is of potential importance for the understanding and treatment of obesity.
ACKNOWLEDGMENTS
Funding was provided by the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement 281854, the ObERStress project (to M.L.), and 245009, the Neurofast project (to C.D. and M.L.), Spanish Ministerio de Economia y Competitividad (SAF2011-30520-C02-01 (to D.S.); BFU2011-29102 (to C.D.); SAF2011-30520-C02-02 (to N.C.), Xunta de Galicia (10PXIB208164PR and 2012-CP070 [to M.L.]); and Fondo de Investigaciones Sanitarias (Instituto de Salud Carlos III; PI12/01814 [to M.L.]).
L.M. is a recipient of a fellowship from Fundação para a Ciência e Tecnologia, Portugal (SFRH/BD/65379/2009). M.P. is a recipient of a fellowship from Agència de Gestió d’Ajuts Universitaris i de la Recerca in Catalunya. CIBER de Fisiopatología de la Obesidad y Nutrición is an initiative of Instituto de Salud Carlos III.
No potential conflicts of interest relevant to this article were reported.
S.R. and L.M. performed collection and assembly of data, data analysis, and interpretation. J.J. performed collection and assembly of data. P.C. performed collection and assembly of data, data analysis, and interpretation. M.P. performed collection of data. J.C. performed data interpretation. D.S. and F.G.H. performed data interpretation and acquired financial support. C.D. performed data interpretation. M.L. and N.C. were responsible for conception and design, data analysis and interpretation, and wrote the manuscript. N.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors emphatically thank Dr. Rubén Nogueiras (University of Santiago de Compostela, Spain), Dr. Andrew J. Whittle (University of Cambridge, UK), and Dr. Silje Skrede (University of Bergen, Norway) for comments and advice.
Footnotes
S.R. and L.M. contributed equally to this work.
REFERENCES
- 1.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656–660 [DOI] [PubMed] [Google Scholar]
- 2.Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000;407:908–913 [DOI] [PubMed] [Google Scholar]
- 3.Anderson KA, Ribar TJ, Lin F, et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab 2008;7:377–388 [DOI] [PubMed] [Google Scholar]
- 4.López M, Lage R, Saha AK, et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab 2008;7:389–399 [DOI] [PubMed] [Google Scholar]
- 5.Andrews ZB, Liu ZW, Walllingford N, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature 2008;454:846–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dietrich MO, Antunes C, Geliang G, et al. Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci 2010;30:11815–11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velásquez DA, Martínez G, Romero A, et al. The central Sirtuin 1/p53 pathway is essential for the orexigenic action of ghrelin. Diabetes 2011;60:1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakkou M, Wiedmer P, Anlag K, et al. A role for brain-specific homeobox factor Bsx in the control of hyperphagia and locomotory behavior. Cell Metab 2007;5:450–463 [DOI] [PubMed] [Google Scholar]
- 9.Sierra AY, Gratacós E, Carrasco P, et al. CPT1c is localized in endoplasmic reticulum of neurons and has carnitine palmitoyltransferase activity. J Biol Chem 2008;283:6878–6885 [DOI] [PubMed] [Google Scholar]
- 10.Price N, van der Leij F, Jackson V, et al. A novel brain-expressed protein related to carnitine palmitoyltransferase I. Genomics 2002;80:433–442 [DOI] [PubMed] [Google Scholar]
- 11.Wolfgang MJ, Kurama T, Dai Y, et al. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proc Natl Acad Sci USA 2006;103:7282–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfgang MJ, Lane MD. The role of hypothalamic malonyl-CoA in energy homeostasis. J Biol Chem 2006;281:37265–37269 [DOI] [PubMed] [Google Scholar]
- 13.Wolfgang MJ, Lane MD. Hypothalamic malonyl-CoA and CPT1c in the treatment of obesity. FEBS J 2011;278:552–558 [DOI] [PubMed] [Google Scholar]
- 14.Gao XF, Chen W, Kong XP, et al. Enhanced susceptibility of Cpt1c knockout mice to glucose intolerance induced by a high-fat diet involves elevated hepatic gluconeogenesis and decreased skeletal muscle glucose uptake. Diabetologia 2009;52:912–920 [DOI] [PubMed] [Google Scholar]
- 15.Gao S, Zhu G, Gao X, et al. Important roles of brain-specific carnitine palmitoyltransferase and ceramide metabolism in leptin hypothalamic control of feeding. Proc Natl Acad Sci USA 2011;108:9691–9696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci USA 2004;101:4679–4684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merrill AH, Jr, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: high-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods 2005;36:207–224 [DOI] [PubMed] [Google Scholar]
- 18.López M, Lelliott CJ, Tovar S, et al. Tamoxifen-induced anorexia is associated with fatty acid synthase inhibition in the ventromedial nucleus of the hypothalamus and accumulation of malonyl-CoA. Diabetes 2006;55:1327–1336 [DOI] [PubMed] [Google Scholar]
- 19.López M, Varela L, Vázquez MJ, et al. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med 2010;16:1001–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whittle AJ, Carobbio S, Martins L, et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 2012;149:871–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martínez de Morentin PB, Whittle AJ, Fernø J, et al. Nicotine induces negative energy balance through hypothalamic AMP-activated protein kinase. Diabetes 2012;61:807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrasco P, Sahún I, McDonald J, et al. Ceramide levels regulated by carnitine palmitoyltransferase 1C control dendritic spine maturation and cognition. J Biol Chem 2012;287:21224–21232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-David O, Futerman AH. The role of the ceramide acyl chain length in neurodegeneration: involvement of ceramide synthases. Neuromolecular Med 2010;12:341–350 [DOI] [PubMed] [Google Scholar]
- 24.Hanada K, Nishijima M, Fujita T, Kobayashi S. Specificity of inhibitors of serine palmitoyltransferase (SPT), a key enzyme in sphingolipid biosynthesis, in intact cells. A novel evaluation system using an SPT-defective mammalian cell mutant. Biochem Pharmacol 2000;59:1211–1216 [DOI] [PubMed] [Google Scholar]
- 25.Mitoma J, Ito M, Furuya S, Hirabayashi Y. Bipotential roles of ceramide in the growth of hippocampal neurons: promotion of cell survival and dendritic outgrowth in dose- and developmental stage-dependent manners. J Neurosci Res 1998;51:712–722 [DOI] [PubMed] [Google Scholar]
- 26.Zigman JM, Elmquist JK. Minireview: From anorexia to obesity—the yin and yang of body weight control. Endocrinology 2003;144:3749–3756 [DOI] [PubMed] [Google Scholar]
- 27.Nogueiras R, Tschöp M. Biomedicine. Separation of conjoined hormones yields appetite rivals. Science 2005;310:985–986 [DOI] [PubMed] [Google Scholar]
- 28.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol 2008;9:139–150 [DOI] [PubMed] [Google Scholar]
- 29.Lelliott C, Vidal-Puig AJ. Lipotoxicity, an imbalance between lipogenesis de novo and fatty acid oxidation. Int J Obes Relat Metab Disord 2004;28(Suppl. 4):S22–S28 [DOI] [PubMed] [Google Scholar]
- 30.Virtue S, Vidal-Puig A. It’s not how fat you are, it’s what you do with it that counts. PLoS Biol 2008;6:e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez de Morentin PB, Varela L, Fernø J, Nogueiras R, Diéguez C, López M. Hypothalamic lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 2010;1801:350–361 [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008;135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hosoi T, Sasaki M, Miyahara T, et al. Endoplasmic reticulum stress induces leptin resistance. Mol Pharmacol 2008;74:1610–1619 [DOI] [PubMed] [Google Scholar]
- 34.Ozcan L, Ergin AS, Lu A, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab 2009;9:35–51 [DOI] [PubMed] [Google Scholar]
- 35.Won JC, Jang PG, Namkoong C, et al. Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity (Silver Spring) 2009;17:1861–1865 [DOI] [PubMed] [Google Scholar]